GWAS-Based Prediction of Genes Regulating Trehalose and Sucrose in Potato Tubers
Abstract
1. Introduction
2. Materials and Methods
2.1. Planting Accessions and Field Experiment Design
2.2. Determination of Trehalose and Sucrose Content in Potato Tubers
2.3. GWAS of Potato Tuber Trehalose and Sucrose
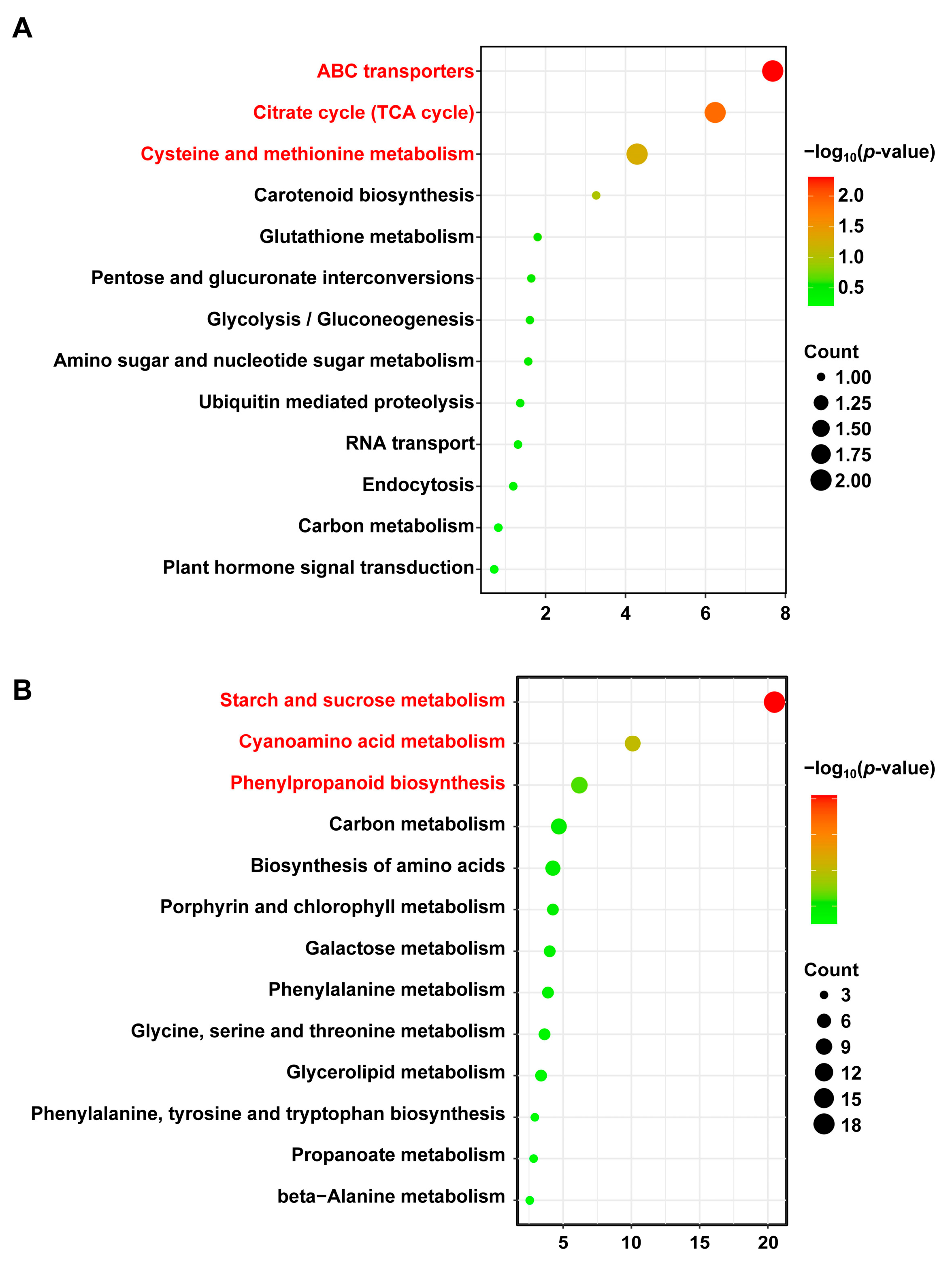
2.4. Candidate Gene Mining and Enrichment Analysis
3. Results
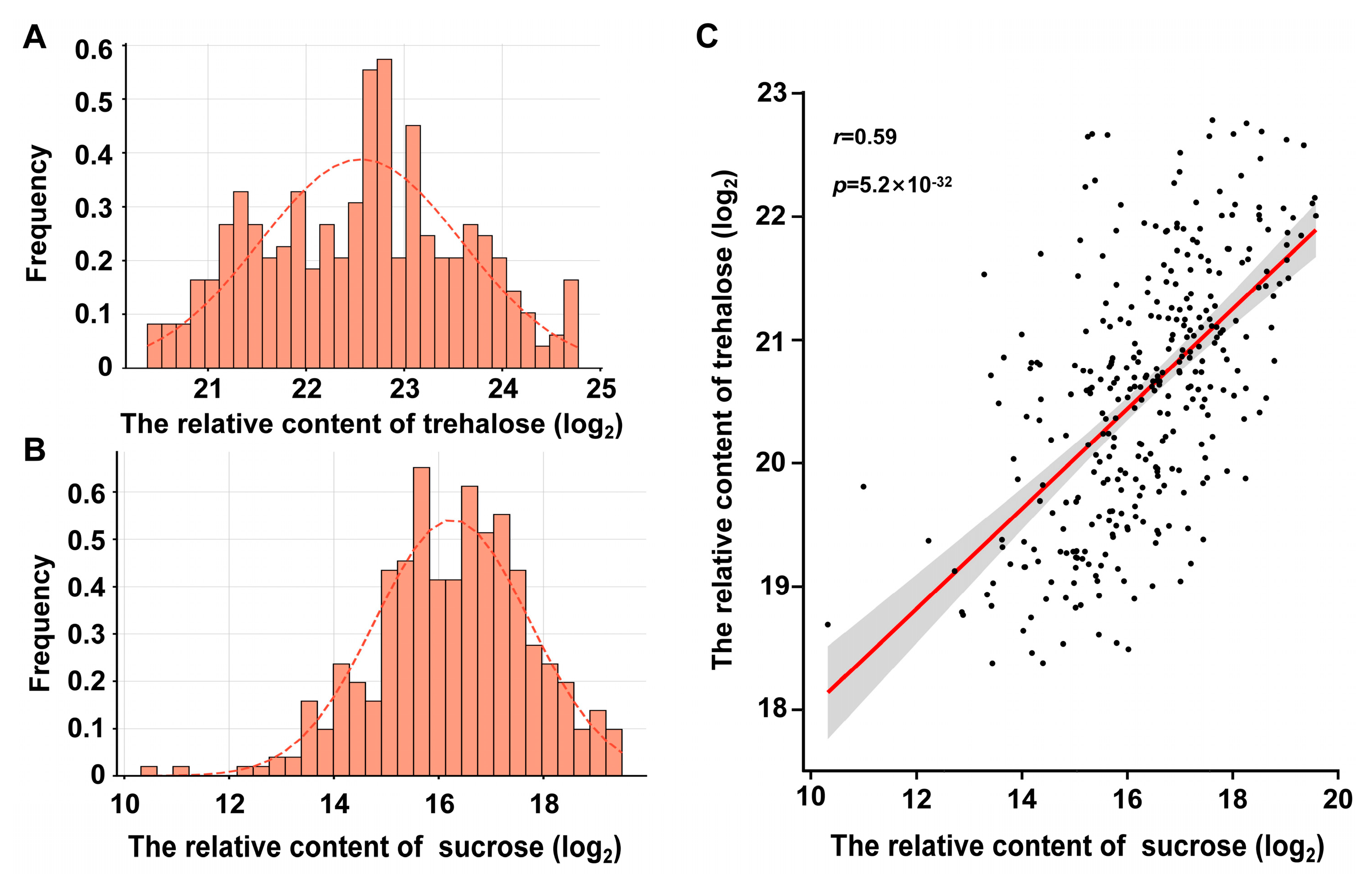
3.1. Phenotypic Analysis of Trehalose and Sucrose
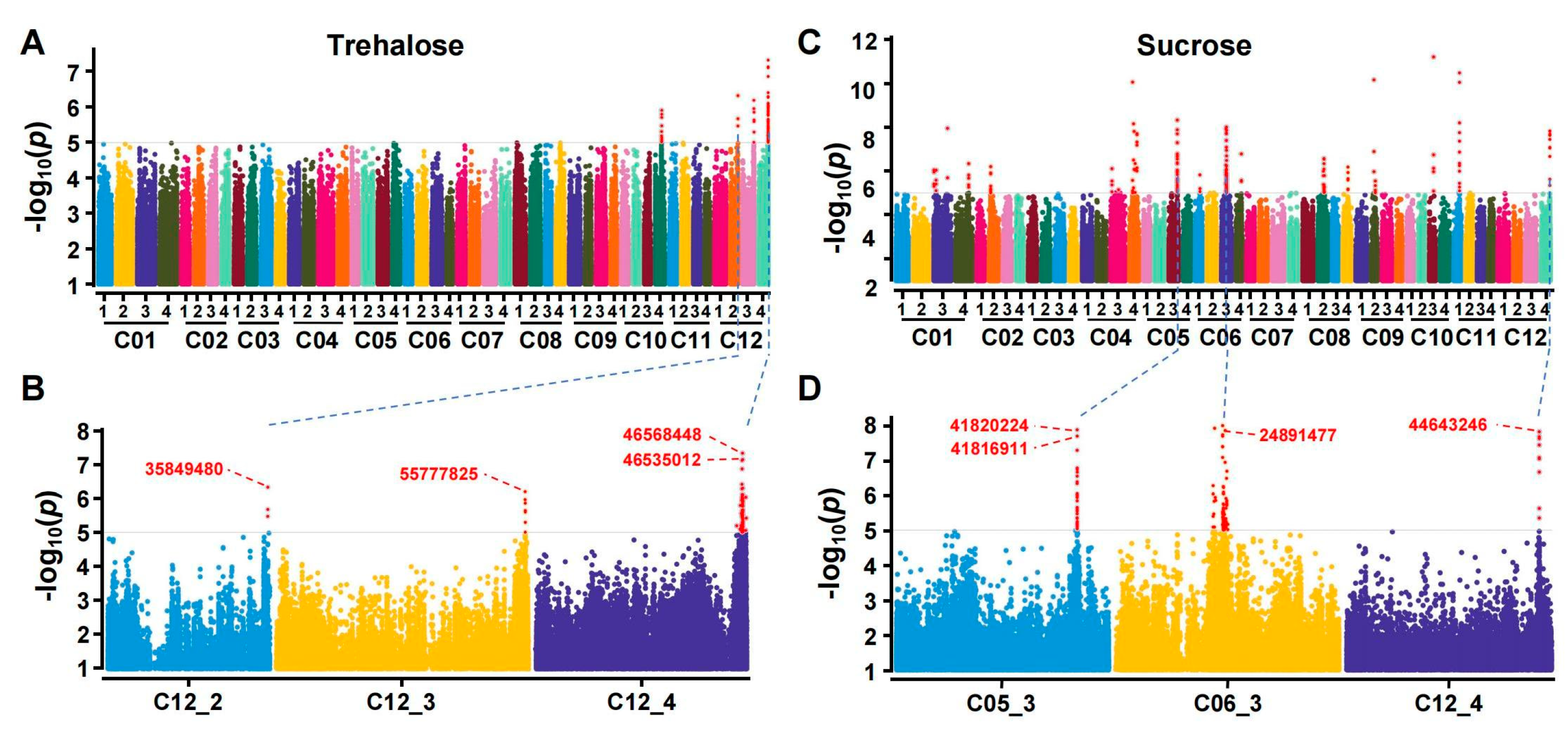
3.2. Genome-Wide Association Analysis of Trehalose and Sucrose
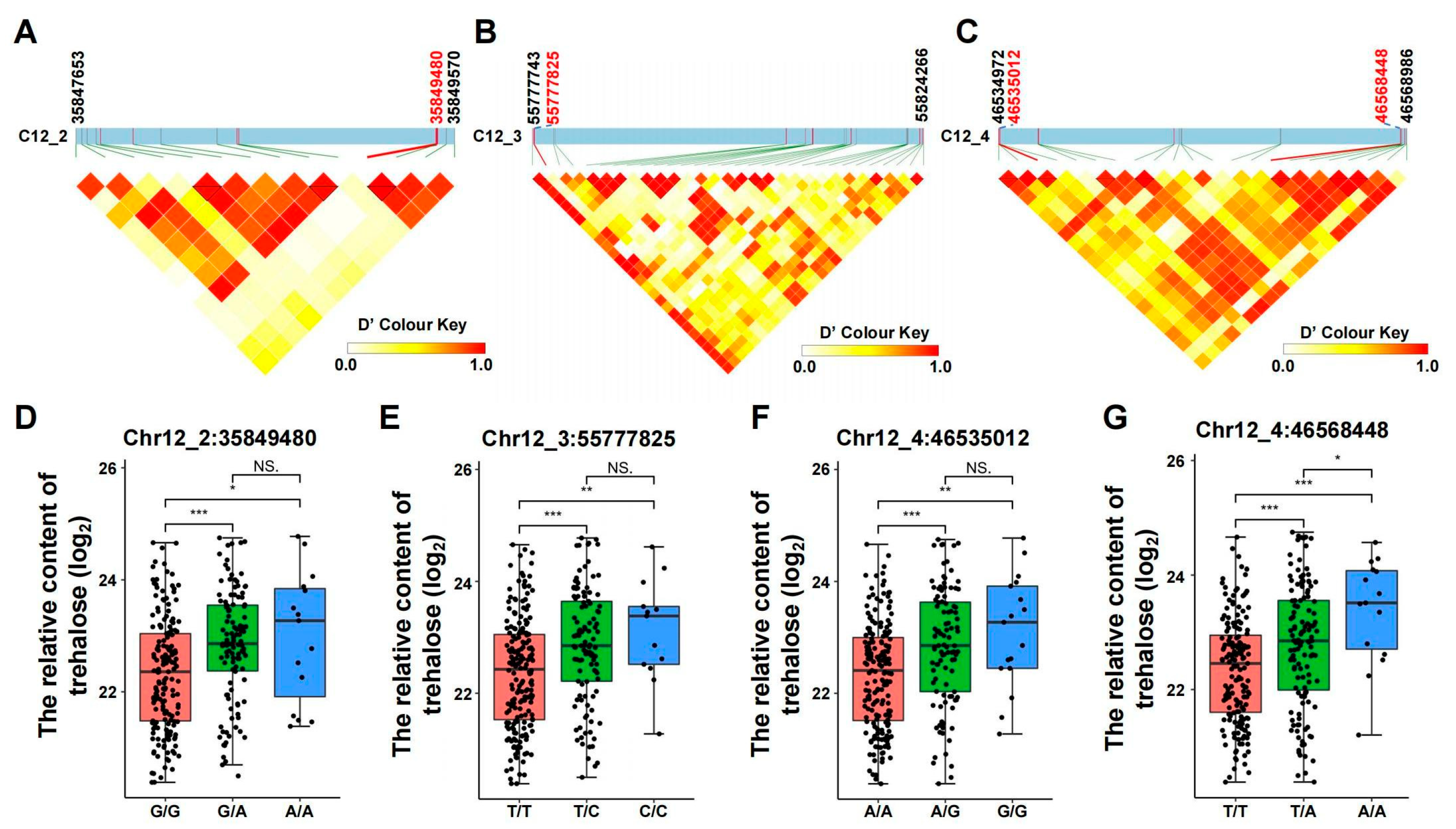
3.3. Analysis of Haplotypes and Allelic Variation Effects of Trehalose
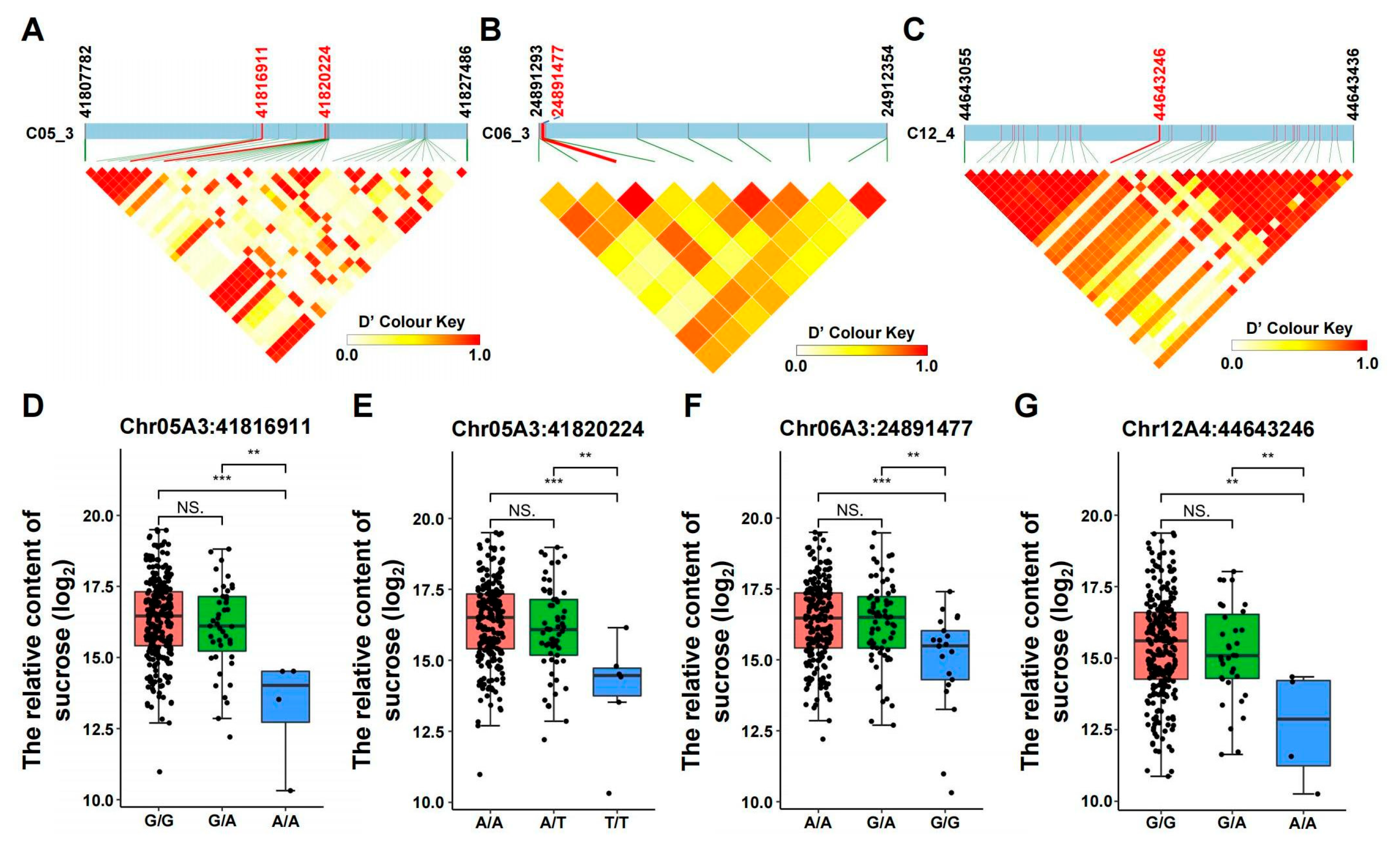
3.4. Analysis of Haplotypes and Allelic Variation Effects of Sucrose
3.5. Candidate Gene Analysis of Trehalose and Sucrose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Navarre, D.A.; Goyer, A.; Shakya, R. Chapter 14—Nutritional value of potatoes: Vitamin, phytonutrient, and mineral content. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 395–424. [Google Scholar]
- Bradshaw, J.E.; Ramsay, G. Chapter 1—Potato origin and production. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 1–26. [Google Scholar]
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The potato of the future: Opportunities and challenges in sustainable agri-food systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P. Regulation of sucrose to starch conversion in growing potato tubers. J. Exp. Bot. 2003, 54, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, F.; Mariotti, M.S.; Granby, K. Current issues in dietary acrylamide: Formation, mitigation and risk assessment. J. Sci. Food Agric. 2014, 94, 9–20. [Google Scholar] [CrossRef]
- Bachir, N.; Haddarah, A.; Sepulcre, F.; Pujola, M. Study the interaction of amino acids, sugars, thermal treatment and cooking technique on the formation of acrylamide in potato models. Food Chem. 2023, 408, 135235. [Google Scholar] [CrossRef]
- Yang, Y.; Achaerandio, I.; Pujolà, M. Influence of the frying process and potato cultivar on acrylamide formation in French fries. Food Control 2016, 62, 216–223. [Google Scholar] [CrossRef]
- Morgan, B.L.; Kakeshpour, T.; Occhialini, A.; King, G.; Sichterman, M.; Harbison, S.A.; Rigoulot, S.B.; Brabazon, H.; Stewart, C.N., Jr.; Lenaghan, S.C. Heterologous expression of OtsB increases tuber yield and phenotypic stability in potato under both abiotic and biotic stresses. Plants 2023, 12, 3394. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef]
- Wiberley-Bradford, A.E.; Bethke, P.C. Rate of cooling alters chip color, sugar contents, and gene expression profiles in stored potato tubers. Am. J. Potato Res. 2017, 94, 534–543. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Rohman, M.M.; Islam, M.R.; Monsur, M.B.; Amiruzzaman, M.; Fujita, M.; Hasanuzzaman, M. Trehalose protects maize plants from salt stress and phosphorus deficiency. Plants 2019, 8, 568. [Google Scholar] [CrossRef]
- Eh, T.J.; Jiang, Y.; Jiang, M.; Li, J.; Lei, P.; Ji, X.; Kim, H.I.; Zhao, X.; Meng, F. The role of trehalose metabolism in plant stress tolerance. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Hille, J.; Woerdenbag, H.J.; Benina, M.; Mehterov, N.; Toneva, V.; Fernie, A.R.; Mueller-Roeber, B. Natural products from resurrection plants: Potential for medical applications. Biotechnol. Adv. 2014, 32, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Fu, L.; Zhang, D.; He, X.; Chen, Q.; Peng, M.; Zhang, J. Interspecies and intraspecies analysis of trehalose contents and the biosynthesis pathway gene family reveals crucial roles of trehalose in osmotic-stress tolerance in cassava. Int. J. Mol. Sci. 2016, 17, 1077. [Google Scholar] [CrossRef] [PubMed]
- Delorge, I.; Janiak, M.; Carpentier, S.; Van Dijck, P. Fine tuning of trehalose biosynthesis and hydrolysis as novel tools for the generation of abiotic stress tolerant plants. Front. Plant Sci. 2014, 5, 147. [Google Scholar] [CrossRef]
- Li, H.W.; Zang, B.S.; Deng, X.W.; Wang, X.P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef]
- Ge, L.F.; Chao, D.Y.; Shi, M.; Zhu, M.Z.; Gao, J.P.; Lin, H.X. Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 2008, 228, 191–201. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, Y.; Huang, L.; Liu, S.; Tian, L.; Dai, Y.; Cao, Z.; Huang, L.; Li, D.; Song, F. Virus-induced gene silencing-based functional analyses revealed the involvement of several putative trehalose-6-phosphate synthase/phosphatase genes in disease resistance against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000 in tomato. Front. Plant Sci. 2016, 7, 1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Luo, J.; Yu, W.; Xiao, Y.; Peng, F. Peach PpSnRK1α interacts with bZIP11 and maintains trehalose balance in plants. Plant Physiol. Biochem. 2021, 160, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, X.; Tong, X.; Zhao, J.; Liu, X.; Wang, H.; Tang, L.; Shu, Y.; Li, G.; Wang, Y.; et al. The OsNAC23-Tre6P-SnRK1a feed-forward loop regulates sugar homeostasis and grain yield in rice. Mol. Plant 2022, 15, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Gao, S.; Yin, M.; Xu, M.; Zhang, H.; Li, S.; Han, Y.; Ji, S.; Li, X.; Du, G. Transcription factors PuPRE6/PuMYB12 and histone deacetylase PuHDAC9-like regulate sucrose levels in pear. Plant Physiol. 2024, 194, 1577–1592. [Google Scholar] [CrossRef]
- Hartmann, L.; Pedrotti, L.; Weiste, C.; Fekete, A.; Schierstaedt, J.; Göttler, J.; Kempa, S.; Krischke, M.; Dietrich, K.; Mueller, M.J.; et al. Crosstalk between two bZIP signaling pathways orchestrates salt-induced metabolic reprogramming in Arabidopsis roots. Plant Cell 2015, 27, 2244–2260. [Google Scholar] [CrossRef]
- Weltmeier, F.; Rahmani, F.; Ehlert, A.; Dietrich, K.; Schütze, K.; Wang, X.; Chaban, C.; Hanson, J.; Teige, M.; Harter, K.; et al. Expression patterns within the Arabidopsis C/S1 bZIP transcription factor network: Availability of heterodimerization partners controls gene expression during stress response and development. Plant Mol. Biol. 2009, 69, 107–119. [Google Scholar] [CrossRef]
- Sagor, G.H.; Berberich, T.; Tanaka, S.; Nishiyama, M.; Kanayama, Y.; Kojima, S.; Muramoto, K.; Kusano, T. A novel strategy to produce sweeter tomato fruits with high sugar contents by fruit-specific expression of a single bZIP transcription factor gene. Plant Biotechnol. J. 2016, 14, 1116–1126. [Google Scholar] [CrossRef]
- Dong, Q.; Xu, Q.; Kong, J.; Peng, X.; Zhou, W.; Chen, L.; Wu, J.; Xiang, Y.; Jiang, H.; Cheng, B. Overexpression of ZmbZIP22 gene alters endosperm starch content and composition in maize and rice. Plant Sci. 2019, 283, 407–415. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, J.; Ren, Y.; Li, M.; Tian, S.; Yu, Y.; Zuo, Y.; Gong, G.; Zhang, H.; et al. The NAC transcription factor ClNAC68 positively regulates sugar content and seed development in watermelon by repressing ClINV and ClGH3.6. Hortic. Res. 2021, 8, 214. [Google Scholar] [CrossRef]
- Du, C.; Sun, W.; Song, Q.; Zuo, K. GhDOFD45 promotes sucrose accumulation in cotton seeds by transcriptionally activating GhSWEET10 expression. Plant J. 2024, 120, 2468–2484. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cheng, M.N.; Ba, L.J.; Zeng, R.X.; Luo, D.L.; Qin, Y.H.; Liu, Z.L.; Kuang, J.F.; Lu, W.J.; Chen, J.Y.; et al. Pitaya HpWRKY3 is associated with fruit sugar accumulation by transcriptionally modulating sucrose metabolic genes HpINV2 and HpSuSy1. Int. J. Mol. Sci. 2019, 20, 1890. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Singh, R.K.; Moehninsi; Navarre, D.A. R2R3-MYB transcription factors, StmiR858 and sucrose mediate potato flavonol biosynthesis. Hortic. Res. 2021, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Yang, C.; Liu, Z.; Shen, S.; Zhan, C.; Lyu, Y.; Zhang, F.; Li, K.; Shi, Y.; et al. The NET locus determines the food taste, cooking and nutrition quality of rice. Sci. Bull. 2022, 67, 2045–2049. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Jiang, S.; Xia, C.; Deng, K.; Liu, B.; Wang, Z.; Liu, Q.; He, M.; Zou, M.; et al. The telomere-to-telomere genome of jaboticaba reveals the genetic basis of fruit color and citric acid content. Int. J. Mol. Sci. 2024, 25, 11951. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W.; et al. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 2022, 15, 258–275. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Y.; Xie, W.; Gong, L.; Lu, K.; Wang, W.; Li, Y.; Liu, X.; Zhang, H.; Dong, H.; et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat. Genet. 2014, 46, 714–721. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhan, C.; Li, Y.; Zhang, R.; Lv, Y.; Yang, Z.; Zhou, J.; Shi, Y.; Liu, X.; et al. Variation in a Poaceae-conserved fatty acid metabolic gene cluster controls rice yield by regulating male fertility. Nat. Commun. 2024, 15, 6663. [Google Scholar] [CrossRef]
- Ding, Z.; Fu, L.; Wang, B.; Ye, J.; Ou, W.; Yan, Y.; Li, M.; Zeng, L.; Dong, X.; Tie, W.; et al. Metabolic GWAS-based dissection of genetic basis underlying nutrient quality variation and domestication of cassava storage root. Genome Biol. 2023, 24, 289. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, K.; Bao, Y.; Lv, C.; Xu, M.; Wang, J.; Chen, F.; Wang, F. StMYB113b is a key regulator of red skin pigmentation in potato tubers. Hortic. Res. 2025, 12, uhaf164. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, M.; Deng, K.; Xia, C.; Jiang, S.; Zhang, C.; Ma, Y.; Dong, X.; He, M.; Na, T.; et al. Insights into the genetic determination of tuber shape and eye depth in potato natural population based on autotetraploid potato genome. Front. Plant Sci. 2023, 14, 1080666. [Google Scholar] [CrossRef]
- Wang, F.; Xia, Z.; Zou, M.; Zhao, L.; Jiang, S.; Zhou, Y.; Zhang, C.; Ma, Y.; Bao, Y.; Sun, H.; et al. The autotetraploid potato genome provides insights into highly heterozygous species. Plant Biotechnol. J. 2022, 20, 1996–2005. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.P.; Kumar, P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar] [CrossRef]
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Blenkinsop, R.W.; Copp, L.J.; Yada, R.Y.; Marangoni, A.G. Changes in compositional parameters of tubers of potato (Solanum tuberosum) during low-temperature storage and their relationship to chip processing quality. Agric. Food Chem. 2002, 50, 4545–4553. [Google Scholar] [CrossRef]
- Hajirezaei, M.R.; Börnke, F.; Peisker, M.; Takahata, Y.; Lerchl, J.; Kirakosyan, A.; Sonnewald, U. Decreased sucrose content triggers starch breakdown and respiration in stored potato tubers (Solanum tuberosum). J. Exp. Bot. 2003, 54, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar] [CrossRef]
- Debast, S.; Nunes-Nesi, A.; Hajirezaei, M.R.; Hofmann, J.; Sonnewald, U.; Fernie, A.R.; Börnke, F. Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting. Plant Physiol. 2011, 156, 1754–1771. [Google Scholar] [CrossRef]
- Sowokinos, J.R. Biochemical and molecular control of cold-induced sweetening in potatoes. Am. J. Potato Res. 2001, 78, 221–236. [Google Scholar] [CrossRef]
- Yu, C.; Li, T.; Shi, X.; Saleem, M.; Li, B.; Liang, W.; Wang, C. Deletion of endo-β-1,4-xylanase VmXyl1 impacts the virulence of Valsa mali in apple tree. Front. Plant Sci. 2018, 9, 663. [Google Scholar] [CrossRef]
- Lai, M.W.; Liou, R.F. Two genes encoding GH10 xylanases are essential for the virulence of the oomycete plant pathogen Phytophthora parasitica. Curr. Genet. 2018, 64, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, J.; Du, J.; Yang, Y.; Bi, C.; Qing, L. Disruption of the gene encoding endo-β-1, 4-xylanase affects the growth and virulence of Sclerotinia sclerotiorum. Front. Microbiol. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.; Espino, J.J.; González, C. The endo-beta-1,4-xylanase xyn11A is required for virulence in Botrytis cinerea. Mol. Plant-Microbe Interact. 2006, 19, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sharples, S.C.; Fry, S.C. Radioisotope ratios discriminate between competing pathways of cell wall polysaccharide and RNA biosynthesis in living plant cells. Plant J. 2007, 52, 252–262. [Google Scholar] [CrossRef]
- Gu, X.; Bar-Peled, M. The biosynthesis of UDP-galacturonic acid in plants. Functional cloning and characterization of Arabidopsis UDP-D-glucuronic acid 4-epimerase. Plant Physiol. 2004, 136, 4256–4264. [Google Scholar] [CrossRef]
- Zhao, S.; Fu, S.; Cao, Z.; Liu, H.; Huang, S.; Li, C.; Zhang, Z.; Yang, H.; Wang, S.; Luo, J.; et al. OsUGT88C3 encodes a UDP-glycosyltransferase responsible for biosynthesis of malvidin 3-o-galactoside in rice. Plants 2024, 13, 697. [Google Scholar] [CrossRef]
- Shimada, T.; Endo, T.; Rodríguez, A.; Fujii, H.; Goto, S.; Matsuura, T.; Hojo, Y.; Ikeda, Y.; Mori, I.C.; Fujikawa, T.; et al. Ectopic accumulation of linalool confers resistance to Xanthomonas citri subsp. citri in transgenic sweet orange plants. Tree Physiol. 2017, 37, 654–664. [Google Scholar] [CrossRef][Green Version]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Hu, L. Integration of multiple volatile cues into plant defense responses. New Phytol. 2022, 233, 618–623. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, D.; Wang, H.; Wu, B.; Cheng, J.; Zhang, B. Identification of UGT85A glycosyltransferases associated with volatile conjugation in grapevine (Vitis vinifera × Vitis labrusca). Hortic. Plant J. 2023, 9, 1095–1107. [Google Scholar] [CrossRef]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol. 2005, 138, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gao, Z.; Wang, J.; Huang, Y.; Chen, Y.; Li, J.; Lv, M.; Wang, J.; Luo, M.; Zuo, K. Cotton fiber elongation requires the transcription factor GhMYB212 to regulate sucrose transportation into expanding fibers. New Phytol. 2019, 222, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; He, M.; Bai, Y.; Xu, H.; Dandekar, A.M.; Fei, Z.; Cheng, L. Decreased sorbitol synthesis leads to abnormal stamen development and reduced pollen tube growth via an MYB transcription factor, MdMYB39L, in apple (Malus domestica). New Phytol. 2018, 217, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Tintor, N.; Mentzel, T.; Kombrink, E.; Boller, T.; Robatzek, S.; Schulze-Lefert, P.; Saijo, Y. Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc. Natl. Acad. Sci. USA 2009, 106, 22522–22527. [Google Scholar] [CrossRef]
- von Numers, N.; Survila, M.; Aalto, M.; Batoux, M.; Heino, P.; Palva, E.T.; Li, J. Requirement of a homolog of glucosidase II beta-subunit for EFR-mediated defense signaling in Arabidopsis thaliana. Mol. Plant 2010, 3, 740–750. [Google Scholar] [CrossRef]
- Vieira, P.S.; Bonfim, I.M.; Araujo, E.A.; Melo, R.R.; Lima, A.R.; Fessel, M.R.; Paixão, D.A.A.; Persinoti, G.F.; Rocco, S.A.; Lima, T.B.; et al. Xyloglucan processing machinery in xanthomonas pathogens and its role in the transcriptional activation of virulence factors. Nat. Commun. 2021, 12, 4049. [Google Scholar] [CrossRef]
- Cumino, A.; Curatti, L.; Giarrocco, L.; Salerno, G.L. Sucrose metabolism: Anabaena sucrose-phosphate synthase and sucrose-phosphate phosphatase define minimal functional domains shuffled during evolution. FEBS Lett. 2002, 517, 19–23. [Google Scholar] [CrossRef]
- Baba, S.A.; Vishwakarma, R.A.; Ashraf, N. Functional characterization of CsBGlu12, a β-glucosidase from Crocus sativus, provides insights into its role in abiotic stress through accumulation of antioxidant flavonols. J. Biol. Chem. 2017, 292, 4700–4713. [Google Scholar] [CrossRef]
- Dong, N.Q.; Sun, Y.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Liu, X.; Fu, L.; Qin, P.; Sun, Y.; Liu, J.; Wang, X. Overexpression of the wheat trehalose 6-phosphate synthase 11 gene enhances cold tolerance in Arabidopsis thaliana. Gene 2019, 710, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Zhang, S.C.; Qi, S.D.; Zheng, C.C.; Wu, C.A. Delayed germination of Arabidopsis seeds under chilling stress by overexpressing an abiotic stress inducible GhTPS11. Gene 2016, 575, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wu, X.; Li, Y.; Liu, G.; Cui, Z.; Jiang, T.; Ma, Q.; Luo, L.; Zhang, P. Cell wall invertase 3 affects cassava productivity via regulating sugar allocation from source to sink. Front. Plant Sci. 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Tong, C.; Li, C.; Cao, X.Y.; Sun, X.D.; Bao, Q.X.; Mu, X.R.; Liu, C.Y.; Loake, G.J.; Chen, H.H.; Meng, L.S. Long-distance transport of sucrose in source leaves promotes sink root growth by the EIN3-SUC2 module. PLoS Genet. 2022, 18, e1010424. [Google Scholar] [CrossRef]
- Wei, L.; Mao, W.; Jia, M.; Xing, S.; Ali, U.; Zhao, Y.; Chen, Y.; Cao, M.; Dai, Z.; Zhang, K.; et al. FaMYB44.2, a transcriptional repressor, negatively regulates sucrose accumulation in strawberry receptacles through interplay with FaMYB10. J. Exp. Bot. 2018, 69, 4805–4820. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Wang, T.; Liu, W.; Zhang, J.; Fang, H.; Zhang, Z.; Peng, F.; Chen, X.; Wang, N. MdMYB305-MdbHLH33-MdMYB10 regulates sugar and anthocyanin balance in red-fleshed apple fruits. Plant J. 2023, 113, 1062–1079. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Li, J.; Yue, P.; Bu, H.; Jiang, J.; Liu, W.; Xu, Y.; Yuan, H.; Li, T.; et al. Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit. Plant Physiol. 2020, 182, 2035–2046. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, K.; Bao, Y.; Xu, M.; Lv, C.; Zhao, L.; Wang, J.; Wang, F. GWAS-Based Prediction of Genes Regulating Trehalose and Sucrose in Potato Tubers. Horticulturae 2025, 11, 1033. https://doi.org/10.3390/horticulturae11091033
Deng K, Bao Y, Xu M, Lv C, Zhao L, Wang J, Wang F. GWAS-Based Prediction of Genes Regulating Trehalose and Sucrose in Potato Tubers. Horticulturae. 2025; 11(9):1033. https://doi.org/10.3390/horticulturae11091033
Chicago/Turabian StyleDeng, Ke, Yuting Bao, Minghao Xu, Chunna Lv, Long Zhao, Jian Wang, and Fang Wang. 2025. "GWAS-Based Prediction of Genes Regulating Trehalose and Sucrose in Potato Tubers" Horticulturae 11, no. 9: 1033. https://doi.org/10.3390/horticulturae11091033
APA StyleDeng, K., Bao, Y., Xu, M., Lv, C., Zhao, L., Wang, J., & Wang, F. (2025). GWAS-Based Prediction of Genes Regulating Trehalose and Sucrose in Potato Tubers. Horticulturae, 11(9), 1033. https://doi.org/10.3390/horticulturae11091033





