Effects of Ozone Treatment on Reactive Oxygen Species Metabolism and Storage Quality of Flat Jujubes (Ziziphus jujuba Mill. cv. Panzao)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample
2.2. Ozone Treatment
2.3. Quality Measurements
2.3.1. Color Change and Degree of Browning
2.3.2. Weight Loss, Respiratory Rate, Firmness, Total Soluble Solid (TSS), Titratable Acid (TA), and Ascorbic Acid (ASA) Content
2.3.3. Total Flavonoid and Total Phenolic Content
2.3.4. 2,2 Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) Scavenging Activities
2.3.5. H2O2 Content, Superoxide Anions (O2−) Generation Rate, and Malondialdehyde (MDA) Content
2.3.6. Enzyme Activity Assay
2.4. Statistical Analyses
3. Results and Discussion
3.1. Color Change and Degree of Browning
3.2. Weight Loss, TA, TSS Content, Respiration Rate, Firmness, ASA Content
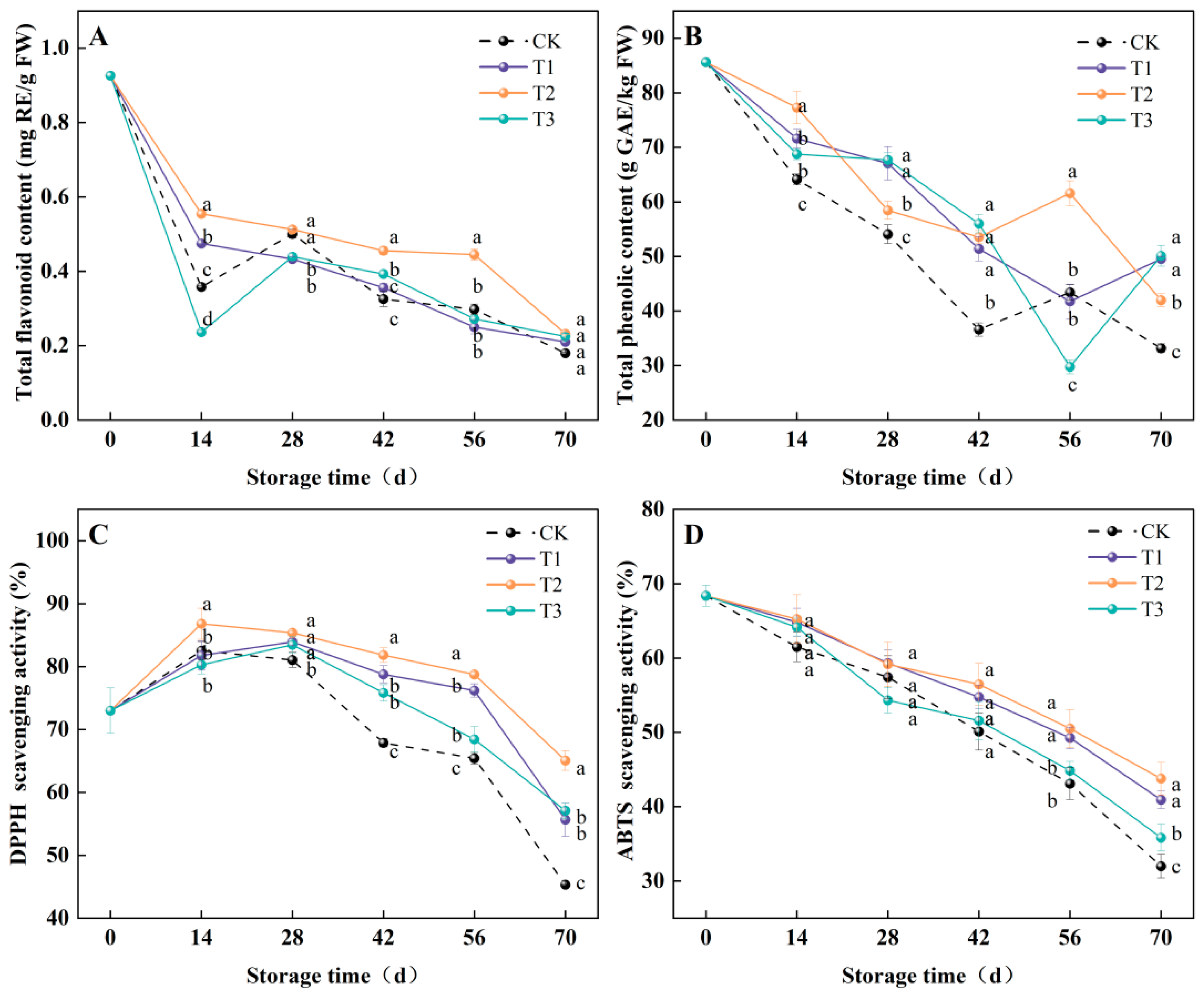
3.3. Total Flavonoid Content, Total Phenolic Content, DPPH, and ABTS Scavenging Activities
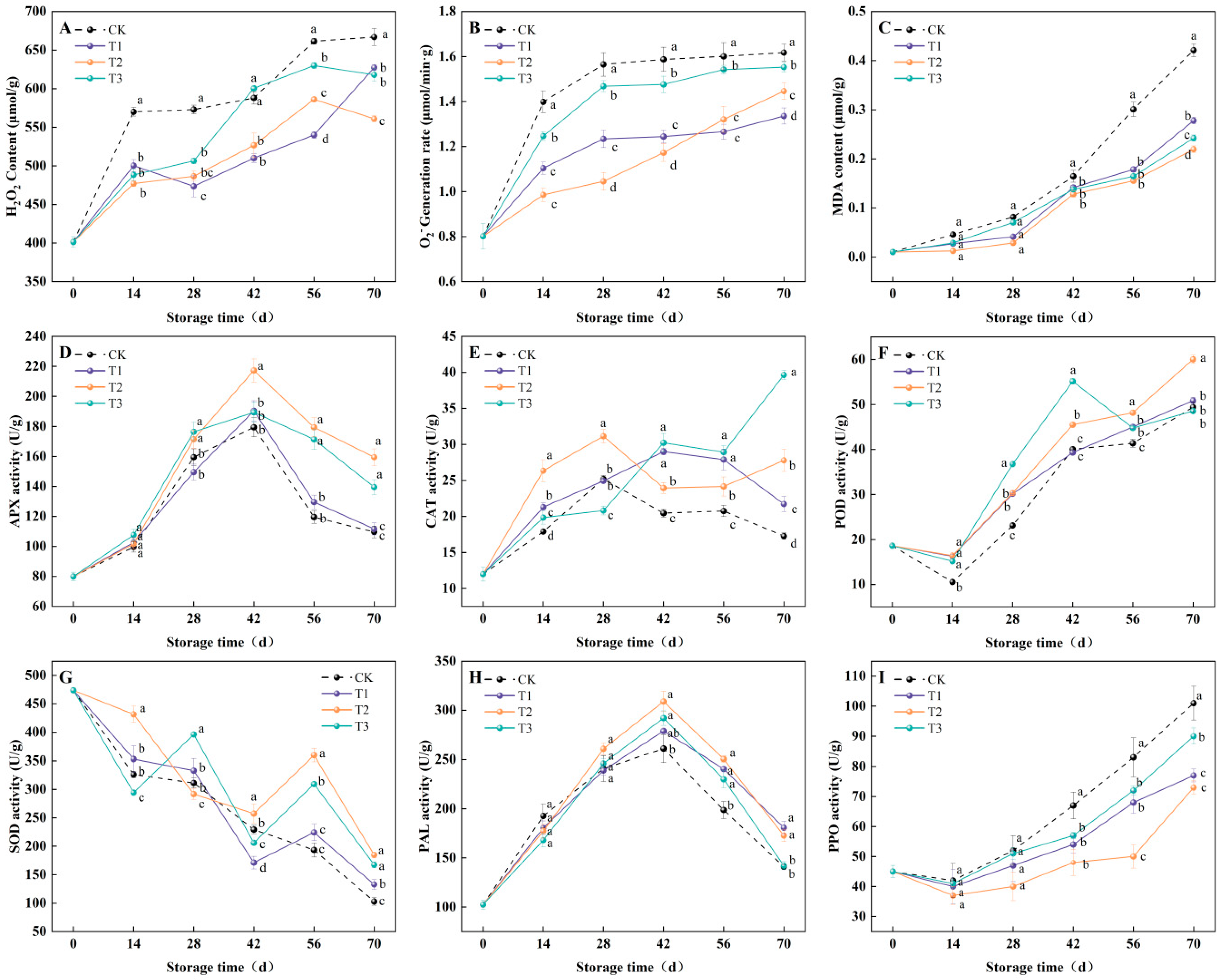
3.4. ROS Metabolism and Defense Enzyme Activity Changes
4. Multi-Way ANOVA Based on Measured Parameters
5. Principal Component Analysis and Correlation Analysis
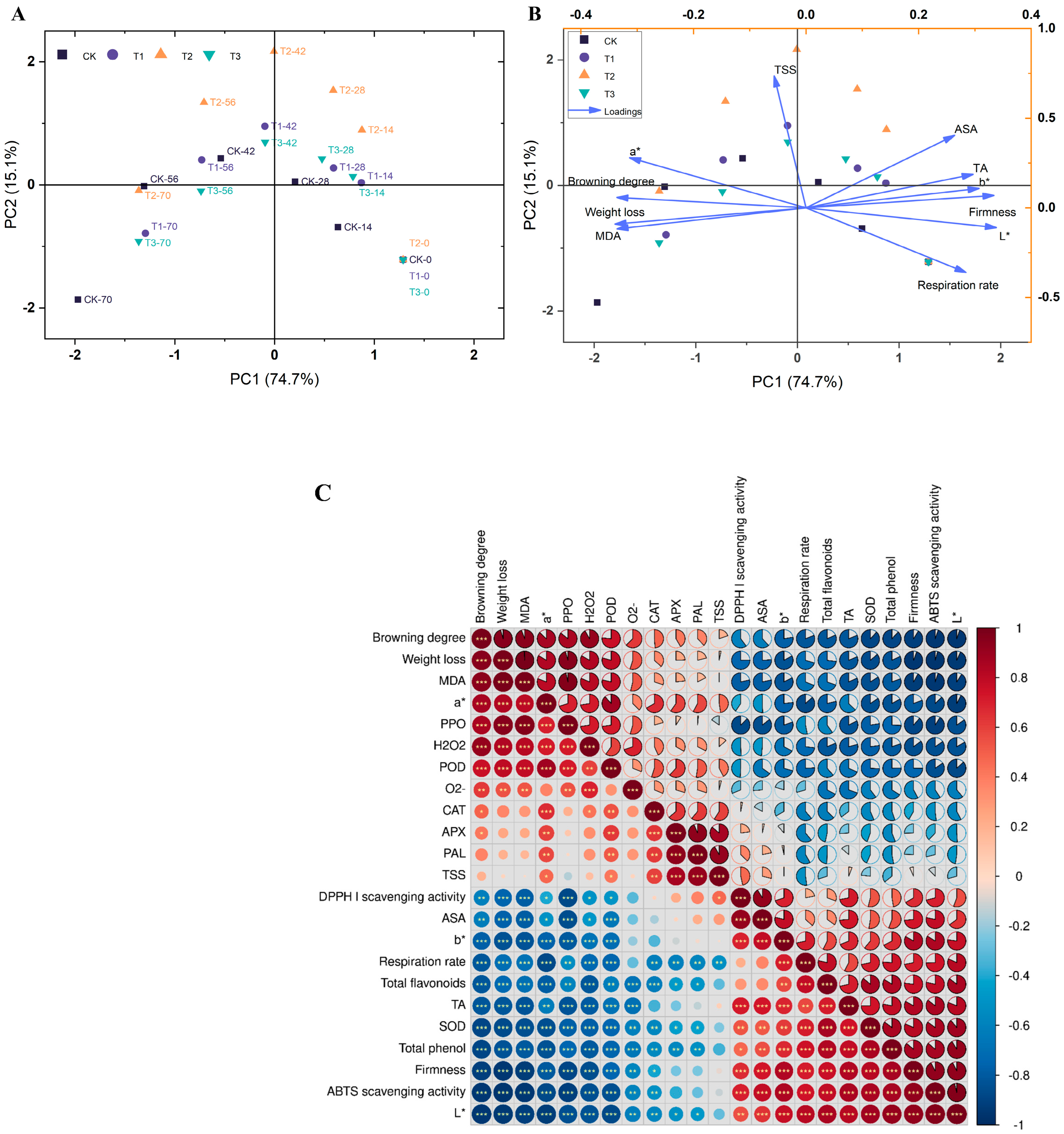
5.1. Principal Component Analysis
5.2. Correlation Analysis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Zhang, S.; Xue, J.; Sun, H. Lightweight Target Detection for the Field Flat Jujube Based on Improved YOLOv5. Comput. Electron. Agric. 2022, 202, 107391. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, Q.; Gou, M.; Liu, Z.; Purcaro, G.; Jin, X.; Wu, X.; Lyu, J.; Bi, J. Elucidation of Baking Induced Changes in Key Odorants of Red Jujube (Ziziphus jujuba Mill. Cv.‘Junzao’). J. Food Compos. Anal. 2023, 120, 105320. [Google Scholar] [CrossRef]
- Zuo, Z.; Jiang, P.; Chen, D.; Zhang, C.; Guo, F.; Nie, X.; Wu, D.; Fan, X.; Zhao, H. Improving the Storage Quality and Antioxidant Capacity of Postharvest Winter Jujube by Laser Microporous Modified Atmosphere Packaging. Sci. Hortic. 2024, 337, 113477. [Google Scholar] [CrossRef]
- Dou, J.-F.; Kou, X.-H.; Wu, C.-E.; Fan, G.-J.; Li, T.-T.; Li, X.-J.; Zhou, D.-D.; Yan, Z.-C.; Zhu, J.-P. Recent Advances and Development of Postharvest Management Research for Fresh Jujube Fruit: A Review. Sci. Hortic. 2023, 310, 111769. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Li, X.; Hu, T.; Jatt, A.-N.; Zhang, C.; Gong, H. Effects of Postharvest Chitosan and Potassium Sorbate Coating on the Storage Quality and Fungal Community of Fresh Jujube. Postharvest Biol. Technol. 2023, 205, 112503. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, S.; Zhu, Z.; Xu, Y.; Qin, G. Effects of 1-Methylcyclopropene(1-MCP) on Ripening and Resistance of Jujube (Zizyphus jujuba Cv. Huping) Fruit against Postharvest Disease. LWT-Food Sci. Technol. 2012, 45, 13–19. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Li, W.; Xu, Y.; Han, X.; Bao, N.; Sheng, Z.; Yuan, Y.; Zhang, X.; Luo, Z. Elevated O2 Alleviated Anaerobic Metabolism in Postharvest Winter Jujube Fruit by Regulating Pyruvic Acid and Energy Metabolism. Postharvest Biol. Technol. 2023, 203, 112397. [Google Scholar] [CrossRef]
- Hou, S.; Yang, Q.; Zheng, J.; Liu, X. A Novel Postharvest Preservation Technology of Fruit—Intermittent High Oxygen Treatment: A Case Study on “Lingwu” Long Jujube. J. Food Process. Preserv. 2022, 46, e17209. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, J.-H.; Sun, D.-W. Cold Plasma-Mediated Treatments for Shelf Life Extension of Fresh Produce: A Review of Recent Research Developments. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1312–1326. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; He, S.; Luo, Y.; Chen, S.; Shan, Y.; Wang, R.; Ding, S. Heat Shock Treatment Maintains the Quality Attributes of Postharvest Jujube Fruits and Delays Their Senescence Process during Cold Storage. J. Food Biochem. 2021, 45, e13937. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, Y.; Tang, Y.; Yang, W.; Guo, M.; Chen, G. Transcriptome Sequencing Reveals Mechanism of Improved Antioxidant Capacity and Maintained Postharvest Quality of Winter Jujube during Cold Storage after Salicylic Acid Treatment. Postharvest Biol. Technol. 2022, 189, 111929. [Google Scholar] [CrossRef]
- Ban, Z.; Niu, C.; Li, L.; Gao, Y.; Liu, L.; Lu, J.; Farouk, A.; Chen, C. Exogenous Brassinolides and Calcium Chloride Synergically Maintain Quality Attributes of Jujube Fruit (Ziziphus jujuba Mill.). Postharvest Biol. Technol. 2024, 216, 113039. [Google Scholar] [CrossRef]
- Kaur, K.; Pandiselvam, R.; Kothakota, A.; Padma Ishwarya, S.; Zalpouri, R.; Mahanti, N.K. Impact of Ozone Treatment on Food Polyphenols—A Comprehensive Review. Food Control 2022, 142, 109207. [Google Scholar] [CrossRef]
- Peng, X.; Dong, C.; Zhang, N.; Zheng, P.; Bai, Y.; Ji, H.; Yu, J.; Ban, Z.; Chen, C.; Hu, Y. Effect of Ozone Treatment on the Decay and Cell Wall Metabolism during the Postharvest Storage of Cantaloupe. Sci. Hortic. 2024, 331, 113119. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Chen, S.; Li, X. Reactive Oxygen Species Signaling in Melatonin-Mediated Plant Stress Response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS Interplay between Plant Growth and Stress Biology: Challenges and Future Perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Batista Silva, W.; Cosme Silva, G.M.; Santana, D.B.; Salvador, A.R.; Medeiros, D.B.; Belghith, I.; da Silva, N.M.; Cordeiro, M.H.M.; Misobutsi, G.P. Chitosan Delays Ripening and ROS Production in Guava (Psidium guajava L.) Fruit. Food Chem. 2018, 242, 232–238. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. One-Time Ozone Treatment Improves the Postharvest Quality and Antioxidant Activity of Actinidia arguta Fruit. Phytochemistry 2022, 203, 113393. [Google Scholar] [CrossRef]
- Huang, X.; Tian, Y.; Xing, J.; Chong, Y.; Chen, C.; Hou, Z. Coexpression Modules Constructed Identifies Regulation Pathways of Winter Jujube (Ziziphus jujuba Mill. ’Dongzao’) Following Postharvest Treatment with Ozone. Postharvest Biol. Technol. 2023, 197, 112183. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Zhang, X.; Dong, C.; Xue, W.; Xu, W. The Effect of Different Doses of Ozone Treatments on the Postharvest Quality and Biodiversity of Cantaloupes. Postharvest Biol. Technol. 2020, 163, 111124. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Zeng, T.; Nie, Q.; Zhang, F.; Zhu, L. Hydrogen Sulfide Inhibits Enzymatic Browning of Fresh-Cut Lotus Root Slices by Regulating Phenolic Metabolism. Food Chem. 2015, 177, 376–381. [Google Scholar] [CrossRef]
- Suo, K.; Zhang, Y.; Feng, Y.; Yang, Z.; Zhou, C.; Chen, W.; Wang, J. Ultrasonic Synergistic Slightly Acidic Electrolyzed Water Processing to Improve Postharvest Storage Quality of Chinese Bayberry. Ultrason. Sonochem. 2023, 101, 106668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, K.; Zhang, X.; Dong, C.; Ji, H.; Ke, R.; Ban, Z.; Hu, Y.; Lin, S.; Chen, C. Effects of Ozone Treatment on the Antioxidant Capacity of Postharvest Strawberry. RSC Adv. 2020, 10, 38142–38157. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, D.; Peng, J.; Pan, L.; Tu, K. Hot Air Treatment Induces Disease Resistance through Activating the Phenylpropanoid Metabolism in Cherry Tomato Fruit. J. Agric. Food Chem. 2017, 65, 8003–8010. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, H.; Fan, Z.; Wang, H.; Lin, M.; Chen, Y.; Hung, Y.-C.; Lin, Y. Inhibitory Effect of Propyl Gallate on Pulp Breakdown of Longan Fruit and Its Relationship with ROS Metabolism. Postharvest Biol. Technol. 2020, 168, 111272. [Google Scholar] [CrossRef]
- Wang, X.; Chao, H.; Ma, W.; Li, Y.; Yang, H.; Chen, W.; Li, L. Preservation Mechanism of Cold Plasma Pretreatment on the Antioxidant Activity and Quality of Prune during Storage. Food Res. Int. 2025, 206, 116081. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, W.; Liu, Y.; Sang, W.; Guo, M.; Cheng, S.; Chen, G. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao) Sci. Hortic. 2021, 288, 110372. [Google Scholar] [CrossRef]
- Liu, J.; Chang, M.; Meng, J.; Liu, J.; Cheng, Y.-F.; Feng, C. Effect of Ozone Treatment on the Quality and Enzyme Activity of Lentinus Edodes during Cold Storage. J. Food Process. Preserv. 2020, 44, e14557. [Google Scholar] [CrossRef]
- Al-Qarni, S.S.M.; Bazzi, M.D. Date Fruit Ripening with Degradation of Chlorophylls, Carotenes, and Other Pigments. Int. J. Fruit Sci. 2020, 20, S827–S839. [Google Scholar] [CrossRef]
- Shi, J.; Xiao, Y.; Jia, C.; Zhang, H.; Gan, Z.; Li, X.; Yang, M.; Yin, Y.; Zhang, G.; Hao, J.; et al. Physiological and Biochemical Changes during Fruit Maturation and Ripening in Highbush Blueberry (Vaccinium corymbosum L.). Food Chem. 2023, 410, 135299. [Google Scholar] [CrossRef]
- Van, T.T.; Tanaka, F.; Wardak, M.H.; Jothi, J.S.; Phuong, N.T.H.; Yan, X.; Zdunek, A.; Tanaka, F. Effect of Coatings Containing 1-Methylcyclopropane or Mandarin Peel Extract on the Freshness and Metabolic Profiles of Cold Stored Strawberry. Food Chem. 2024, 461, 140819. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, L.; Xu, Q.; Tian, X.; Sun, L.; Cai, J. Synergistic Treatment with Ozone Water and Morpholine Fatty Acid Salts Improves Postharvest Quality in Mandarin Oranges. Foods 2025, 14, 1346. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Yang, W.; Liu, Y.; Zhang, W.; Guo, T.; Shen, P.; Tang, Y.; Guo, M.; Chen, G. Influences of Low Temperature on the Postharvest Quality and Antioxidant Capacity of Winter Jujube (Zizyphus jujuba Mill. Cv. Dongzao). LWT 2022, 154, 112876. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Zhang, Y.; Jiang, A.; Hu, W. Aqueous Ozone Treatment Inhibited Degradation of Cellwall Polysaccharides in Fresh-Cut Apple during Cold Storage. Innov. Food Sci. Emerg. Technol. 2021, 67, 102550. [Google Scholar] [CrossRef]
- Minas, I.S.; Vicente, A.R.; Dhanapal, A.P.; Manganaris, G.A.; Goulas, V.; Vasilakakis, M.; Crisosto, C.H.; Molassiotis, A. Ozone-Induced Kiwifruit Ripening Delay Is Mediated by Ethylene Biosynthesis Inhibition and Cell Wall Dismantling Regulation. Plant Sci. 2014, 229, 76–85. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Wani, A.W.; Bakshi, M.; Dhiman, Z. Exploring Phenolic Compounds as Natural Stress Alleviators in Plants—A Comprehensive Review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Rodoni, L.; Casadei, N.; Concellón, A.; Chaves Alicia, A.R.; Vicente, A.R. Effect of Short-Term Ozone Treatments on Tomato (Solanum lycopersicum L.) Fruit Quality and Cell Wall Degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A.; Forney, C.F. Effects of Ozone on Major Antioxidants and Microbial Populations of Fresh-Cut Papaya. Postharvest Biol. Technol. 2014, 89, 56–58. [Google Scholar] [CrossRef]
- Soares, C.; Carvalho, M.E.A.; Azevedo, R.A.; Fidalgo, F. Plants Facing Oxidative Challenges—A Little Help from the Antioxidant Networks. Environ. Exp. Bot. 2019, 161, 4–25. [Google Scholar] [CrossRef]
- Nie, M.; Wu, C.; Xiao, Y.; Song, J.; Zhang, Z.; Li, D.; Liu, C. Efficacy of Aqueous Ozone Combined with Sodium Metasilicate on Microbial Load Reduction of Fresh-Cut Cabbage. Int. J. Food Prop. 2020, 23, 2065–2076. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Balawejder, M. Effects of Post-Harvest Ozone Treatment on Some Molecular Stability Markers of Amelanchier Alnifolia Nutt. fruit during Cold Storage. Int. J. Mol. Sci. 2022, 23, 11152. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Y.; Ye, L.; Shi, Y.; Luo, A. Ozone Treatment Modulates Reactive Oxygen Species Levels in Kiwifruit through the Antioxidant System: Insights from Transcriptomic Analysis. J. Plant Physiol. 2023, 291, 154135. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of Nitric Oxide and Melatonin in Improving Postharvest Fruit Quality and the Separate and Crosstalk Biochemical Mechanisms. Trends Food Sci. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of Ozone Pre-Conditioning on Quality and Antioxidant Capacity of Papaya Fruit during Ambient Storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Modesti, M.; Petriccione, M.; Forniti, R.; Zampella, L.; Scortichini, M.; Mencarelli, F. Methyl Jasmonate and Ozone Affect the Antioxidant System and the Quality of Wine Grape during Postharvest Partial Dehydration. Food Res. Int. 2018, 112, 369–377. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, G.; Han, Z.; Li, Q.; Chen, Y.; Li, D. Effect of Ozone on the Antioxidant Capacity of “Qiushui” Pear (Pyrus pyrifolia Nakai Cv. Qiushui) during Postharvest Storage. J. Food Qual. 2013, 36, 190–197. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, L.; Zhang, Z.; Li, J.; Zhao, C. Impact of Ozone on Quality of Strawberry during Cold Storage. Front. Agric. China 2011, 5, 356–360. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of Phenylalanine Ammonia-Lyase (PAL) in Increased Phenolic Compounds in Fresh Cold Stressed Walnut (Juglans regia L.) Kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Qu, W.; Ruan, W.; Feng, Y.; Liu, Y.; Tuly, J.A.; Zhou, C. Assessing the Dry Inactivation Mechanism of Polyphenol Oxidase (PPO) and Peroxidase (POD) Employing Catalytic Infrared Treatment Based on Experiments and Molecular Simulations. Innov. Food Sci. Emerg. Technol. 2025, 100, 103943. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, W.; Murtaza, A.; Iqbal, A.; Li, J.; Zhang, J.; Li, J.; Kong, M.; Xu, X.; Pan, S. Eugenol Treatment Delays the Flesh Browning of Fresh-Cut Water Chestnut (Eleocharis tuberosa) through Regulating the Metabolisms of Phenolics and Reactive Oxygen Species. Food Chem. X 2022, 14, 100307. [Google Scholar] [CrossRef]


| Source of Variation | Ozone Treatment (A) | Storage Time (B) | (A) × (B) |
|---|---|---|---|
| L* | 1.73 | 30.07 *** | 0.17 |
| a* | 44.96 ** | 209.40 *** | 4.50 ** |
| b* | 2.60 | 22.49 *** | 0.319 |
| Weight loss | 99.16 *** | 737.19 *** | 14.71 *** |
| TA content | 6.48 | 13.41 *** | 0.83 |
| TSS content | 2.70 | 5.92 *** | 0.22 |
| Respiration rate | 16.94 *** | 99.13 *** | 3.66 ** |
| Firmness | 8.06 *** | 52.52 *** | 0.54 |
| ASA content | 16.25 *** | 146.91 *** | 2.69 * |
| Total flavonoid content | 19.78 ** | 383.94 *** | 5.48 *** |
| Total phenolic content | 12.60 *** | 118.08 *** | 5.66 *** |
| DPPH scavenging activities | 7.86 *** | 29.33 *** | 1.10 |
| ABTS scavenging activities | 4.66 | 63.65 *** | 0.53 |
| H2O2 content | 9.00 *** | 34.53 *** | 1.06 |
| O2− generation rate | 29.60 *** | 60.23 *** | 1.97 |
| MDA content | 102.41 *** | 728.70 *** | 20.84 *** |
| APX activity | 19.93 *** | 135.43 *** | 3.38 *** |
| CAT activity | 32.53 *** | 89.26 *** | 16.10 *** |
| PPO activity | 31.38 *** | 130.31 *** | 3.82 ** |
| SOD activity | 17.63 *** | 204.70 *** | 7.48 *** |
| PAL activity | 5.10 ** | 159.36 *** | 1.77 |
| POD activity | 14.354 ** | 276.32 *** | 4.50 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Zheng, P.; Li, X.; Chen, C.; Dong, C.; Zhang, N.; Ji, H.; Yu, J.; Gao, Y.; Ju, T.; et al. Effects of Ozone Treatment on Reactive Oxygen Species Metabolism and Storage Quality of Flat Jujubes (Ziziphus jujuba Mill. cv. Panzao). Horticulturae 2025, 11, 1142. https://doi.org/10.3390/horticulturae11091142
Peng X, Zheng P, Li X, Chen C, Dong C, Zhang N, Ji H, Yu J, Gao Y, Ju T, et al. Effects of Ozone Treatment on Reactive Oxygen Species Metabolism and Storage Quality of Flat Jujubes (Ziziphus jujuba Mill. cv. Panzao). Horticulturae. 2025; 11(9):1142. https://doi.org/10.3390/horticulturae11091142
Chicago/Turabian StylePeng, Xuyang, Pufan Zheng, Xiaoxue Li, Cunkun Chen, Chenghu Dong, Na Zhang, Haipeng Ji, Jinze Yu, Ying Gao, Tinghu Ju, and et al. 2025. "Effects of Ozone Treatment on Reactive Oxygen Species Metabolism and Storage Quality of Flat Jujubes (Ziziphus jujuba Mill. cv. Panzao)" Horticulturae 11, no. 9: 1142. https://doi.org/10.3390/horticulturae11091142
APA StylePeng, X., Zheng, P., Li, X., Chen, C., Dong, C., Zhang, N., Ji, H., Yu, J., Gao, Y., Ju, T., Zhang, Y., Yan, R., & Chen, A. (2025). Effects of Ozone Treatment on Reactive Oxygen Species Metabolism and Storage Quality of Flat Jujubes (Ziziphus jujuba Mill. cv. Panzao). Horticulturae, 11(9), 1142. https://doi.org/10.3390/horticulturae11091142







