The Effect of Ethephon, Abscisic Acid, and Methyl Jasmonate on Fruit Ripening in Rabbiteye Blueberry (Vaccinium virgatum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and PGRs
2.2. Rate of Ripening
2.3. Postharvest Fruit Quality and Disease Incidence
3. Results
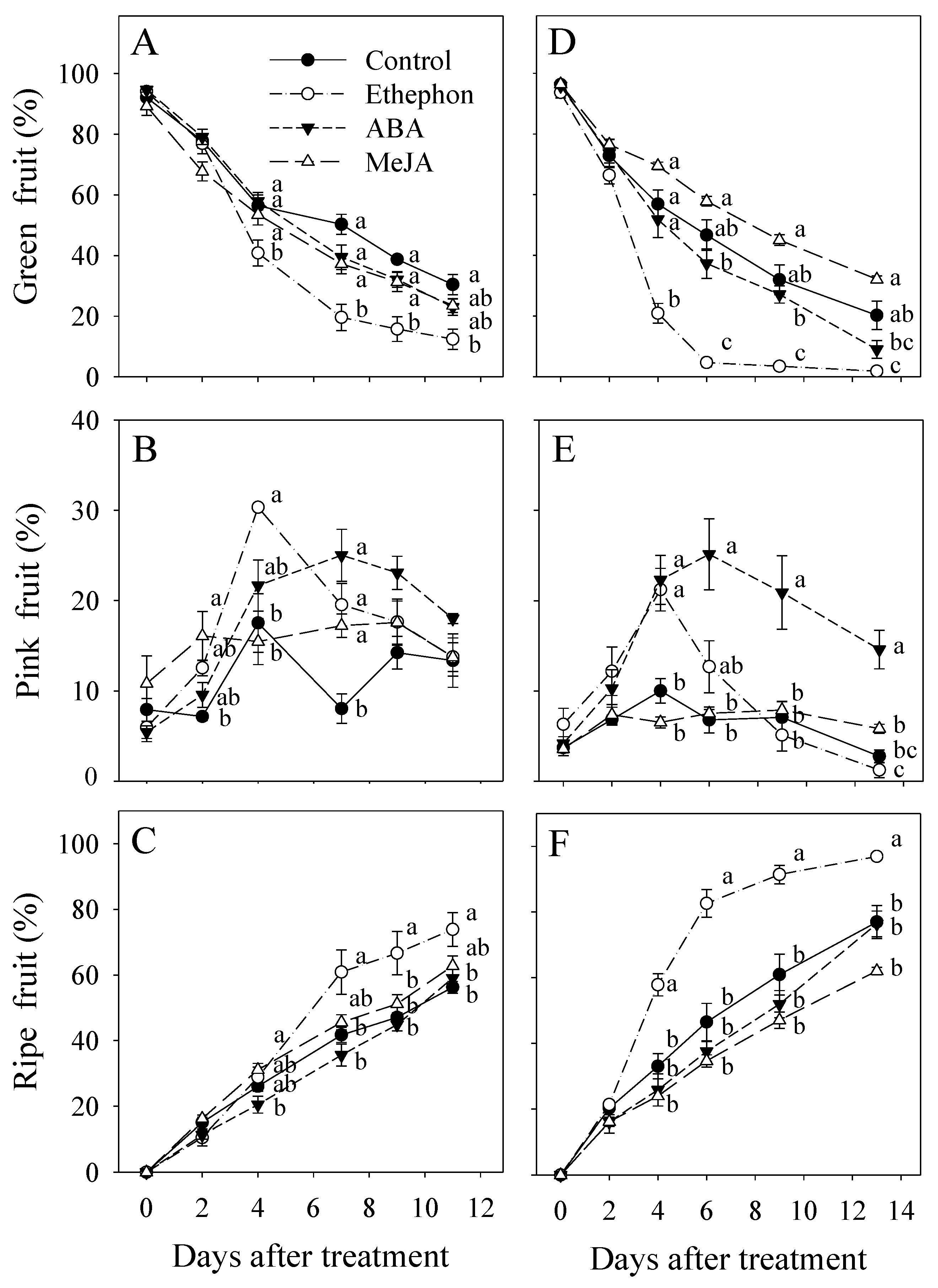
3.1. Effect of PGR Application on Fruit Ripening
3.2. Effect of PGR Application on Fruit Color
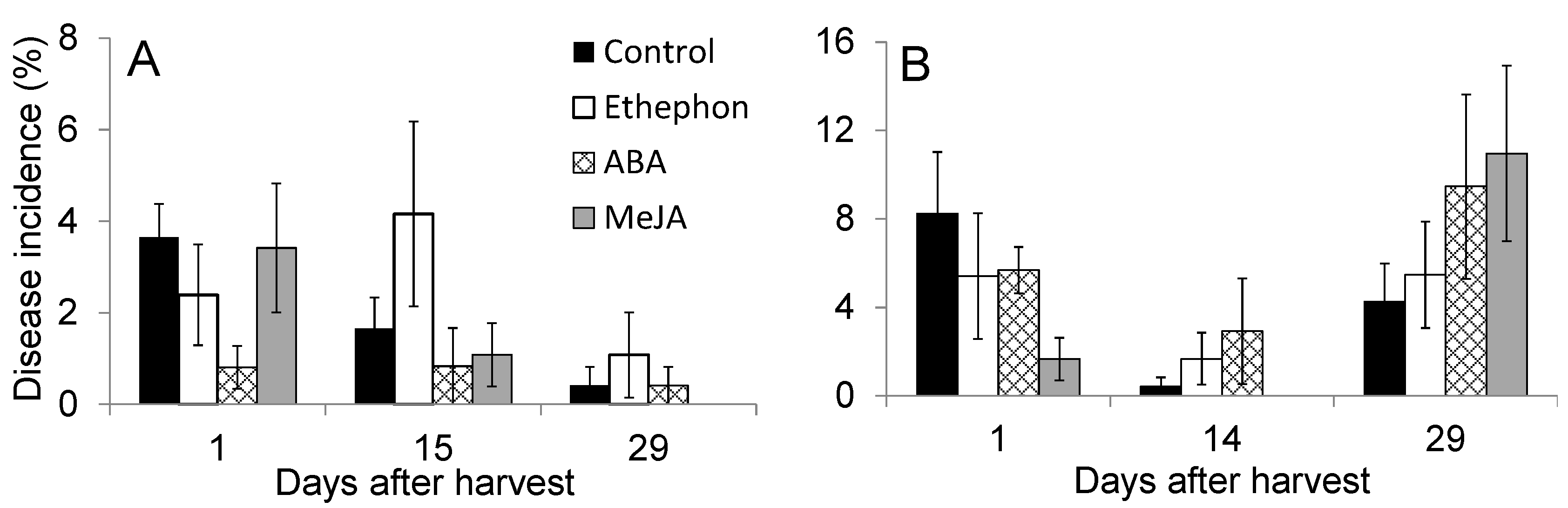
3.3. Effect of PGR Application on Fruit Quality during Postharvest Storage
3.4. Effect of PGR Application on Postharvest Disease Incidence During Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A. metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Evans, E.A.; Ballen, F.H. An Overview of US Blueberry Production, Trade, and Consumption, with Special Reference to Florida; Publication FE952; University of Florida, Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2014. [Google Scholar]
- Lang, G.A. Southern highbush blueberries: Physiological and cultural factors important for optimal cropping of these complex hybrids. Acta Hortic. 1993, 346, 72–80. [Google Scholar] [CrossRef]
- Boches, P.S.; Bassil, N.V.; Rowland, L.J. Genetic diversity in the highbush blueberry evaluated with microsatellite markers. J. Am. Soc. Hort. Sci. 2006, 131, 674–686. [Google Scholar]
- Rowland, L.J.; Ogden, E.L.; Bassil, N.; Buck, E.J.; McCallum, S.; Graham, J.; Brown, A.; Wiedow, C.; Campbell, A.M.; Haynes, K.G.; et al. Construction of a genetic linkage map of an interspecific diploid blueberry population and identification of QTL for chilling requirement and cold hardiness. Mol. Breed. 2014, 34, 2033–2048. [Google Scholar] [CrossRef]
- Suzuki, A.; Kikuchi, T.; Aoba, K. Changes of ethylene activity evolution, ACC content, in fruits ethylene forming blueberry enzyme and respiration of highbush blueberry. J. Jpn Soc. Hort. Sci. 1997, 66, 23–27. [Google Scholar] [CrossRef]
- Brown, G.K.; Marshall, D.E.; Tennes, B.R.; Booster, D.E.; Chen, P.; Garrett, R.E.; O’Brien, M.; Studer, H.E.; Kepner, R.A.; Hedden, S.L.; et al. Status of harvest mechanization of horticultural crops. Am. Soc. Agric. Eng. 1983, 83, 3. [Google Scholar]
- Brown, G.K.; Shulte, N.L.; Timm, E.J.; Beaudry, R.M.; Peterson, D.L.; Hancock, J.F.; Takeda, F. Estimates of mechanization effects on fresh blueberry quality. Appl. Eng. Agric. 1996, 12, 21–26. [Google Scholar] [CrossRef]
- Safley, C.D.; Cline, W.O.; Mainland, C.M. Estimated costs of producing, harvesting, and marketing blueberries in the southeastern United States. In Proceedings of the 12th Biennial Southeast Blueberry Conference, Savannah, GA, USA, 6–9 January 2005; pp. 33–49. [Google Scholar]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Genetic regulation of fruit development and ripening. Plant Cell 2004, 16, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Symons, G.M.; Chua, Y.J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Expt. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Abdul Shukor, A.R.; Yulianingsih, N.H.; Acedo, A.L.; Teng, K.C. Regulation of ripening in banana. In Banana: Fruit Development, Postharvest Physiology, Handling and Marketing in ASEAN; Abdullah, H., Pantastico, E.B., Eds.; ASEAN Food Handling Bureau: Kuala Lumpur, Malaysia, 1990; pp. 72–84. [Google Scholar]
- Cherian, S.; Figueroa, C.R.; Nair, H. ‘Movers and shakers’ in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Expt. Bot. 2014, 65, 4705–4722. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J. Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Windus, N.D.; Shutak, V.G.; Gough, R.E. CO2 and C2H4 evolution by highbush blueberry fruit. HortScience 1976, 11, 515–517. [Google Scholar]
- El-Agamy, S.; Aly, M.M.; Biggs, R.H. Fruit maturity as related to ethylene in ‘Delite’ blueberry. Proc. Fla. State Hortic. Soc. 1982, 95, 245–246. [Google Scholar]
- Eck, P. Influence of ethrel upon highbush blueberry fruit ripening. HortScience 1970, 5, 23–25. [Google Scholar]
- Dekazos, E.D. Effects of preharvest applications of ethephon and SADH on ripening, firmness and storage quality of rabbiteye blueberries (cv ‘T.-19’). Proc. Fla. State Hort. Soc. 1976, 89, 266–270. [Google Scholar]
- Ban, T.; Kugishima, M.; Ogata, T.; Shiozaki, S.; Horiuchi, S.; Ueda, H. Effect of ethephon (2-chloroethylphosphonic acid) on the fruit ripening characters of rabbiteye blueberry. Sci. Hortic. 2007, 112, 278–281. [Google Scholar] [CrossRef]
- Frenkel, C. Involvement of peroxidase and indole-3-acetic acid oxidase isozymes from pear, tomato, and blueberry fruit in ripening. Plant Physiol. 1972, 49, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Buesa, C.; Dominguez, M.; Vendrell, M. Abscisic acid effects on ethylene production and respiration rate in detached apple fruits at different stages of development. Revista Española de Ciencia y Tecnología de Alimentos 1994, 34, 495–506. [Google Scholar]
- Rodrigo, M.; Marcos, J.F.; Alferez, F.; Mallent, M.D.; Zacarias, L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J. Exp. Bot. 2003, 54, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Inoue, K. Abscisic acid (ABA) and 1-aminocyclopropane-1-carboxylic acid (ACC) content during growth of ‘Satohnishiki’ cherry fruit, and effect of ABA and ethephon application on fruit quality. J. Hortic. Sci. 1997, 72, 221–227. [Google Scholar] [CrossRef]
- Chai, Y.M.; Jia, H.F.; Li, C.L.; Dong, Q.H.; Shen, Y.Y. FaPYR1 is involved in strawberry fruit ripening. J. Expt. Bot. 2011, 62, 5079–5089. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.F.; Chai, Y.M.; Li, C.L.; Lu, D.; Luo, J.J.; Qin, L.; Shen, Y.Y. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011, 157, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Gagné, S.; Esteve, K.; Deytieux-Belleau, C.; Saucier, C.; Geny, L. Influence of abscisic acid in triggering véraison in grape berry skins of Vitis vinifera L. cv. Cabernet-Sauvignon. J. Int. Sci. 2006, 40, 7–14. [Google Scholar] [CrossRef]
- Jeong, S.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Peppi, M.C.; Fidelibus, M.W.; Dokoozlian, N. Abscisic acid application timing and concentration affect firmness, pigmentation, and color of ‘Flame Seedless’ grapes. HortScience 2006, 41, 1440–1445. [Google Scholar]
- Cantín, C.M.; Fidelibus, M.W.; Crisosto, C.H. Application of abscisic acid (ABA) at Veraison advanced red color development and maintained postharvest quality of “Crimson Seedless” grapes. Postharvest Biol. Technol. 2007, 46, 237–241. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, B.; Leng, P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 2009, 60, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Mou, W.; Li, D.; Bu, J.; Jiang, Y.; Khan, Z.U.; Luo, Z.; Mao, L.; Ying, T. Comprehensive analysis of ABA effects on ethylene biosynthesis and signaling during tomato fruit ripening. PLoS ONE 2016, 11, e0154072. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Joyce, D.; Macnish, A. Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J. Plant Growth Regul. 2000, 19, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, K.; Hirvelä, E.; Nevala, T.; Sipari, N.; Suokas, M.; Jaakola, L. Changes in the abscisic acid levels and related gene expression during fruit development and ripening in bilberry (Vaccinium myrtillus L.). Phytochemistry 2013, 95, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zifkin, M.; Jin, A.; Ozga, J.A.; Zaharia, L.I.; Schernthaner, J.P.; Gesell, A.; Abrams, S.R.; Kennedy, J.A.; Constabel, C.P. Gene expression and metabolite profiling of developing Highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012, 158, 200–224. [Google Scholar] [CrossRef] [PubMed]
- Buran, T.J.; Sandhu, A.K.; Azeredo, A.M.; Bent, A.H.; Williamson, J.G.; Gu, L. Effects of exogenous abscisic acid on fruit quality, antioxidant capacities, and phytochemical contents of southern high bush blueberries. Food Chem. 2012, 132, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates: A review. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P.; Fellman, J.K. A role for jasmonates in climacteric fruit ripening. Planta 1998, 204, 444–449. [Google Scholar] [CrossRef]
- Kondo, S.; Setha, S.; Rudell, D.R.; Buchanan, D.; Mattheis, J.P. Aroma volatile biosynthesis in apples affected by 1-MCP and methyl jasmonate. Postharvest Biol. Technol. 2005, 36, 61–68. [Google Scholar] [CrossRef]
- Ziosi, V.; Bonghi, C.; Bregoli, A.M.; Trainotti, L.; Biondi, S.; Sutthiwal, S.; Kondo, S.; Costa, G.; Torrigiani, P. Jasmonate-induced transcriptional changes suggest a negative interference with the ripening syndrome in peach fruit. J. Exp. Bot. 2008, 59, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wen, X.; Tang, L. Effect of methyl jasmonic acid on peach fruit ripening progress. Sci. Hortic. 2017, 220, 206–213. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Olías, R.; Olías, J.M. Effect of methyl jasmonate on in vitro strawberry ripening. J. Agric. Food Chem. 1997, 45, 3733–3737. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zheng, W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Int. J. Food Sci. Technol. 2005, 40, 187–195. [Google Scholar] [CrossRef]
- Cocetta, G.; Rossoni, M.; Gardana, C.; Mignani, I.; Ferrante, A.; Spinardi, A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum). Physiol. Plant. 2015, 153, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Mehra, L.K.; MacLean, D.D.; Savelle, A.T.; Scherm, H. Postharvest disease development on southern highbush blueberry fruit in relation to berry flesh type and harvest method. Plant Dis. 2013, 97, 213–221. [Google Scholar] [CrossRef]
- Forsyth, F.R.; Craig, D.L.; Stark, R. Ethephon as a chemical harvesting aid for the late season highbush blueberry Coville. Can. J. Plant Sci. 1977, 57, 1099–1102. [Google Scholar] [CrossRef]
- MacLean, D.D.; NeSmith, D.S. Rabbiteye blueberry postharvest fruit quality and stimulation of ethylene production by 1-Methylcylopropene. HortScience 2011, 46, 1278–1281. [Google Scholar]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Shelf life determination of fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. Int. J. Food Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.S.; Stergios, B.G.; Stackhouse, S.S.; Bittenbender, H.C.; Burton, C.L. Ethephon as a mechanical harvesting aid for highbush blueberries (Vaccinium austral Small). J. Am. Soc. Hort. Sci. 1976, 101, 111–115. [Google Scholar]
- Gupta, V.; Estrada, A.D.; Blakley, I.; Reid, R.; Patel, K.; Meyer, M.D.; Andersen, S.U.; Brown, A.F.; Lila, M.A.; Loraine, A.E. RNA-Seq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. GigaScience 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Malladi, A.; Vashisth, T.; Johnson, L.K. Ethephon and methyl jasmonate affect fruit detachment in rabbiteye and southern highbush blueberry. HortScience 2012, 47, 1745–1749. [Google Scholar]
- Vashisth, T.; Malladi, A. Fruit detachment in rabbiteye blueberry: Abscission and physical separation. J. Am. Soc. Hortic. Sci. 2013, 138, 95–101. [Google Scholar]
- Vashisth, T.; Malladi, A. Fruit abscission in rabbiteye blueberry in response to organ removal and mechanical wounding. HortScience 2014, 49, 1403–1407. [Google Scholar]
| Cultivar/Treatment z | L* | a* | b* | c* | h* |
|---|---|---|---|---|---|
| Premier | |||||
| Control | 38.0 | −1.1 | −6.3 | 6.4 | 260.0 |
| Ethephon | 38.3 | −1.0 | −6.3 | 6.4 | 261.2 |
| ABA | 37.9 | −1.0 | −5.9 | 6.1 | 260.2 |
| MeJA | 38.1 | −1.0 | −6.3 | 6.5 | 260.9 |
| Significance | NS | NS | NS | NS | NS |
| Powderblue | |||||
| Control | 40.9b | −1.2 | −6.37a | 6.6ab | 260.5 |
| Ethephon | 43.3ab | −1.3 | −6.44ab | 6.6ab | 258.1 |
| ABA | 40.7b | −1.1 | −6.16a | 6.3b | 260.1 |
| MeJA | 44.0a | −1.4 | −6.77b | 6.9a | 258.6 |
| Significance | 0.0078 | NS | 0.0066 | 0.0063 | NS |
| Defective Fruit (%) z | |||
|---|---|---|---|
| Cultivar/Treatment | 1 Day | 15 Days | 29 Days |
| Premier | |||
| Control | 3.3 | 19.2 | 23.3b |
| Ethephon | 2.2 | 7.8 | 10.0c |
| ABA | 3.3 | 16.7 | 43.3a |
| MeJA | 11.7 | 15.0 | 28.3ab |
| Significance | NS | NS | 0.0003 |
| Powderblue | |||
| Control | 5.0 | 13.3 | 20.0 |
| Ethephon | 5.0 | 6.7 | 19.2 |
| ABA | 4.2 | 8.3 | 18.3 |
| MeJA | 5.8 | 11.7 | 21.7 |
| Significance | NS | NS | NS |
| Berry Texture z | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compression (kgF) | Pressure (kgF) | Berry Weight (g) z | |||||||
| Cultivar/Treatment | 1 d | 15 d | 29 d | 1 d | 15 d | 29 d | 1 d | 15 d | 29 d |
| Premier | |||||||||
| Control | 0.22 | 0.20 | 0.18a | 0.15 | 0.15 | 0.12 | 0.81ab | 0.82ab | 0.81 |
| Ethephon | 0.23 | 0.20 | 0.19a | 0.15 | 0.15 | 0.12 | 0.77ab | 0.80ab | 0.76 |
| ABA | 0.20 | 0.19 | 0.15b | 0.14 | 0.14 | 0.11 | 0.86a | 0.88a | 0.79 |
| MeJA | 0.23 | 0.21 | 0.19a | 0.15 | 0.16 | 0.13 | 0.70b | 0.72b | 0.70 |
| Significance | NS | NS | 0.0166 | NS | NS | NS | 0.0229 | 0.0067 | NS |
| Powderblue | |||||||||
| Control | 0.23 | 0.19b | 0.19ab | 0.19 | 0.15 | 0.14 | 0.87 | 0.83 | 0.82 |
| Ethephon | 0.26 | 0.22a | 0.21a | 0.18 | 0.15 | 0.15 | 0.64 | 0.68 | 0.69 |
| ABA | 0.24 | 0.20b | 0.18b | 0.18 | 0.15 | 0.14 | 0.84 | 0.83 | 0.89 |
| MeJA | 0.24 | 0.21ab | 0.17b | 0.19 | 0.16 | 0.15 | 0.80 | 0.77 | 0.78 |
| Significance | NS | 0.0077 | 0.0120 | NS | NS | NS | NS | NS | NS |
| Total Soluble Solids (Brix) z | Titratable Acidity (%) z | Juice pH z | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cultivar/Treatment | 1 d | 15 d | 29 d | 1 d | 15 d | 29 d | 1 d | 15 d | 29 d |
| Premier | |||||||||
| Control | 11.2 | 10.6 | 10.6 | 0.40 | 0.34 | 0.27 | 3.48 | 3.60 | 3.70 |
| Ethephon | 9.7 | 9.8 | 9.4 | 0.46 | 0.37 | 0.35 | 3.47 | 3.60 | 3.53 |
| ABA | 10.9 | 9.6 | 9.9 | 0.37 | 0.34 | 0.29 | 3.53 | 3.60 | 3.60 |
| MeJA | 10.5 | 9.8 | 9.9 | 0.44 | 0.36 | 0.30 | 3.43 | 3.58 | 3.70 |
| Significance | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Powderblue | |||||||||
| Control | 12.7 | 13.1a z | 13.2 | 0.48 | 0.45b | 0.36 | 3.48a | 3.48a | 3.45 |
| Ethephon | 12.0 | 12.1ab | 12.6 | 0.56 | 0.54a | 0.41 | 3.35b | 3.38a | 3.45 |
| ABA | 11.4 | 11.6b | 12.4 | 0.49 | 0.50ab | 0.36 | 3.40ab | 3.40a | 3.48 |
| MeJA | 12.1 | 13.0a | 13.2 | 0.60 | 0.53ab | 0.41 | 3.35b | 3.38a | 3.43 |
| Significance | NS | 0.0166 | NS | NS | 0.0490 | NS | 0.0150 | 0.0486 | NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-W.; Malladi, A.; Doyle, J.W.; Scherm, H.; Nambeesan, S.U. The Effect of Ethephon, Abscisic Acid, and Methyl Jasmonate on Fruit Ripening in Rabbiteye Blueberry (Vaccinium virgatum). Horticulturae 2018, 4, 24. https://doi.org/10.3390/horticulturae4030024
Wang Y-W, Malladi A, Doyle JW, Scherm H, Nambeesan SU. The Effect of Ethephon, Abscisic Acid, and Methyl Jasmonate on Fruit Ripening in Rabbiteye Blueberry (Vaccinium virgatum). Horticulturae. 2018; 4(3):24. https://doi.org/10.3390/horticulturae4030024
Chicago/Turabian StyleWang, Yi-Wen, Anish Malladi, John W. Doyle, Harald Scherm, and Savithri U. Nambeesan. 2018. "The Effect of Ethephon, Abscisic Acid, and Methyl Jasmonate on Fruit Ripening in Rabbiteye Blueberry (Vaccinium virgatum)" Horticulturae 4, no. 3: 24. https://doi.org/10.3390/horticulturae4030024
APA StyleWang, Y.-W., Malladi, A., Doyle, J. W., Scherm, H., & Nambeesan, S. U. (2018). The Effect of Ethephon, Abscisic Acid, and Methyl Jasmonate on Fruit Ripening in Rabbiteye Blueberry (Vaccinium virgatum). Horticulturae, 4(3), 24. https://doi.org/10.3390/horticulturae4030024






