Exogenous Spermidine Enhances Drought Resistance of Mango Seedlings by Regulating Physiological and Biochemical Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Osmotic Potential
- Applicable range of concentration: The formula is applicable to a concentration range of 50–250 g/kg H2O (i.e., 5–25% w/w). Accuracy decreases when it exceeds this range.
- The importance of temperature correction: The formula contains a temperature correction factor (T), so it must be accurately calculated based on the actual cultivation temperature. For example, at 25 °C, T = 273 + 25 = 298 K.
- Unit conversion example (taking 20% concentration and 25 °C as an example):
≈ −[2.36 − 0.472 + 0.02136] × 298
≈ −1.909 × 298
2.3. Application of Treatments
2.4. Leaf Phenotype Measurement
2.5. Determination of the Relative Chlorophyll Content in Leaves
2.6. Determination of Antioxidant Enzyme Activities
2.7. Determination of Malondialdehyde (MDA) Content
2.8. Determination of Soluble Sugar Content
2.9. Determination of Proline Content
2.10. Determination of Soluble Protein Content
2.11. Statistical Analysis
3. Results
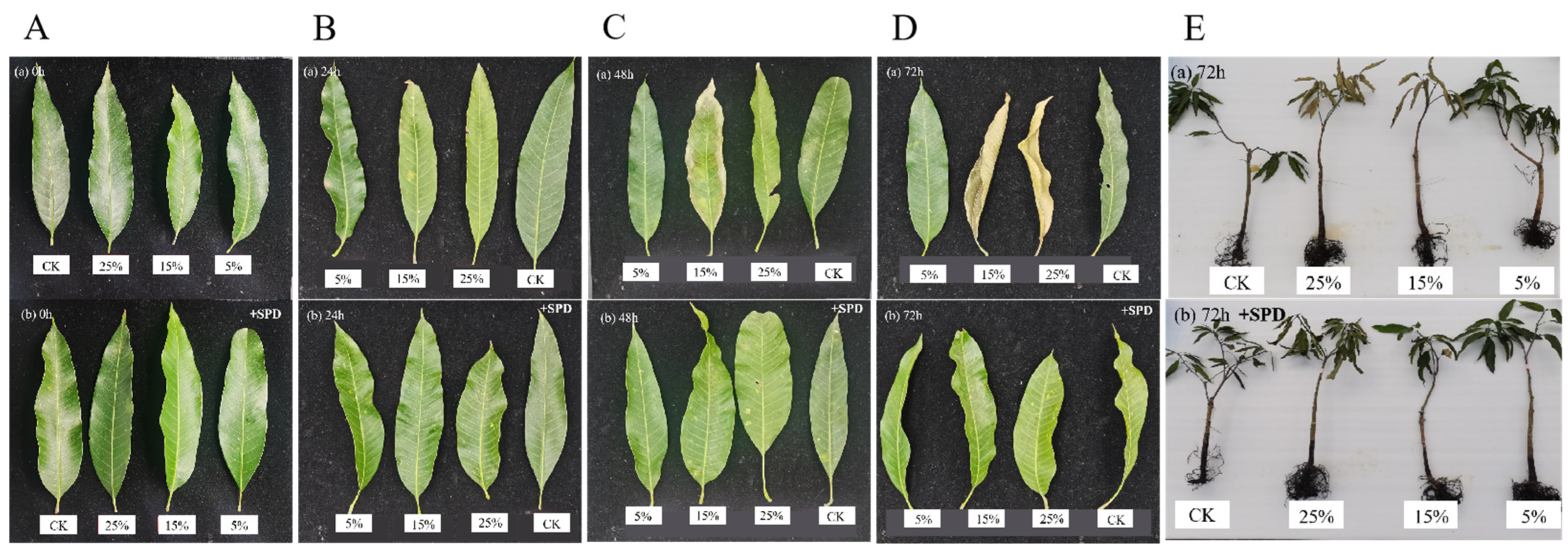
3.1. Effect of Exogenous Spermidine on Leaf Phenotypes of Mango Under Different Drought Stresses
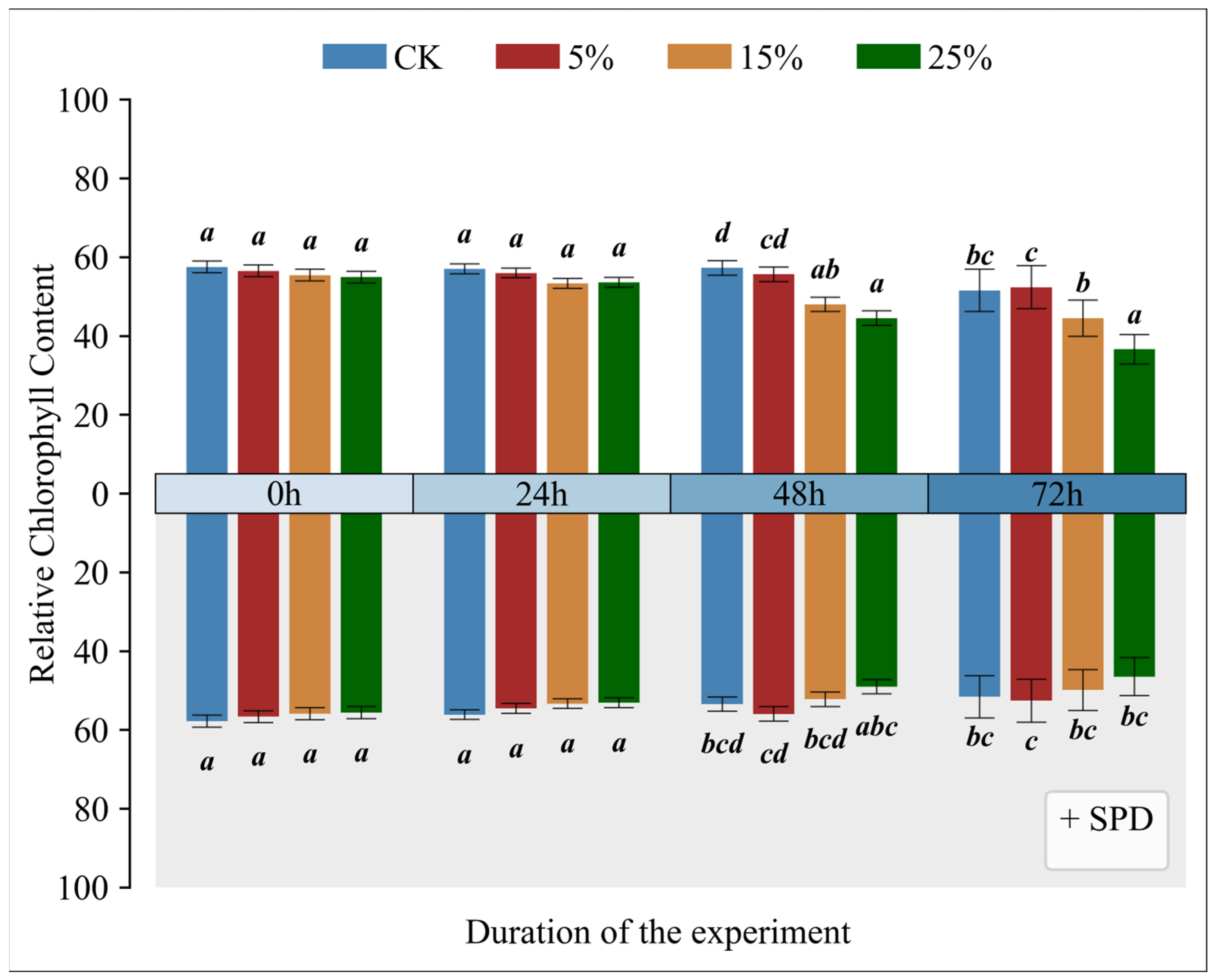
3.2. Impact of Exogenous Spermidine on Relative Chlorophyll Content in Mango Leaves Across Varying Drought Conditions
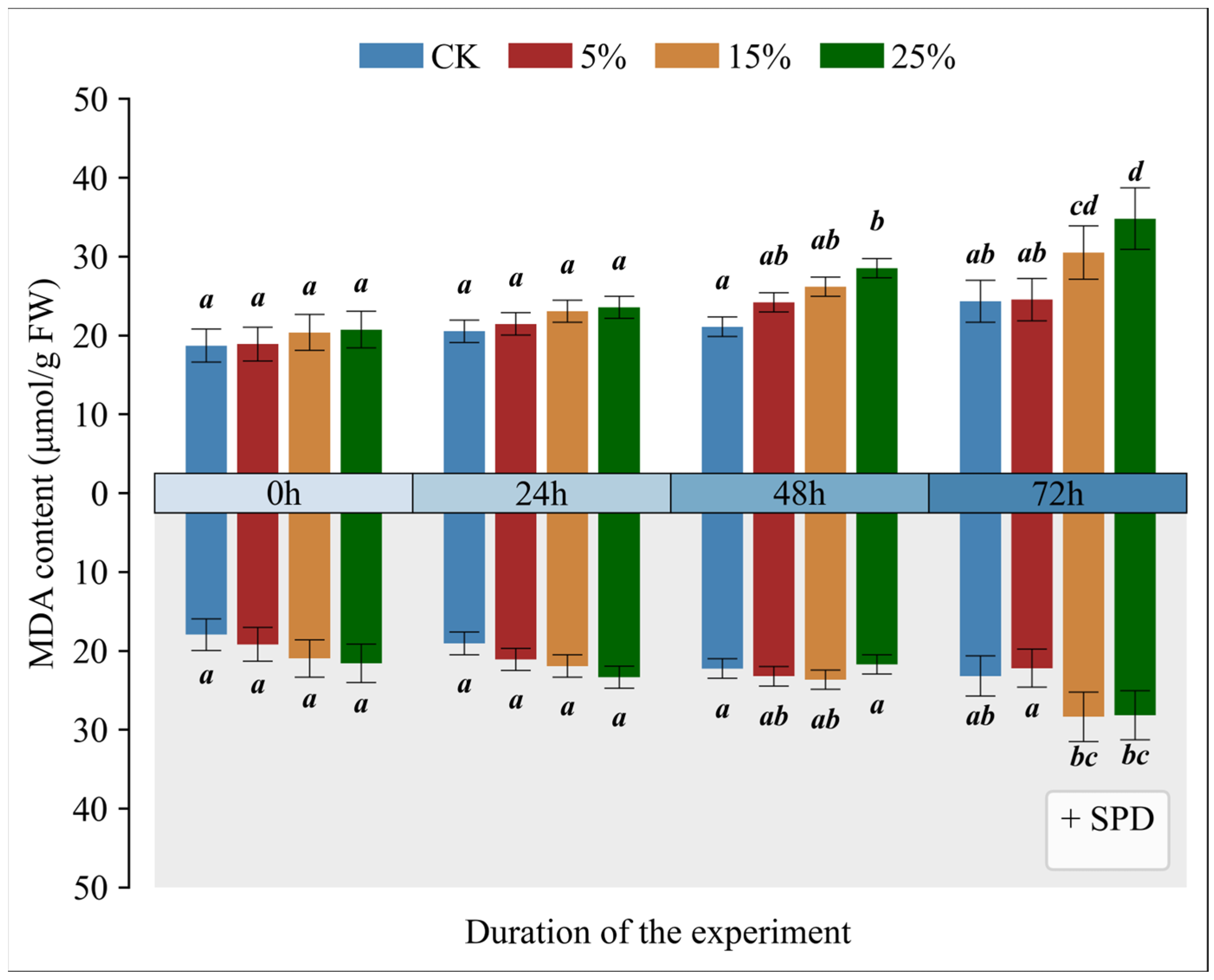
3.3. Impact of Exogenous Spermidine on Malondialdehyde Levels in Mango Leaves Under Varying Drought Conditions
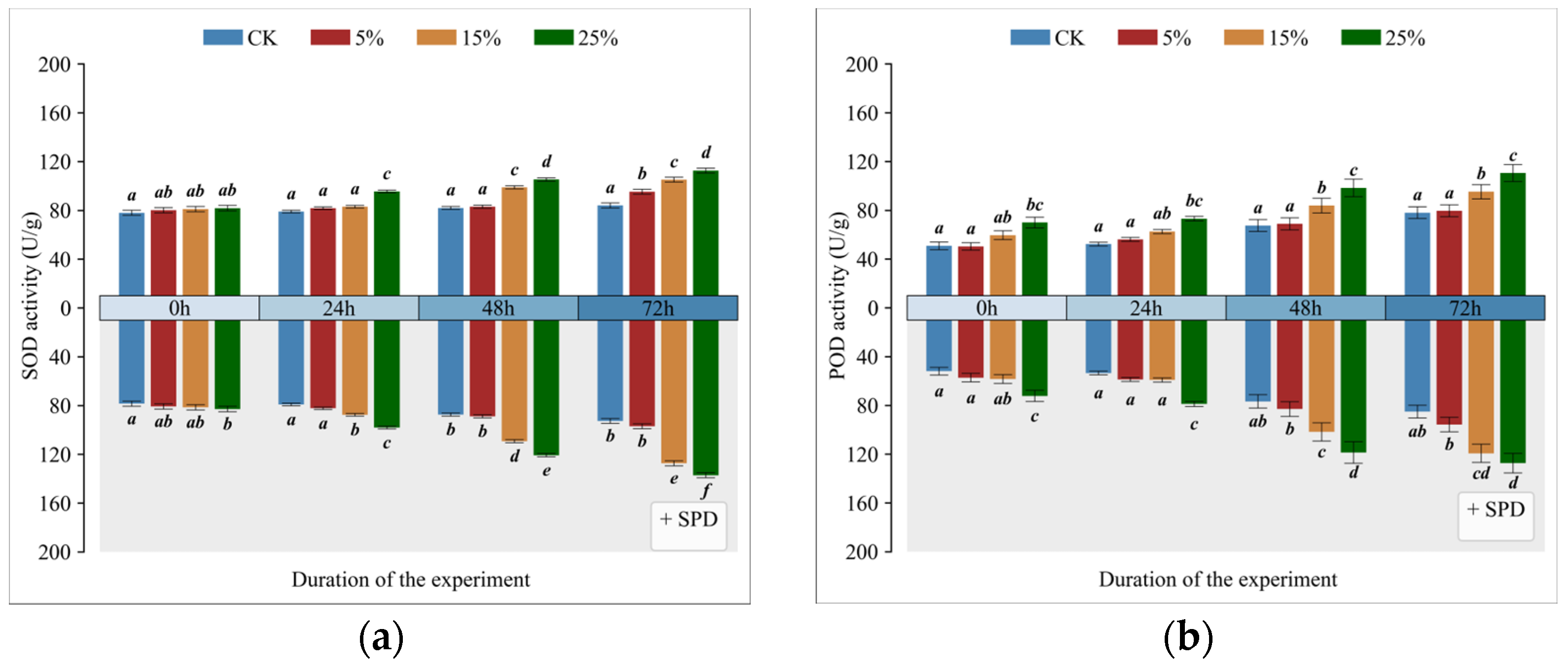
3.4. Impact of Exogenous Spermidine on Superoxide Dismutase (SOD) Activity in Mango Leaves Under Varying Drought Conditions
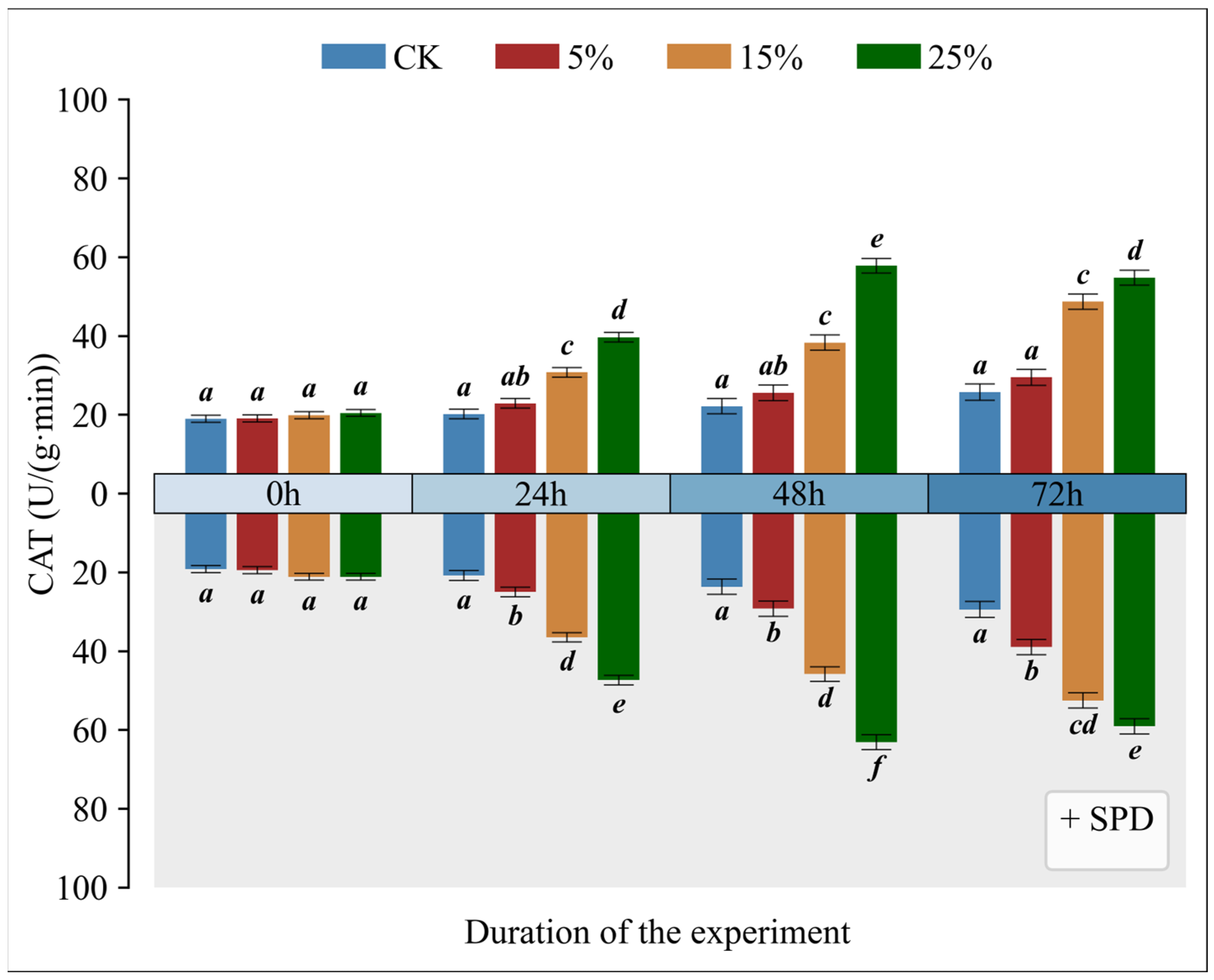
3.5. Impact of Exogenous Spermidine on Catalase Activity in Mango Leaves Under Varying Drought Conditions
3.6. Effect of Exogenous Spermidine on Proline Content of Mango Leaves Under Different Drought Stresses
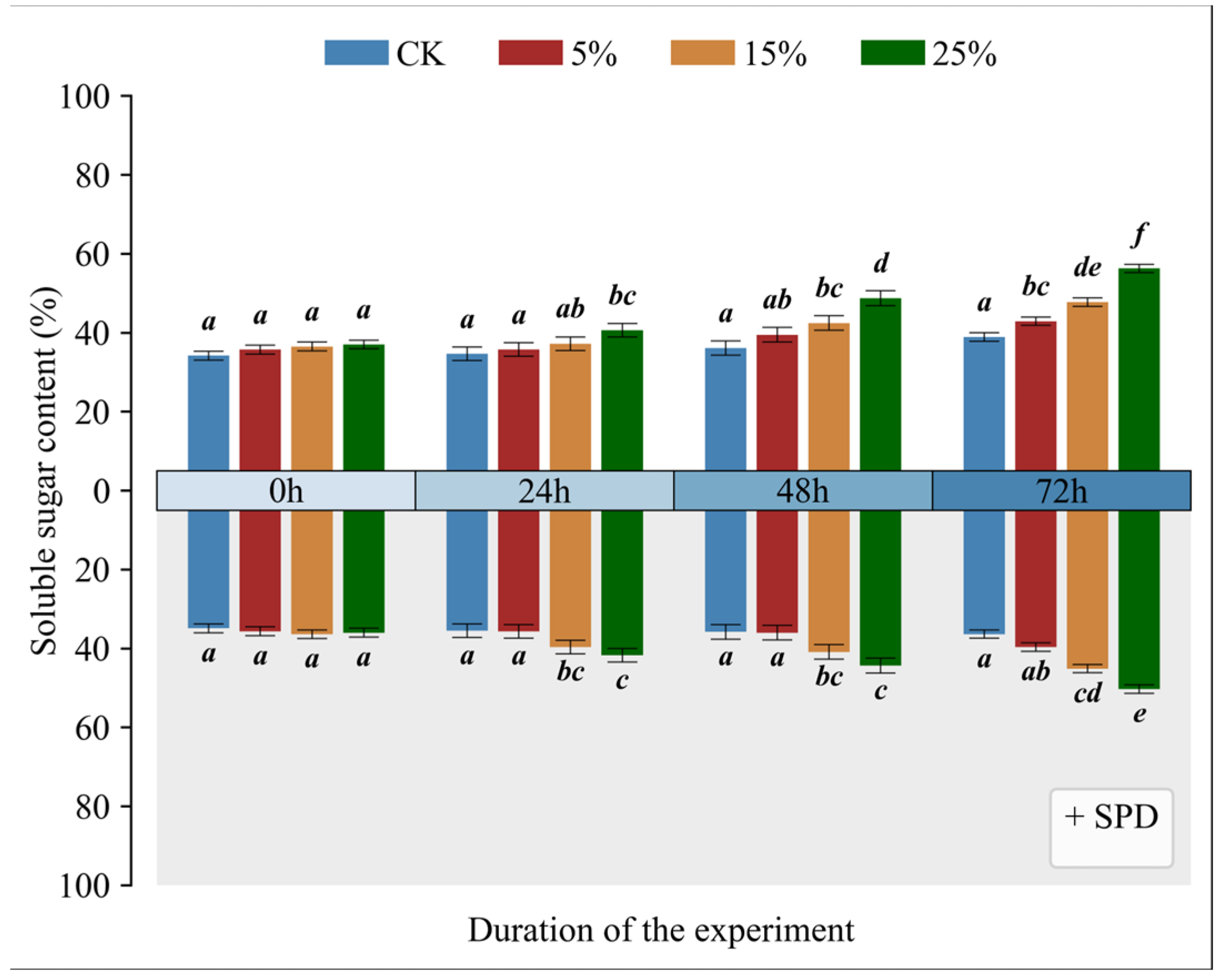
3.7. Impact of Exogenous Spermidine on Soluble Sugar Levels in Mango Leaves Under Varying Drought Conditions
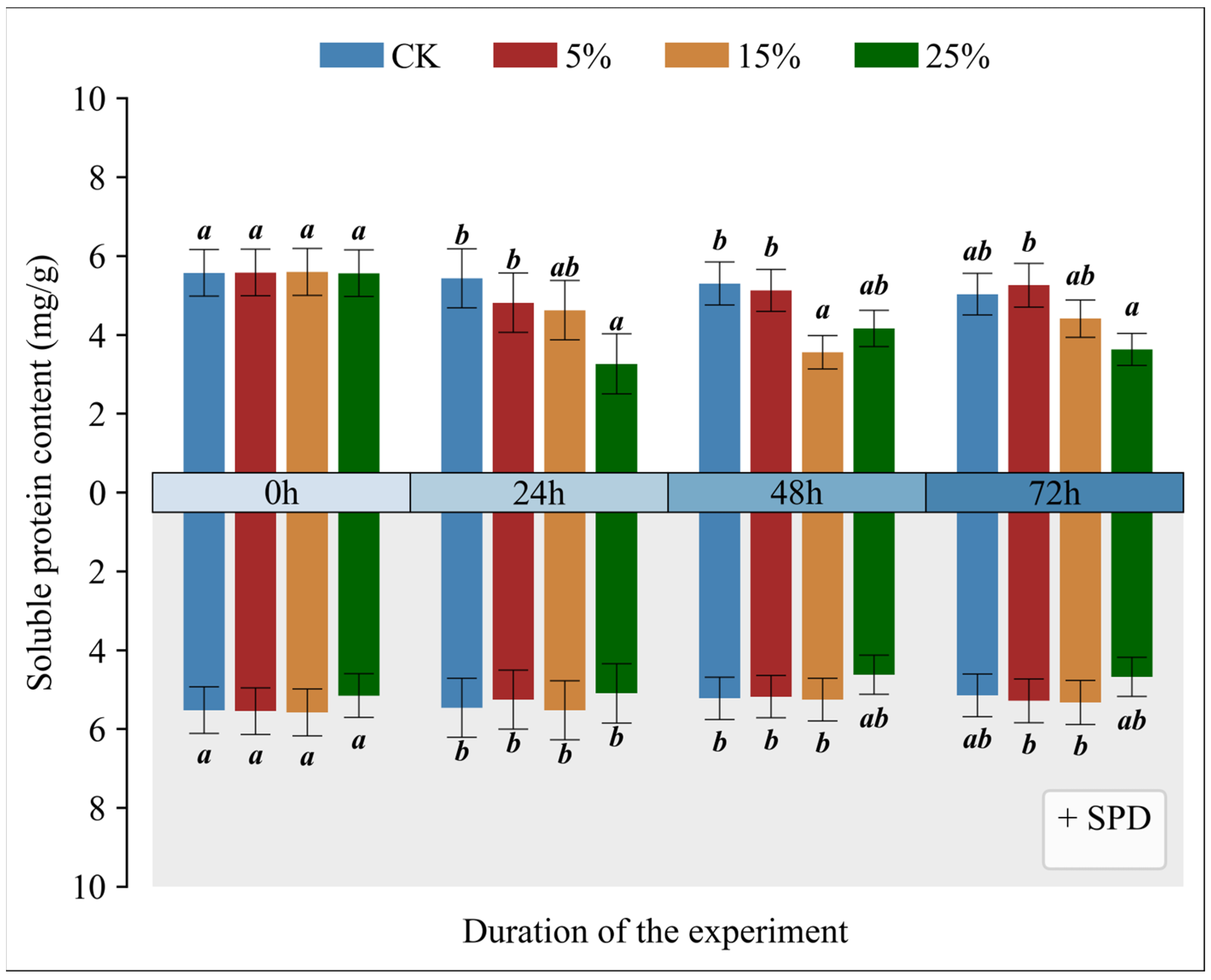
3.8. Impact of Exogenous Spermidine on Soluble Protein Levels in Mango Leaves Under Varying Drought Conditions
4. Discussion
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Jiang, H.; Liu, Z.; Gao, M.; Liu, G.; Tian, S.; Zeng, F. The global potato-processing industry: A review of production, products, quality and sustainability. Foods 2025, 14, 1758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.R.; Liu, X.Y.; Leng, X.C.; Zhang, M.; Yang, Z.Y.; Xu, W.; Wang, S.B.; Wu, H.X.; Liang, Q.Z. Genome-Wide Identification and Expression Analysis of the Mango (Mangifera indica L.) SWEET Gene Family. Horticulturae 2025, 11, 675. [Google Scholar] [CrossRef]
- Tharanathan, R.; Yashoda, H.; Prabha, T. Mango (Mangifera indica L.), “The king of fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; von Wettberg, E.J. Population genomic analysis of mango (Mangifera indica) suggests a complex history of domestication. New Phytol. 2019, 222, 2023–2037. [Google Scholar] [CrossRef]
- Víctor, G.S. El Cultivo del Mango; Ediciones Mundi-Prensa: Barcelona, Spain, 2009. [Google Scholar]
- Grajal-Martín, M.; Rosell, P.; Galán-Saúco, V.; Fernández, D. Filipino, un nuevo tetraploide de mango. In Proceedings of the XI Congreso de la Sociedad Española de Ciencias Hortícolas (SECH), Albacete, Spain, 7–9 November 2007. [Google Scholar]
- Parrotta, J.A. Mangifera Indica L.: Mango, Anacardiaceae, Cashew Family; International Institute of Tropical Forestry, US Department of Agriculture: San Juan, PR, USA, 1993.
- Yadav, D.; Singh, S. Mango: History origin and distribution. J. Pharmacogn. Phytochem. 2017, 6, 1257–1262. [Google Scholar]
- Zuazo, V.H.D.; García-Tejero, I.F.; Rodríguez, B.C.; Tarifa, D.F.; Ruiz, B.G.; Sacristán, P.C. Deficit irrigation strategies for subtropical mango farming. A review. Agron. Sustain. Dev. 2021, 41, 13. [Google Scholar] [CrossRef]
- Pavel, E.; Vanassche, F.; Grossman, Y. Optimisation of irrigation management in mango trees by determination of water and carbon demands to improve water use efficiency and fruit quality. In Final Report to the Water Research Commission on the Project; WRC Report No.: 1136/1/03; WRC: Pretoria, South Africa, 2003. [Google Scholar]
- Habiba, U.; Shaw, R.; Takeuchi, Y. Farmers’ adaptive practices for drought risk reduction in the northwest region of Bangladesh. Nat. Hazards 2014, 72, 337–359. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Z.; Zi, N.; Shen, S.; Chen, P. Effects of Different Soil Amendments on Yield and Quality of Mango Fruit. J. Henan Agric. Sci. 2024, 53, 118. [Google Scholar]
- Chartzoulakis, K.; Bertaki, M. Sustainable water management in agriculture under climate change. Agric. Agric. Sci. Procedia 2015, 4, 88–98. [Google Scholar] [CrossRef]
- Elliottii Selections, V. Oral Session Abstracts. Hortscience 2009, 44, 1002. [Google Scholar]
- Jia, H.; Cai, S.; Li, D.; Han, Y. Effect on photosynthesis of mango seedlings treated with calcium under soil drying stress. J. Fruit Sci. 2000, 17, 52–56. [Google Scholar]
- Kumar, A.; Rai, A.C.; Rai, A.; Rai, K.K.; Rai, V.P. Stress Tolerance in Horticultural Crops: Challenges and Mitigation Strategies; Woodhead Publishing: London, UK, 2021. [Google Scholar]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, X.; Peng, Y.; Merewitz, E.; Ma, X.; Huang, L.; Yan, Y. The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ. Exp. Bot. 2016, 124, 22–38. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. ; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Jiang, X.M.; Qin, Y.; Guo, G.X. Effects of Exogenous Spermidine on Seed Germination and seedling growth of pepper under low temperature stress. Xinjiang Agric. Sci. 2013, 50, 2266–2273, (In Chinese with English abstract). [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence:correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jia, M.X.; Shi, Y.; Di, W.; Jiang, X.R.; Xu, J.; Liu, Y. ROS-induced oxidative stress is closely related to pollen deterioration following cryopreservation. In Vitr. Cell. Dev. Biol. Plant 2017, 53, 433–439. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Hu, X.J. Study on Drought Resistance Characteristics of 10 Tree Species. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2005. (In Chinese with English abstract). [Google Scholar]
- Guo, H.; Hong, C.; Chen, X.; Xu, Y.; Liu, Y.; Jiang, D.; Zheng, B. Different growth and physiological responses to cadmium of the three Miscanthus species. PLoS ONE 2016, 11, e0153475. [Google Scholar] [CrossRef]
- Qiu, Y.; An, K.; Sun, J.; Chen, X.; Gong, X.; Ma, L.; Wu, S.; Jiang, S.; Zhang, Z.; Wang, Y. Investigating the effect of methyl jasmonate and melatonin on resistance of Malus crabapple ‘Hong Jiu’ to ozone stress. Environ. Sci. Pollut. Res. 2019, 26, 27761–27768. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Wang, P.; Zhao, H. Determination of the content of free proline in wheat leaves. Plant Physiol. Commun. 1990, 4, 62–65. [Google Scholar]
- Gao, J. Experimental Techniques of Plant Physiology; World Publishing Corporation: Xi’an, China, 2000. [Google Scholar]
- Xie, X.; Gu, Y.; Wang, W.; Abbas, F.; Qin, S.; Fu, S.; Mei, J.; Wang, J.; Ma, D.; Wen, G.; et al. Exogenous spermidine improved drought tolerance in Ilex verticillata seedlings. Front. Plant Sci. 2023, 14, 1065208. [Google Scholar] [CrossRef] [PubMed]
- Alijani, S.; Raji, M.-R.; Bistgani, Z.E.; Nia, A.E.; Farajpour, M. Spermidine-induced improvements in water relations and antioxidant defense enhance drought tolerance in yarrow (Achillea millefolium L.). Heliyon 2025, 11, e41482. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.T.; Lv, A.; Chen, D.D.; Zhang, Z.L.; Wang, X.M.; Zhou, A.C.; Xu, X.W.; Shao, Q.S.; Zheng, Y. Exogenous spermidine enhanced the water deficit tolerance of Anoectochilus roxburghii by modulating plant antioxidant enzymes and polyamine metabolism. Agric. Water Manag. 2023, 289, 108538. [Google Scholar] [CrossRef]
- Blázquez, M.A. Polyamines: Their Role in Plant Development and Stress. Annu. Rev. Plant Biol. 2024, 75, 95–117. [Google Scholar] [CrossRef]
- Xue, R.R.; Guo, R.Q.; Li, Q.; Lin, T.H.; Wu, Z.C.; Gao, N.; Wu, F.; Tong, L.; Zeng, R.S.; Song, Y.Y.; et al. Rice responds to Spodoptera frugiperda infestation via epigenetic regulation of H3K9ac in the jasmonic acid signaling and phenylpropanoid biosynthesis pathways. Plant Cell Rep. 2024, 43, 78. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Nawaz, M.; Shah, A.N.; Raza, A.; Barbanti, L.; Skalicky, M.; Hashem, M.; Brestic, M.; Pandey, S.; Alamri, S.; et al. Trehalose: A Key Player in Plant Growth Regulation and Tolerance to Abiotic Stresses. J. Plant Growth Regul. 2023, 42, 4935–4957. [Google Scholar] [CrossRef]
- Lu, Z.D.; Jiang, Y.J.; Liu, Y.; Fan, Z.; Liu, M.X. Physiological Responses of Centipedegrass to Exogenous Spermidine under Drought Stress. Mol. Plant Breed. 2022, 18, 6207–6215. [Google Scholar]
- Wen, J.K.; Xing, C.J.; Wang, Y.P.; Yang, L.; Zheng, W.; Li, W.Y. Effects of Different Drought Stress and Spermidine Rehydration on Physiological Characteristics of Taxus chinensis var. mairei. Water Sav. Irrig. 2023, 1, 109–115. [Google Scholar]
- Zhang, M.W.; Qiao, J.F.; Song, S.H.; Ma, J.; Zhang, P.P.; Li, C.; Niu, J.; Guo, H.X. Effect of Drought Stress on Grain-Filling and Physiological Properties of Summer Maize and the Exogenous Spermidine Regulation. J. Nucl. Agric. Sci. 2022, 36, 2501–2509, (In Chinese with English abstract). [Google Scholar]

| Group | Treatments |
|---|---|
| 5% | Mild drought stress (50 g/L PEG) |
| 15% | Moderate drought stress (150 g/L PEG) |
| 25% | Severe drought stress (250 g/L PEG) |
| CK | CK |
| 5% + SPD | Mild drought stress (50 g/L PEG) + Spermidine (1 mmol/L) |
| 15% + SPD | Moderate drought stress (150 g/L PEG) + Spermidine (1 mmol/L) |
| 25% + SPD | Severe drought stress (250 g/L PEG) + Spermidine (1 mmol/L) |
| CK + SPD | CK + Spermidine (1 mmol/L) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, M.; Yan, J.; Cheng, F.; Liao, W.; Xiao, Y.; Zhou, L.; Zhang, M.; Leng, X.; Liang, Q. Exogenous Spermidine Enhances Drought Resistance of Mango Seedlings by Regulating Physiological and Biochemical Metabolism. Horticulturae 2025, 11, 1102. https://doi.org/10.3390/horticulturae11091102
Liu X, Wang M, Yan J, Cheng F, Liao W, Xiao Y, Zhou L, Zhang M, Leng X, Liang Q. Exogenous Spermidine Enhances Drought Resistance of Mango Seedlings by Regulating Physiological and Biochemical Metabolism. Horticulturae. 2025; 11(9):1102. https://doi.org/10.3390/horticulturae11091102
Chicago/Turabian StyleLiu, Xinyu, Mingtian Wang, Jing Yan, Feng Cheng, Wei Liao, Yunhe Xiao, Lirong Zhou, Meng Zhang, Xiangchi Leng, and Qingzhi Liang. 2025. "Exogenous Spermidine Enhances Drought Resistance of Mango Seedlings by Regulating Physiological and Biochemical Metabolism" Horticulturae 11, no. 9: 1102. https://doi.org/10.3390/horticulturae11091102
APA StyleLiu, X., Wang, M., Yan, J., Cheng, F., Liao, W., Xiao, Y., Zhou, L., Zhang, M., Leng, X., & Liang, Q. (2025). Exogenous Spermidine Enhances Drought Resistance of Mango Seedlings by Regulating Physiological and Biochemical Metabolism. Horticulturae, 11(9), 1102. https://doi.org/10.3390/horticulturae11091102








