Dissipation of Pre-Harvest Pesticides on ‘Clementine’ Mandarins after Open Field Application, and Their Persistence When Stored under Conventional Postharvest Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
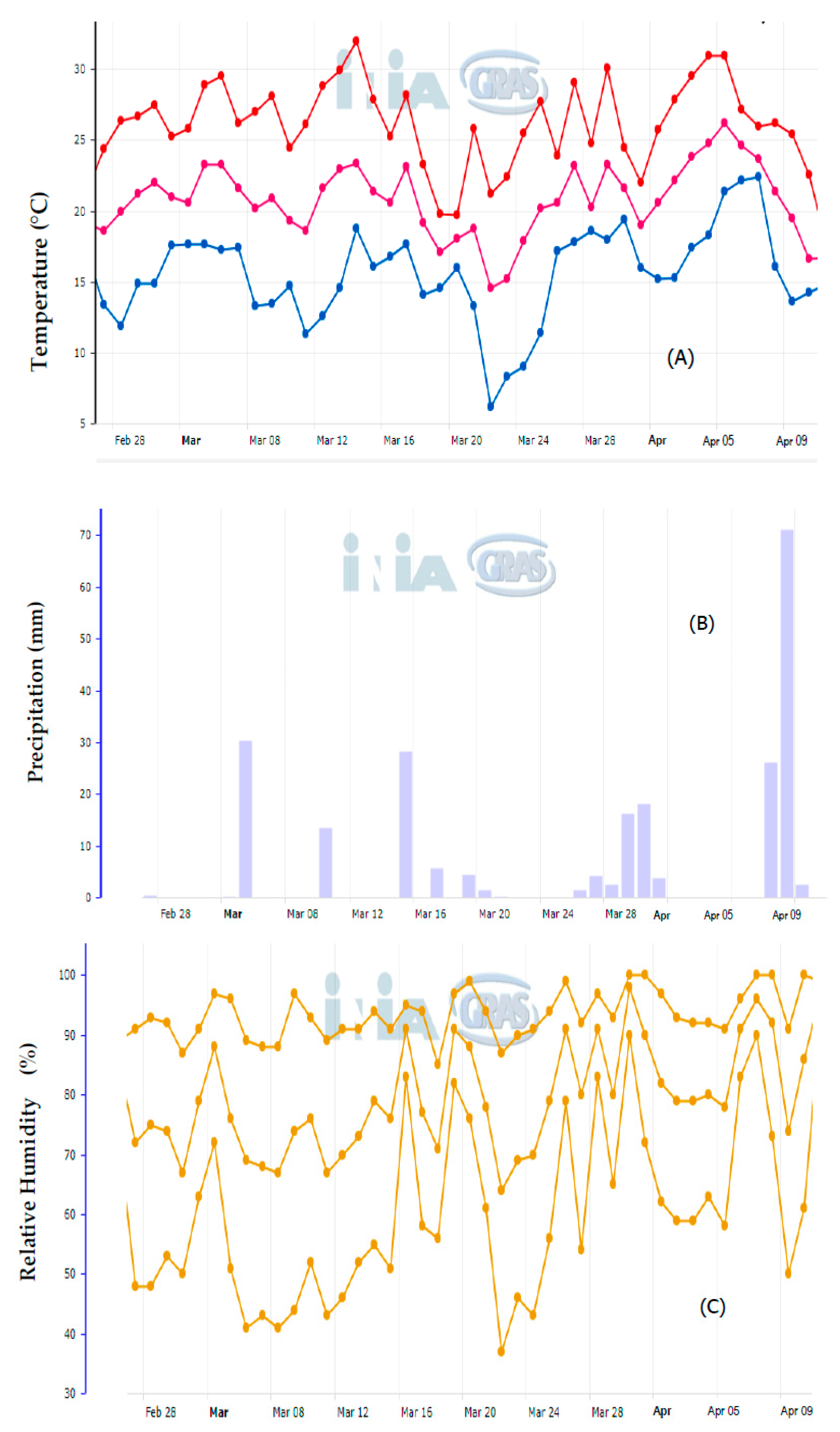
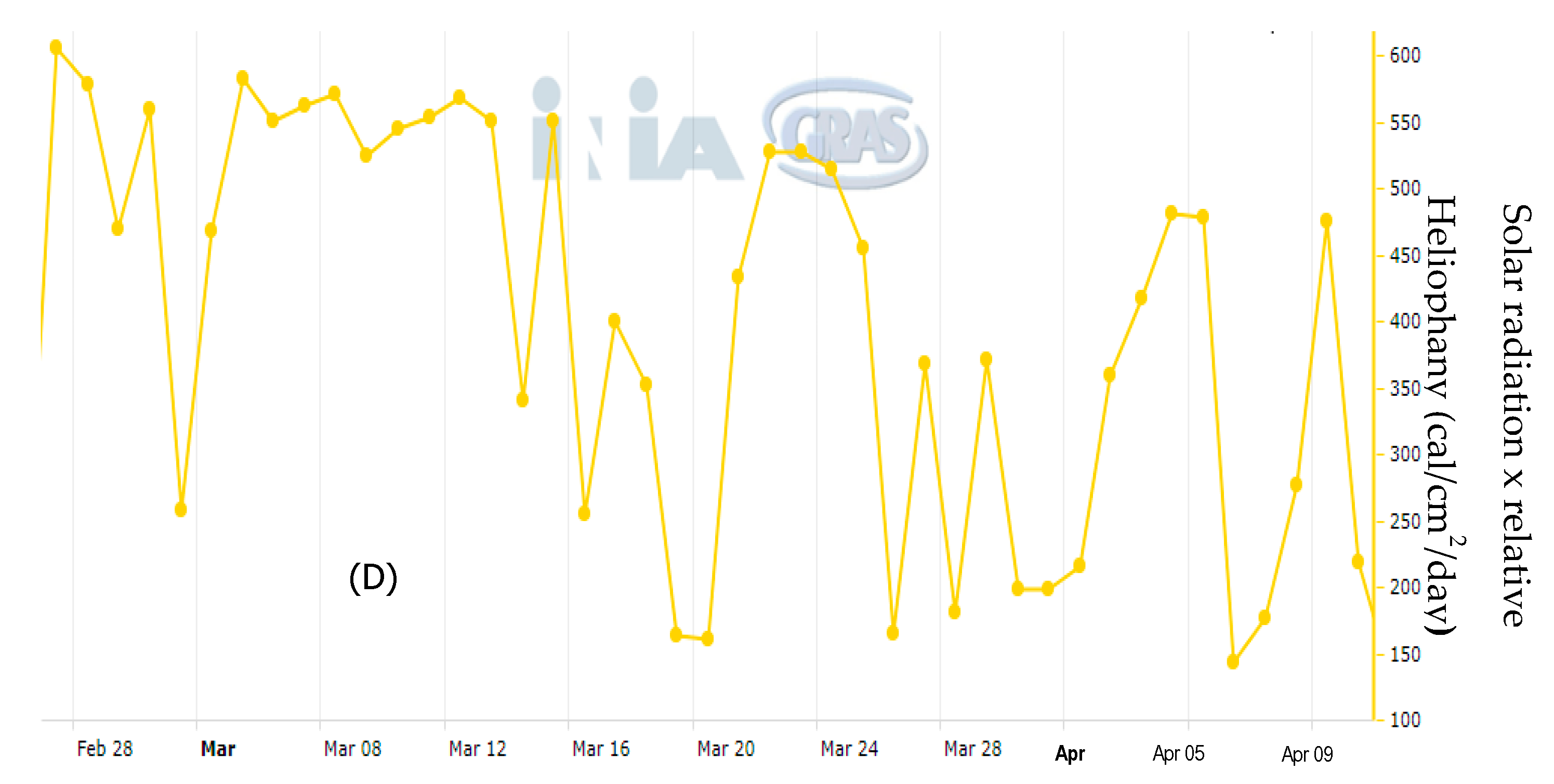
2.2. Field and Postharvest Experiment
2.3. Fruit Sampling
2.4. LC-MS/MS Operating Conditions
2.5. Residue Analysis and Method Validation
2.6. Dissipation Models
2.7. Data Management and Statistical Analysis
3. Results and Discussion
3.1. Method Performance
3.2. Dissipation Studied under Field Conditions
Best Fitting Model
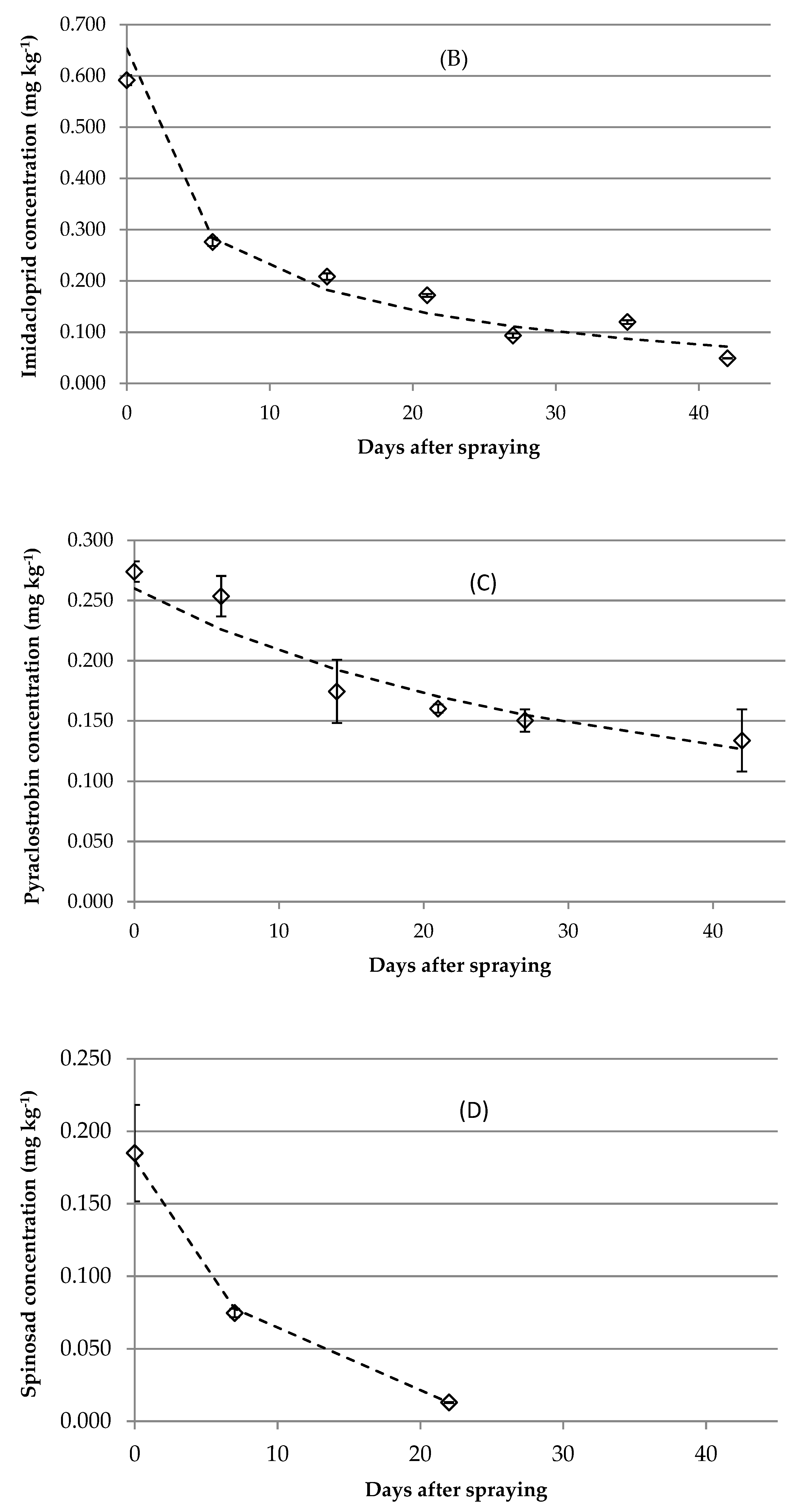
3.3. Initial Residues, Dissipation Dynamics and Postharvest Storage
3.3.1. Difenoconazole
3.3.2. Imidacloprid
3.3.3. Pyraclostrobin
3.3.4. Spinosad
3.4. Half-Lives
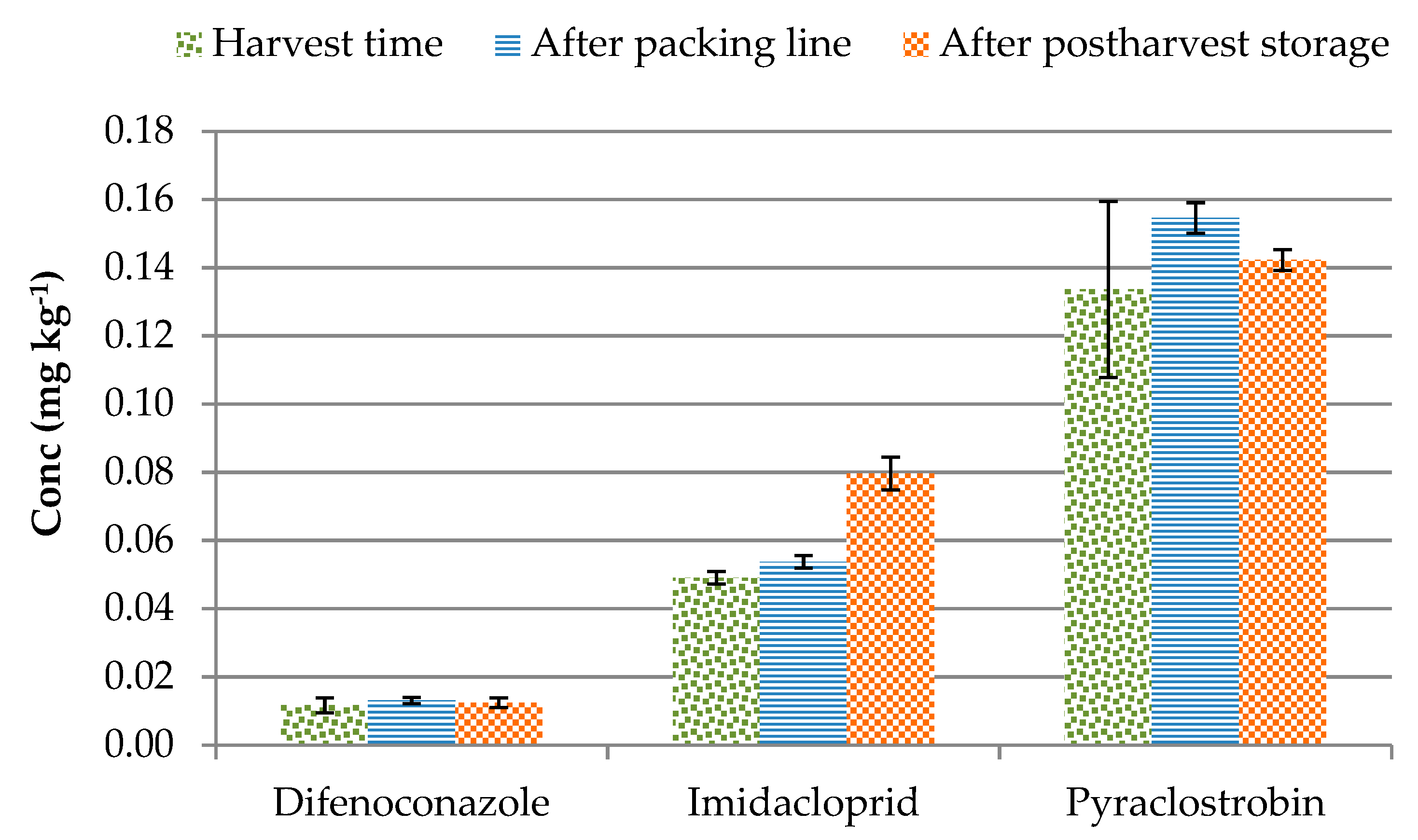
3.5. Estimating Storage Effect on Pesticide Level
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef] [Green Version]
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 6 September 2018).
- DIEA. Anuario Estadístico Agropecuario, 20th ed.; MGAP: Montevideo, Uruguay, 2017. [Google Scholar]
- Shinde, S.; Neharkar, P.; Dhurve, N.; Sawai, H.; Lavhe, N.; Masolkar, D. Evaluation of different insecticides against citrus leaf miner on Nagpur mandarin. J. Entomol. Zool. Stud. 2017, 5, 1889–1892. [Google Scholar]
- Rodríguez, V.A.; Avanza, M.M.; Mazza, S.M.; Giménez, L.I. Pyraclostrobin effect to control of citrus black spot. Summa Phytopathol. 2010, 36, 334–337. [Google Scholar] [CrossRef]
- Timmer, L.W.; Peever, T.; Solel, Z.; Akimitsu, K. Alternaria Diseases of Citrus—Novel Pathosystems. Phytopathol. Mediter. 2003, 42, 99–112. [Google Scholar]
- Altieri, G.; Di Renzo, G.C.; Genovese, F.; Calandra, M.; Strano, M.C. A new method for the postharvest application of imazalil fungicide to citrus fruit. Biosyst. Eng. 2013, 115, 434–443. [Google Scholar] [CrossRef]
- Lado, J.; Manzi, M.; Silva, G.; Luque, E.; Blanco, O.; Pérez, E. Effective alternatives for the postharvest control of imazalil resistant Penicillium digitatum strains. Acta Hortic. 2010, 877, 1449–1456. [Google Scholar] [CrossRef]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Farha, W.; Abd El-Aty, A.M.; Rahman, M.M.; Shin, H.C.; Shim, J.H. An overview on common aspects influencing the dissipation pattern of pesticides: A review. Environ. Monit. Assess. 2016, 188. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Oliva, J.; Navarro, G.; Barba, A. Dissipation of chlorpyrifos, fenarimol, mancozeb, metalaxyl, penconazole, and vinclozolin in grapes. Am. J. Enol. Vitic. 2001, 52, 35–40. [Google Scholar]
- Karmakar, R.; Kulshrestha, G. Persistence, metabolism and safety evaluation of thiamethoxam in tomato crop. Pest Manag. Sci. 2009, 65, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; El-Sheikh, M.A.; Alfarhan, A.H.; Barceló, D. Target vs non-target analysis to determine pesticide residues in fruits from Saudi Arabia and influence in potential risk associated with exposure. Food Chem. Toxicol. 2018, 111, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tao, L.; McLean, J.; Lu, C. Quantitative Analysis of Neonicotinoid Insecticide Residues in Foods: Implication for Dietary Exposures. J. Agric. Food Chem. 2014, 62, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Jacobo, A.; Alcantar-Rosales, V.M.; Alonso-Segura, D.; Heras-Ramírez, M.; Elizarragaz-De La Rosa, D.; Lugo-Melchor, O.; Gaspar-Ramirez, O. Pesticide residues in orange fruit from citrus orchards in Nuevo Leon State, Mexico. Food Addit. Contam. Part B 2017, 10, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ortelli, D.; Patrick, E.; Corvi, C. Pesticide residues survey in citrus fruits. Food Addit. Contam. 2005, 22, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating Half-Lives for Pesticide Dissipation from Plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantke, P.; Juraske, R. Variability of pesticide dissipation half-lives in plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; Bond, C.; Buhl, K.; Stone, D. Pesticide Half-life Fact Sheet. Available online: http://npic.orst.edu/factsheets/half-life.html (accessed on 24 August 2018).
- Lewis, K.; Tzilivakis, J. Development of a data set of pesticide dissipation rates in/on various plant matrices for the Pesticide Properties Database (PPDB). Data 2017, 2, 28. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, L.; Zhao, H.; Wang, K.; Zhang, H. Dissipation dynamic and residue distribution of flusilazole in mandarin. Environ. Monit. Assess. 2013, 185, 9169–9176. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiao, B.; Su, X.; Zhao, Q.; Sun, D. Dissipation and residue of 2,4-d in citrus under field condition. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Wang, S.; Yu, Y.; Lu, P.; Hu, D.; Yang, Z. Dissipation, residues and risk assessment of spirotetramat and its four metabolites in citrus and soil under field conditions by LC-MS/MS. Biomed. Chromatogr. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhu, Y.; Pang, J.; Zhou, Z.; Jiao, B. Residue level, persistence and safety of spirodiclofen-pyridaben mixture in citrus fruits. Food Chem. 2016, 194, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Payá, P.; Oliva, J.; Cámara, M.A.; Barba, A. Dissipation of insect growth regulators in fresh orange and orange juice. Commun. Agric. Appl. Biol. Sci. 2007, 72, 161–169. [Google Scholar] [PubMed]
- Waks, J.; Schiffmann-Nadel, M.; Lomaniec, E.; Chalutz, E. Relation between fruit waxing and development of rots in citrus fruit during storage. Plant Dis. 1985, 69, 869–870. [Google Scholar] [CrossRef]
- Vicent, A.; Armengol, J.; García-Jiménez, J. Rain fastness and persistence of fungicides for control of alternaria brown spot of citrus. Plant Dis. 2007, 91, 393–399. [Google Scholar] [CrossRef]
- Miles, A.K.; Willingham, S.L.; Cooke, A.W. Field evaluation of strobilurins and a plant activator for the control of citrus black spot. Aust. Plant Pathol. 2004, 33, 371–378. [Google Scholar] [CrossRef]
- Dong, F.; Li, J.; Chankvetadze, B.; Cheng, Y.; Xu, J.; Liu, X.; Li, Y.; Chen, X.; Bertucci, C.; Tedesco, D.; et al. Chiral Triazole Fungicide Difenoconazole: Absolute Stereochemistry, Stereoselective Bioactivity, Aquatic Toxicity, and Environmental Behavior in Vegetables and Soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health Part B 2007, 42, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Miles, M. The effects of spinosad, a naturally derived insect control agent to the honeybee. Bull. Insectol. 2003, 56, 119–124. [Google Scholar]
- West, S.D.; Turner, L.G. Determination of spinosad and its metabolites in citrus crops and orange processed commodities by HPLC with UV detection. J. Agric. Food Chem. 2000, 48, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Phartiyal, T.; Srivastava, R.M. Dissipation of imidacloprid on lemon fruit rind under tarai agro-climatic condition of Uttarakhand. J. Entomol. Res. 2014, 38, 285–288. [Google Scholar]
- Singh, N.; Bisht, S.; Yadav, G.; Kamari, B. Dissipation of imidacloprid on sweet orange fruits. Int. J. Chem. Stud. 2017, 5, 683–686. [Google Scholar]
- Saunt, J. Citrus Varieties of the World; Sinclair Intl Business Resources: Norwich, UK, 2000. [Google Scholar]
- Codex. Recommended Methods of Sampling for the Determination of Pesticide Residues for Compliance with MRLs CAC/GL 33-199; Codex: New Delhi, India, 1999. [Google Scholar]
- Besil, N.; Pareja, L.; Colazzo, M.; Niell, S.; Rodríguez, A.; Cesio, V.; Heinzen, H. GC-MS method for the determination of 44 pesticides in mandarins and blueberries. In Proceedings of the 3rd Latin American Pesticide Residue Workshop, Food and Environment, Montevideo, Uruguay, 8–11 May 2011; p. 156. [Google Scholar]
- Besil, N.; Pérez-Parada, A.; Cesio, V.; Varela, P.; Rivas, F.; Heinzen, H. Degradation of imazalil, orthophenylphenol and pyrimethanil in Clementine mandarins under conventional postharvest industrial conditions at 4 °C. Food Chem. 2016, 194, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Timme, G.; Frehse, H.; Laska, V. Statistical interpretation and graphic representation of the degradation behavior of pesticide residues II. Pflanzenschutz-Nachrichten Bayer 1986, 39, 187–203. [Google Scholar]
- Timme, G.; Frehse, H. Statistical interpretation and graphic representation of the degradation behaviour of pesticide residues. 1. Pflanzenschutz-Nachrichten Bayer 1980, 33, 47–60. [Google Scholar]
- European. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; Document SANTE/11945/2015; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Besil, N.; Cesio, V.; Heinzen, H.; Fernandez-Alba, A.R. Matrix Effects and Interferences of Different Citrus Fruit Coextractives in Pesticide Residue Analysis Using Ultrahigh-Performance Liquid Chromatography-High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 4819–4829. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Modification of the existing MRLs for pyraclostrobin in various crops. EFSA J. 2011, 9, 2120. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Zhang, J.; Rashid, M.; Zhong, G.; Liu, J. The control effect of fungicide pyraclostrobin against freckle disease of banana and its residue dynamics under field conditions. J. Environ. Sci. Health Part B 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cang, T.; Qi, P.; Zhao, X.; Xu, H.; Wang, X.; Zhang, H.; Wang, X. Dissipation of four fungicides on greenhouse strawberries and an assessment of their risks. Food Control 2015, 55, 215–220. [Google Scholar] [CrossRef]
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of six fungicides in greenhouse-grown tomatoes with processing and health risk. Environ. Sci. Pollut. Res. 2016, 23, 11885–11900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adak, T.; Mukherjee, I. Investigating Role of Abiotic Factors on Spinosad Dissipation. Bull. Environ. Contam. Toxicol. 2016, 96, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.E.; Fantke, P.; Trapp, S. Analysing half-lives for pesticide dissipation in plants. SAR QSAR Environ. Res. 2015, 26, 325–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sun, H.; Wang, S. Dissipation and residue of spinosad in zucchini under field conditions. Bull. Environ. Contam. Toxicol. 2013, 91, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.; Shawir, M.; El-Bakary, A.; Abdelgaleil, S. Dissipation of four insecticides in tomato fruit using high performance liquid chromatography and QuECHERS methodology. Chilean J. Agric. Res. 2016, 76, 129–133. [Google Scholar] [CrossRef]
- Singh, S.; Battu, R.S. Dissipation kinetics of spinosad in cabbage (Brassica oleracea L.var. capitata). Toxicol. Environ. Chem. 2012, 94, 319–326. [Google Scholar] [CrossRef]


| Commercial Formulation/Manufacturer | Active (g L−1) | Vol (cc) | EU MRL (mg kg−1) | Codex MRL (mg kg−1) |
|---|---|---|---|---|
| SCORE 250 EC/Syngenta | Difenoconazole 250 | 20 | 0.6 | 0.6 |
| SPINGARD 35F/Compañía Cibeles SA | Imidacloprid 350 | 50 | 1 | 1 |
| COMET/BASF | Pyraclostrobin 250 | 30 | 1 | 2 |
| TRACER I/Rutilan SA | Spinosad 480 | 20 | 0.3 z | 0.3 z |
| Function | Transformation | |
|---|---|---|
| For y (residue) | For x (time) | |
| 1st-order | logR | none |
| 1.5th-order | 1/√R | none |
| 2nd-order | 1/R | none |
| 1st-order RF z | logR | √t |
| Concentration Level (µg kg−1) | |||||
|---|---|---|---|---|---|
| 10 | 100 | ||||
| Rec (%) | RSD (%) | Rec 1 (%) | RSD (%) | ME (%) | |
| Difenoconazole | 71.8 z | 8.0 | 88.7 | 7.4 | 8.6 |
| Imidacloprid | 113.4 | 6.6 | 80.9 | 2.5 | −63.5 |
| Pyraclostrobin | 72.1 | 2.1 | 100.0 | 2.2 | 7.2 |
| Spinosad | 86.5 | 3.9 | 98.3 | 15.9 | −55.1 |
| Difenoconazole | Imidacloprid | Pyraclostrobin | Spinosad | |
|---|---|---|---|---|
| 1st-order model | ||||
| Dissipation curve | 10−(0.016t+1.140) | 10−(0.022t+0.334) | 10−(0.008t+0.591) | 10−(0.052t+0.744) |
| r2 | 0.857 | 0.883 | 0.890 | 0.987 |
| D | 0.198 | 0.197 | 0.124 | 0.003 |
| 1.5th-order model | ||||
| Dissipation curve | 1/(0.116t + 3.353)2 | 1/(0.064t + 1.312)2 | 1/(0.021t + 1.970)2 | 1/(0.301t + 2.011)2 |
| r2 | 0.509 | 0.951 | 0.906 | 0.871 |
| D | 0.167 | 0.178 | 0.137 | <0 |
| 2nd-order model | ||||
| Dissipation curve | 1/(1.491t + 5.617) | 1/(0.362t + 0.421) | 1/(0.096t + 3.846) | 1/(3.420t-1.062) |
| r2 | <0 | <0 | 0.918 | <0 |
| D | 0.13 | 0.124 | 0.149 | <0 |
| RF 1st-order model | ||||
| Dissipation curve | 10−(0.103√t + 1.086) | 10−(0.148√t + 0.185) | 10−(0.053√t + 0.535) | 10−(0.242√t + 0.658) |
| r2 | 0.598 | 0.962 | 0.893 | 0.963 |
| D | 0.108 | 0.198 | 0.147 | <0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besil, N.; Cesio, V.; Luque, E.; Pintos, P.; Rivas, F.; Heinzen, H. Dissipation of Pre-Harvest Pesticides on ‘Clementine’ Mandarins after Open Field Application, and Their Persistence When Stored under Conventional Postharvest Conditions. Horticulturae 2018, 4, 55. https://doi.org/10.3390/horticulturae4040055
Besil N, Cesio V, Luque E, Pintos P, Rivas F, Heinzen H. Dissipation of Pre-Harvest Pesticides on ‘Clementine’ Mandarins after Open Field Application, and Their Persistence When Stored under Conventional Postharvest Conditions. Horticulturae. 2018; 4(4):55. https://doi.org/10.3390/horticulturae4040055
Chicago/Turabian StyleBesil, Natalia, Verónica Cesio, Eleana Luque, Pedro Pintos, Fernando Rivas, and Horacio Heinzen. 2018. "Dissipation of Pre-Harvest Pesticides on ‘Clementine’ Mandarins after Open Field Application, and Their Persistence When Stored under Conventional Postharvest Conditions" Horticulturae 4, no. 4: 55. https://doi.org/10.3390/horticulturae4040055




