The Use of Spectral Vegetation Indices to Evaluate the Effect of Grafting and Salt Concentration on the Growth Performance of Different Tomato Varieties Grown Hydroponically
Abstract
1. Introduction
- (i)
- To determine selected vegetation indices as key parameters for evaluating the effect of grafting and salinity levels on the growth performance of selected commercial tomato varieties grown hydroponically,
- (ii)
- To study the response of tomato plant growth performance to the variety, grafting rootstock, and salt concentration, and their interactive effects.
2. Materials and Methods
2.1. Experimental Layout
2.2. Spectral Data Collection
2.3. Spectral Vegetation Indices
2.4. Statistical Analysis
3. Results and Discussion
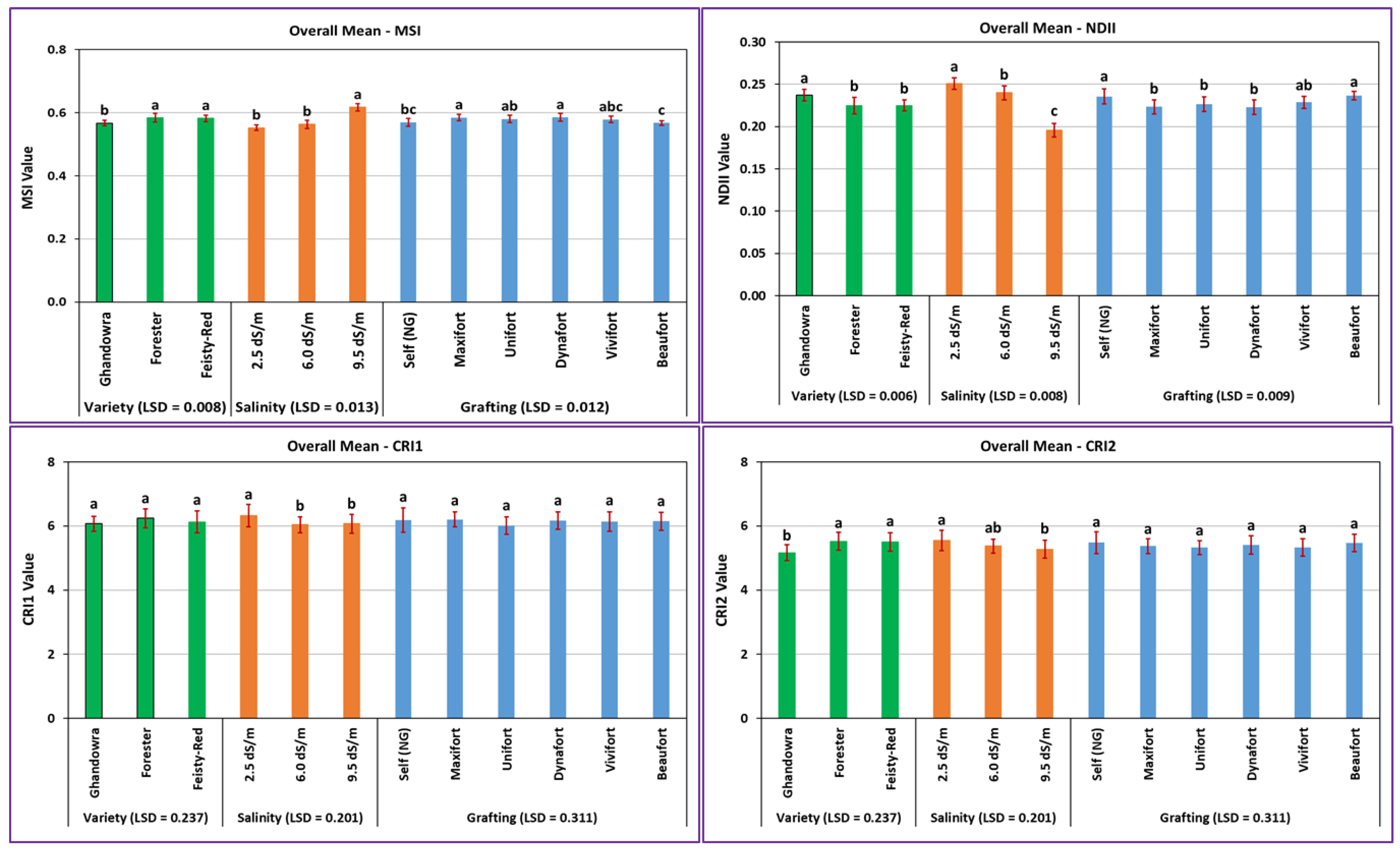
3.1. The Response of Vegetation Growth to Tomato Variety, Salinity Level, and Grafting Rootstock
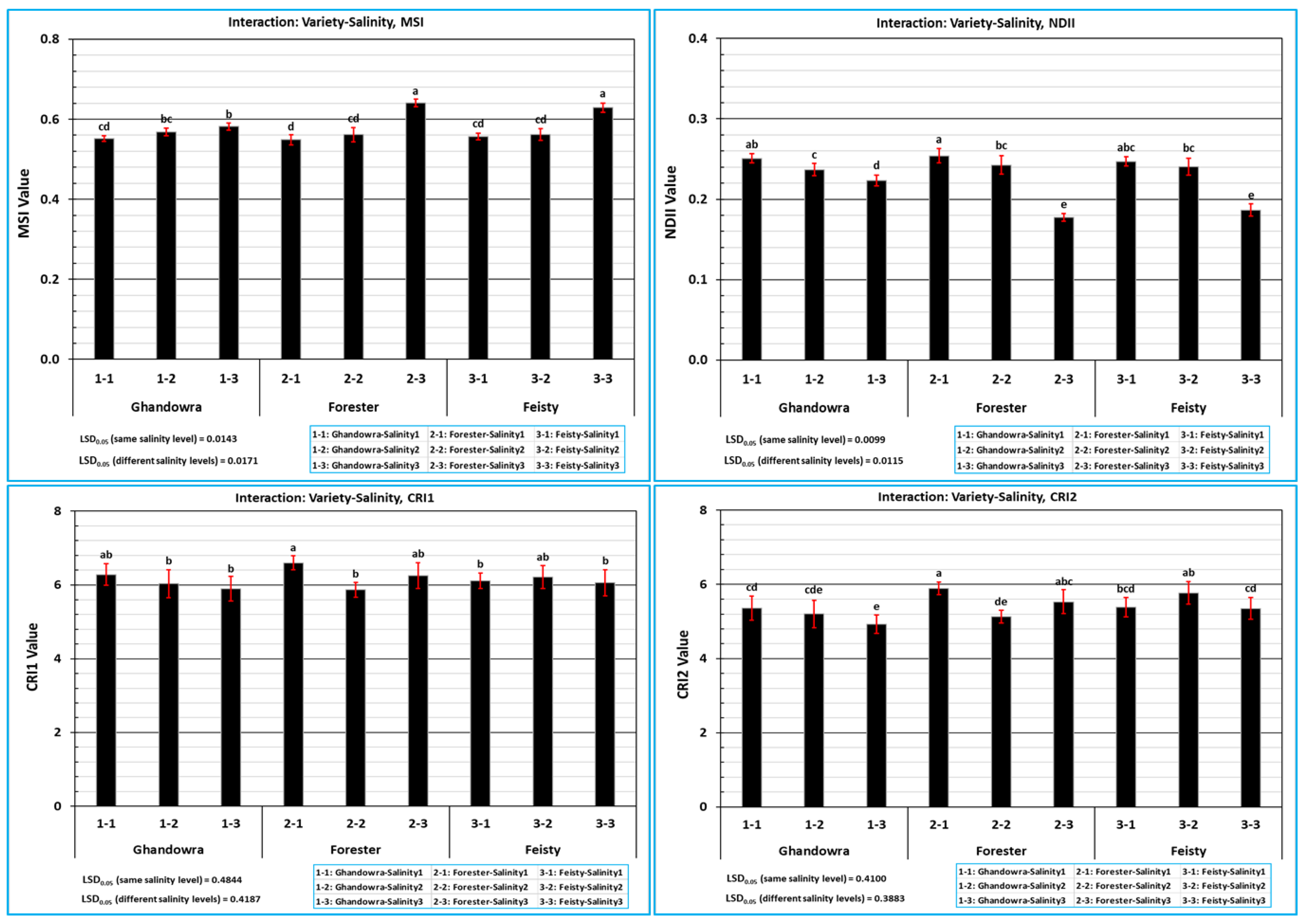
3.2. The Response of the Vegetative Growth of Tomato Plant to the Interactive Effect of Variety and Salinity
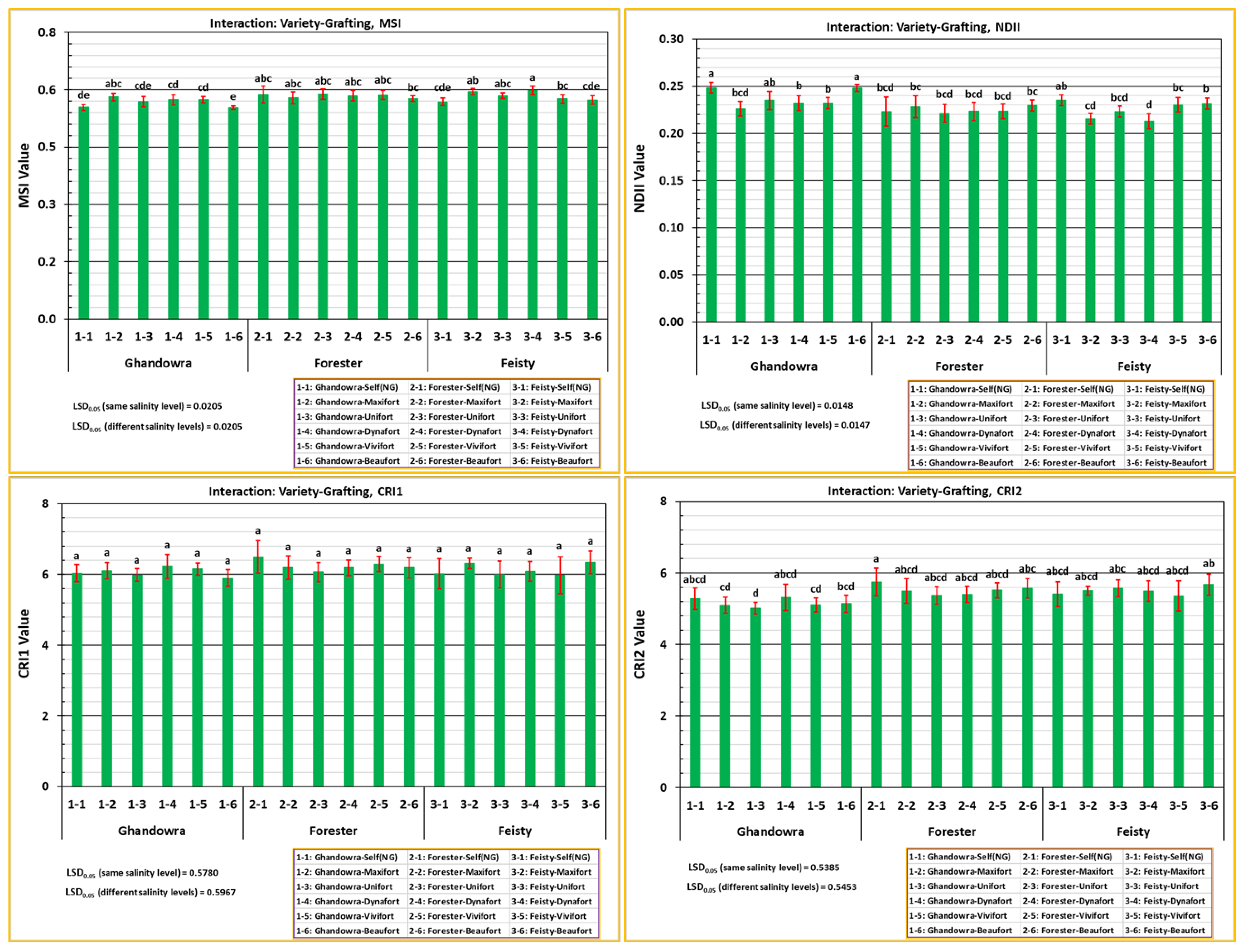
3.3. The Response of the Vegetative Growth of Tomato Plant to the Interactive Effect of Variety and Grafting
3.4. The Response of the Vegetative Growth of Tomato Plants to the Interactive Effect of Salinity and Grafting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S1, S2, S3 | Salinity1, Salinity2, Salinity3 |
| VIs | Vegetation Indices |
| MSI | Moisture Stress Index |
| NDII | Normalized Difference Infrared Index |
| CRI1 | Carotenoid Reflectance Index 1 |
| CRI2 | Carotenoid Reflectance Index 2 |
| DAT | Days After Transplanting |
| LSD | Least Significant Difference |
References
- Ntinas, G.K.; Kadoglidou, K.; Tsivelika, N.; Krommydas, K.; Kalivas, A.; Ralli, P.; Irakli, M. Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae 2019, 5, 42. [Google Scholar] [CrossRef]
- Cowan, N.; Ferrier, L.; Spears, B.; Drewer, J.; Reay, D.; Skiba, U. CEA Systems: The Means to Achieve Future Food Security and Environmental Sustainability? Front. Sustain. Food Syst. 2022, 6, 891256. [Google Scholar] [CrossRef]
- Wang, L.; Lian, G.; Harris, Z.; Horler, M.; Wang, Y.; Chen, T. The controlled environment agriculture: A sustainable agrifood production paradigm empowered by systems engineering. In Computer Aided Chemical Engineering; Kokossis, A.C., Georgiadis, M.C., Pistikopoulos, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 52, pp. 2167–2172. [Google Scholar] [CrossRef]
- Rajendran, S.; Domalachenpa, T.; Arora, H.; Li, P.; Sharma, A.; Rajauria, G. Hydroponics: Exploring innovative sustainable technologies and applications across crop production, with Emphasis on potato mini-tuber cultivation. Heliyon 2024, 10, e26823. [Google Scholar] [CrossRef] [PubMed]
- Rajaseger, G.; Chan, K.L.; Yee, K.T.; Ramasamy, S.; Khin, M.C.; Amaladoss, A.; Kadamb, H.P. Hydroponics: Current trends in sustainable crop production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef]
- Farvardin, M.; Taki, M.; Gorjian, S.; Shabani, E.; Sosa-Savedra, J.C. Assessing the Physical and Environmental Aspects of Greenhouse Cultivation: A Comprehensive Review of Conventional and Hydroponic Methods. Sustainability 2024, 16, 1273. [Google Scholar] [CrossRef]
- Aydin, A. Effects of grafting with wild tomato (Solanum pimpinellifolium and Solanum habrochaites) rootstocks on growth and leaf mineral accumulation in salt stress. Hortic. Environ. Biotechnol. 2024, 10, 785–801. [Google Scholar] [CrossRef]
- Albornoz, F.; Nario, A.; Saavedra, M.; Videla, X. Rootstock x Environment Interactions on Nitrogen-Use Efficiency in Grafted Tomato Plants at Different Phenological Stages. Agronomy 2020, 10, 350. [Google Scholar] [CrossRef]
- Dash, P.K.; Guo, B.; Leskovar, D.I. Optimizing Hydroponic Management Practices for Organically Grown Greenhouse Tomato under Abiotic Stress Conditions. HortScience 2023, 58, 1129–1138. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Jiménez-Benavente, E.; Gil-Villar, D.; Cebolla-Cornejo, J.; Romero-Hernández, G.; Carrillo, L.; Vicente-Carbajosa, J.; Medina, J.; Victoria Molina, R.; González Nebauer, S. Arabidopsis CDF3 transcription factor increases carbon and nitrogen assimilation and yield in trans-grafted tomato plants. Plant Physiol. Biochem. 2024, 210, 108607. [Google Scholar] [CrossRef]
- Al-Gaadi, K.A.; Tola, E.; Madugundu, R.; Zeyada, A.M.; Alameen, A.A.; Edrris, M.K.; Edrees, H.F.; Mahjoop, O. Response of leaf photosynthesis, chlorophyll content and yield of hydroponic tomatoes to different water salinity levels. PLoS ONE 2024, 19, e0293098. [Google Scholar] [CrossRef]
- Ormazabal, M.; Prudencio, Á.S.; Martínez-Melgarejo, P.A.; Martín-Rodríguez, J.Á.; Ruiz-Pérez, L.; Martínez-Andújar, C.; Jiménez, A.R.; Pérez-Alfocea, F. Rootstock Effects on Tomato Fruit Composition and Pollinator Preferences in Tomato. Horticulturae 2024, 10, 992. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, D.; Bian, X.; Huo, R.; Lü, G.; Gong, B.; Li, J.; Liu, S.; Gao, H. Transcriptome analysis showed that tomato-rootstock enhanced salt tolerance of grafted seedlings was accompanied by multiple metabolic processes and gene differences. Front. Plant Sci. 2023, 14, 1167145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Grafting Enhances the Photosynthesis and Nitrogen Absorption of Tomato Plants under Low-Nitrogen Stress. J. Plant Growth Regul. 2021, 41, 1714–1725. [Google Scholar] [CrossRef]
- Djidonou, D.; Zhao, X.; Koch, K.E.; Zotarelli, L. Nitrogen Accumulation and Root Distribution of Grafted Tomato Plants as Affected by Nitrogen Fertilization. HortScience 2019, 54, 1907–1914. [Google Scholar] [CrossRef]
- Ignat, T.; Shavit, Y.; Rachmilevitch, S.; Karnieli, A. Spectral monitoring of salinity stress in tomato plants. Biosyst. Eng. 2022, 217, 26–40. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Alzhrani, A.I.; Alanazi, H.H. Local Climate Zones and Thermal Characteristics in Riyadh City, Saudi Arabia. Remote Sens. 2021, 13, 4526. [Google Scholar] [CrossRef]
- Hochmuth, G.J.; Hochmuth, R.C. Nutrient Solution Formulation for Hydroponic (Perlite, Rockwool, NFT) Tomatoes in Florida; Publication #HS796; University of Florida, IFAS Extension: Gainesville, FL, USA, 2018; Available online: https://edis.ifas.ufl.edu/pdf/CV/CV216/CV216-2297144.pdf (accessed on 10 November 2024).
- Zhang, F.; Zhou, G. Estimation of vegetation water content using hyperspectral vegetation indices: A comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019, 19, 18. [Google Scholar] [CrossRef]
- Zhou, J.-J.; Zhang, Y.-H.; Han, Z.-M.; Liu, X.-Y.; Jian, Y.-F.; Hu, C.-G.; Dian, Y.-Y. Evaluating the Performance of Hyperspectral Leaf Reflectance to Detect Water Stress and Estimation of Photosynthetic Capacities. Remote Sens. 2021, 13, 2160. [Google Scholar] [CrossRef]
- Sriwongsitanon, N.; Gao, H.; Savenije, H.H.G.; Maekan, E.; Saengsawang, S.; Thianpopirug, S. The Normalized Difference Infrared Index (NDII) as a proxy for soil moisture storage in hydrological modelling. Hydrol. Earth Syst. Sci. 2015, 12, 8419–8457. [Google Scholar] [CrossRef]
- Sriwongsitanon, N.; Jandang, W.; Williams, J.; Suwawong, T.; Maekan, E.; Savenije, H.H.G. Using normalized difference infrared index patterns to constrain semi-distributed rainfall–runoff models in tropical nested catchments. Hydrol. Earth Syst. Sci. 2023, 27, 2149–2171. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Singh, R.K.; Chouhan, S.; Bhunia, U.; Paul, A.; Jeyaram, A.; Krihna Murthy, Y.V.N. Assessment of vegetation health quality parameters using hyperspectral indices and decision tree classification. In Proceedings of the ISRS Symposium, Nagpur, Maharashtra, India, 17 September 2009; Available online: https://www.ijcstjournal.org/volume-11/issue-5/IJCST-V11I5P3.pdf (accessed on 1 December 2024).
- Hajer, A.S.; Malibari, A.A.; Al-Zahrani, H.S.; Almaghrabi, O.A. Responses of three tomato cultivars to sea water salinity 1. Effect of salinity on the seedling growth. Afr. J. Biotechnol. 2006, 5, 855–861. Available online: https://www.ajol.info/index.php/ajb/article/view/42896 (accessed on 15 December 2024).
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Di Gioia, F.; Signore, A.; Serio, F.; Santamaria, P. Grafting Improves Tomato Salinity Tolerance through Sodium Partitioning within the Shoot. HortScience 2013, 48, 855–862. [Google Scholar] [CrossRef]
- Dudhat, M.A.; Bhanderi, D.R.; Tandel, B.M. Response of intra and inter specific grafts on plant growth and yield of tomato plants under salinity stress. J. Pharm. Innov. 2022, 11, 982–986. Available online: https://www.thepharmajournal.com/archives/2022/vol11issue8/PartL/11-7-462-448.pdf (accessed on 25 December 2024).
- Martinez-Rodriguez, M.M.; Estañ, M.T.; Moyano, E.; Garcia-Abellan, J.O.; Flores, F.B.; Campos, J.F.; Al-Azzawi, M.J.; Flowers, T.J.; Bolarín, M.C. The effectiveness of grafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Environ. Exp. Bot. 2008, 63, 392–401. [Google Scholar] [CrossRef]
- Soare, R.; Dinu, M.; Babeanu, C. The effect of using grafted seedlings on the yield and quality of tomatoes grown in greenhouses. Hort. Sci. 2018, 45, 76–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Hussain, A.; Arif, M.; Alkahtani, J.; AlMunqedhi, B.M.; Song, C. Genetic variability for salinity tolerance of tomato (Solanum lycopersicon MILL.) genotypes determined by stress tolerance indices. J. King Saud Univ. Sci. 2024, 36, 103386. [Google Scholar] [CrossRef]
- Abou El Salehein, E.H.; El Hamadi, M.M.; Al Gosabi, W.M.A. Tolerance response of some tomato cultivars to salinity stress. Middle East J. Appl. Sci. 2020, 10, 368–378. [Google Scholar] [CrossRef]
- Rajametov, S.N.; Jeong, H.B.; Yang, E.Y.; Cho, M.C. The effect of grafting on vegetative and reproductive traits of tomato. Veg. Crops Russ. 2024, 2, 12–20. [Google Scholar] [CrossRef]
- Semiz, G.D.; Suarez, D.L. Tomato salt tolerance: Impact of grafting and water composition on yield and ion relations. Turk. J. Agric. For. 2015, 39, 876–886. [Google Scholar] [CrossRef]
- Wahb-Allah, M.A. Effectiveness of Grafting for the Improvement of Salinity and Drought Tolerance in Tomato (Solanum lycopersicon L.). Asian J. Crop Sci. 2014, 6, 112–122. [Google Scholar] [CrossRef]

| Nutrient (ppm) | Transplant to 1st Cluster | 1st to 2nd Cluster | 2nd to 3rd Cluster | 3rd to 5th Cluster | 5th Cluster to Termination |
|---|---|---|---|---|---|
| N | 70 | 80 | 100 | 120 | 150 |
| P | 50 | 50 | 50 | 50 | 50 |
| K | 120 | 120 | 150 | 150 | 200 |
| Ca | 150 | 150 | 150 | 150 | 150 |
| Mg | 40 | 40 | 40 | 50 | 50 |
| S | 50 | 50 | 50 | 60 | 60 |
| Fe | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 |
| Cu | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Mn | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Zn | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| B | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| Mo | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tola, E.; Al-Gaadi, K.A.; Madugundu, R.; Zeyada, A.M.; Edrris, M.K.; Edrees, H.F.; Mahjoop, O. The Use of Spectral Vegetation Indices to Evaluate the Effect of Grafting and Salt Concentration on the Growth Performance of Different Tomato Varieties Grown Hydroponically. Horticulturae 2025, 11, 368. https://doi.org/10.3390/horticulturae11040368
Tola E, Al-Gaadi KA, Madugundu R, Zeyada AM, Edrris MK, Edrees HF, Mahjoop O. The Use of Spectral Vegetation Indices to Evaluate the Effect of Grafting and Salt Concentration on the Growth Performance of Different Tomato Varieties Grown Hydroponically. Horticulturae. 2025; 11(4):368. https://doi.org/10.3390/horticulturae11040368
Chicago/Turabian StyleTola, Elkamil, Khalid A. Al-Gaadi, Rangaswamy Madugundu, Ahmed M. Zeyada, Mohamed K. Edrris, Haroon F. Edrees, and Omer Mahjoop. 2025. "The Use of Spectral Vegetation Indices to Evaluate the Effect of Grafting and Salt Concentration on the Growth Performance of Different Tomato Varieties Grown Hydroponically" Horticulturae 11, no. 4: 368. https://doi.org/10.3390/horticulturae11040368
APA StyleTola, E., Al-Gaadi, K. A., Madugundu, R., Zeyada, A. M., Edrris, M. K., Edrees, H. F., & Mahjoop, O. (2025). The Use of Spectral Vegetation Indices to Evaluate the Effect of Grafting and Salt Concentration on the Growth Performance of Different Tomato Varieties Grown Hydroponically. Horticulturae, 11(4), 368. https://doi.org/10.3390/horticulturae11040368








