Hybridization Efficiency and Genetic Diversity in Cut Chrysanthemum: Integration of Morphological and iPBS Marker Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Method
2.2.1. Ploidy Levels, Pollen Viability and Germination
2.2.2. Hybridization
2.2.3. Seed Set and Assessment of Qualitative and Quantitative Characteristics
2.3. Molecular Characterization Analysis
3. Results and Discussion
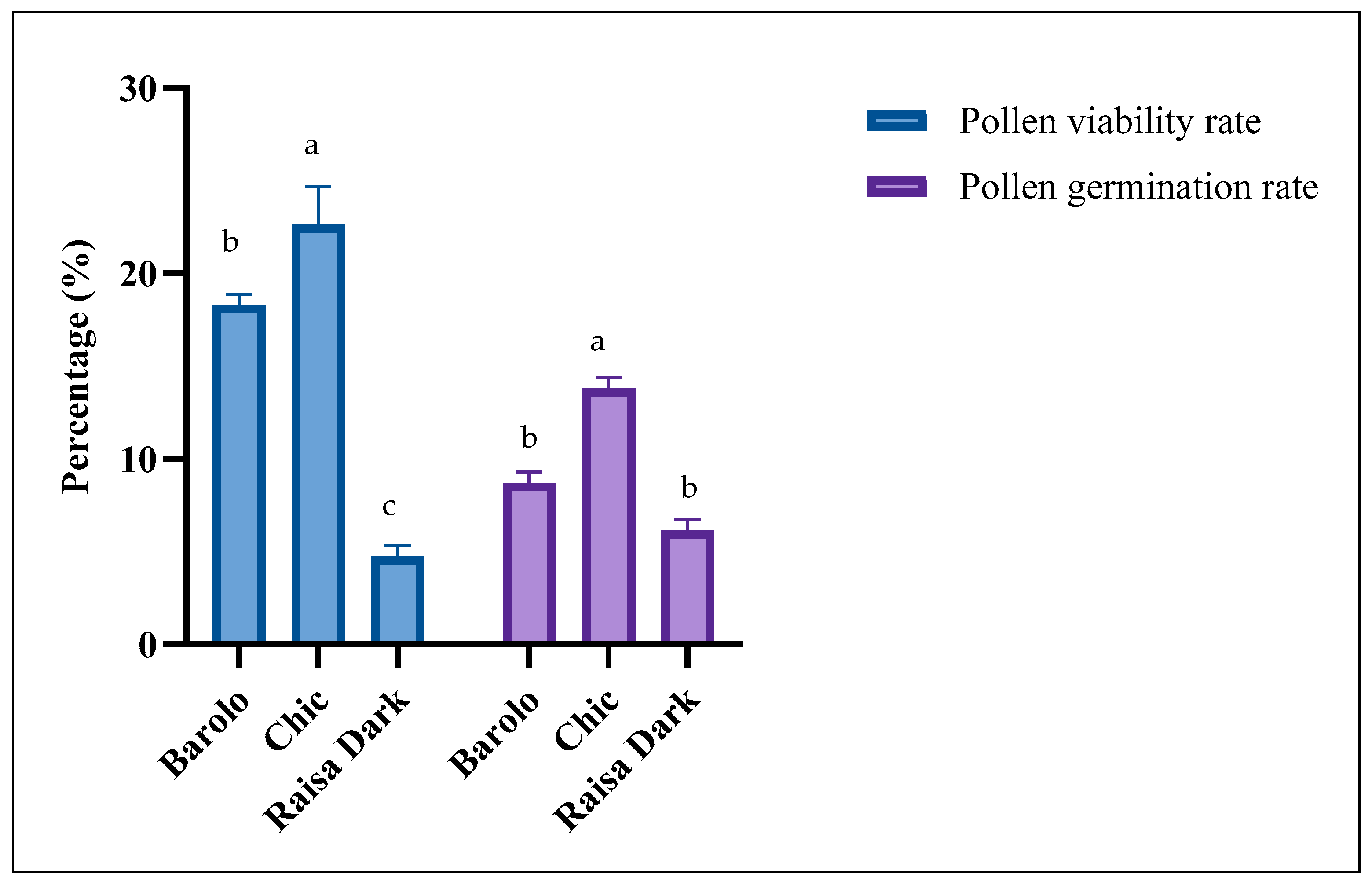
3.1. Ploidy Levels, Pollen Viability and Germination Rates
3.2. Total Seed Number, Total Seed Weight, Average Seed Number per Flower Head, Germinating Seed Number
3.3. Qualitative and Quantitative Traits of F1 Progenies
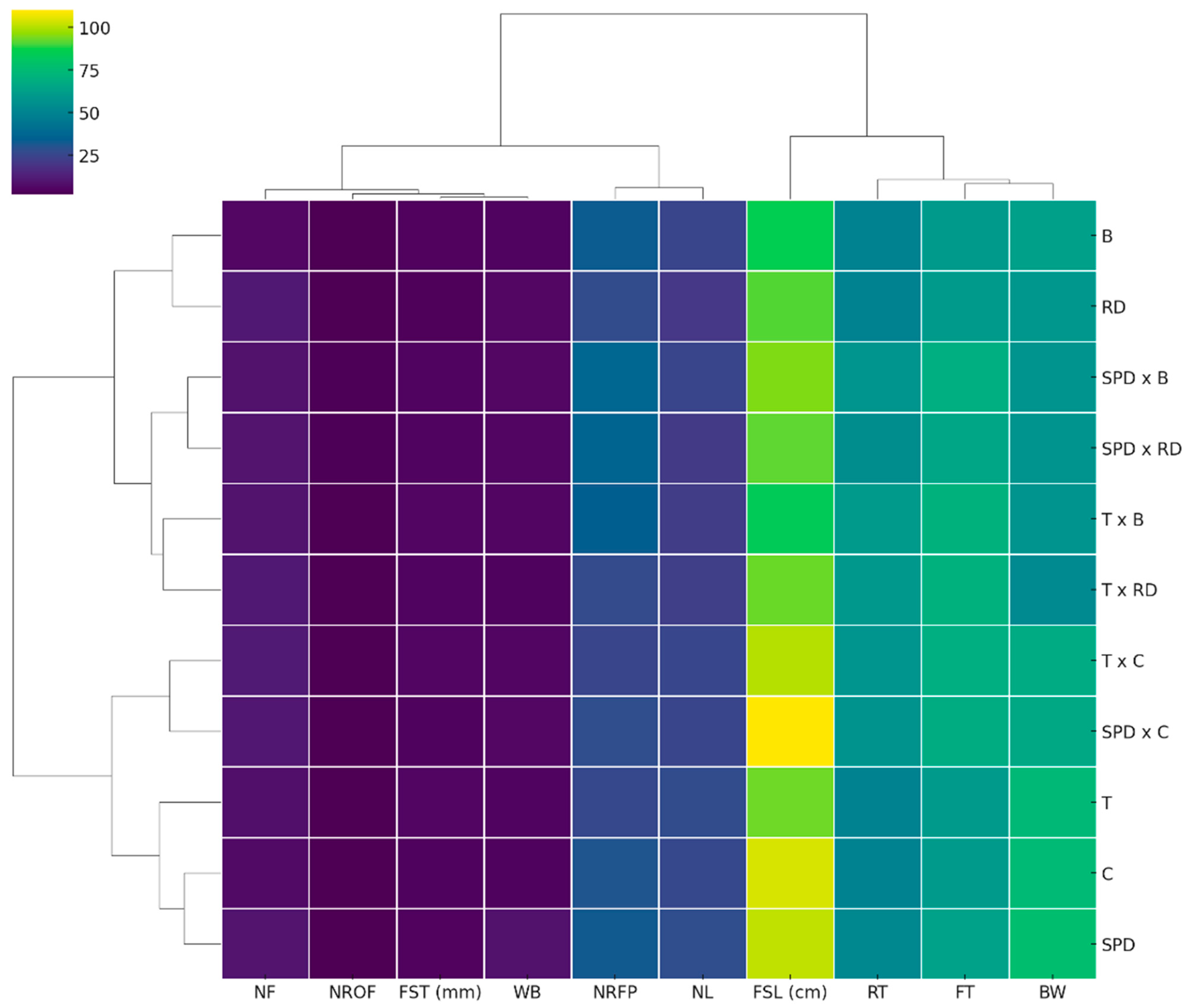
3.4. Heat Map of the Examined Traits with Hierarchical Clustering
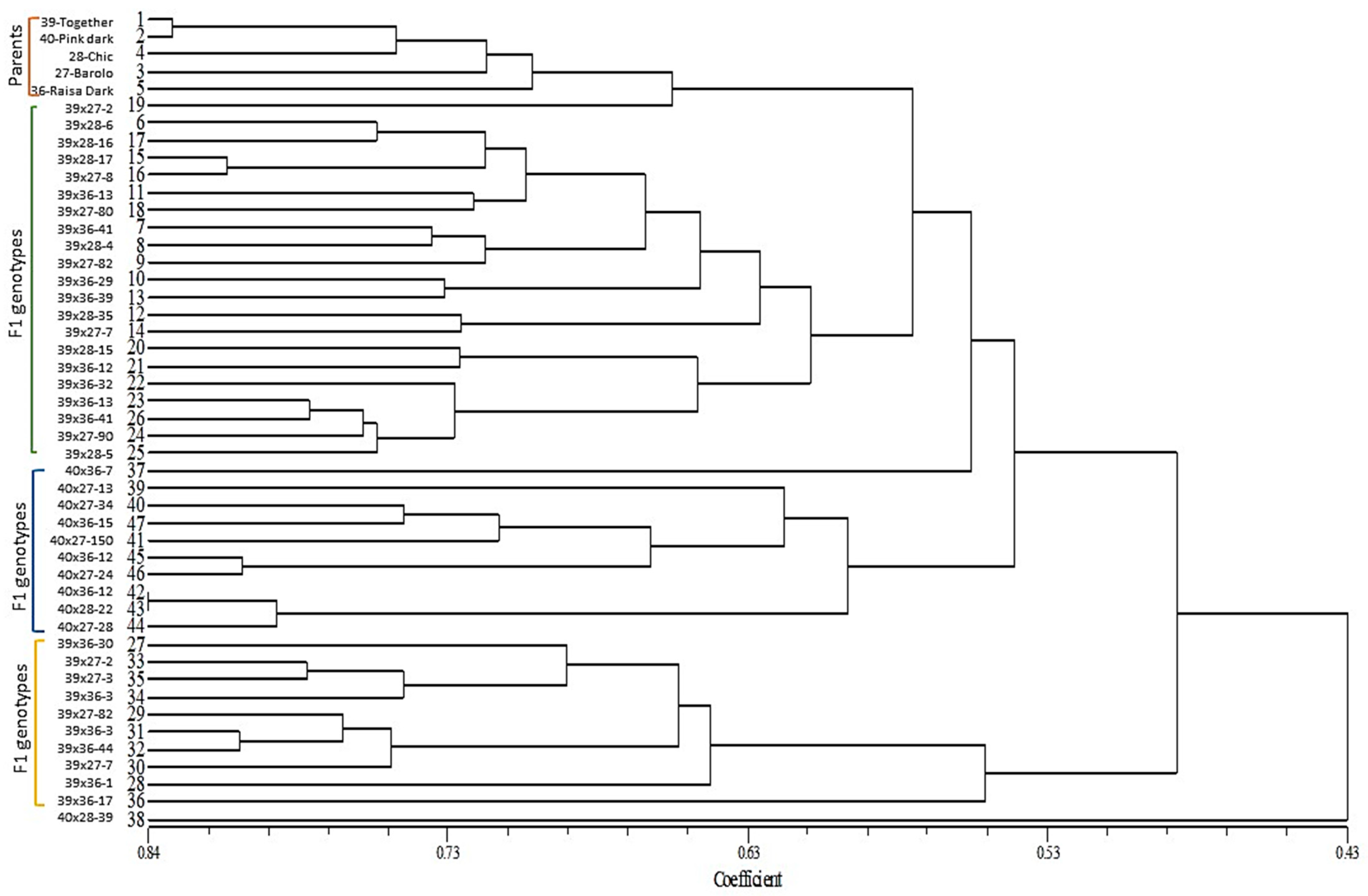
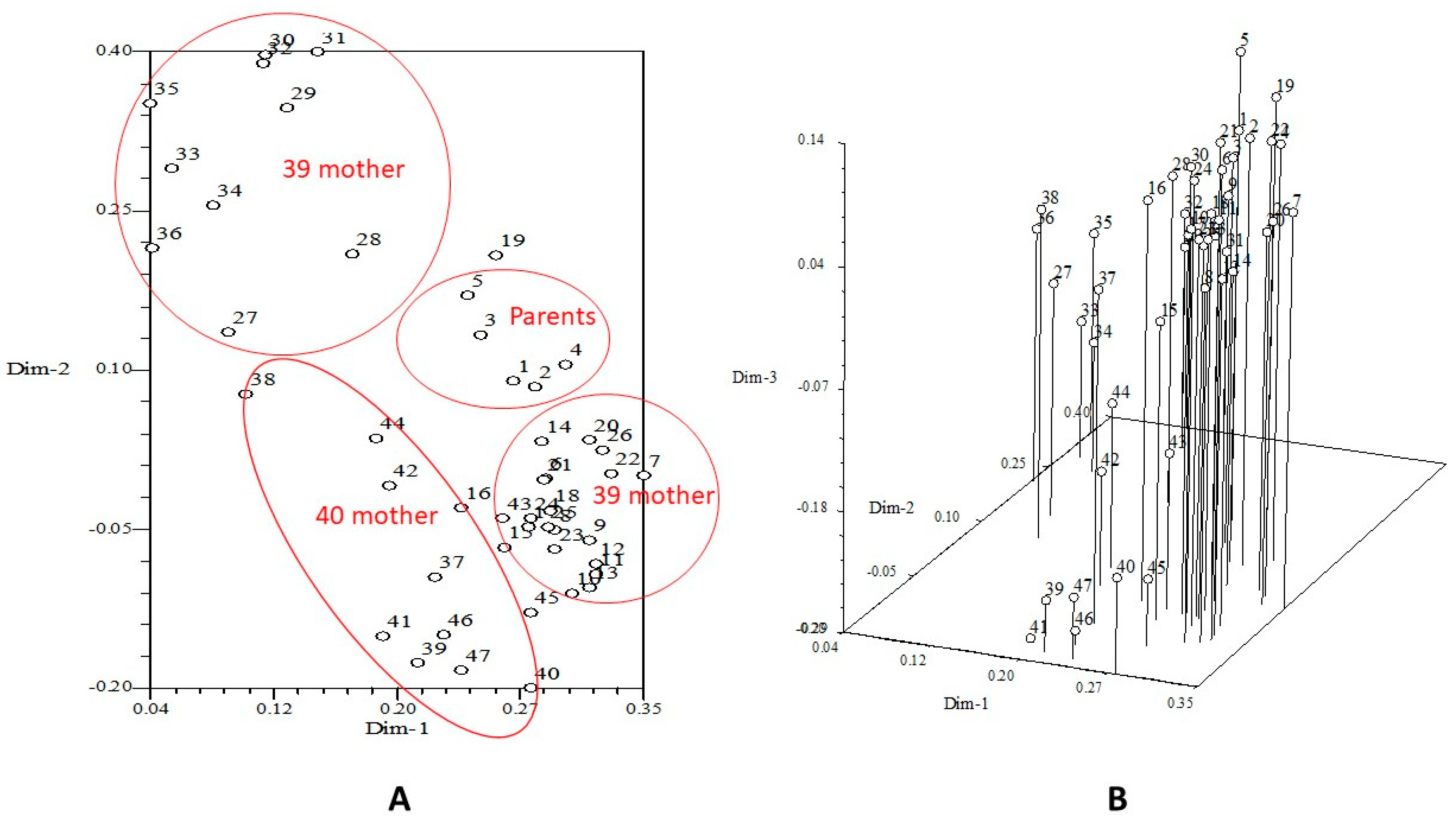
3.5. Molecular Characterization of F1 Genotypes and Parental Lines
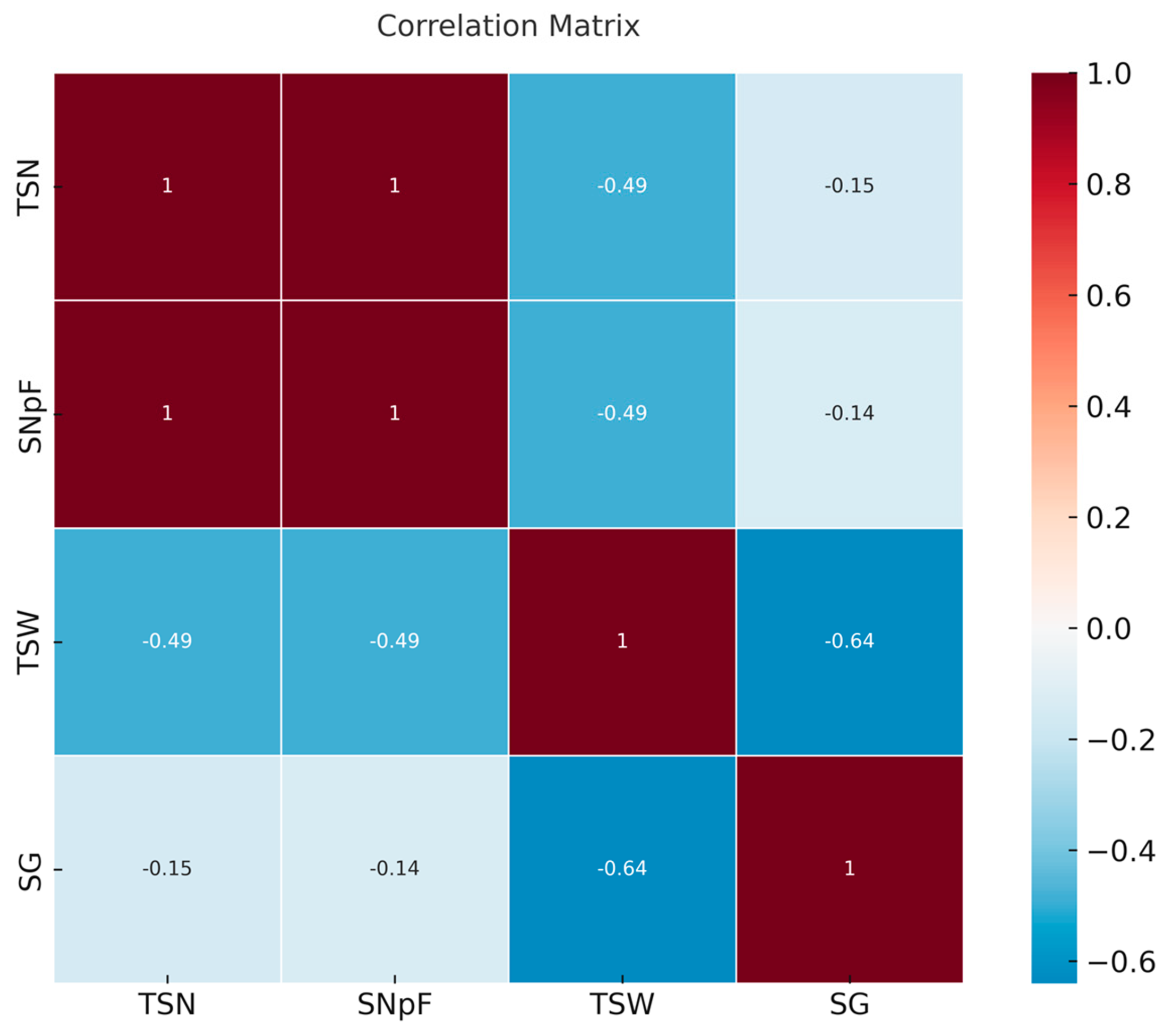
3.6. Relationship Between Molecular and Morphological Traits
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekapogu, M.; Kwon, O.K.; Song, H.Y.; Jung, J.A. Towards the improvement of ornamental attributes in chrysanthemum: Recent progress in biotechnological advances. Int. J. Mol. Sci. 2022, 23, 12284. [Google Scholar] [CrossRef]
- Randhava, G.S.; Mukhopadhyay, A. Chrysanthemum. In Floriculture in India; Allied Publishers: Lahore, Pakistan, 1986; pp. 362–367. [Google Scholar]
- Hadizadeh, H.; Samiei, S.; Hakeri, A. Chrysanthemum, an ornamental genus with considerable medicinal value: A comprehensive review. S. Afr. J. Bot. 2022, 144, 23–43. [Google Scholar] [CrossRef]
- AIPH/Union Fleurs. International Statistics Flowers and Plants 2020; AIPH/Union Fleurs International Flower Trade Association: Ixelles, Belgium, 2021; 226p. [Google Scholar]
- Haider, S.; Gao, Y.; Gao, Y. Standardized Genetic Transformation Protocol for Chrysanthemum cv. ‘Jinba’ with TERMINAL FLOWER 1 Homolog CmTFL1a. Genes 2020, 11, 860. [Google Scholar] [CrossRef]
- Su, J.; Jiang, J.; Zhang, F.; Liu, Y.; Ding, L.; Chen, S.; Chen, F. Current achievements and future prospects in the genetic breeding of chrysanthemum: A review. Hortic. Res. 2019, 6, 109. [Google Scholar] [CrossRef]
- Chong, X.; Su, J.; Wang, F.; Wang, H.; Song, A.; Guan, Z.; Fang, W.; Jiang, J.; Chen, S.; Chen, F.; et al. Identification of favorable SNP alleles and candidate genes responsible for inflorescence-related traits via GWAS in chrysanthemum. Plant Mol. Biol. 2019, 99, 407–420. [Google Scholar] [CrossRef]
- Yan, W.; Jung, J.A.; Lim, K.B.; Cabahug, R.A.M.; Hwang, Y. Cytogenetic studies of chrysanthemum: A review. Flower. Res. J. 2015, 4, 242–253. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Zhao, H.; Gao, T.; Song, A.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum Cm HSFA 4 gene positively regulates salt stress tolerance in transgenic chrysanthemum. Plant Biotechnol. J. 2018, 16, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Jaishreepriyanka, R.; Sinha, S.; Mandal, S.S.; Singh, B.; Rashmi, K. Diversity Analysis of Maize Inbred Lines. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2765–2773. [Google Scholar] [CrossRef]
- Luo, C.; Chen, D.; Cheng, X.; Liu, H.; Li, Y.; Huang, C. SSR Analysis of genetic relationship and classification in chrysanthemum germplasm collection. Hortic. Plant J. 2018, 4, 73–82. [Google Scholar] [CrossRef]
- Olejnik, A.; Parkitna, K.; Kozak, B.; Florczak, S.; Matkowski, J.; Nowosad, K. Assessment of the Genetic diversity of chrysanthemum cultivars using SSR markers. Agronomy 2021, 11, 2318. [Google Scholar] [CrossRef]
- Mekapogu, M.; Lim, S.H.; Choi, Y.J.; Lee, S.Y.; Jung, J.A. Evaluation of Genetic Diversity and Identification of Cultivars in Spray-Type Chrysanthemum Based on SSR Markers. Genes 2025, 16, 81. [Google Scholar] [CrossRef]
- Huang, S.C.; Tsai, C.C.; Sheu, C.S. Genetic analysis of Chrysanthemum hybrids based on RAPD molecular markers. Bot. Bull. Acad. Sin. 2000, 41, 257–262. [Google Scholar]
- Martin, C.; Uberhuaga, E.; Pérez, C. Application of RAPD markers in the characterisation of Chrysanthemum varieties and the assessment of somaclonal variation. Euphytica 2002, 127, 247–253. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, M.; Yadav, H.K.; Sharma, S. Genetic diversity and population structure analysis of chrysanthemum (Dendranthema grandiflora Tzvelev) germplasm based on RAPD markers. J. Environ. Biol. 2017, 38, 457. [Google Scholar] [CrossRef]
- Shao, Q.S.; Guo, Q.S.; Deng, Y.M.; Guo, H.P. A comparative analysis of genetic diversity in medicinal Chrysanthemum morifolium based on morphology, ISSR and SRAP markers. Biochem. Syst. Ecol. 2010, 38, 1160–1169. [Google Scholar] [CrossRef]
- Dalda-Sekerci, A. Comprehensive assessment of genetic diversity in chrysanthemum germplasm using morphological, biochemical and retrotransposon-based molecular markers. Genet. Resour. Crop Evol. 2023, 70, 2321–2336. [Google Scholar] [CrossRef]
- Kalendar, R.; Flavell, A.J.; Ellis, T.H.N.; Sjakste, T.; Moisy, C.; Schulman, A.H. Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 2011, 106, 520–530. [Google Scholar] [CrossRef]
- Kalendar, R.; Schulman, A.H. Transposon-Based Tagging: IRAP, REMAP, and iPBS. In Molecular Plant Taxonomy: Methods and Protocols; Besse, P., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 233–255. [Google Scholar] [CrossRef]
- Tuna, M.; Cabi, E. Bazı Buğdaygil yem Bitkisi Türlerine ait Populasyonların Çekirdek DNA Içeriklerinin Flow Sitometri Yöntemiyle Belirlenmesi ve Ploidy Analizi ile tür Teşhisinde Kullanımı. 2014. Available online: https://acikerisim.nku.edu.tr:8080/xmlui/handle/20.500.11776/2119 (accessed on 21 January 2020).
- Eti, S. Çiçek tozu miktarını belirlemede kullanılan pratik bir yöntem. Çukurova Üniv. Ziraat Fak. Derg. 1990, 5, 49–58. [Google Scholar]
- Eti, S. Bazı meyve tür ve çeşitlerinde değişik in vitro testler yardımıyla çiçek tozu canlılık ve çimlenme yeteneklerinin belirlenmesi. Çukurova Üniv. Ziraat Fak. Derg. 1991, 6, 69–80. (In Turkish) [Google Scholar]
- Meral, E.D.; Kırbay, E.; Haspolat, G.; Kazaz, S. Evaluation of pollen vivability in some spray Chrysanthemum varieties on storage period. Ornam. Hortic. 2024, 30, e242740. [Google Scholar] [CrossRef]
- Yang, J.; Endo, M. In vitro germination and viability of Dendranthema Pollen. Asian J. Plant Sci. 2005, 4, 673–677. [Google Scholar] [CrossRef][Green Version]
- Anderson, N. Chrysanthemum (Dendranthema x grandiflora Tzvelz.). In Flower Breeding and Genetics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 389–437. [Google Scholar]
- Wang, X.G.; Wang, H.; Chen, F.; Jiang, J.; Fang, W.; Liao, Y.; Teng, N. Factors affecting quantity of pollen dispersal of spray cut chrysanthemum (Chrysanthemum morifolium). BMC Plant Biol. 2014, 14, 5. [Google Scholar] [CrossRef]
- Boeder, M. (Armada Young Plants BV. Nieuwveens Jaagpad 47, 2441 EK Nieuwveen The Netherlands). Oral communication, 2020.
- Post, A. (Deliflor Chrysanthemum B.V., Postbus 77, 2676 ZH Maasdijk, The Netherlands). Oral communication, 2017.
- Carvalho, S.M.P. Effects of Growth Conditions on External Quality of Cut Chrysanthemum; Analysis and Simulation. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2003. [Google Scholar]
- National Agriculture and Food Research Organization. Dus Test for Chrysanthemum Naktuinbouw and NCSS(/NARO). 2022. Available online: https://www.naro.go.jp/publicity_report/publication/files/Chrysanthemum_calibration_manual_formalized.pdf (accessed on 10 October 2022).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar][Green Version]
- Dice, L.R. Measures of the amount of ecologic association between species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Guo, X.; Luo, C.; Wu, Z.; Zhang, X.; Cheng, X.; Huang, C. Polyploidy levels of Chinese large-flower chrysanthemum determined by flow cytometry. Afr. J. Biotechnol. 2012, 11, 7789–7794. [Google Scholar] [CrossRef]
- Chang, L.I.; Chen, S.M.; Chen, F.D.; Zhen, L.I.; Fan, W.M. Karyomorphological studies on chinese pot Chrysanthemum cultivars with large inflorescences. Agric. Sci. China 2009, 8, 793–802. [Google Scholar] [CrossRef]
- Abd El-Twab, M.H.; Kondo, K. Hybridity and relationship between Chrysanthemum shiwogiku Kitam. and C. Vestitum (Hemsl.) Stapf. Chromosome Bot. 2009, 4, 65–70. [Google Scholar] [CrossRef]
- Shibata, M. Importance of genetic transformation in ornamental plant breeding. Plant Biotechnol. 2008, 25, 3–8. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, F.; Chen, S.; Teng, N.; Li, F. Seed set of inbred, meiosis and pollen capacity of 5 Chrysanthemum cultivars. Acta Bot. Boreali-Occident. Sin. 2009, 29, 469–474. [Google Scholar]
- Zhu, P.; Liu, N.; Zhou, Z.; Song, W.; Mao, H. Different pollination method on improving the self-compatibility and cross-compatibility in cut-flower Chrysanthemum. Chin. Agric. Sci. Bull. 2011, 27, 114–118. [Google Scholar]
- Zhang, Y.; Wang, C.; Ma, H.; Dai, S. Assessing the genetic diversity of chrysanthemum cultivars with microsatellites. J. Am. Soc. Hortic. Sci. 2013, 138, 479–486. [Google Scholar] [CrossRef]
- Mekapogu, M.; Song, H.; Lim, S.; Jung, J. Assessment of genetic diversity in the white-colored variants of spray-type chrysanthemum cultivars using ssr markers. Horticulturae 2023, 9, 798. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Park, C.; Kim, D.; Kim, J.; Kim, S.; Jin Jeong, K.; Pak, H.; Jung, J.A.; Kim, T. Assessing amounts of genetic variability in key horticultural traits underlying core Korean breeding lines of cut chrysanthemums. Plants 2024, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, W.; Ren, Y.; Chen, R.; Xing-lian, L.; Yang, Y.; Wu, H. In vitro cloning potential and phytochemical evaluations of aneuploid individuals produced from reciprocal crosses between diploid and triploid in Echinacea purpurea L. Acta Soc. Bot. Pol. 2017, 86, 3556. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, S.; Hong, Y.; Song, X. Application of genomic SSR locus polymorphisms on the identification and classification of chrysanthemum cultivars in China. PLoS ONE 2014, 9, e104856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Chen, F.D.; Fang, W.M. Pollen germination in vitro of Chrysanthemum cultivars with small inflorescences and several species of Dendranthema. J. Nanjing Agric. Univ. 2005, 28, 22–27. [Google Scholar]
- Wang, F.; Zhong, X.; Wang, H.; Song, A.; Chen, F.; Fang, W.; Jiang, J.; Teng, N. Investigation of differences in fertility among progenies from self-pollinated Chrysanthemum. Int. J. Mol. Sci. 2018, 19, 832. [Google Scholar] [CrossRef]
- Dursun, H.B.; Kazaz, S.; Kılıç, T. Farklı sıcaklık ve sürelerde bekletilen kasımpatı polenlerinde polen kalitesinin belirlenmesi. Iğdır Üniv. Fen Bilim. Enst. Derg. 2023, 13, 2303–2314. (In Turkish) [Google Scholar] [CrossRef]
- Kazaz, S.; Yanmaz, R.; Karagüzel, Ö.; Haspolat, G. Melezleme Islahı ile Kesme Çiçek Kasımpatı Çeşitlerinin Geliştirilmesi ve Sektöre Kazandırılması; Proje No: 119O082; TÜBİTAK ULAKBİM TR Dizin: Ankara, Turkey, 2023. (In Turkish) [Google Scholar]
- Sun, C.; Huang, Z.; Wang, Y.; Chen, F.; Teng, N.; Fang, W.; Liu, Z. Overcoming pre-fertilization barriers in the wide cross between chrysanthemum grandiflorum (ramat.) kitamura and c. nankingense (nakai) tzvel. by using special pollination techniques. Euphytica 2010, 178, 195–202. [Google Scholar] [CrossRef]
- Xu, L.; Liu, C.L.; Wang, H.D.; Chen, K.L. Study on the pollen viability and stigma receptivity of Chrysanthemum morifolium ‘Fubaiju’. Zhong yao cai J. Chin. Med. Mater. 2012, 35, 1546–1550. [Google Scholar]
- Miler, N.; Wozny, A. Effect of pollen genotype, temperature and period of storage on in vitro germinability and in vivo seed set in chrysanthemum—Preliminary study. Agronomy 2021, 11, 2395. [Google Scholar] [CrossRef]
- Jie, Y.; Ziran, S.; Mingfang, Y.; Deying, L.; Yubin, N. The breeding of early-blossoming cut Chrysanthemum (Dendranthema morifolium Tzvcl.). Acta Hortic. 1995, 404, 131–138. [Google Scholar] [CrossRef]
- Anderson, N.O.; Ascher, P.D. Inheritance of seed set, germination, and day neutrality/heat delay insensitivity of garden Chrysanthemums (Dendranthema x grandiflora) under glasshouse and field conditions. J. Am. Soc. Hortic. Sci. 2004, 129, 509–516. [Google Scholar] [CrossRef]
- Miler, N.; Kulus, D. Effect of parental components and pollination frequency on the setting and germination of Chrysanthemum seeds. Horticulturae 2022, 8, 827. [Google Scholar] [CrossRef]
- Deng, Y.; Teng, N.; Chen, S. Reproductive barriers in the intergeneric hybridization between Chrysanthemum grandiflorum (Ramat.) Kitam. and Ajania przewalskii Poljak. (Asteraceae). Euphytica 2010, 174, 41–50. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, J.; Chen, S.; Teng, N.; Song, A.; Guan, Z.; Chen, F. Combination of multiple resistance traits from wild relative species in chrysanthemum via trigeneric hybridization. PLoS ONE 2012, 7, e44337. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; Chen, F.D.; Teng, N.J.; Liu, Z.L.; Fang, W.M.; Hou, X.L. Factors affecting seed set in the crosses between Dendranthema grandiflorum (Ramat.) Kitamura and its wild species. Euphytica 2010, 171, 181–192. [Google Scholar] [CrossRef]
- Kim, H.; Seong, J.; Han, Y.Y.; Choi, D.J.; Woo, J. Breeding of a new light pink spray-mum ‘Noble ND’ as cut flower. Korean J. Hortic. Sci. Technol. 2010, 28, 895–898. [Google Scholar]
- Hwang, J.C.; Chin, Y.D.; Kim, J.K.; Kim, S.K. A new early flowering, spray Crysanthemum cultivar for cut flower, “Green witch” with pompon type and green petals. Korean J. Breed. Sci. 2009, 41, 529–533. [Google Scholar]
- Hwang, J.C.; Chin, Y.D.; Chung, Y.M.; Kim, S.K.; Ro, C.W.; Jeong, B.R. A new spray Chrysanthemum cultivar, ’Blue Hope’with anemone type and white petals for cut flower. Hortic. Sci. Technol. 2013, 31, 123–127. [Google Scholar]
- Lim, J.H.; Shin, H.K.; Park, S.K.; Cho, H.R.; Rhee, H.K.; Kim, M.S.; Joung, H.Y. A new spray chrysanthemum cultivar, “Cherry Blossom” with resistant to white rust, single flower type and bright pink petals for cut flower. Korean J. Breed. Sci. 2008, 40, 439–442. [Google Scholar]
- Lang, A. Physiology of flower initiation. Encycl. Plant Physiol. 1965, 15, 1380–1444. [Google Scholar]
- Karlsson, M.G.; Heins, R.D. Influence of temperature on growth and development of chrysanthemum. J. Am. Soc. Hortic. Sci. 1994, 119, 438–445. [Google Scholar]
- Cockshull, K.E. Environmental control of flower initiation in chrysanthemum. Acta Hortic. 1985, 174, 73–80. [Google Scholar]
- Armitage, A.M.; Laushman, J.M. Specialty Cut Flowers: The Production of Annuals, Perennials, Bulbs, and Woody Plants for Fresh and Dried Cut Flowers; Timber Press: Portland, OR, USA, 2003; ISBN 9780881922257. [Google Scholar]
- Anonymous. Dekkerchrysanten. 2023. Available online: https://dekkerchrysanten.com (accessed on 5 September 2023).
- Anonymous. Deliforchrysanten. 2023. Available online: https://www.delishow.nl (accessed on 5 September 2023).
- Anonymous. Royalvanzanten. 2023. Available online: https://www.royalvanzanten.com/en/product-category/cut-flowers/ (accessed on 5 September 2023).
- Anonymous. Dummenorange. 2023. Available online: https://emea.dummenorange.com/app/en/products/netherlands?category=Cut%20Flowers&productgroup=Cut%20Chrysanthemum&surplus (accessed on 5 September 2023).
- De Jong, J. Selection for wide temperature adaptation in Chrysanthemum morifolium. Neth. J. Agric. Sci. 1978, 26, 110–118. [Google Scholar] [CrossRef]
- Mulford, F.L. Result of selfing twenty-four early blooming Chrysanthemums. Proc. Am. Soc. Hortic. Sci. 1937, 35, 818–821. [Google Scholar]
- Jung, Y.K.; Lim, J.W.; Kim, S.K.; Lee, Y.S.; Yu, Y.Y. A new spray Chrysanthemum cultivar, ’Dream Water’with single type for cut flower. Hortic. Sci. Technol. 2012, 30, 220–223. [Google Scholar] [CrossRef]
- Carvalho, S.M.P.; Heuvelink, E. Influence of greenhouse climate and plant density on external quality of chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura): First steps towards a quality model. J. Hortic. Sci. Biotechnol. 2001, 76, 249–258. [Google Scholar] [CrossRef]
- Zhang, C.; Mao, H.; Wu, D.; Liu, Y.; Yang, Q.; Deng, B.; Chen, F. Climatic heterogeneity in a large chrysanthemum greenhouse resolved by a LoRa-based wireless sensor network. Sci. Hortic. 2025, 344, 114109. [Google Scholar] [CrossRef]


| Varieties | FT | RT | FSL | FST | WB | NF | BW | NRFS | NP | NL | Color |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Barolo | 60 | 49 | 86 | 4.5 | 5.05 | 6.0 | 62.0 | 2.0 | 33.0 | 25.0 | Red |
| Chic | 60 | 49 | 104 | 4.8 | 4.78 | 7.0 | 75.0 | 2.0 | 31.0 | 26.0 | White |
| Raisa Dark | 60 | 49 | 91 | 3.5 | 6.00 | 11.0 | 59.0 | 2.0 | 27.0 | 21.0 | Bicolor (Orange-Yellow) |
| Together | 60 | 49 | 93 | 5.5 | 5.30 | 8.0 | 74.0 | 2.0 | 26.0 | 27.0 | Bicolor (Pink-White) |
| Stylist Pink Dark | 63 | 52 | 101 | 4.4 | 9.10 | 10.0 | 77.0 | 2.0 | 32.0 | 28.0 | Bicolor (Pink-White) |
| Varieties | Sample Peak | Standard Peak | Amount of DNA | DNA (pg/2C) | Level of Ploidy |
|---|---|---|---|---|---|
| Together | 200.37 | 113.28 | 16.65 | 18.84 | 2n = 6x = 54 |
| Stylist Pink Dark | 214.96 | 121.79 | 16.65 | 18.80 | 2n = 6x = 54 |
| Barolo | 203.92 | 118.69 | 16.65 | 18.30 | 2n = 6x = 54 |
| Chic | 229.61 | 122.8 | 16.65 | 19.91 | 2n = 6x = 54 |
| Raisa Dark | 200.35 | 111.28 | 16.65 | 19.17 | 2n = 6x = 54 |
| Combination | Total Seed Number | Average Seed Number per Flower Head | Total Seed Weight (g) | Germination of Seed (%) |
|---|---|---|---|---|
| Together × Barolo | 5233 | 209 | 3.39 | 2.69 |
| Together × Chic | 4961 | 198 | 2.83 | 3.93 |
| Together × Raisa Dark | 3680 | 147 | 3.09 | 5.92 |
| Stylist Pink Dark × Barolo | 7226 | 289 | 2.13 | 6.38 |
| Stylist Pink Dark × Chic | 4772 | 191 | 2.61 | 10.73 |
| Stylist Pink Dark × Raisa Dark | 4519 | 181 | 2.25 | 9.58 |
| Hybrid Combination | FT (day) | RT (day) | FSL (cm) | FST (mm) | BW (g) | NL | WB (cm) | NF | NRF | NRFP |
|---|---|---|---|---|---|---|---|---|---|---|
| Together × Barolo | 70.75 ± 0.65 a | 59.75 ± 0.65 a | 86.60 ± 3.39 b | 5.57 ± 0.23 a | 58.68 ± 3.38 ab | 22.58 ± 0.93 a | 5.48 ± 0.16 ab | 9.68 ± 0.48 a | 2.25 ± 0.16 a | 32.97 ± 2.48 ab |
| Together × Chic | 69.20 ± 0.78 ab | 57.98 ± 0.80 ab | 98.73 ± 2.10 ab | 5.48 ± 0.18 ab | 66.10 ± 4.43 a | 24.99 ± 0.87 a | 5.54 ± 0.31 ab | 11.77 ± 1.28 a | 1.99 ± 0.10 a | 25.19 ± 0.81 c |
| Together × Raisa Dark | 70.95 ± 1.72 a | 59.81 ± 1.71 a | 93.97 ± 2.40 ab | 5.17 ± 0.26 ab | 54.58 ± 3.48 b | 23.30 ± 0.76 a | 4.85 ± 0.15 b | 11.10 ± 0.64 a | 2.13 ± 0.15 a | 26.86 ± 0.91 c |
| Stylist Pink Dark × Barolo | 68.71 ± 0.75 ab | 57.52 ± 0.80 ab | 93.21 ± 3.60 ab | 4.92 ± 0.15 ab | 57.33 ± 3.36 ab | 24.81 ± 1.20 a | 6.05 ± 0.22 a | 9.42 ± 0.62 a | 2.70 ± 0.28 a | 37.67 ± 1.89 a |
| Stylist Pink Dark × Chic | 68.89 ± 1.09 ab | 56.44 ± 1.95 ab | 111.54 ± 2.31 a | 4.78 ± 0.14 b | 65.67 ± 3.47 a | 24.65 ± 1.67 a | 6.13 ± 0.30 a | 9.55 ± 0.50 a | 2.11 ± 0.20 a | 30.87 ± 3.70 bc |
| Stylist Pink Dark × Raisa Dark | 65.80 ± 1.91 b | 54.66 ± 1.80 b | 90.09 ± 1.44 ab | 5.16 ± 0.28 ab | 55.97 ± 6.01 ab | 23.83 ± 0.96 a | 5.64 ± 0.28 ab | 10.03 ± 0.94 a | 2.55 ± 0.24 a | 34.19 ± 1.91 ab |
| Primary | Sequence 5′–3′ | Bp | TNF | NPF | MP (%) | PIC | p | q | Ne | I | He | uHe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| iPBS-2252 | TCATGGCTCATGATACCA | 175–1000 | 22 | 22 | 100 | 0.59 | 0.419 | 0.581 | 1.813 | 0.624 | 0.436 | 0.441 |
| iPBS-2221 | ACCTAGCTCACGATCA | 150–1100 | 15 | 14 | 93.33 | 0.65 | 0.395 | 0.605 | 1.692 | 0.565 | 0.389 | 0.393 |
| iPBS-2270 | ATCCTGGCAATGGAACCA | 160–990 | 16 | 16 | 100 | 0.48 | 0.504 | 0.496 | 1.811 | 0.630 | 0.440 | 0.445 |
| iPBS-2272 | GGCTCAGATGCCA | 100–1200 | 17 | 15 | 88.24 | 0.57 | 0.479 | 0.521 | 1.639 | 0.506 | 0.351 | 0.355 |
| iPBS-2376 | TAGATGGCACCA | 100–1090 | 17 | 13 | 76.47 | 0.67 | 0.454 | 0.546 | 1.372 | 0.372 | 0.239 | 0.242 |
| iPBS-2239 | ACCTAGGCTCGGATGCCA | 110–700 | 11 | 11 | 100 | 0.86 | 0.242 | 0.758 | 1.445 | 0.414 | 0.266 | 0.269 |
| iPBS-2399 | AAACTGGCAACGGCGCCA | 150–1000 | 15 | 15 | 100 | 0.60 | 0.417 | 0.583 | 1.797 | 0.624 | 0.435 | 0.440 |
| iPBS-2383 | GCATGGCCTCCA | 150–1400 | 27 | 27 | 100 | 0.49 | 0.478 | 0.522 | 1.895 | 0.660 | 0.469 | 0.474 |
| Total | - | - | 140 | 133 | - | - | - | - | - | - | - | - |
| Mean | - | 100–1400 | 17.5 | 16.6 | 93.81 | 0.614 | 0.424 | 0.577 | 1.683 | 0.549 | 0.378 | 0.382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kırbay, E.; Kazaz, S.; Doğan Meral, E.; Dalda Şekerci, A. Hybridization Efficiency and Genetic Diversity in Cut Chrysanthemum: Integration of Morphological and iPBS Marker Analysis. Horticulturae 2025, 11, 1101. https://doi.org/10.3390/horticulturae11091101
Kırbay E, Kazaz S, Doğan Meral E, Dalda Şekerci A. Hybridization Efficiency and Genetic Diversity in Cut Chrysanthemum: Integration of Morphological and iPBS Marker Analysis. Horticulturae. 2025; 11(9):1101. https://doi.org/10.3390/horticulturae11091101
Chicago/Turabian StyleKırbay, Emine, Soner Kazaz, Ezgi Doğan Meral, and Akife Dalda Şekerci. 2025. "Hybridization Efficiency and Genetic Diversity in Cut Chrysanthemum: Integration of Morphological and iPBS Marker Analysis" Horticulturae 11, no. 9: 1101. https://doi.org/10.3390/horticulturae11091101
APA StyleKırbay, E., Kazaz, S., Doğan Meral, E., & Dalda Şekerci, A. (2025). Hybridization Efficiency and Genetic Diversity in Cut Chrysanthemum: Integration of Morphological and iPBS Marker Analysis. Horticulturae, 11(9), 1101. https://doi.org/10.3390/horticulturae11091101





