Yield, Quality, Antioxidants and Elemental Composition of New Leek Cultivars under Organic or Conventional Systems in a Greenhouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Dry Matter
2.3. Total Soluble Solids (TSS) and Sugars
2.4. Polyphenols
2.5. Ascorbic Acid
2.6. Antioxidant Activity
2.7. Nitrates
2.8. Elemental Composition
2.9. Statistical Analysis
3. Results and Discussion
3.1. Yield, Dry Matter, Sugars and Nitrates of Pseudo-Stems
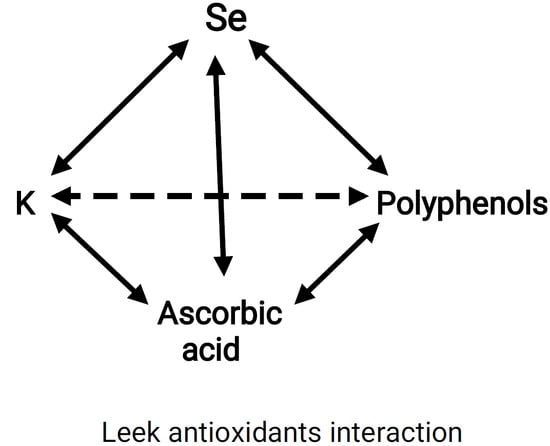
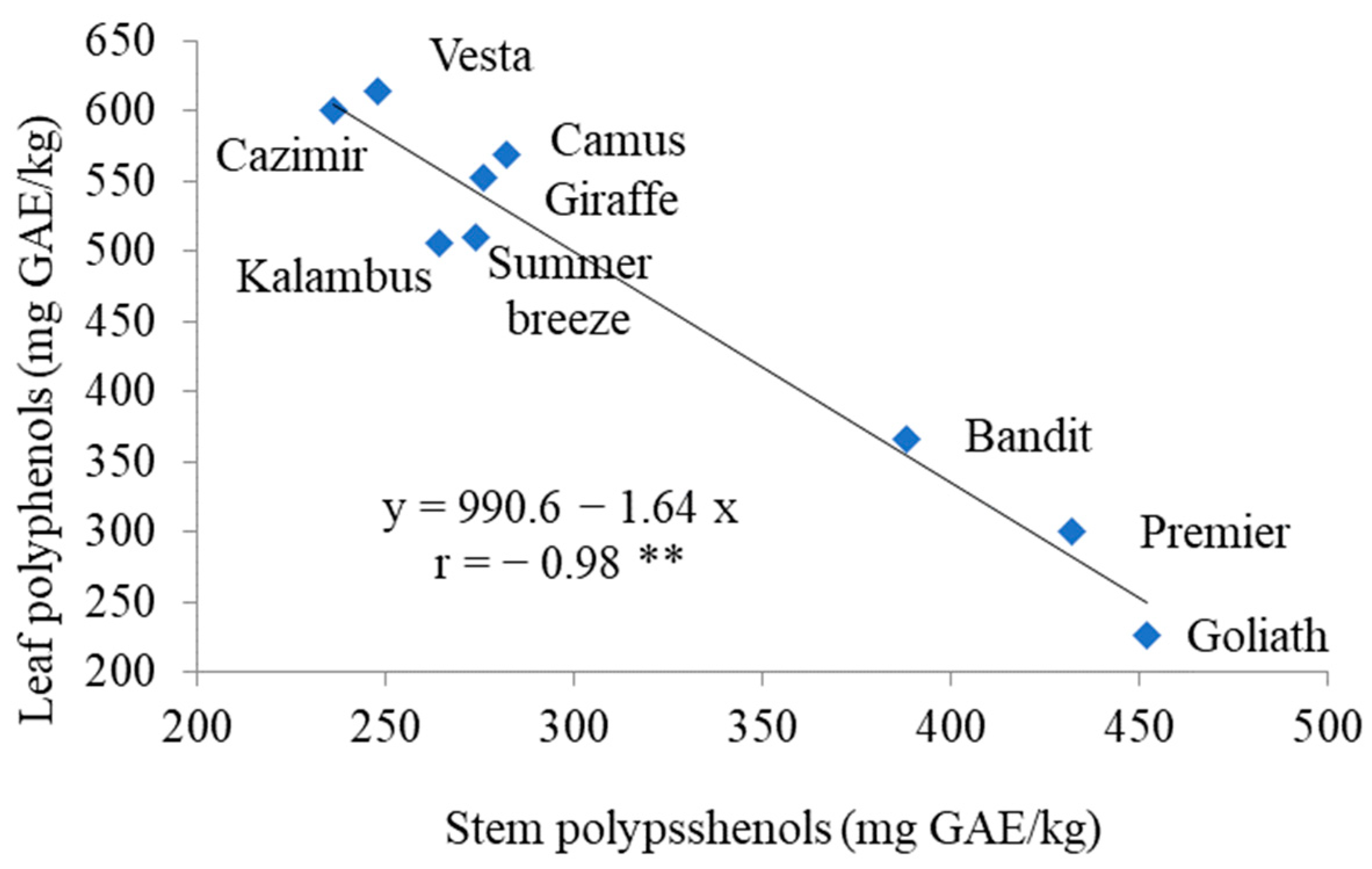
3.2. Antioxidants
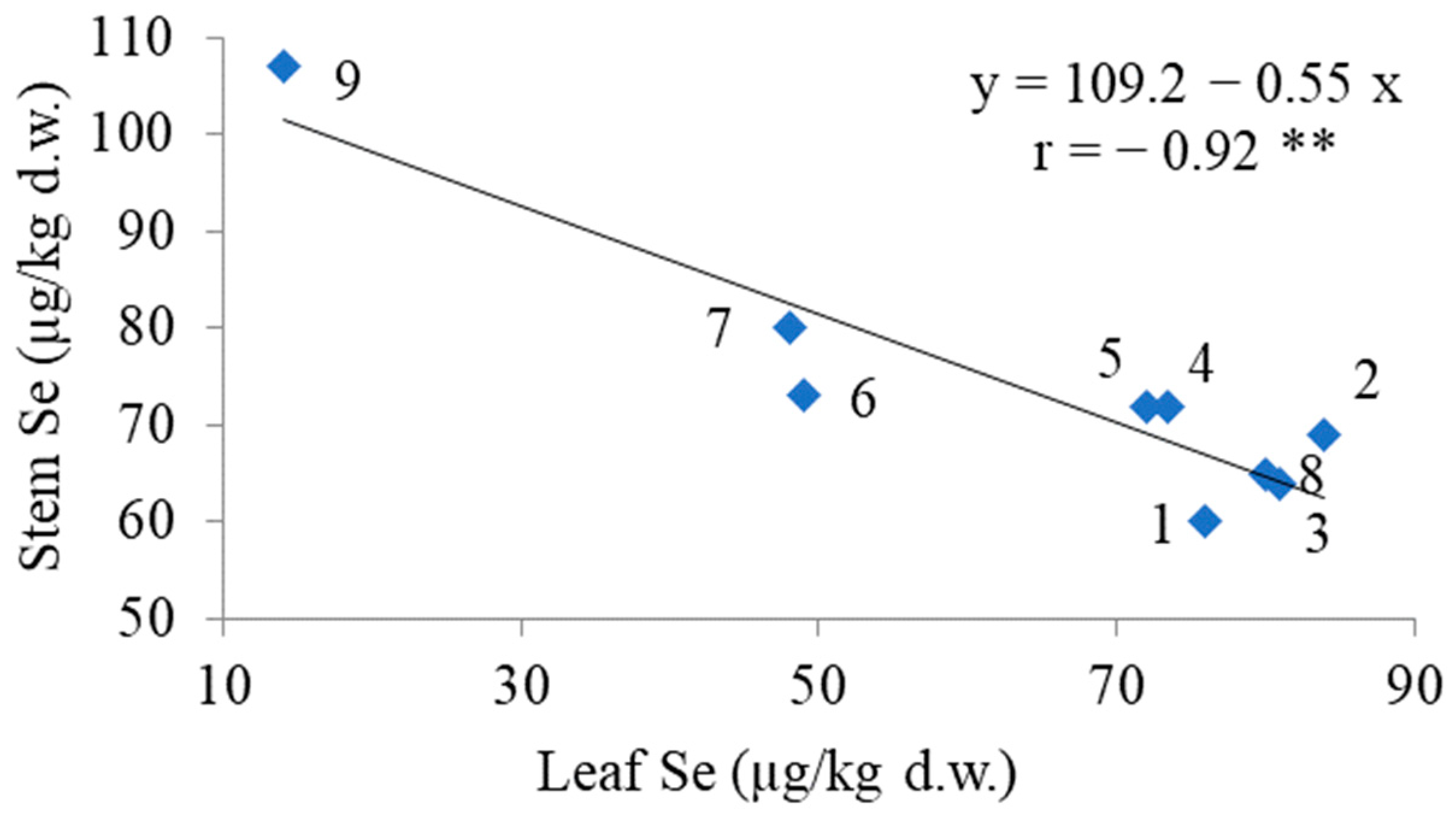
3.3. Elemental Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ozgur, M.; Akpinar-Bayaziy, A.; Ozcan, T.; Afolayan, A.J. Effect of dehydration on several physic-chemical properties and the antioxidant activity of leeks (Allium porrum L.). Not. Bot. Hort. Agrobot. Cluj-Napoca 2011, 39, 144–151. [Google Scholar] [CrossRef]
- Steinmetz, A.K.; Potter, D.J. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Cicala, C. The flavonoids of leek, Allium porrum. Phytochemistry 2001, 57, 565–569. [Google Scholar] [CrossRef]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Fraceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Najjaa, H.; Yahia, B.; Tlig, A.; Neffati, M. Antioxidant capacity and phenolic composition as a function of genetic diversity of wild Tunisian leek (Allium ampeloprasum L.). Acad. J. Biotechnol. 2015, 3, 15–26. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions as global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, B.; Mladenović, J.; Radovanović, A.; Pavlović, R.; Nikolić, V. Phenolic composition, antioxidant, antimicrobial and cytotoxic activites of Allium porrum L. (Serbia) extracts. J. Food Nutr. Res. 2015, 3, 564–569. [Google Scholar] [CrossRef]
- Bianchini, F.; Vainio, H. Allium vegetables and organosulfur compounds: Do they help prevent cancer? Environ. Health Perspect. 2001, 109, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Chokkalingam, A.P.; Gao, Y.T.; Madigan, M.P.; Deng, J.; Gridley, G.; Fraumeni, J.F. Allium vegetables and risk of prostate cancer: A population based study. J. Natl. Cancer Inst. 2002, 94, 1648–1651. [Google Scholar] [CrossRef] [PubMed]
- Kyoung-Hee, K.; Hye-Joung, K.; Myung-Woo, B.; Hong-Sun, Y. Antioxidant and antimicrobial activities of ethanol extract from 6 vegetables containing different sulfur compounds. J. Korean Soc. Food Sci. Nutr. 2012, 41, 577–583. [Google Scholar]
- Vergawen, R.; Van Leuven, F.; Van Laere, A. Purification and characterization of strongly chitin-binding chitinases from salicylic acid-treated leek (Allium porrum). Physiol. Plant. 1998, 104, 175–182. [Google Scholar] [CrossRef]
- Yin, M.C.; Tsao, S.M. Inhibitory effect of seven Allium plants upon three Aspergillus species. Int. J. Food Microbiol. 1999, 49, 49–56. [Google Scholar] [CrossRef]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essential oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef] [PubMed]
- Lundegardh, B.; Botek, P.; Schulzo, V.; Hajšlo, V.J.; Stromberg, V.A.; Andersson, H.C. Impact of different green manures on the content of S-Alk(en)yl-l-cysteine sulfoxides and l-Ascorbic acid in leek (Allium porrum). J. Agric. Food Chem. 2008, 56, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Naem-Rana, K.; Had-Noora, A. The antimicrobial activity of Allium porrum water extract against pathogenic bacteria. J. Kerbala Univ. 2012, 10, 45–49. [Google Scholar]
- Sekara, A.; Pokluda, R.; Del Vacchio, L.; Somma, S.; Caruso, G. Interactions among genotype, environment and agronomic practices on production and quality of storage onion (Allium cepa L.). Rev. Hortic. Sci. 2017, 44, 21–42. [Google Scholar] [CrossRef]
- Koca, I.; Tasci, B. Mineral composition of leek. Acta Hortic. 2016, 1143, 147–151. [Google Scholar] [CrossRef]
- Conti, S.; Villari, G.M.; Amico, E.; Caruso, G. Effects of production system and transplanting time on yield, quality and antioxidant content of organic winter squash (Cucurbita moschata Duch.). Sci. Hortic. 2015, 183, 136–143. [Google Scholar] [CrossRef]
- Bernaert, N.; De Paepe, D.; Bouten, C.; De Clercq, H.; Stewart, D.; Van Bockstaele, E.; De Loose, M.; Van Droogenbroeck, B. Antioxidant capacity, total phenolic and ascorbate content as a function of the genetic diversity of leek (Allium ampeloprasum var. porrum). Food Chem. 2012, 134, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Swamy, P.M. Laboratory Manual on Biotechnology; Rastogi Publications: Meerut, India, 2008; 617p. [Google Scholar]
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Nadezhkin, S.M.; Dobrutskaya, H.G.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- AOAC. The Official Methods of Analysis of AOAC (Association Official Analytical Chemists) International; AOAC: Arlington, VA, USA, 2012; Volume 22. [Google Scholar]
- Maximova, T.V.; Nikulina, I.N.; Pakhomov, V.P.; Shkarina, H.I.; Chumakova, Z.V.; Arzamastsev, A.P. Method of Antioxidant Activity Determination. RF Patent No. 2.170,930, 20 July 2001. [Google Scholar]
- Srivastava, S.; Adholeya, A.; Conlan, X.A.; Cahill, D.M. Acidic potassium permanganate chemiluminescence for the determination of antioxidant potential in three cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2015, 70. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.G.; Liu, N.; Liu, H. Determination of the total mass of antioxidant substances and antioxidant capacity per unit mass in serum using redox titration. Bioinorg. Chem. Appl. 2014, 928595. [Google Scholar] [CrossRef]
- Skalny, A.V.; Lakarova, H.V.; Kuznetsov, V.V.; Skalnaya, M.G. Analytical Methods in Bioelementology; Saint Petersburg-Science: St. Petersburg, Russia, 2009. [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 65, 187–194. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, G.; Borrelli, C.; Russo, G. Effects of crop method and harvest seasons on yield and quality of green asparagus under tunnel in southern Italy. Adv. Hort. Sci. 2012, 26, 51–58. [Google Scholar]
- Biswas, S.K.; Khair, A.; Sarker, P.K.; Alom, M.S. Yield and storability of onion (Allium cepa L.) as affected by various levels of irrigation. Bangladesh J. Agric. Res. 2010, 35, 247–255. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kekina, H.G.; Antoshkina, M.S.; Agafonov, A.F.; Nadezhkin, S.M. Inter varietal differences in accumulation of biologically active compounds by Allium cepa L. Messenger Russ. Agric. Sci. 2016, 2, 51–55. [Google Scholar]
- Sinclair, P.J.; Blakeney, A.B.; Barlow, E.W. Relationships between bulb dry matter content, soluble solids concentration and non-structural carbohydrate composition in the onion (Allium cepa). J. Sci. Food Agric. 1995, 69, 203–209. [Google Scholar] [CrossRef]
- Caruso, G.; Conti, S.; La Rocca, G. Influence of crop cycle and nitrogen fertilizer form on yield and nitrate content in different species of vegetables. Adv. Hort. Sci. 2011, 25, 81–89. [Google Scholar]
- Santamaria, P. Nitrates in vegetables: Toxicity content, intake and EC regulation. J. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Pannala, A.S.; Pagana, G.; Van Buren, L.; Wagner, E.; Wiseman, S. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Villari, G.; Faugno, S.; Melchionna, G.; Somma, S.; Caruso, G. Effects of organic vs. conventional management system on yield and quality of strawberry grown as an annual or biennial crop in southern Italy. Sci. Hortic. 2014, 180, 63–71. [Google Scholar] [CrossRef]
- Biesiada, A.; Kolota, E.; Adamczewska-Sowinska, K. The effect of maturity stage on nutritional value of leek, zucchini and kohlrabi. Res. Bull. 2007, 66, 39–45. [Google Scholar] [CrossRef]
- Malagoli, M.; Schiavon, M.; dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Miholová, D.; Pivec, V.; Jírů, K.; Janovská, D. Content of phenolic antioxidants and selenium in grain of einkorn (Triticum monococcum), emmer (Triticum dicoccum) and spring wheat (Triticum aestivum) varieties. Plant Soil Environ. 2011, 57, 235–243. [Google Scholar] [CrossRef]
- Kavalcová, P.; Bystrická, J.; Trebichalský, P.; Volnová, B.; Kopernická, M. The influence of selenium on content of total polyphenols and antioxidant activity of onion (Allium cepa L.). J. Microbiol. Biotechnol. Food Sci. 2014, 3, 238–240. [Google Scholar]
- Golubkina, N.; Kekina, H.; Caruso, G. Foliar biofortification of Indian mustard (Brassica juncea L.) with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Butt, M.S.; Shehzad, A.; Tanwer, S. Chemical and mineral analysis of garlic, a golden herb. Pak. J. Food Sci. 2014, 24, 108–110. [Google Scholar]
- Seredin, Т.М.; Agafonov, A.F.; Gerasimova, L.I.; Krivenkov, L.V. Element composition of winter garlic. Veg. Crop. 2015, 3–4, 81–85. [Google Scholar]
- Ahn, S.-J.; Matsumoto, H. The role of the plasma membrane in the response of plant roots to aluminum toxicity. Plant Signal Behav. 2006, 1, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Yalamanchali, R.C. Lithium, an Emerging Environmental Contaminant, Is Mobile in the Soil-Plant System. Ph.D. Thesis, Lincoln University, Lincoln, UK, 2012. [Google Scholar]
- Essiett, U.A.; Effiong, G.S.; Ogbemudia, F.O.; Bruno, E.J. Heavy metal concentrations in plants growing in crude oil contaminated soil in AkwaIbom State, South-Eastern Nigeria. Afr. J. Pharm. Pharmacol. 2010, 4, 465–470. [Google Scholar]
- Crush, J.R.; Evans, J.P.M.; Cosgrove, G.P. Chemical composition of ryegrass (Lolium perenne L.) and prairie grass (Bromus willdenowii Kunth) pastures. N. Z. J. Agric. Res 1989, 32, 461–468. [Google Scholar] [CrossRef]
- Bozokalfa, K.; Yağmur, B.; Aşçıoğul, T.; Eşiyok, D. Diversity in nutritional composition of Swiss chard (Beta vulgaris subsp. L. var. cicla) accessions revealed by multivariate analysis. Plant Genet. Resour. 2011, 9, 557–566. [Google Scholar] [CrossRef]
- Põldma, P.; Tõnutare, T.; Viitak, A.; Luik, A.; Moor, U. Effect of Selenium treatment on mineral nutrition, bulb size, and antioxidant properties of garlic (Allium sativum L.). J. Agric. Food Chem. 2011, 59, 5498–5503. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Mudau, F.N.; Soundy, P.; du Toit, E.S. Effects of nitrogen, phosphorus, and potassium nutrition on total polyphenol content of bush tea (Athrixia phylicoides L.) leaves in shaded nursery environment. Hort Sci. 2007, 42, 334–338. [Google Scholar]
- Qing, X.; Zhao, X.; Hu, C.; Wang, P.; Zhang, Y.; Zhang, X.; Wang, P.; Shi, H.; Jia, F.; Qu, C. Selenium alleviates chromium toxicity by preventing oxidative stress in cab-bage (Brassica campestris L. ssp. Pekinensis) leaves. Cotoxicol. Environ. Saf. 2015, 114, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Belokobylsky, A.I.; Ginturi, E.I.; Kuchava, N.E.; Kirkesali, E.I.; Mosulishvili, L.M.; Frontasyeva, M.V.; Pavlov, S.S.; Aksenova, N.G. Accumulation of selenium and chromium in the growth dynamics of Spirulina platensis. J. Radioanal. Nucl. Chem. 2004, 259, 65–68. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Selenium in plants. In Progress in Botany; Luttge, U., Beyschlag, W., Eds.; Springer International Publishing: Basel, Switzerland, 2015; pp. 93–107. [Google Scholar]
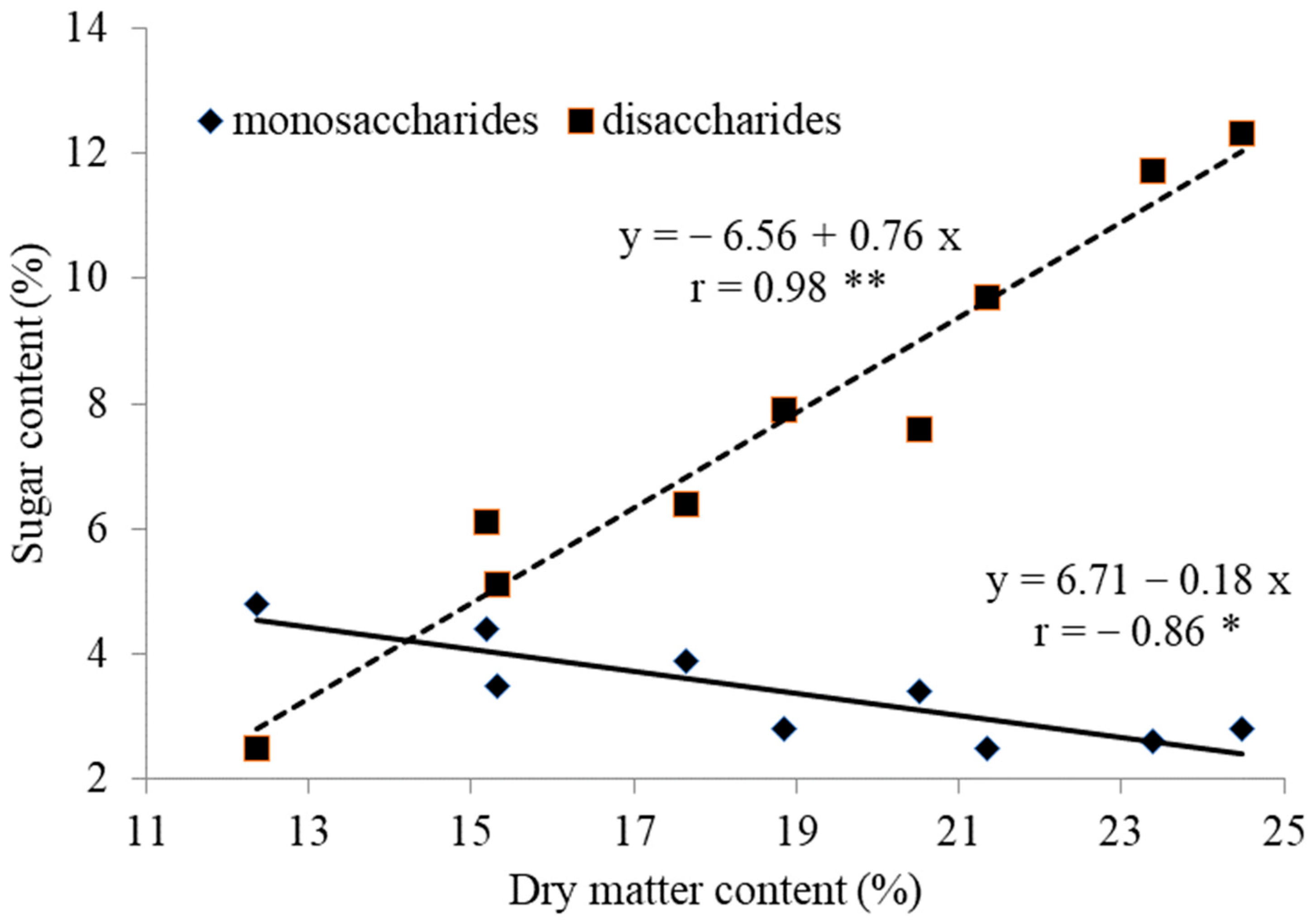
| Treatment | Yield 103 kg ha−1 | Mean Pseudo- Stem Weight g | Dry Matter % | Sugars g/100 g d.w. | Nitrates mg/kg f.w. | |
|---|---|---|---|---|---|---|
| Mono- | Total | |||||
| Crop management | ||||||
| Organic | 30.9 | 185.5 | 19.7 | 3.6 | 11.7 | 76 |
| Conventional | 31.2 | 187.0 | 17.8 | 3.2 | 10.4 | 102 |
| n.s.z | n.s. | * | * | * | * | |
| Cultivar | ||||||
| Goliath | 31.1 d,e,y | 188.0 d,e | 12.4 ± 0.4 e | 4.8 ± 0.3 a | 7.3 ± 0.5 e | 77 ± 3 d |
| Premier | 23.8 g | 142.4 g | 15.2 ± 0.5 d | 4.4 ± 0.3 a,b | 10.5 ± 0.7 c | 74 ± 3 d |
| Bandit | 35.0 bc | 209.8 b,c | 15.3 ± 0.6 d | 3.5 ± 0.2 c | 8.6 ± 0.5 d | 103 ± 4 a |
| Kalambus | 24.0 g | 143.6 g | 17.6 ± 0.6 c | 3.9 ± 0.2 b,c | 10.3 ± 0.7 c | 70 ± 3 d |
| Cazimir | 26.9 f | 160.9 f | 18.9 ± 0.6 c | 2.8 ± 0.2 d | 10.7 ± 0.7 b,c | 90 ± 4 b,c |
| Giraffe | 40.2 a | 242.5 a | 20.5 ± 0.8 b | 3.4 ± 0.2 c | 11.0 ± 0.7 bc | 86 ± 3 c |
| Camus | 33.3 c,d | 201.7 c,d | 21.4 ± 0.7 b | 2.5 ± 0.2 d | 12.2 ± 0.8 b | 105 ± 5 a |
| Vesta | 29.0 e,f | 175.0 e,f | 23.4 ± 0.8 a | 2.6 ± 0.2 d | 14.3 ± 0.8 a | 98 ± 4 ab |
| Summer Breeze | 36.7 b | 221.3 b | 24.3 ± 0.9 a | 2.8 ± 0.2 d | 15.1 ± 0.9 a | 98 ± 4 ab |
| M | 31.1 | 187.2 | 18.8 | 3.86 | 11.11 | 89 |
| SD | 5.7 | 34.8 | 3.2 | 1.23 | 1.84 | 11 |
| CV (%) | 18.3 | 18.6 | 17.3 | 31.9 | 16.8 | 12.4 |
| Concentration range | 23.8–40.2 | 142.4–242.5 | 12.4–24.3 | 2.5–8.4 | 7.3–15.1 | 70–105 |
| Treatment | Ascorbic Acid in Pseudo-Stems mg/100 g f.w. | Polyphenols mg GAE/100 g d.w. | Selenium µg/kg d.w. | ||
|---|---|---|---|---|---|
| Pseudo-Stems | Leaves | Pseudo-Stems | Leaves | ||
| Crop management | |||||
| Organic | 47.2 | 375.4 | 696.7 | 76.3 | 64.4 |
| Conventional | 37.0 | 356.2 | 674.9 | 73.1 | 60.8 |
| * z | n.s. | n.s. | n.s. | n.s. | |
| Cultivar | |||||
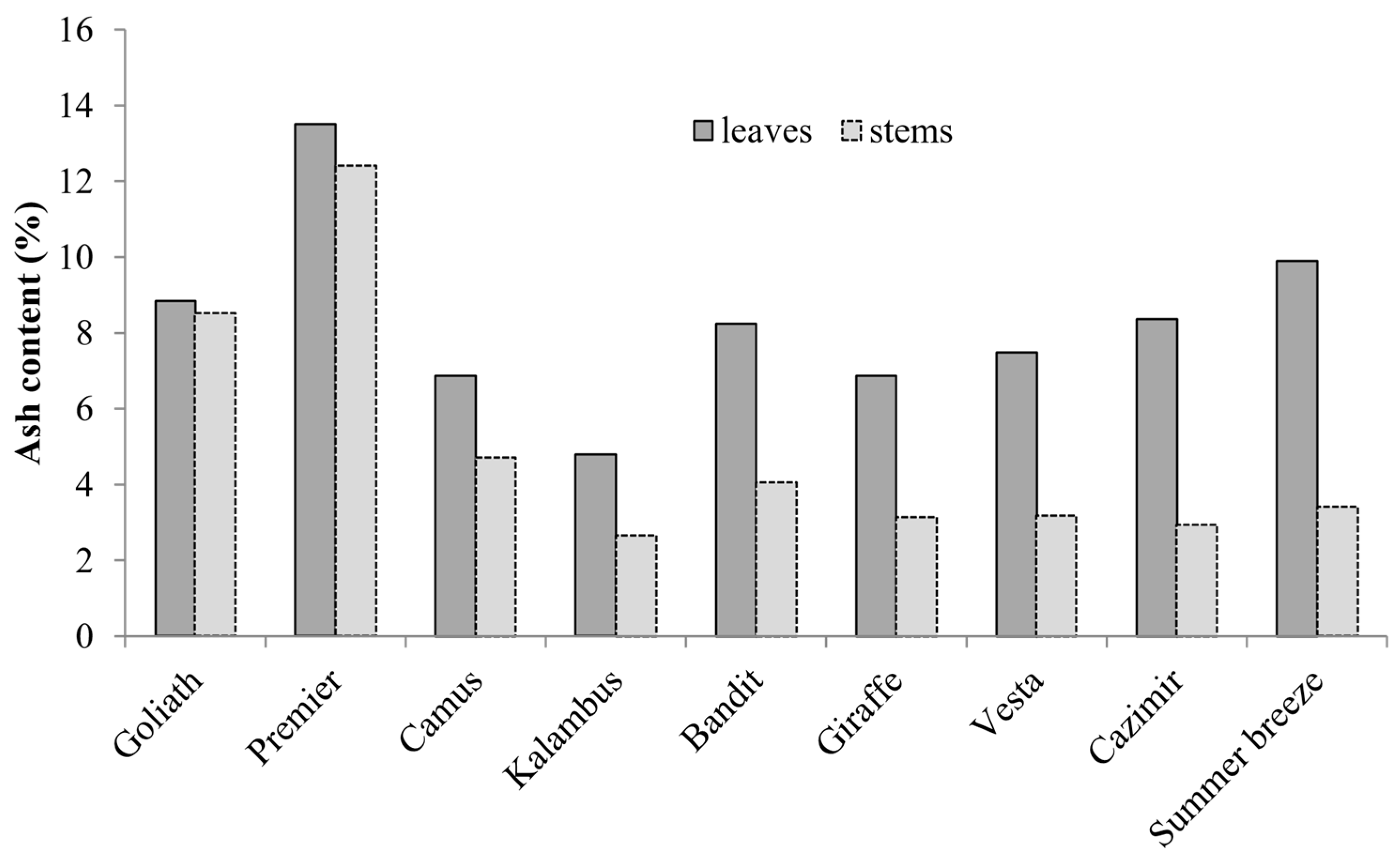
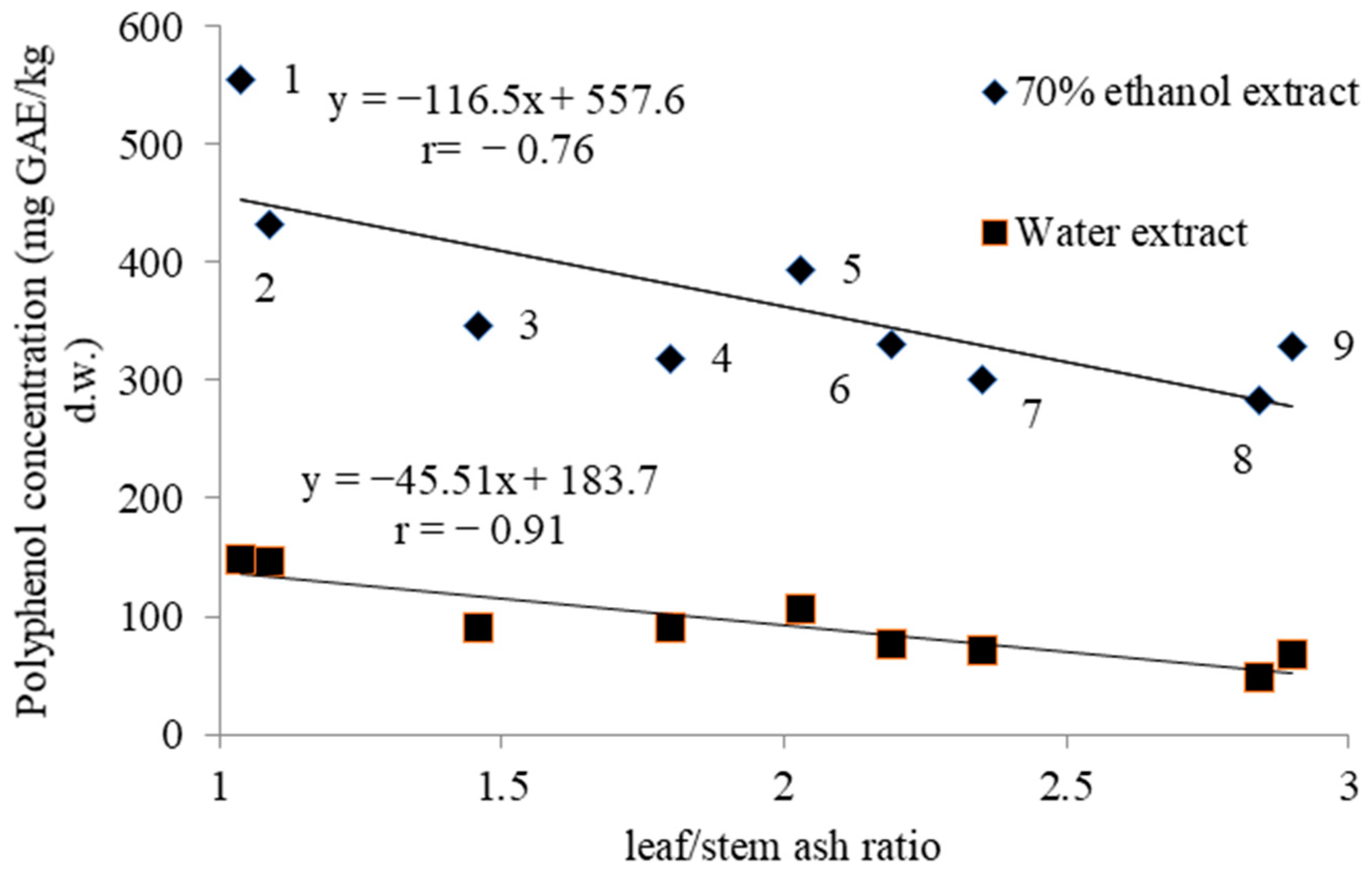
| Goliath | 136.8 ± 8.1 a,y | 555 ± 52 a | 711 ± 19 a | 107 ± 7 a | 14 ± 1 e |
| Premier | 58.6 ± 3.3 b | 432 ± 34 b | 650 ± 21 b | 80 ± 5 b | 65 ± 3 c |
| Bandit | 40.5 ± 2.6 c | 394 ± 26 b,c | 647 ± 26 b | 75 ± 5 b,c | 48 ± 2 d |
| Kalambus | 25.5 ± 2.3 d,e | 319 ± 19 d,e | 731 ± 46 a | 72 ± 4 b,c | 74 ± 3 b |
| Cazimir | 30.2 ± 2.6 d | 284 ± 20 e | 728 ± 53 a | 60 ± 3 e | 76 ± 4 a,b |
| Giraffe | 24.9 ± 2.0 e,f | 331 ± 19 d | 665 ± 41 a,b | 73 ± 4 b,c | 49 ± 2 d |
| Camus | 21.1 ± 1.4 f,g | 347 ± 21 c,d | 684 ± 43 a,b | 64 ± 3 d,e | 81 ± 5 a,b |
| Vesta | 19.2 ± 1.3 g | 301 ± 19 d,e | 740 ± 55 a | 69 ± 3 c,d | 84 ± 5 a |
| Summer Breeze | 22.2 ± 1.9 f,g | 329 ± 20 d,e | 616 ± 42 b | 72 ± 3 b,c | 72 ± 4 b,c |
| M | 42.1 | 317 | 686 | 74.7 | 62.5 |
| SD | 24.7 | 71 | 37 | 8.4 | 17 |
| CV, % | 58.7 | 22.4 | 5.4 | 11.2 | 27.2 |
| Concentration range | 19.2–136.8 | 284–555 | 616–740 | 60–107 | 14–84 |
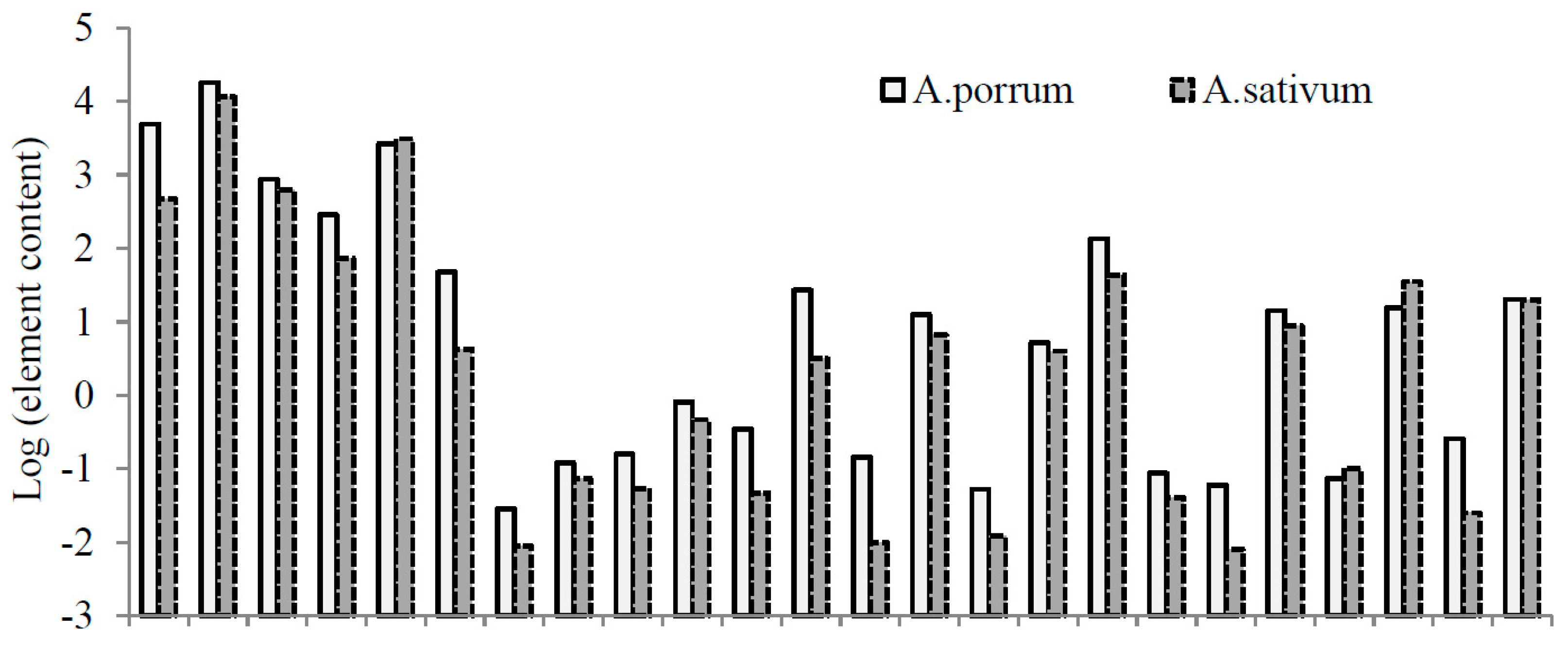
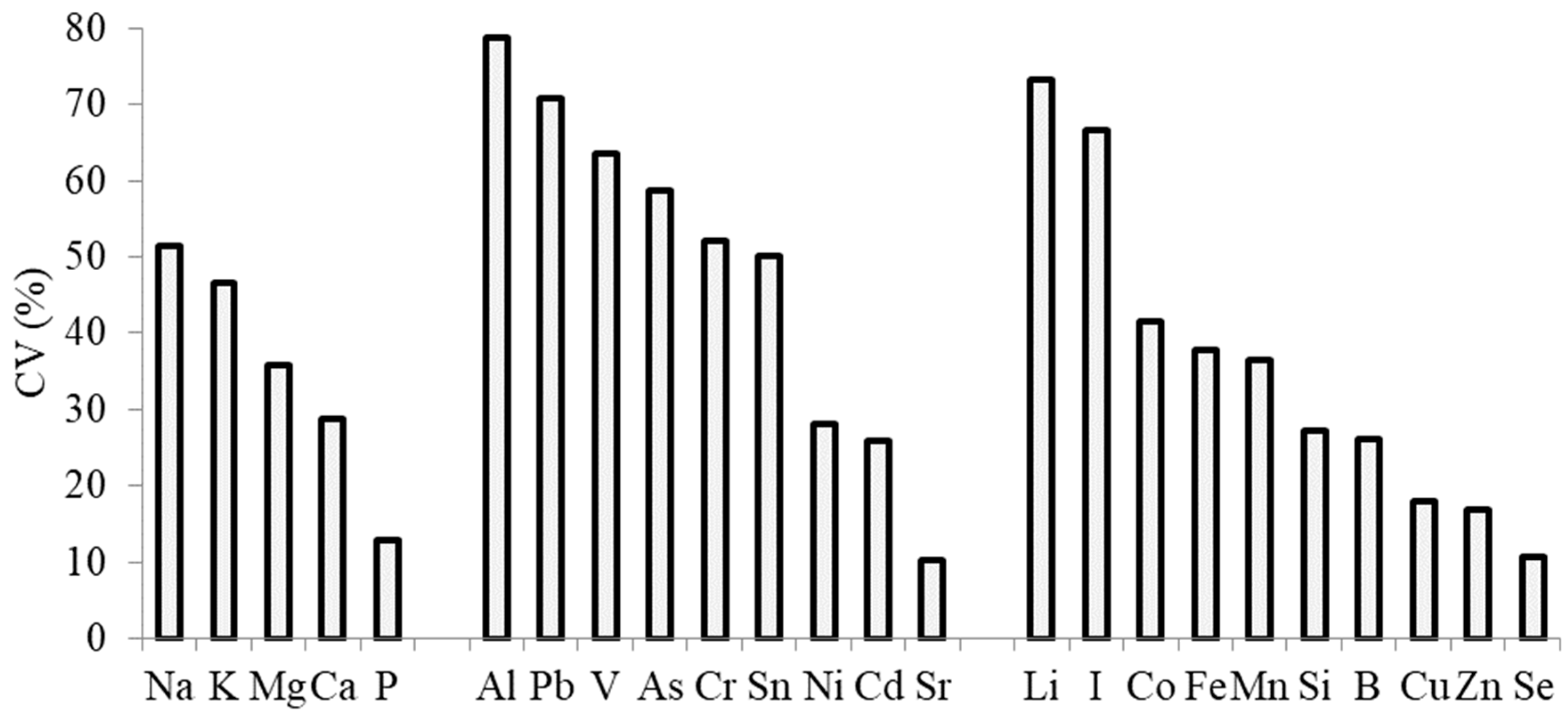
| Element | Goliath | Cazimir | Premier | Vesta | Kalambus | Summer Breeze | Bandit | Giraffe | Camus |
|---|---|---|---|---|---|---|---|---|---|
| Macro elements | |||||||||
| Ca | 3.98 b,c,z | 3.40 c,d | 11.32 a | 4.35 b | 4.68 b | 2.81 d | 4.82 b | 4.24 b,c | 4.73 b |
| K | 51.76 a | 4.71 e | 23.39 b | 13.50 c | 8.00 d | 15.94 c | 16.95 c | 15.05 c | 15.82 c |
| Mg | 0.78 c | 0.76 c | 2.02 a | 0.56 d | 0.53 d | 0.65 c,d | 1.00 b | 0.70 c,d | 0.80 c |
| Na | 0.34 b,c | 0.39 b | 0.81 a | 0.16 e | 0.16 e | 0.12 e | 0.17 e | 0.19 d,e | 0.28 c,d |
| P | 2.95 b | 2.61 bc | 2.41 c,d | 2.30 c,d | 1.97 d | 2.37 cd | 3.85 a | 2.64 bc | 2.65 b,c |
| Element | Goliath | Cazimir | Premier | Vesta | Kalambus | Summer Breeze | Bandit | Giraffe | Camus |
|---|---|---|---|---|---|---|---|---|---|
| B | 21.25 a,z | 15.21 b,c | 16.75 b | 9.68 d,e | 8.61 e | 9.55 d,e | 9.73 d,e | 12.79 c,d | 11.14 d |
| Co | 0.070 c | 0.050 d | 0.091 b | 0.034 d | 0.290 a | 0.035 d | 0.035 d | 0.035 d | 0.099 b |
| Cu | 4.81 d,f | 4.52 e,f | 3.46 g | 5.90 b,c | 6.66 a,b | 5.15 c,e | 7.18 a | 4.08 f,g | 5.53 c,d |
| Fe | 221 a | 116 c | 178 b | 101 b,d | 77 e | 84 d,e | 98 c,e | 104 c,d | 235 a |
| I | 0.060 a | 0.040 d | 0.353 a | 0.042 c,d | 0.055 b,d | 0.057 b,d | 0.038 d | 0.071 b,c | 0.073 b |
| Li | 0.110 b | 0.040 c | 0.160 a | 0.025 c | 0.014 c | 0.032 c | 0.023 c | 0.029 c | 0.109 b |
| Mn | 12.57 c | 12.18 c | 23.15 a | 9.87 c | 6.39 d | 9.69 c | 10.93 c | 19.97 b | 22.51 a,b |
| Si | 14.62 c | 10.74 b | 28.78 a | 9.50 e | 13.43 c,d | 13.86 c,d | 11.41 d,e | 20.00 b | 16.17 c |
| Sn | 0.160 e | 0.240 c,d | 0.023 f | 0.171 e | 0.519 b | 0.193 d,e | 0.574 a | 0.245 c | 0.247 c |
| Zn | 23.97 a,b | 27.27 a | 11.96 f | 18.45 de | 16.26 e | 19.58 ce | 21.96 b,c | 22.83 b,c | 21.72 b,d |
| Element | Goliath | Cazimir | Premier | Vesta | Kalambus | Summer Breeze | Bandit | Giraffe | Camus |
|---|---|---|---|---|---|---|---|---|---|
| Heavy metals | |||||||||
| Al | 84.0 c,z | 31.3 d | 137.0 a | 21.9 d,f | 8.0 g | 25.3 d,e | 12.6 f,g | 20.2 e,f | 96.2 b |
| As | 0.030 b | 0.020 c,d | 0.066 a | 0.015 c,e | 0.013 d,e | 0.017 c,e | 0.009 e | 0.023 b,c | 0.066 a |
| Cd | 0.090 c,d | 0.110 b,c | 0.196 a | 0.110 b | 0.085 d | 0.073 d | 0.113 b | 0.182 a | 0.122 b |
| Cr | 0.130 c | 0.080 g | 0.524 a | 0.104 d,f | 0.095 e,g | 0.122 c,d | 0.085 f,g | 0.161 b | 0.111 c,e |
| Ni | 1.100 a | 0.480 c | 1.000 a,b | 1.010 a,b | 0.578 c | 0.588 c | 0.875 b | 0.621 c | 1.140 a |
| Pb | 0.360 b | 0.290 b,c | 0.894 a | 0.108 e | 0.096 e | 0.143 d,e | 0.220 c,d | 0.117 e | 0.878 a |
| Sr | 29.0 a,b | 25.7 c | 31.3 a | 25.0 c | 28.7 a,b | 17.8 d | 29.3 a,b | 26.8 b,c | 29.1 a,b |
| V | 0.230 b | 0.090 c,d | 0.311 a | 0.079 c,d | 0.043 e | 0.097 c | 0.066 d,e | 0.073 c,d | 0.299 a |
| Al | As | B | Ca | Cd | Co | Cr | Cu | Fe | I | K | Li | Mg | Mn | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.93 * | 1 | 0.38 | ||||||||||||
| Ca | 0.71 * | 0.66 | 0.29 | 1 | |||||||||||
| Cd | 0.47 | 0.54 | 0.26 | 0.72 * | 1 | ||||||||||
| Co | 0.95 *** | 0.95 *** | 0.53 | 0.55 | 0.37 | 1 | |||||||||
| Cr | 0.74 * | 0.66 | 0.40 | 0.94 *** | 0.73 | 0.53 | 1 | ||||||||
| Fe | 0.85 ** | 0.80 ** | 0.64 | 0.31 | 0.20 | 0.92 *** | 0.30 | −0.36 | 1 | ||||||
| I | 0.77 ** | 0.70 * | 0.37 | 0.96 *** | 0.70 * | 0.58 | 0.99 *** | −0.60 | 0.33 | 1 | |||||
| K | 0.50 | 0.23 | 0.75 e | 0.13 | −0.03 | 0.39 | 0.21 | −0.23 | 0.63 | 0.17 | 1 | ||||
| Li | 0.99 *** | 0.90 *** | 0.68 | 0.70 * | 0.46 | 0.93 *** | 0.73 * | −0.59 | 0.86 ** | 0.76 * | 0.56 | 1 | |||
| Mg | 0.75 * | 0.65 | 0.41 | 0.94 *** | 0.69 | 0.59 | 0.93 *** | −0.50 | 0.36 | 0.94 *** | 0.21 | 0.75 * | 1 | ||
| Mn | 0.74 * | 0.86 ** | 0.36 | 0.58 | 0.81 ** | 0.77 * | 0.62 | −0.65 | 0.64 | 0.62 | 0.14 | 0.72 * | 0.62 | 1 | |
| Na | 0.83 ** | 0.71 * | 0.64 | 0.85 ** | 0.63 | 0.70 | 0.88 ** | −0.59 | 0.50 | 0.90 *** | 0.25 | 0.83 ** | 0.90 *** | 0.61 | |
| Ni | 0.65 | 0.58 | 0.32 | 0.38 | 0.16 | 0.66 | 0.27 | 0.01 | 0.76 * | 0.29 | 0.60 | 0.65 | 0.34 | 0.42 | |
| Pb | 0.92 *** | 0.97 *** | 0.38 | 0.67 | 0.47 | 0.97 *** | 0.63 | −0.42 | 0.80 ** | 0.68 | 0.22 | 0.90 *** | 0.71 * | 0.79 ** | 1 |
| Se | 0.35 | 0.05 | 0.69 | 0.14 | −0.06 | 0.19 | −0.72 * | −0.16 | 0.42 | −0.71 * | 0.95 *** | 0.42 | 0.18 | −0.03 | 0.04 |
| Si | 0.72 * | 0.72 * | 0.39 | 0.83 ** | 0.80 ** | 0.57 | 0.91 *** | −0.70 | 0.37 | 0.90 *** | 0.23 | 0.72 * | 0.81 ** | 0.77 * | 0.64 |
| Sn | −0.66 | −0.57 | −0.57 | −0.37 | −0.39 | −0.53 | −0.58 | 0.844 c | −0.47 | −0.55 | 0.26 | −0.64 | −0.42 | −0.52 | −0.45 |
| V | 0.98 *** | 0.94 *** | 0.56 | 0.60 | 0.38 | 0.98 *** | 0.61 | −0.50 | 0.91 *** | 0.65 | 0.51 | 0.97 *** | 0.64 | 0.75 * | 0.94 *** |
| Zn | −0.34 | −0.33 | 0.21 | −0.74 * | −0.31 | −0.15 | −0.69 | 0.05 | 0.07 | −0.71 * | 0.05 | −0.31 | −0.54 | −0.14 | −0.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.A.; Seredin, T.M.; Antoshkina, M.S.; Kosheleva, O.V.; Teliban, G.C.; Caruso, G. Yield, Quality, Antioxidants and Elemental Composition of New Leek Cultivars under Organic or Conventional Systems in a Greenhouse. Horticulturae 2018, 4, 39. https://doi.org/10.3390/horticulturae4040039
Golubkina NA, Seredin TM, Antoshkina MS, Kosheleva OV, Teliban GC, Caruso G. Yield, Quality, Antioxidants and Elemental Composition of New Leek Cultivars under Organic or Conventional Systems in a Greenhouse. Horticulturae. 2018; 4(4):39. https://doi.org/10.3390/horticulturae4040039
Chicago/Turabian StyleGolubkina, Nadezhda A., Timofey M. Seredin, Marina S. Antoshkina, Olga V. Kosheleva, Gabriel C. Teliban, and Gianluca Caruso. 2018. "Yield, Quality, Antioxidants and Elemental Composition of New Leek Cultivars under Organic or Conventional Systems in a Greenhouse" Horticulturae 4, no. 4: 39. https://doi.org/10.3390/horticulturae4040039
APA StyleGolubkina, N. A., Seredin, T. M., Antoshkina, M. S., Kosheleva, O. V., Teliban, G. C., & Caruso, G. (2018). Yield, Quality, Antioxidants and Elemental Composition of New Leek Cultivars under Organic or Conventional Systems in a Greenhouse. Horticulturae, 4(4), 39. https://doi.org/10.3390/horticulturae4040039