Advances in Cold Stress Response Mechanisms of Cucurbits
Abstract
1. Introduction
2. Phytohormone-Mediated Cold Stress Response in Cucurbits
2.1. Abscisic Acid (ABA)
2.2. Brassinosteroids (BRs)
2.3. Jasmonic Acid (JA)
2.4. Salicylic Acid (SA)
2.5. Auxin (AUX)
3. Signaling Molecules Involved in Cold Stress Response in Cucurbits
3.1. Calcium (Ca2+)
3.2. Hydrogen Peroxide (H2O2)
3.3. Nitric Oxide (NO) and Hydrogen Sulfide (H2S)
4. Soluble Sugars Involved in Cold Stress Response in Cucurbits
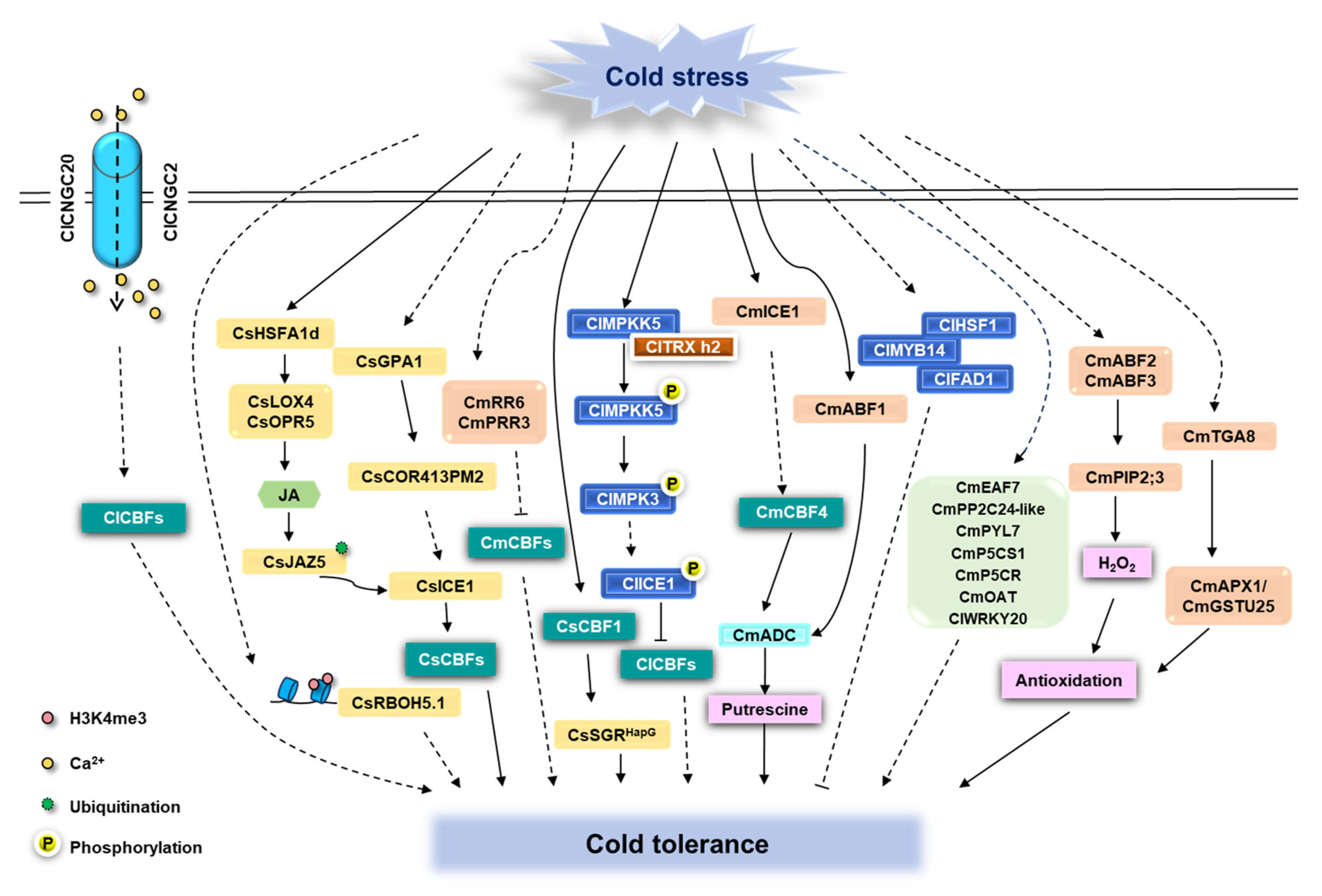
5. Molecular Regulatory Networks of Cucurbits in Response to Cold Stress
5.1. CBF-Dependent Pathways
5.2. Non-CBF Regulatory Factors
5.3. Post-Transcriptional Regulation
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, M.; Xiao, X.; Khan, K.; Lyu, J.; Yu, J. Characterization and functions of myeloblastosis (MYB) transcription factors in cucurbit crops. Plant Sci. 2024, 348, 112235. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, L. Cucurbitaceae. In Identification and Control of Common Weeds: Volume 3; Springer: Singapore, 2017; pp. 417–432. [Google Scholar]
- Grumet, R.; McCreight, J.D.; McGregor, C.; Weng, Y.Q.; Mazourek, M.; Reitsma, K.; Labate, J.; Davis, A.; Fei, Z.J. Genetic resources and vulnerabilities of major cucurbit crops. Genes 2021, 12, 1222. [Google Scholar] [CrossRef]
- Kerje, T.; Grum, M.J.A. The origin of melon, Cucumis melo: A review of the literature. Acta. Hortic. 2000, 510, 37–44. [Google Scholar] [CrossRef]
- Salah, R.; Zhang, R.; Xia, S.; Song, S.; Hao, Q.; Hashem, M.H.; Li, H.; Li, Y.; Li, X.; Lai, Y. Higher phytohormone contents and weaker phytohormone signal transduction were observed in cold-tolerant cucumber. Plants 2022, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Paris, H.S. Origin and emergence of the sweet dessert watermelon. Ann. Bot. 2015, 116, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Browse, J. Cold comfort farm: The acclimation of plants to freezing temperatures. Plant Cell Environ. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, S.; Wang, Y.; Lu, L.; Sun, M.; He, C.; Wang, J.; Li, Y.; Yu, X.; Li, Q.; et al. Physiological and molecular mechanisms of ABA and CaCl2 regulating chilling tolerance of cucumber seedlings. Plants 2021, 10, 2746. [Google Scholar] [CrossRef]
- Hou, J.; Zhou, Y.; Gao, L.; Wang, Y.; Yang, L.; Zhu, H.; Wang, J.; Zhao, S.; Ma, C.; Sun, S.; et al. Dissecting the genetic architecture of melon chilling tolerance at the seedling stage by association mapping and identification of the elite alleles. Front. Plant Sci. 2018, 9, 1577. [Google Scholar] [CrossRef]
- Chang, J.; Guo, Y.; Li, J.; Su, Z.; Wang, C.; Zhang, R.; Wei, C.; Ma, J.; Zhang, X.; Li, H. Positive interaction between H2O2 and Ca2+ mediates melatonin-induced CBF pathway and cold tolerance in watermelon (Citrullus lanatus L.). Antioxidants 2021, 10, 1457. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Y.; Li, X.; Xu, S.; An, L.; Liu, D. Enhancement of low-temperature tolerance in watermelon (Citrullus lanatus) seedlings by cool-hardening germination. Aust. J. Exp. Agric. 2007, 47, 749–754. [Google Scholar] [CrossRef]
- Wen, D.; Yang, N.; Zhang, W.; Wang, X.; Zhang, J.; Nie, W.; Song, H.; Sun, S.; Zhang, H.; Han, Y.; et al. GATA3-COMT1-melatonin as upstream signaling of ABA participated in Se-enhanced cold tolerance by regulate iron uptake and distribution in Cucumis sativus L. J. Pineal Res. 2025, 77, e70028. [Google Scholar] [CrossRef]
- Li, M.; Duan, X.; Liu, T.; Qi, H. Short-term suboptimal low temperature has short- and long-term effects on melon seedlings. Sci. Hortic. 2022, 297, 110967. [Google Scholar] [CrossRef]
- Qin, Y.; Dong, X.; Dong, H.; Wang, X.; Ye, T.; Wang, Q.; Duan, J.; Yu, M.; Zhang, T.; Du, N.; et al. γ-aminobutyric acid contributes to a novel long-distance signaling in figleaf gourd rootstock-induced cold tolerance of grafted cucumber seedlings. Plant Physiol. Biochem. 2024, 216, 109168. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C.; Shi, J.; Zhang, Y.; Liu, T.; Qi, H. Abscisic acid and putrescine synergistically regulate the cold tolerance of melon seedlings. Plant Physiol. Biochem. 2021, 166, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, M.; Liu, G.; Yang, X.; Hou, X. Comparative transcriptome profiling of chilling stress responsiveness in grafted watermelon seedlings. Plant Physiol. Biochem. 2016, 109, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Zheng, S.; Su, M.; Wang, L.; Zhang, T.; Wang, J.; Xie, H.; Wu, X.; Ul Haq, S.I.; Qiu, Q. Small signaling molecules in plant response to cold stress. J. Plant Physiol. 2021, 266, 153534. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Ding, X.; Wang, W.; Zhai, S.; Bai, L.; Guo, Z. Gene regulation and signal transduction in the ICE-CBF-COR signaling pathway during cold stress in plants. Biochemistry 2017, 82, 1103–1117. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Y.; Yang, S. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Integr. Plant Biol. 2018, 60, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.L.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR signaling cascade and its regulation in plants responding to cold stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory networks underlying plant responses and adaptation to cold stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, Z.; Kong, X.; Khan, A.; Ullah, N.; Zhang, X. Plant coping with cold stress: Molecular and physiological adaptive mechanisms with future perspectives. Cells 2025, 14, 110. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, L.; Long, Y.; Zhang, M.; Yao, Y.; Deng, H.; Quan, C.; Lu, M.; Cui, B.; Wang, D. An ABA biosynthesis enzyme gene OsNCED4 regulates NaCl and cold stress tolerance in rice. Sci. Rep. 2024, 14, 26711. [Google Scholar] [CrossRef]
- Yang, T.; Zhuang, Z.; Bian, J.; Ren, Z.; Ta, W.; Peng, Y. Mechanisms of exogenous brassinosteroids and abscisic acid in regulating maize cold stress tolerance. Int. J. Mol. Sci. 2025, 26, 3326. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, J.; Su, Z.; Chang, J.; Yang, J.; Wei, C.; Zhang, Y.; Ma, J.; Zhang, X.; Li, H. Abscisic acid mediates grafting-induced cold tolerance of watermelon interaction with melatonin and methyl jasmonate. Front. Plant Sci. 2021, 12, 785317. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, K.I.; Khan, A.L.; Waqas, M.; Lee, I.J. Exogenous application of abscisic acid regulates endogenous gibberellins homeostasis and enhances resistance of oriental melon (Cucumis melo var L.) against low temperature. Sci. Hortic. 2016, 207, 41–47. [Google Scholar] [CrossRef]
- Li, M.; Zhao, W.; Du, Q.; Xiao, H.; Li, J.; Wang, J.; Shang, F. Abscisic acid and hydrogen peroxide regulate proline homeostasis in melon seedlings under cold stress by forming a bidirectional closed loop. Environ. Exp. Bot. 2023, 205, 105102. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Chong, L.; Xu, R.; Huang, P.; Guo, P.; Zhu, M.; Du, H.; Sun, X.; Ku, L.; Zhu, J.; Zhu, Y. The tomato OST1-VOZ1 module regulates drought-mediated flowering. Plant Cell 2022, 34, 2001–2018. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, L.; Zhang, M.; Yao, Y.; Qian, Q.; Wei, Z.; Cui, B.; Wang, D.; Quan, C.; Lu, M.; et al. OsNCED5 confers cold stress tolerance through regulating ROS homeostasis in rice. Plant Physiol. Biochem. 2025, 220, 109455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, L.; Zhang, L.; Li, J.; Fang, Y.; Zhao, L.; Ren, Y.; Chen, F. Abscisic acid enhances tolerance to spring freeze stress and regulates the expression of ascorbate-glutathione biosynthesis-related genes and stress-responsive genes in common wheat. Mol. Breed. 2020, 40, 108. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.; Zhu, C.; Wu, S.; Li, J.; Yu, J.; Hu, Z. A transcriptional regulation of ERF15 contributes to ABA-mediated cold tolerance in tomato. Plant Cell Environ. 2024, 47, 1334–1347. [Google Scholar] [CrossRef]
- Liu, W.; Lv, Y.; Zhang, L.; Jiang, Y.; Liu, S.; Wang, Z.; Zhang, J.; He, M. The gene CmPYL6 strongly contributes to cold tolerance in oriental melon. Plant Biol. 2024, 26, 1033–1046. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Lv, Y.; Zhang, L.; Liu, S.; Wang, Z.; He, M.; Zhang, J. CmPYL7 positively regulates the cold tolerance via interacting with CmPP2C24-like in oriental melon. Physiol. Plant. 2024, 176, e14628. [Google Scholar] [CrossRef]
- Li, M.; Duan, X.; Gao, G.; Liu, T.; Qi, H. CmABF1 and CmCBF4 cooperatively regulate putrescine synthesis to improve cold tolerance of melon seedlings. Hortic. Res. 2022, 9, uhac002. [Google Scholar] [CrossRef]
- He, Z.; Zhou, M.; Feng, X.; Di, Q.; Meng, D.; Yu, X.; Yan, Y.; Sun, M.; Li, Y. The role of brassinosteroids in plant cold stress response. Life 2024, 14, 1015. [Google Scholar] [CrossRef]
- Yao, T.; Xie, R.; Zhou, C.; Wu, X.; Li, D. Roles of brossinosteroids signaling in biotic and abiotic stresses. J. Agric. Food Chem. 2023, 71, 7947–7960. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, X.; Shi, Z.; He, F.; Qi, G.; Li, X.; Niu, Y.; Zhou, W. Exogenous 24-epibrassinolide improves low-temperature tolerance of maize seedlings by influencing sugar signaling and metabolism. Int. J. Mol. Sci. 2025, 26, 585. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.; Chang, J.; Wei, H.; Li, H.; Li, C.; Yang, J.; Song, Z.; Wang, Z.; Lun, J.; et al. Foliar application of 24-epibrassinolide enhances leaf nicotine content under low temperature conditions during the mature stage of flue-cured tobacco by regulating cold stress tolerance. BMC Plant Biol. 2025, 25, 77. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Hou, Y.; Zhao, L.; Zheng, Y.; Jin, P. Exogenous 24-epibrassinolide alleviates chilling injury in peach fruit through modulating PpGATA12-mediated sucrose and energy metabolisms. Food Chem. 2023, 400, 133996. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Mao, M.; Xue, X.; Yao, G.; Zhang, Q.; Hu, S. 24-Epibrassinolide treatment alleviates frost damage of apple flower via regulating proline, ROS, and energy metabolism. Plant Physiol. Biochem. 2025, 220, 109507. [Google Scholar] [CrossRef]
- Dong, F.; Li, X.; Liu, C.; Zhao, B.; Ma, Y.; Ji, W. Exogenous 24-epibrassinolide mitigates damage in grape seedlings under low-temperature stress. Front. Plant Sci. 2025, 16, 1487680. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zou, Z.; Zhang, J.; Zhao, Y.; Yan, F. 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). Funct. Integr. Genom. 2016, 16, 29–35. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, L.; Cheng, F.; Zhou, Y.; Xia, X.; Mao, W.; Shi, K.; Yu, J. Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning, carboxylation and redox homeostasis in cucumber. Physiol. Plant. 2013, 148, 133–145. [Google Scholar] [CrossRef]
- Ofoe, R. Signal transduction by plant heterotrimeric G-protein. Plant Biol. 2021, 23, 3–10. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, M.; Ma, S.; Feng, Q.; Wang, Y.; Di, Q.; Zhou, M.; He, C.; Li, Y.; Gao, L.; et al. Mechanism of CsGPA1 in regulating cold tolerance of cucumber. Hortic. Res. 2022, 9, uhac109. [Google Scholar] [CrossRef]
- Theune, M.L.; Bloss, U.; Brand, L.H.; Ladwig, F.; Wanke, D. Phylogenetic analyses and GAGA-motif binding studies of BBR/BPC proteins lend to clues in GAGA-motif recognition and a regulatory role in brassinosteroid signaling. Front. Plant Sci. 2019, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Miao, L.; Huang, B.; Gao, L.; He, C.; Yan, Y.; Wang, J.; Yu, X.; Li, Y. Genome-wide identification and characterization of cucumber BPC transcription factors and their responses to abiotic stresses and exogenous phytohormones. Int. J. Mol. Sci. 2019, 20, 5048. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Li, S.; Feng, X.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 is essential for cucumber survival under cold stress. BMC Plant Biol. 2023, 23, 566. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, M.; Li, Y.; Feng, X.; Li, M.; Shi, A.; He, C.; Yan, Y.; Wang, J.; Yu, X. Sodium nitrophenolate mediates brassinosteroids signaling to enhance cold tolerance of cucumber seedling. Plant Physiol. Biochem. 2024, 206, 108317. [Google Scholar]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Niu, D.; Wang, Z.; Yan, X.; Yang, X.; Yang, Y.; Cui, H. Tobacco transcription repressors NtJAZ: Potential involvement in abiotic stress response and glandular trichome induction. Plant Physiol. Biochem. 2019, 141, 388–397. [Google Scholar] [CrossRef]
- Sharma, M.; Laxmi, A. Jasmonates: Emerging players in controlling temperature stress tolerance. Front. Plant Sci. 2016, 6, 1129. [Google Scholar] [CrossRef]
- Yu, M.; Wang, R.; Xia, J.; Li, C.; Xu, Q.; Cang, J.; Wang, Y.; Zhang, D. JA-induced TaMPK6 enhanced the freeze tolerance of Arabidopsis thaliana through regulation of ICE-CBF-COR module and antioxidant enzyme system. Plant Sci. 2023, 329, 111621. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.; Zhang, X.; You, C.; Hao, Y. Apple B-box protein BBX37 regulates jasmonic acid mediated cold tolerance through the JAZ-BBX37-ICE1-CBF pathway and undergoes MIEL1-mediated ubiquitination and degradation. New Phytol. 2021, 229, 2707–2729. [Google Scholar] [CrossRef]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate positively regulates cold tolerance by promoting ABA biosynthesis in tomato. Plants 2023, 12, 60. [Google Scholar] [CrossRef]
- Qi, C.; Dong, D.; Li, Y.; Wang, X.; Guo, L.; Liu, L.; Dong, X.; Li, X.; Yuan, X.; Ren, S.; et al. Heat shock-induced cold acclimation in cucumber through CsHSFA1d-activated JA biosynthesis and signaling. Plant J. 2022, 111, 85–102. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Liu, L.; Liu, J.; Li, C.; Yuan, L.; Wei, C.; Zhang, X.; Li, H. The Ca2+ channels CNGC2 and CNGC20 mediate methyl jasmonate-induced calcium signaling and cold tolerance. Plant Physiol. 2025, 198, kiaf219. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, Y.; Li, Y.; Zhou, J.; Shi, H. Improving photosynthesis and drought tolerance in Nicotiana tabacum L. by foliar application of salicylic acid. All Life 2023, 16, 2224936. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, S.; Amin, F.; Al-Hawadi, J.S.; Okla, M.K.; Alaraidh, I.A.; AbdElgawad, H.; Liu, K.; Harrison, M.T.; Saud, S.; et al. Salicylic acid and tocopherol improve wheat (Triticum aestivum L.) physio-biochemical and agronomic features grown in deep sowing stress: A way forward towards sustainable production. BMC Plant Biol. 2024, 24, 477. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.; Wang, D.; Fu, Z. Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol. Plant 2018, 11, 1427–1439. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Chai, X.; Xue, D.; Zheng, W.; Shi, Y.; Wang, A. The involvement of jasmonic acid, ethylene, and salicylic acid in the signaling pathway of clonostachys rosea-induced resistance to gray mold disease in tomato. Phytopathology 2019, 109, 1102–1114. [Google Scholar] [CrossRef]
- Li, A.; Sun, X.; Liu, L. Action of salicylic acid on plant growth. Front. Plant Sci. 2022, 13, 878076. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid and salicylic acid induce the accumulation of sucrose and increase resistance to chilling injury in peach fruit. J. Sci. Food Agric. 2021, 101, 4250–4255. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Grewal, S.K. Composite coating of chitosan with salicylic acid retards pear fruit softening under cold and supermarket storage. Food Res. Int. 2022, 160, 111724. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Bodaghi, H.; Hagh, Z.G. Alleviation of postharvest chilling injury in sweet pepper using salicylic acid foliar spraying incorporated with caraway oil coating under cold storage. Front. Plant Sci. 2022, 13, 999518. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, S.; Shojaie, A.; Koy, R.A.M. Foliar application of salicylic acid and calcium chloride delays the loss of chlorophyll and preserves the quality of broccoli during storage. J. Food Biochem. 2022, 46, e14154. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef]
- Fu, X.; Feng, Y.; Zhang, X.; Zhang, Y.; Bi, H.; Ai, X. Salicylic acid is involved in rootstock-scion communication in improving the chilling tolerance of grafted cucumber. Front. Plant Sci. 2021, 12, 693344. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Dong, C.; Li, L.; Shang, Q.; Liu, X.; Zhang, Z. Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 2014, 240, 687–700. [Google Scholar] [CrossRef]
- Fu, X.; Feng, Y.; Zhang, Y.; Bi, H.; Ai, X. Salicylic acid improves chilling tolerance via CsNPR1-CsICE1 interaction in grafted cucumbers. Hortic. Res. 2024, 11, uhae231. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Feng, Y.; Zhang, X.; Bi, H.; Ai, X. Abscisic acid mediates salicylic acid induced chilling tolerance of grafted cucumber by activating H2O2 biosynthesis and accumulation. Int. J. Mol. Sci. 2022, 23, 16057. [Google Scholar] [CrossRef]
- Pan, D.; Fu, X.; Zhang, X.; Liu, F.; Bi, H.; Ai, X. Hydrogen sulfide is required for salicylic acid-induced chilling tolerance of cucumber seedlings. Protoplasma 2020, 257, 1543–1557. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Wang, Y.; Zheng, X.; Zhang, H.; Zhang, L.; Tan, B.; Ye, X.; Wang, W.; Li, J.D.; et al. PpPIF8, a DELLA2-interacting protein, regulates peach shoot elongation possibly through auxin signaling. Plant Sci. 2022, 323, 111409. [Google Scholar] [CrossRef]
- Jia, H.; Song, Z.; Wu, F.; Ma, M.; Li, Y.; Han, D.; Yang, Y.; Zhang, S.; Cui, H. Low selenium increases the auxin concentration and enhances tolerance to low phosphorous stress in tobacco. Environ. Exp. Bot. 2018, 153, 127–134. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and abiotic stress responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, F.; Zhai, J.; Li, F.; Bi, H.; Ai, X. Auxin acts as a downstream signaling molecule involved in hydrogen sulfide-induced chilling tolerance in cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, X.; Liu, F.; Wang, Y.; Bi, H.; Ai, X. Hydrogen sulfide improves the cold stress resistance through the CsARF5-CsDREB3 module in cucumber. Int. J. Mol. Sci. 2021, 22, 13229. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Zhang, X.; Ai, X.; Bi, H. Auxin as a downstream signal positively participates in melatonin-mediated chilling tolerance of cucumber. Physiol. Plant. 2024, 176, e14526. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Xu, C.; Liu, K.; Bi, H.; Ai, X. H2O2 functions as a downstream signal of IAA to mediate H2S-induced chilling tolerance in cucumber. Int. J. Mol. Sci. 2021, 22, 12910. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Y.; Zhong, M.; Hussian, J.; Tang, Y.; Liu, S.; Qi, G. Calcium signaling-mediated transcriptional reprogramming during abiotic stress response in plants. Theor. Appl. Genet. 2023, 136, 210. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, H.; Wang, Y.; Wang, H.; Yang, S.; Li, C.; Chen, N.; Yang, H.; Zhang, Y.; Zhu, Y.; et al. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J. Integr. Plant Biol. 2021, 63, 510–527. [Google Scholar] [CrossRef]
- Iqbal, Z.; Memon, A.G.; Ahmad, A.; Iqbal, M.S. Calcium mediated cold acclimation in plants: Underlying signaling and molecular mechanisms. Front. Plant Sci. 2022, 13, 855559. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, C.; Yang, X.; Chen, H.; Yang, Y.; Mo, Y.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; et al. Genome-wide identification and expression analysis of calcium-dependent protein kinase and its related kinase gene families in melon (Cucumis melo L.). PLoS ONE 2017, 12, e0176352. [Google Scholar] [CrossRef]
- Xu, X.; Liu, M.; Lu, L.; He, M.; Qu, W.Q.; Xu, Q.; Qi, X.; Chen, X. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber. Mol. Genet. Genom. 2015, 290, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Pei, Z.; Zhu, Q.; Chai, C.; Xu, T.; Dong, C.; Wang, J.; Zheng, S.; Zhang, T. Melatonin-mediated low-temperature tolerance of cucumber seedlings requires Ca2+/CPKs signaling pathway. Plant Physiol. Biochem. 2024, 214, 108962. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Guo, Y.; Li, J.; Liu, L.; Liu, J.; Yuan, L.; Wei, C.; Ma, J.; Zhang, Y.; Ahammed, G.J.; et al. Cyclic nucleotide-gated ion channel 20 regulates melatonin-induced calcium signaling and cold tolerance in watermelon. Plant Physiol. 2025, 197, kiae630. [Google Scholar] [CrossRef] [PubMed]
- Cerny, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohaty, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Khedia, J.; Agarwal, P.; Agarwal, P.K. Deciphering hydrogen peroxide-induced signalling towards stress tolerance in plants. 3 Biotech. 2019, 9, 395. [Google Scholar] [CrossRef]
- Meng, L.; Feng, Y.; Zhao, M.; Jang, T.; Bi, H.; Ai, X. Hydrogen peroxide mediates melatonin-induced chilling tolerance in cucumber seedlings. Plant Cell Rep. 2024, 43, 279. [Google Scholar] [CrossRef]
- Han, Y.Q.; Luo, F.; Liang, A.D.; Xu, D.D.; Zhang, H.Y.; Liu, T.; Qi, H.Y. Aquaporin CmPIP2; 3 links H2O2 signal and antioxidation to modulate trehalose-induced cold tolerance in melon seedlings. Plant Physiol. 2025, 197, kiae477. [Google Scholar] [CrossRef]
- Puyaubert, J.; Baudouin, E. New clues for a cold case: Nitric oxide response to low temperature. Plant Cell Environ. 2014, 37, 2623–2630. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, X.; Zhang, L.; Zhang, Y.; Wei, L.; Liu, Y. The roles of nitric oxide in improving postharvest fruits quality: Crosstalk with phytohormones. Food Chem. 2024, 455, 139977. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ma, Z.; Zheng, Y.; Jin, P. Hydrogen sulfide treatment alleviates chilling injury in cucumber fruit by regulating antioxidant capacity, energy metabolism and proline metabolism. Foods 2022, 11, 2749. [Google Scholar] [CrossRef]
- Cui, J.; Li, C.; Qi, J.; Yu, W.; Li, C. Hydrogen sulfide in plant cold stress: Functions, mechanisms, and challenge. Plant Mol. Biol. 2025, 115, 12. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, L.; Zhang, L.; Zhang, W. Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Xue, S.; Cai, Y.; Qi, W.; Jian, C.; Xu, S.; Wang, T.; Ren, H. Cucumber (Cucumis sativus L.) nitric oxide synthase associated gene1 (CsNOA1) plays a role in chilling stress. Front. Plant Sci. 2016, 7, 1652. [Google Scholar] [CrossRef] [PubMed]
- Reda, M.; Kabala, K.; Stanislawski, J.; Szczepski, K.; Janicka, M. Regulation of NO-generating system activity in cucumber root response to cold. Int. J. Mol. Sci. 2025, 26, 1599. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, X.; Gao, C.; Wu, P.; Ahammed, G.J.; Liu, H.; Chen, S.; Cui, J. Glutathione is required for nitric oxide-induced chilling tolerance by synergistically regulating antioxidant system, polyamine synthesis, and mitochondrial function in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2024, 214, 108878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Ding, H.; Ma, D.; Huang, X.; Hou, J.; Wang, C.; Xie, Y.; Wang, Y.; Qin, H.; Guo, T. Exogenous hydrogen sulfide alleviates salt stress by improving antioxidant defenses and the salt overly sensitive pathway in wheat seedlings. Acta. Physiol. Plant 2019, 41, 123. [Google Scholar] [CrossRef]
- Wang, H.; Moussa, M.G.; Huang, W.; Han, D.; Dang, B.; Hao, H.; Zhang, L.; Xu, Z.; Jia, W. Exogenous hydrogen sulfide increased Nicotiana tabacum L. resistance against drought by the improved photosynthesis and antioxidant system. Sci. Rep. 2024, 14, 25534. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Cai, B.; Pan, D.; Fu, X.; Bi, H.; Ai, X. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020, 291, 110363. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cao, C.; Liang, S.; Ma, Y.; Liu, X.; Pei, Y. The role of H2S in low temperature-induced cucurbitacin C increases in cucumber. Plant Mol. Biol. 2019, 99, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fu, X.; Han, L.; Xu, C.; Liu, C.; Bi, H.; Ai, X. Nitric oxide functions as a downstream signal for melatonin-induced cold tolerance in cucumber seedlings. Front. Plant Sci. 2021, 12, 686545. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Liu, L.; Liu, J.; Yang, W.; Chen, Y.; Li, C.; Yuan, L.; Wei, C.; Ma, J.; et al. A self-amplifying NO-H2S loop mediates melatonin-induced CBF-responsive pathway and cold tolerance in watermelon. Plant J. 2025, 121, e70025. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Lu, J.; Shao, H. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Zhang, C.; Zhang, W.; Deng, N.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Raffinose positively regulates maize drought tolerance by reducing leaf transpiration. Plant J. 2023, 114, 55–67. [Google Scholar] [CrossRef]
- Song, L.; Xu, C.; Zhang, L.; Li, J.; Jiang, L.; Ma, D.; Guo, Z.; Wang, Q.; Wang, X.; Zheng, H.L. Trehalose along with ABA promotes the salt tolerance of Avicennia marina by regulating Na+ transport. Plant J. 2024, 119, 2349–2362. [Google Scholar] [CrossRef]
- Gu, H.; Lu, M.; Zhang, Z.; Xu, J.; Cao, W.; Miao, M. Metabolic process of raffinose family oligosaccharides during cold stress and recovery in cucumber leaves. J. Plant Physiol. 2018, 224, 112–120. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Parween, S.; Majumder, A.L. Galactinol synthase across evolutionary diverse taxa: Functional preference for higher plants? FEBS Lett. 2012, 586, 1488–1496. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, Z.; Wang, Z.; Zhang, Z.; Kong, W.; Miao, M. Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation. Hortic. Res. 2022, 9, uhab063. [Google Scholar] [CrossRef]
- Lu, J.; Sui, X.; Ma, S.; Li, X.; Liu, H.; Zhang, Z. Suppression of cucumber stachyose synthase gene (CsSTS) inhibits phloem loading and reduces low temperature stress tolerance. Plant Mol. Biol. 2017, 95, 1–15. [Google Scholar] [CrossRef]
- Feng, Z.; Zheng, F.; Wu, S.; Li, R.; Li, Y.; Zhong, J.; Zhao, H. Functional characterization of a cucumber (Cucumis sativus L.) vacuolar invertase, CsVI1, involved in hexose accumulation and response to low temperature stress. Int. J. Mol. Sci. 2021, 22, 9365. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, F.; Song, S.; Yu, X.; Ren, Y.; Zhao, X.; Liu, H.; Liu, G.; Wang, Y.; He, H. CsSWEET2, a hexose transporter from cucumber (Cucumis sativus L.), affects sugar metabolism and improves cold tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 3886. [Google Scholar] [CrossRef]
- Li, M.; Yue, T.; Han, J.; Wang, J.; Xiao, H.; Shang, F. Exogenous glucose irrigation alleviates cold stress by regulating soluble sugars, ABA and photosynthesis in melon seedlings. Plant Physiol. Biochem. 2024, 217, 109214. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Han, Y.; Zhang, Y.; Khan, A.; Dong, L.; Shao, L.; Liang, A.; Liu, T.; Qi, H. CmTGA8-CmAPX1/CmGSTU25 regulatory model involved in trehalose induced cold tolerance in oriental melon seedlings. Plant Physiol. Biochem. 2025, 220, 109432. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Li, N.; Wang, Y.; Yu, X.; Yang, L.; Cao, R.; Ye, X. Integrated physiological and transcriptomic analyses revealed improved cold tolerance in cucumber (Cucumis sativus L.) by exogenous chitosan oligosaccharide. Int. J. Mol. Sci. 2023, 24, 6202. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, F.; Cai, W.; Peng, B.; Ning, M.; Shan, C.; Yang, X. Chitosan treatment reduces softening and chilling injury in cold-stored Hami melon by regulating starch and sucrose metabolism. Front. Plant Sci. 2022, 13, 1096017. [Google Scholar] [CrossRef]
- Lin, D.; Yan, R.; Xing, M.; Liao, S.; Chen, J.; Gan, Z. Fucoidan treatment alleviates chilling injury in cucumber by regulating ROS homeostasis and energy metabolism. Front. Plant Sci. 2022, 13, 1107687. [Google Scholar] [CrossRef]
- Hashim, N.F.A.; Ahmad, A.; Bordoh, P.K. Effect of chitosan coating on chilling injury, antioxidant status and postharvest quality of Japanese cucumber during cold storage. Sains Malays. 2018, 47, 287–294. [Google Scholar]
- Liu, T.; Zhang, A.; Zhang, Y.; Shao, L.; Xia, H.; Miao, M.; Qi, H. Sucrose catabolism play vital roles in seed germination of melon at low temperature. Veg. Res. 2024, 4, e020. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, X.; Xia, Y.; Ban, Q.; Cao, L.; Li, S.; Li, Y. CsCBF5 depletion impairs cold tolerance in tea plants. Plant Sci. 2022, 325, 111463. [Google Scholar] [CrossRef]
- Xia, H.; Chen, M.; Ren, P.; Sun, T.; Zhao, D.; Qin, X.; Li, F.; Liu, W.; Qu, Y.; Li, Y.; et al. Heterologous expression of the TaCBF2 gene improves cold resistance in Begonia semperflorens. Plant Cell Tiss. Organ. Cult. 2024, 159, 71. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef]
- Dong, C.; Agarwal, M.; Zhang, Y.; Xie, Q.; Zhu, J. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 2006, 103, 8281–8286. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642. [Google Scholar] [CrossRef]
- Miura, K.; Jin, J.; Lee, J.; Yoo, C.Y.; Stirm, V.; Miura, T.; Ashworth, E.N.; Bressan, R.A.; Yun, D.; Hasegawa, P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 2007, 19, 1403–1414. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Ding, F.; Hou, J.; Wang, J.; Luo, R.; Mao, W.; Li, X.; Zhu, H.; Yang, L.; et al. Genome-wide identification of the melon (Cucumis melo L.) response regulator gene family and functional analysis of CmRR6 and CmPRR3 in response to cold stress. J. Plant Physiol. 2024, 292, 154160. [Google Scholar] [CrossRef]
- Dong, S.; Li, C.; Tian, H.; Wang, W.; Yang, X.; Beckles, D.M.; Liu, X.; Guan, J.; Gu, X.; Sun, J.; et al. Natural variation in STAYGREEN contributes to low-temperature tolerance in cucumber. J. Integr. Plant Biol. 2023, 65, 2552–2568. [Google Scholar] [CrossRef]
- Ma, X.; Xu, J.; Han, D.; Huang, W.; Dang, B.; Jia, W.; Xu, Z. Combination of β-aminobutyric acid and Ca2+ alleviates chilling stress in tobacco (Nicotiana tabacum L.). Front. Plant Sci. 2020, 11, 556. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; Xu, G.; Dai, Z.; Chen, P.; Zhang, T.; Zhang, H. The Ca2+-CaM signaling pathway mediates potassium uptake by regulating reactive oxygen species homeostasis in tobacco roots under low-K+ stress. Front. Plant Sci. 2021, 12, 658609. [Google Scholar] [CrossRef]
- Wang, Q.; Cang, X.; Yan, H.; Zhang, Z.; Li, W.; He, J.; Zhang, M.; Lou, L.; Wang, R.; Chang, M. Activating plant immunity: The hidden dance of intracellular Ca2+ stores. New Phytol. 2024, 242, 2430–2439. [Google Scholar] [CrossRef]
- Knight, M.R.; Knight, H. Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 2012, 195, 737–751. [Google Scholar] [CrossRef]
- Peng, Y.; Ming, Y.; Jiang, B.; Zhang, X.; Fu, D.; Lin, Q.; Zhang, X.; Wang, Y.; Shi, Y.; Gong, Z.; et al. Differential phosphorylation of Ca2+-permeable channel CYCLIC NUCLEOTIDE-GATED CHANNEL20 modulates calcium-mediated freezing tolerance in Arabidopsis. Plant Cell. 2024, 36, 4356–4371. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Liu, L.; Yang, W.; Zhou, Y.; Wei, C.; Ma, J.; Zhang, Y.; Yang, J.; Liu, Y.; et al. Nitric oxide cross-links calcium signals to enhance cold tolerance via inhibiting calmodulin expression in watermelon. Plant Physiol. 2025, 198, kiaf243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, S.; Wang, K.; Tian, H.; Gao, J.; Zhao, Q.; Du, C. A leucine-rich repeat receptor-like kinase, OsSTLK, modulates salt tolerance in rice. Plant Sci. 2020, 296, 110465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Y.; Qiu, X.; Fu, J.X.; Wang, G.R.; Wei, L.; Wang, T.C. Systematic analysis of differentially expressed ZmMYB genes related to drought stress in maize. Physiol. Mol. Biol. Plants. 2021, 27, 1295–1309. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Chen, B.; Wang, J.; Ding, F.; Wang, P.; Zhang, X.; Hou, J.; Luo, R.; Li, X.; et al. Identification of candidate genes that regulate the trade-off between seedling cold tolerance and fruit quality in melon (Cucumis melo L.). Hortic. Res. 2023, 10, uhad093. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Zhu, H.; Song, P.; Guo, L.; Yang, L. Genome-wide analysis of the WRKY family genes and their responses to cold stress in watermelon. Czech J. Genet. Plant Breed. 2018, 54, 168–176. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Ouyang, M.; Yang, L.; Sun, S.; Wang, Y.; Cai, X.; Wu, G.; Li, Y. Overexpression of watermelon ClWRKY20 in transgenic Arabidopsis improves salt and low-temperature tolerance. Sci. Hortic. 2022, 295, 110848. [Google Scholar] [CrossRef]
- Wang, J.; Wei, M.; Wang, H.; Mo, C.; Zhu, Y.; Kong, Q. A time-course transcriptome reveals the response of watermelon to low-temperature stress. J. Integr. Agric. 2025, 24, 1786–1799. [Google Scholar] [CrossRef]
- Luo, P.; Chen, L.; Chen, Y.; Shen, Y.; Cui, Y. RmZAT10, a novel Cys2/His2 zinc finger transcription factor of Rosa multiflora, functions in cold tolerance through modulation of proline biosynthesis and ROS homeostasis. Environ. Exp. Bot. 2022, 198, 104845. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Shi, H.; Wang, H.; Ji, K.; Zhang, L.; Wang, Y.; Dong, Y.; Li, Y. ZmMPK6, a mitogen-activated protein kinase, regulates maize kernel weight. J. Exp. Bot. 2024, 75, 3287–3299. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, J.; Du, L.; Wang, P.; Sun, B.; Zhang, C.; Shi, Y.; Li, H.; Sun, H. Activation of MAPK-mediated immunity by phosphatidic acid in response to positive-strand RNA viruses. Plant Commun. 2024, 5, 100659. [Google Scholar] [CrossRef]
- Fang, J.; Chun, Y.; Zhang, F.; Guo, T.; Ren, M.; Zhao, J.; Yuan, S.; Wang, W.; Li, Y.; Li, X. A novel OsMPK6-OsMADS47-PPKL1/3 module controls grain shape and yield in rice. Adv. Sci. 2025, 12, e01946. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.; et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell. 2017, 43, 618–629. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev. Cell. 2017, 43, 731–743. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Z.; Shi, Y.; Fu, D.; Yin, P.; Cheng, J.; Jiang, C.; Yang, S. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat. Commun. 2021, 12, 4713. [Google Scholar] [CrossRef]
- Xu, A.; Wei, N.; Hu, H.; Zhou, S.; Huang, Y.; Kong, Q.; Bie, Z.; Nie, W.F.; Cheng, F. Thioredoxin h2 inhibits the MPKK5-MPK3 cascade to regulate the CBF-COR signaling pathway in Citrullus lanatus suffering chilling stress. Hortic. Res. 2023, 10, uhac256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Xing, Q.; Yue, L.; Qi, H. Genome-wide identification of mitogen-activated protein kinase (MAPK) cascade and expression profiling of CmMAPKs in melon (Cucumis melo L.). PLoS ONE 2020, 15, e0232756. [Google Scholar] [CrossRef]
- Moustafa, K.; AbuQamar, S.; Jarrar, M.; Al-Rajab, A.J.; Trémouillaux-Guiller, J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014, 33, 1217–1225. [Google Scholar] [CrossRef]
- Hereme, R.; Galleguillos, C.; Morales-Navarro, S.; Molina-Montenegro, M.A. What if the cold days return? Epigenetic mechanisms in plants to cold tolerance. Planta 2021, 254, 46. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, Y.; He, Y. Molecular epigenetic mechanisms for the memory of temperature stresses in plants. J. Genet. Genom. 2022, 49, 991–1001. [Google Scholar] [CrossRef]
- Liu, N.; Fromm, M.; Avramova, Z. H3K27me3 and H3K4me3 chromatin environment at super-induced dehydration stress memory genes of Arabidopsis thaliana. Mol. Plant. 2014, 7, 502–513. [Google Scholar] [CrossRef]
- Di, Q.; Zhou, M.; Li, Y.; Yan, Y.; He, C.; Wang, J.; Wang, X.; Yu, X.; Sun, M. RESPIRATORY BURST OXIDASE HOMOLOG 5.1 regulates H3K4me3 deposition and transcription after cold priming in cucumber. Plant Physiol. 2025, 197, kiae461. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, M.; Yang, K.; Liu, B.; Liu, H.; Liu, J. Genetic variation for adaptive evolution in response to changed environments in plants. J. Integr. Plant Biol. 2025. [Google Scholar] [CrossRef] [PubMed]
| Phytohormone | Plant Species | Phytohormone Functions | Regulation of Cold-Tolerance Genes | References |
|---|---|---|---|---|
| Abscisic acid | Cucumber | Upregulates antioxidant enzyme activities; Reduces the chilling injury index, relative electrical conductivity, and MDA content; Positively regulates seedling cold tolerance | [8] | |
| Melon | Increases activities of SOD, CAT, and APX; Reduces membrane lipid peroxidation; Positively regulates chilling tolerance | [16] | ||
| Melon | Increases endogenous GA4 and SA content; Positively regulates seedling cold tolerance | [29] | ||
| Melon | Enhances activities of antioxidant enzymes (SOD, CAT, and APX) and limited H2O2; Reduces electrolyte leakage and MDA content; Increases proline and soluble sugar content; Positively regulates seedling cold tolerance | CmPYL7 and CmPYL6 positively regulate seedling cold tolerance; CmPP2C24-like negatively regulates seedling cold tolerance | [37,38] | |
| Melon | Positively regulates early-stage cold stress resistance | CmABF1/3/4/5, CmCBF1/2/4, and CmADC positively regulate seedling cold tolerance | [39] | |
| Watermelon | Induces antioxidant potential; Mediates grafting-induced cold tolerance | [28] | ||
| Brassinosteroids | Cucumber | Exogenous EBR upregulates endogenous EBR levels; Increases the activities of SOD, POD, GR, CAT, and APX; reduces ROS and MDA content; Positively regulates chilling tolerance | [15] | |
| Cucumber | Activates of enzymes in Calvin cycle; Increases the antioxidant capacity; Accelerates the recovery of PSII; Positively regulates seedling cold tolerance | [48] | ||
| Cucumber | CsGPA1 positively regulates the brassinolide signal to affect cold stress | CsGPA1 and CsCOR413PM2 positively regulate seedling cold tolerance | [51] | |
| Cucumber | CsBPC2 is associated with BR signaling transduction | CsBPC2 positively regulates seedling cold tolerance | [54] | |
| Cucumber | Exogenous EBR promotes BR synthesis and expression of CsICE-CsCBF-CsCOR genes under cold stress; Positively regulates early-stage cold stress resistance | [55] | ||
| Jasmonic acid | Cucumber | CsHSFA1d positively regulates endogenous JA content after cold treatment; JA positively regulates seedling cold tolerance | CsHSFA1d positively regulates seedling cold tolerance | [62] |
| Watermelon | Induces H2O2 accumulation and activates the antioxidant system; Positively regulates chilling tolerance | [63] | ||
| Watermelon | Upregulates the expression of ClCNGC2 and ClCNGC20; Triggers Ca2+ influx; Positively regulates seedling cold tolerance | ClJMT, ClCNGC2, and ClCNGC20 positively regulate seedling cold tolerance | [64] | |
| Salicylic acid | Cucumber | Increases antioxidant enzymes concentrations; Alleviates fruit chilling injury during cold storage | [75] | |
| Cucumber | Enhances actual photochemical efficiency, maximum photochemical efficiency, and photosynthetic rate; Decreases EL, MDA, and CI; Upregulates the expression level of COR genes; Improving the chilling tolerance of grafted cucumber | [76] | ||
| Cucumber | Precise induction of cellular H2O2 levels; Enhances the expression of cold-responsive genes; Positively regulates seedling cold tolerance | [78] | ||
| Cucumber | Decreases EL, H2O2, and O2− contents; Upregulates the expression of cold-responsive genes; Positively regulates the cold tolerance of grafted plants | CsPAL and CsNPR1 positively regulate the cold tolerance of grafted plants | [79] | |
| Cucumber | Stimulates the biosynthesis of ABA and H2O2; Upregulates the expression of CBF1, COR47, NCED, and RBOH1; Induces chilling tolerance in grafted cucumber plants | [80] | ||
| Cucumber | Induces endogenous H2S content; Improves the activities and mRNA level of L-/D-cysteine desulfhydrase and antioxidant enzymes (SOD, POD, CAT, APX, and GR); Upregulates the expression of ICE, CBF1, and COR; Induces chilling tolerance of cucumber seedlings | [81] | ||
| Auxin | Cucumber | Endogenous IAA system is triggered by cold stress; Acts as a downstream signaling molecule in H2S-induced cold tolerance; Positively regulates early-stage cold stress resistance | [85] | |
| Cucumber | H2S regulates cold stress response by mediating auxin signaling; Positively regulates seedling cold tolerance | CsARF5 and CsDREB3 positively regulate seedling cold tolerance | [86] | |
| Cucumber | Decreases MDA and ROS contents; Upregulates the expression of cold response genes; Improves Pn, Jmax, Vcmax, P700(I/I0), and photosynthetic electron transport; Acts as a downstream signaling molecule in MT-induced cold tolerance; Positively regulates seedling cold tolerance | CsASMT and CsYUCCA10 positively regulate seedling cold tolerance | [87] | |
| Cucumber | Decreases CI, EL, and MDA content; Improves photosynthesis and the expression of COR genes under cold stress; Positively regulates chilling tolerance | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Hou, J.; Hu, J.; Mao, W. Advances in Cold Stress Response Mechanisms of Cucurbits. Horticulturae 2025, 11, 1032. https://doi.org/10.3390/horticulturae11091032
Li L, Hou J, Hu J, Mao W. Advances in Cold Stress Response Mechanisms of Cucurbits. Horticulturae. 2025; 11(9):1032. https://doi.org/10.3390/horticulturae11091032
Chicago/Turabian StyleLi, Lili, Juan Hou, Jianbin Hu, and Wenwen Mao. 2025. "Advances in Cold Stress Response Mechanisms of Cucurbits" Horticulturae 11, no. 9: 1032. https://doi.org/10.3390/horticulturae11091032
APA StyleLi, L., Hou, J., Hu, J., & Mao, W. (2025). Advances in Cold Stress Response Mechanisms of Cucurbits. Horticulturae, 11(9), 1032. https://doi.org/10.3390/horticulturae11091032





