Assessment of Nutritional Components, Mineral Profiles, and Aroma Compounds in Zanthoxylum armatum Fruit from Different Harvest Times, Tree Age and Fruiting Position
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant Material and Fruit Collection
2.3. Determination of Physical Characteristics
2.4. Determination of Mineral Elements
2.5. Determination of Protein and Amino Acids
2.6. Determination of Total Polyphenol and Total Flavonoid Contents
2.7. Determination of Total Carbohydrates
2.8. Determination of Sanshool
2.9. Determination of Volatile Organic Compounds
2.10. Assay of ABTS Radical Scavenging Activity
2.11. Assay of DPPH Radical Scavenging Activity
2.12. Assay of Reducing Capacity
2.13. Statistical Analysis
3. Results and Discussion
3.1. Physical Characteristics
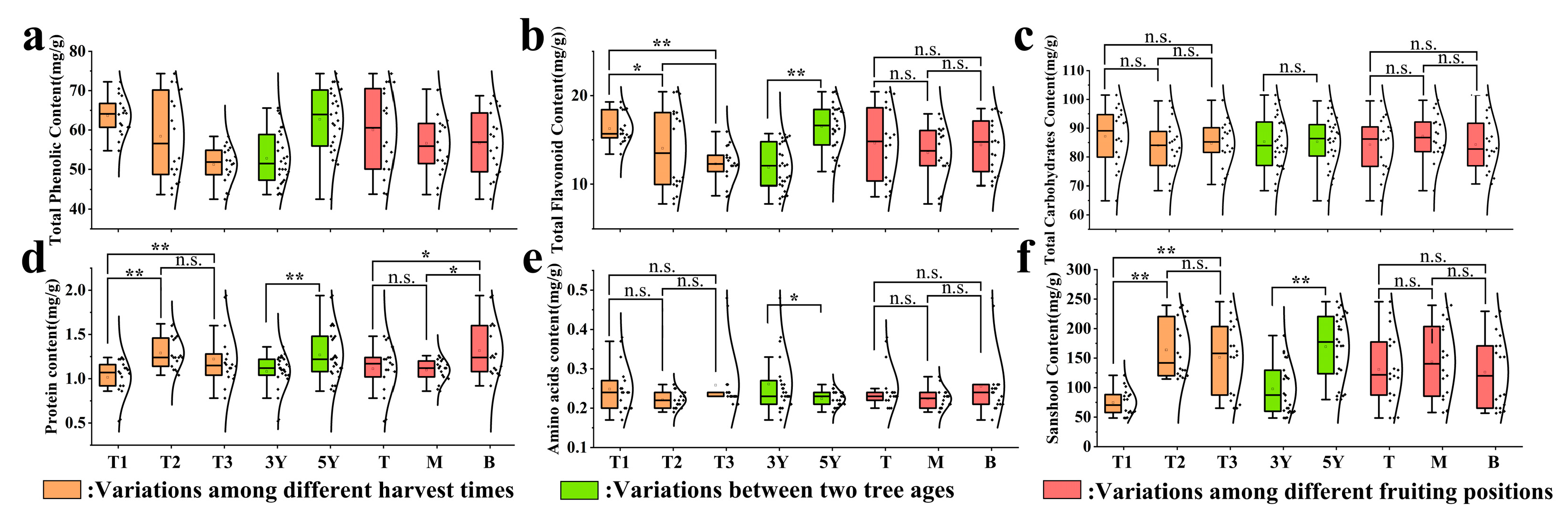
3.2. Nutrient Profiles
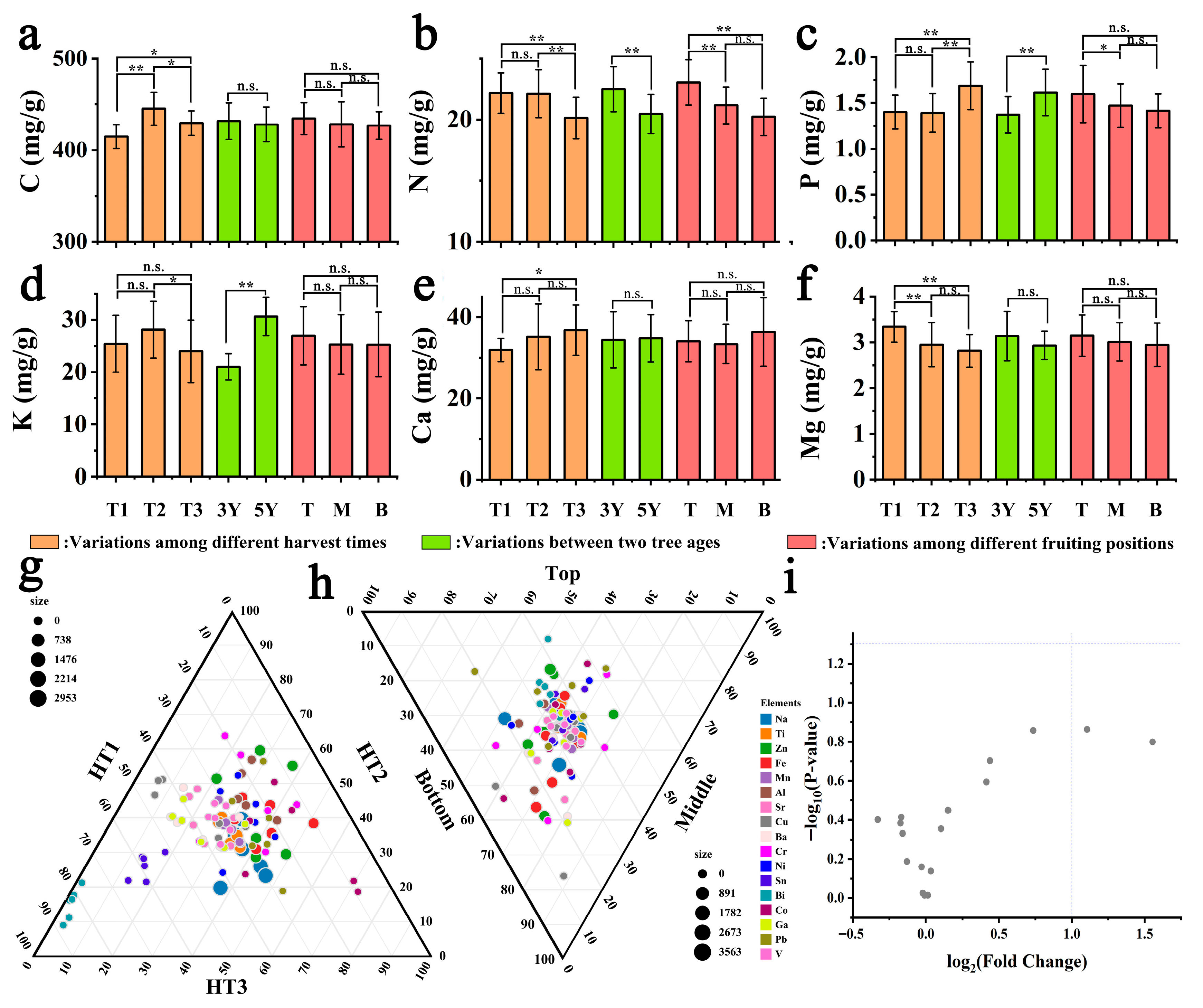
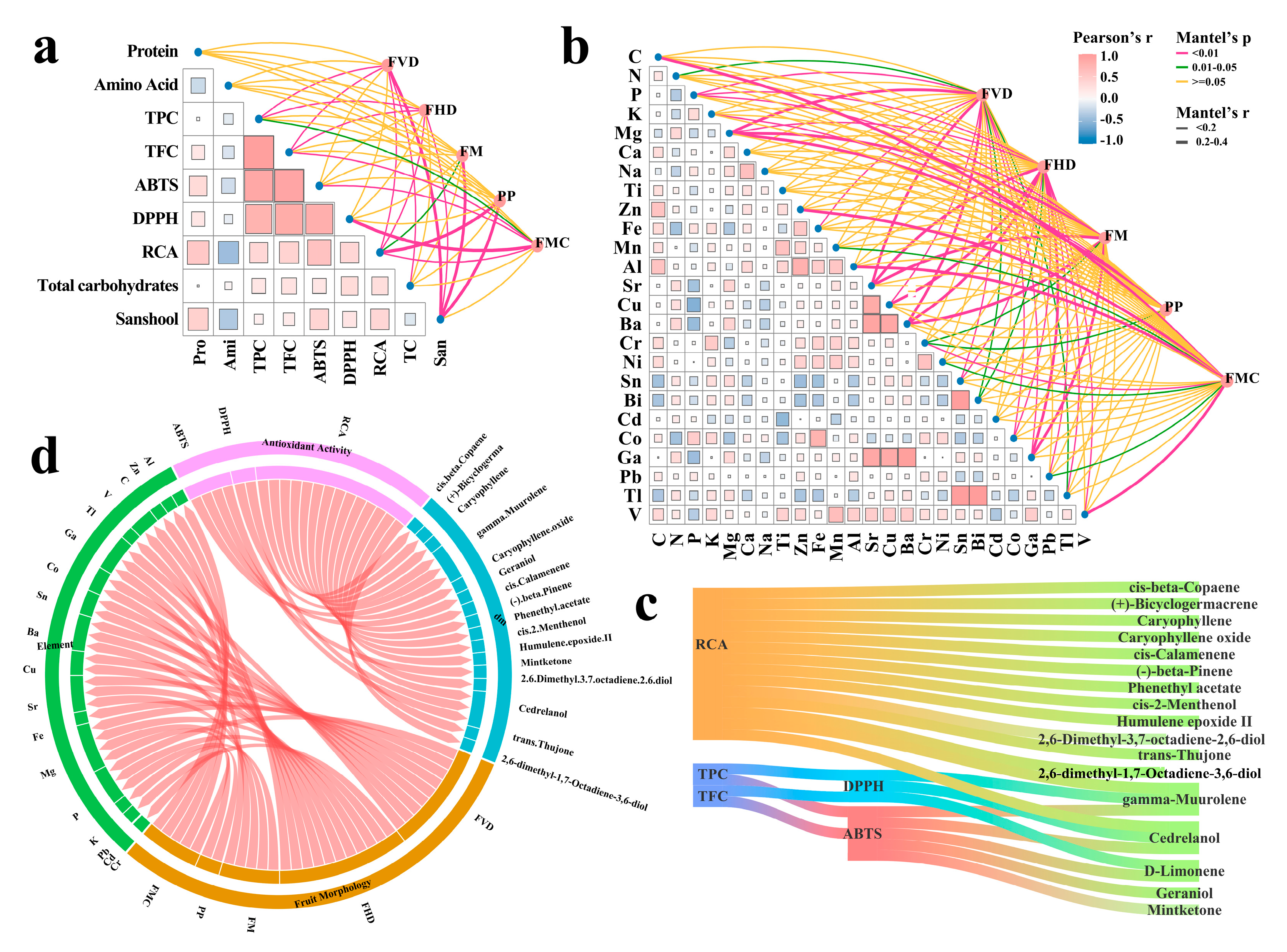
3.3. Mineral Profile
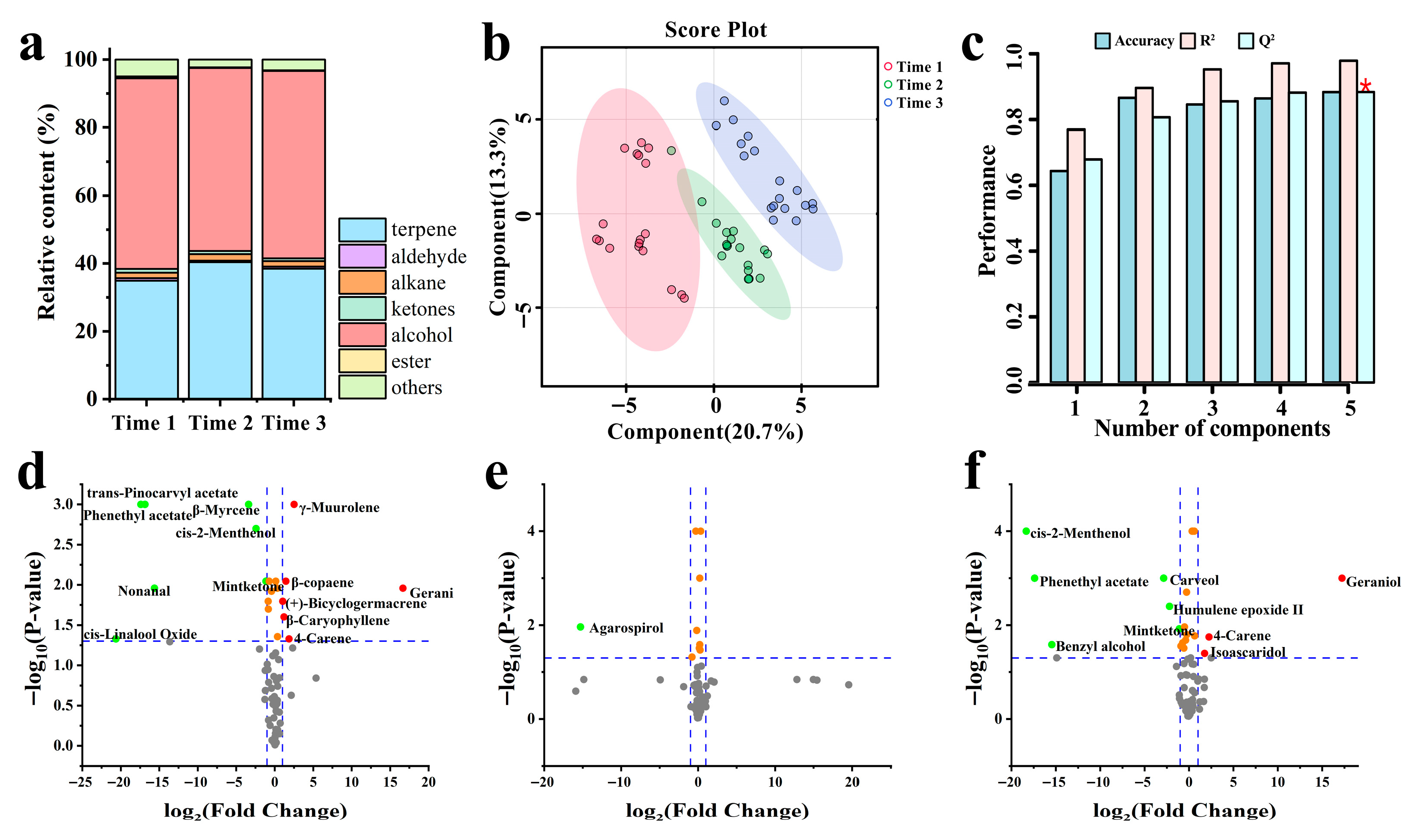
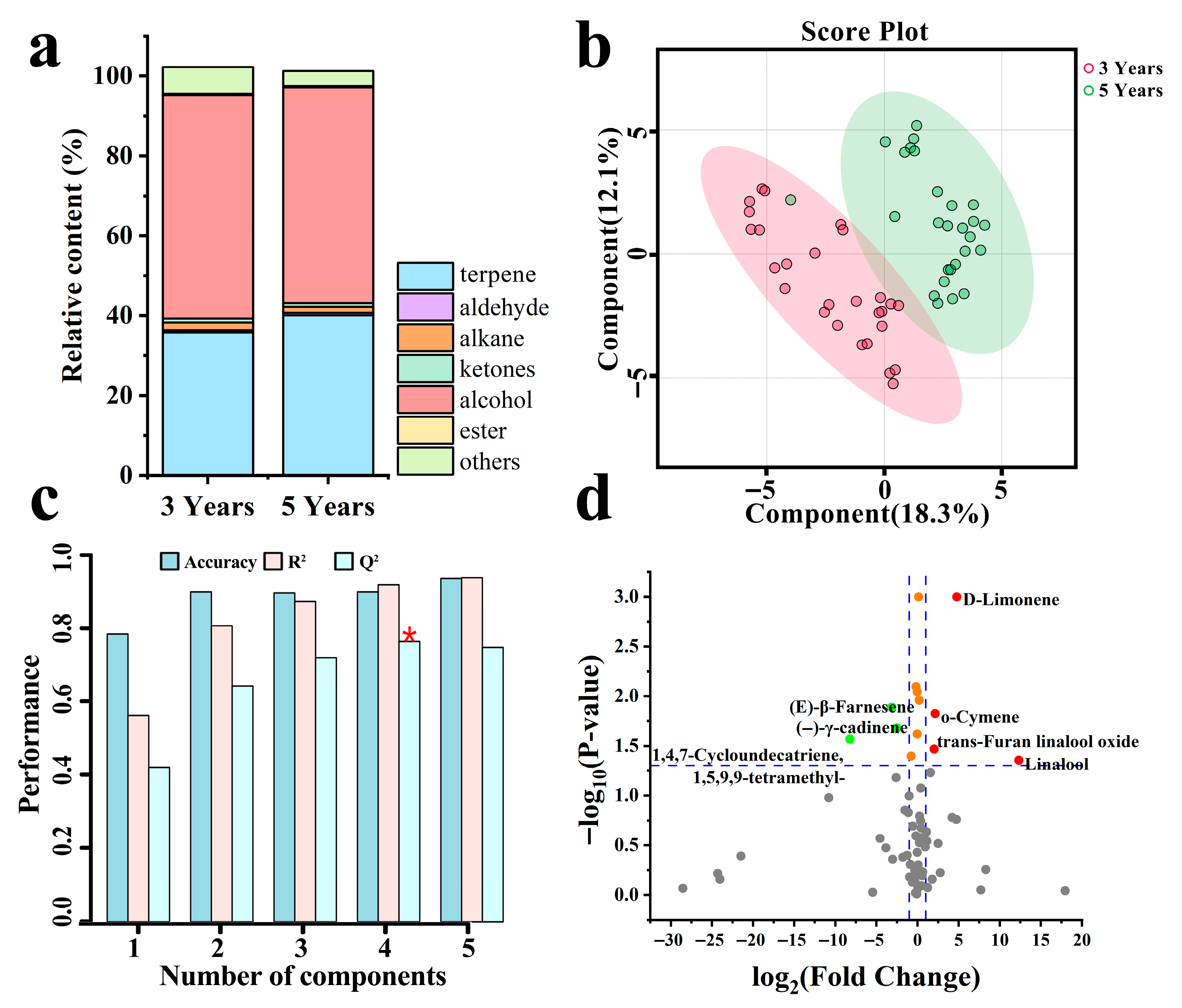
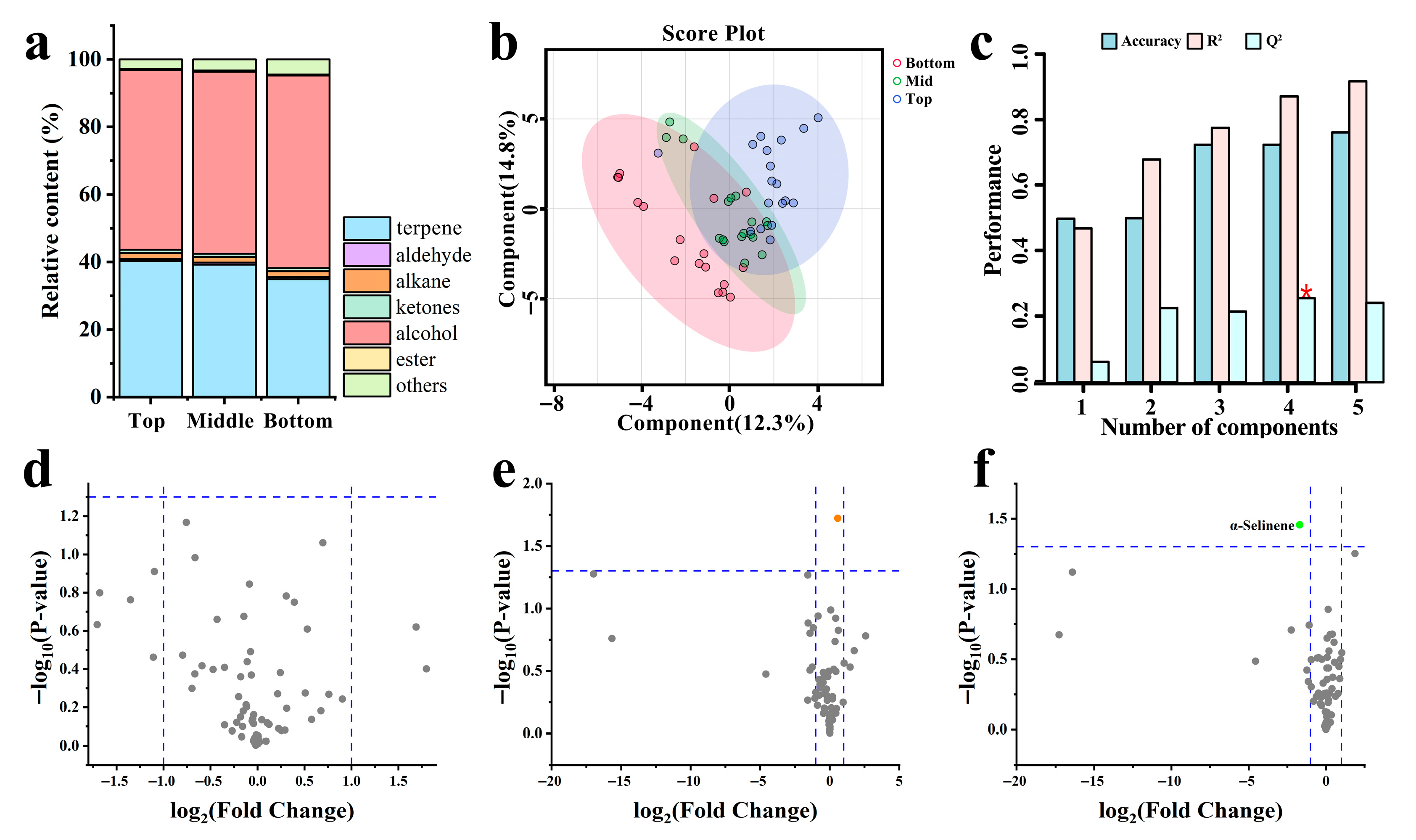
3.4. Variations in VOCs
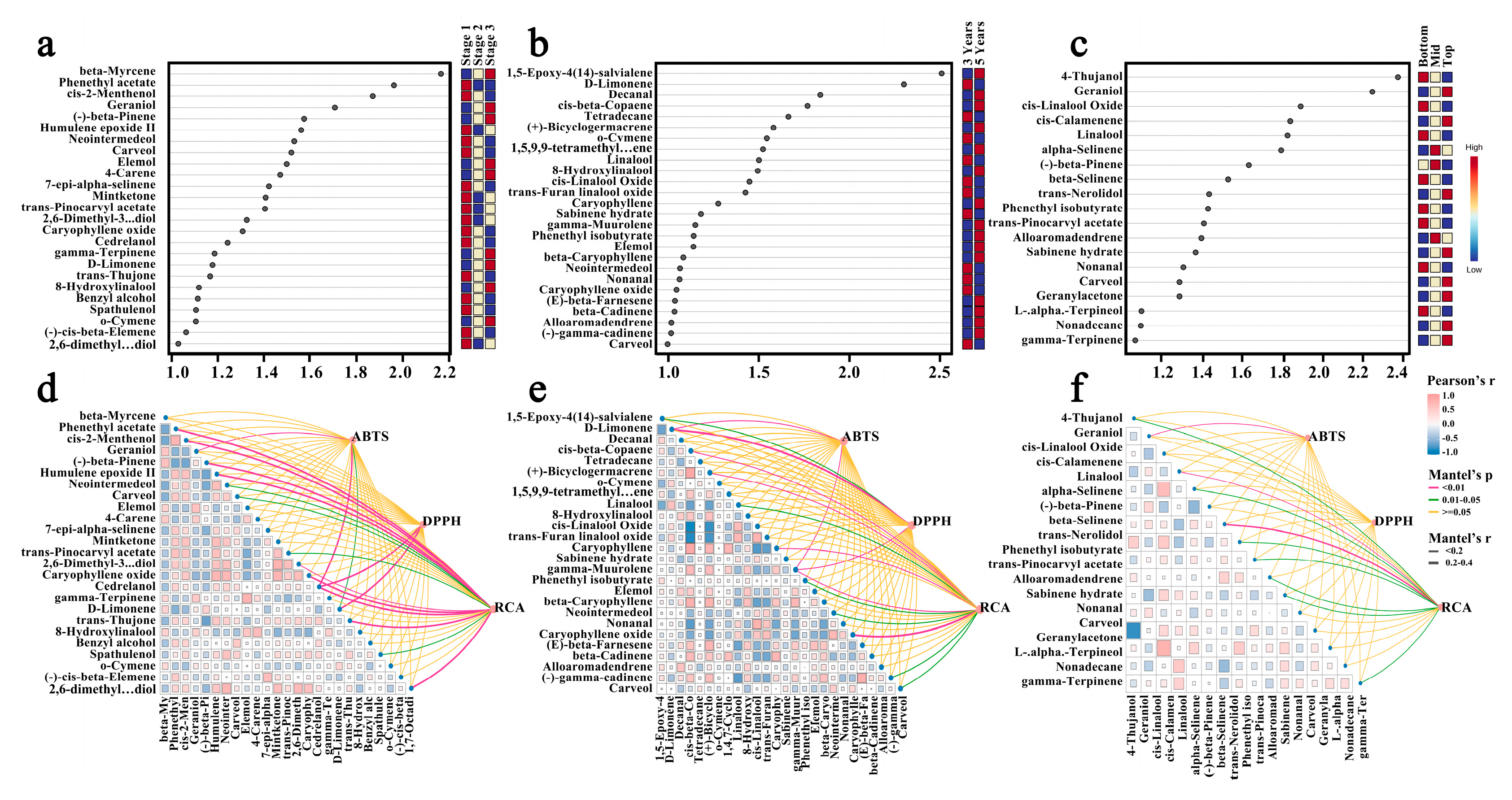
3.5. Screening of Dominant-Materials with Key Activity
3.6. Mantel Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, Y.; Li, S.; Ho, C.-T. Chemical Composition, Sensory Properties and Application of Sichuan Pepper (Zanthoxylum Genus). Food Sci. Human Well. 2019, 8, 115–125. [Google Scholar] [CrossRef]
- Shah, S.S.; Ahmed, S.; Zhou, B.; Shi, L. A Review on Pharmacological Activities and Phytochemical Constituents of Zanthoxylum armatum DC. Nat. Prod. Res. 2025, 39, 3240–3259. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, A.; Semwal, B.C. PRISMA Based Systematic Review: Pharmacognostic Study of Zanthoxylum armatum DC. Mini Rev. Med. Chem. 2021, 21, 1965–1997. [Google Scholar] [CrossRef]
- Liu, J.; Wan, J.; Zhang, Y.; Hou, X.; Shen, G.; Li, S.; Luo, Q.; Li, Q.; Zhou, M.; Liu, X.; et al. The Establishment of Comprehensive Quality Evaluation Model for Flavor Characteristics of Green Sichuan Pepper (Zanthoxylum armatum DC.) in Southwest China. Food Chem. X 2023, 18, 100721. [Google Scholar] [CrossRef]
- Gu, T.; Ren, H.; Wang, M.; Qian, W.; Hu, Y.; Yang, Y.; Yu, T.; Zhao, K.; Gao, S. Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types. Horticulturae 2024, 10, 261. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Qian, W.-Z.; Yi, L.; Mao, Y.-D.; Ye, Y.-L.; Ren, H.-Y.; Gu, T.; Zhang, D.-J.; Cao, G.-X.; Gao, S. Chemical Composition and Antioxidant Activity of Zanthoxylum armatum Leaves in Response to Plant Age, Shoot Type and Leaf Position. Forests 2023, 14, 1022. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and Characterization of the Volatile Organic Compounds in Eight Kinds of Huajiao with Geographical Indication of China Using Electronic Nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Han, M.; Tu, T.; Chen, S.; Hu, W.; Dong, L.; Zhang, F.; Zhao, Y.; Li, Z. Comparative Analysis of Fatty Acids, Volatile and Non-Volatile Components in Red Huajiao (Zanthoxylum bungeanum Maxim.) and Green Huajiao (Zanthoxylum armatum DC.) Using GC-MS, UPLC-LTQ-Orbitrap-MS/MS and HPLC-DAD. Ind. Crop. Prod. 2023, 204, 117371. [Google Scholar] [CrossRef]
- Feng, X.; Huang, P.; Duan, P.; Wang, H.; Kan, J. Dynamic Zanthoxylum Pungency Characteristics and Their Correlation with Sanshool Composition and Chemical Structure. Food Chem. 2023, 407, 135138. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.M.A.; Geraldi, M.V.; Junior, M.R.M.; Silvestre, A.J.D.; Rocha, S.M. Purple Passion Fruit (Passiflora rdulis f. Edulis): A Comprehensive Review on the Nutritional Value, Phytochemical Profile and Associated Health Effects. Food Res. Int. 2022, 160, 111665. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, H.-P.; Shao, C.-Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.-D.; Tan, J.-F.; Peng, Q.-H.; Lin, Z. Identification of Key Odorants Responsible for Chestnut-like Aroma Quality of Green Teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, B.; Zhang, H.; Wu, Z.; Li, M.; Wang, D.; Wang, C. Combining with E-Nose, GC-MS, GC-IMS and Chemometrics to Explore Volatile Characteristics during the Different Stages of Zanthoxylum bungeanum Maxim Fruits. Food Res. Int. 2024, 195, 114964. [Google Scholar] [CrossRef]
- Wenkai, H.; Jingyan, W.; Lexun, M.; Feiyan, Z.; Luping, J.; Yu, Z.; Shaobo, Z.; Wei, G. Identification of Key Genes in the Biosynthesis Pathways Related to Terpenoids, Alkaloids and Flavonoids in Fruits of Zanthoxylum armatum. Sci. Hort. 2021, 290, 110523. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.A.; Kang, H.M.; Lee, Y.T.; Islam, M.Z. Grape Terpenoids: Flavor Importance, Genetic Regulation, and Future Potential. Crit. Rev. Food Sci. Nutri. 2021, 61, 1429–1447. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Y.; Wei, A. Geographical Variations in the Fatty Acids of Zanthoxylum Seed Oils: A Chemometric Classification Based on the Random Forest Algorithm. Ind. Crop. Prod. 2019, 134, 146–153. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. Effects of Location within the Tree Canopy on Carbohydrates, Organic Acids, Amino Acids and Phenolic Compounds in the Fruit Peel and Flesh from Three Apple (Malus × Domestica) Cultivars. Hortic Res. 2014, 1, 14019. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.K.; Asrey, R. Tree Age Affects Physicochemical, Functional Quality and Storability of Amrapali Mango (Mangifera indica L.) Fruits. J. Sci. Food Agric. 2018, 98, 3255–3262. [Google Scholar] [CrossRef]
- Prasad, K.; Jacob, S.; Siddiqui, M.W. Chapter 2—Fruit Maturity, Harvesting, and Quality Standards. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 41–69. ISBN 978-0-12-809807-3. [Google Scholar]
- Zhang, F.; Wang, Q.; Li, H.; Zhou, Q.; Tan, Z.; Zu, X.; Yan, X.; Zhang, S.; Ninomiya, S.; Mu, Y.; et al. Study on the Optimal Leaf Area-to-Fruit Ratio of Pear Trees on the Basis of Bearing Branch Girdling and Machine Learning. Plant Phenomics 2024, 6, 0233. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Liang, G.; Xiang, S.; Han, G. Volatile Composition Changes in Lemon during Fruit Maturation by HS-SPME-GC-MS. J. Sci. Food Agric. 2022, 102, 3599–3606. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Wang, F.; Wang, S.; Feng, H.; Xie, X.; Hao, F.; Zhang, L.; Fang, C. Volatile Constituents and Ellagic Acid Formation in Strawberry Fruits of Selected Cultivars. Food Res. Int. 2020, 138, 109767. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yan, C.; Zhang, T.; Ji, M.; Tao, R.; Gao, H. Comparative Study of Volatile Compounds and Expression of Related Genes in Fruit from Two Apple Cultivars during Different Developmental Stages. Molecules 2021, 26, 1553. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.-Y.; Zhao, S.-M.; Fan, Y.-T.; Huang, J.-W.; Yu, T.; Zhuang, G.-Q.; Gao, S. Variations in the Mineral Composition of Houpoea officinalis Flowers at Different Stages of Development. Horticulturae 2025, 11, 387. [Google Scholar] [CrossRef]
- Snyder, J.C.; Desborough, S.L. Rapid Estimation of Potato Tuber Total Protein Content with Coomassie Brilliant Blue G-250. Theoret. Appl. Genet. 1978, 52, 135–139. [Google Scholar] [CrossRef]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total Phenolic Compounds in Milk from Different Species. Design of an Extraction Technique for Quantification Using the Folin–Ciocalteu Method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef]
- Mammen, D.; Daniel, M. A Critical Evaluation on the Reliability of Two Aluminum Chloride Chelation Methods for Quantification of Flavonoids. Food Chem. 2012, 135, 1365–1368. [Google Scholar] [CrossRef]
- Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Analysis of the Chemical Composition of Natural Carbohydrates—An Overview of Methods. Food Chem. 2022, 394, 133466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Z.; Yang, B.; Yang, Q.; Kan, J. Determination of Main Alkylamides Responsible for Zanthoxylum bungeanum Pungency through Quantitative Analysis of Multi-Components by a Single Marker. Food Chem. 2022, 396, 133645. [Google Scholar] [CrossRef]
- Feng, J.; Hao, L.; Zhu, H.; Li, M.; Liu, Y.; Duan, Q.; Jia, L.; Wang, D.; Wang, C. Combining with Volatilomic Profiling and Chemometrics to Explore the Volatile Characteristics in Five Different Dried Zanthoxylum bungeanum Maxim. Food Res. Int. 2024, 175, 113719. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Fruit Growth Dynamics, Respiration Rate and Physico-Textural Properties during Pomegranate Development and Ripening. Sci. Hort. 2013, 157, 90–98. [Google Scholar] [CrossRef]
- Morandi, B.; Manfrini, L.; Losciale, P.; Zibordi, M.; Corelli Grappadelli, L. Changes in Vascular and Transpiration Flows Affect the Seasonal and Daily Growth of Kiwifruit (Actinidia deliciosa) Berry. Ann. Bot. 2010, 105, 913–923. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Stinco, C.M.; Meléndez-Martínez, A.J. Effect of the Fruit Position on the Cluster on Fruit Quality, Carotenoids, Phenolics and Sugars in Cherry Tomatoes (Solanum lycopersicum L.). Food Res. Int. 2017, 100, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutri. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, Bioactivity and Impact on Health of Dietary Flavonoids and Related Compounds: An Update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of Bioactive Compounds in Foods, with Focus on Fruits and Vegetables. Crit. Rev. Food Sci. Nutri. 2015, 55, 1324–1339. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- De Wilde, T.; De Meulenaer, B.; Mestdagh, F.; Govaert, Y.; Vandeburie, S.; Ooghe, W.; Fraselle, S.; Demeulemeester, K.; Van Peteghem, C.; Calus, A.; et al. Influence of Fertilization on Acrylamide Formation during Frying of Potatoes Harvested in 2003. J. Agric. Food Chem. 2006, 54, 404–408. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Chen, X.; Peng, W.; Liu, Y.; Yu, L.; Liang, F.; Wu, C. Comparative Studies on Flavor Substances of Leaves and Pericarps of Zanthoxylum Bungeanum Maxim. at Different Harvest Periods. Trop. J. Pharm. Res. 2019, 18, 279–286. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, W.; Sun, L.; Wei, A.; Wang, D. Accumulation and Biosynthesis of Hydroxyl-α-Sanshool in Varieties of Zanthoxylum bungeanum Maxim. by HPLC-Fingerprint and Transcriptome Analyses. Ind. Crop. Prod. 2020, 145, 111998. [Google Scholar] [CrossRef]
- Tahir, I.I.; Johansson, E.; Olsson, M.E. Improvement of Quality and Storability of Apple Cv. Aroma by Adjustment of Some Pre-Harvest Conditions. Sci. Hort. 2007, 112, 164–171. [Google Scholar] [CrossRef]
- Grbić, V.; Bleecker, A.B. Ethylene Regulates the Timing of Leaf Senescence in Arabidopsis. Plant J. 1995, 8, 595–602. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorgan. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Qi, Y.; Liu, S. Remobilization of Storage Nitrogen in Young Pear Trees Grafted onto Vigorous Rootstocks (Pyrus betulifolia). Horticulturae 2021, 7, 148. [Google Scholar] [CrossRef]
- San-Martino, L.; Sozzi, G.O.; San-Martino, S.; Lavado, R.S. Isotopically-Labelled Nitrogen Uptake and Partitioning in Sweet Cherry as Influenced by Timing of Fertilizer Application. Sci. Hort. 2010, 126, 42–49. [Google Scholar] [CrossRef]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-Ferrière, N.; Thibaud, J.-B.; Sentenac, H. Identification and Disruption of a Plant Shaker-like Outward Channel Involved in K+ Release into the Xylem Sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Bodo, A.V.; Arain, M.A. Radial Variations in Xylem Sap Flux in a Temperate Red Pine Plantation Forest. Ecol. Process 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cossani, C.M.; Sadras, V.O.; Yang, Q.; Wang, Z. The Interaction Between Nitrogen Supply and Light Quality Modulates Plant Growth and Resource Allocation. Front. Plant Sci. 2022, 13, 864090. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Hu, M.; Wang, S.; Liu, Q.; Cao, R.; Xue, Y. Flavor Profile of Dried Shrimp at Different Processing Stages. LWT 2021, 146, 111403. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, F.; Wu, W.; Wang, P.; Ye, N. Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis. Molecules 2020, 25, 380. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Characterization of Key Odorants in Hanyuan and Hancheng Fried Pepper (Zanthoxylum bungeanum) Oil. J. Agric. Food Chem. 2020, 68, 6403–6411. [Google Scholar] [CrossRef]
- Li, X.-J.; Yang, Y.-J.; Li, Y.-S.; Zhang, W.K.; Tang, H.-B. α-Pinene, Linalool, and 1-Octanol Contribute to the Topical Anti-Inflammatory and Analgesic Activities of Frankincense by Inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for Classification of High-Dimensional (HD) Data: A Review of Contemporary Practice Strategies and Knowledge Gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef]
- Scavarda, C.; Cordero, C.; Strocchi, G.; Bortolini, C.; Bicchi, C.; Liberto, E. Cocoa Smoky Off-Flavour: A MS-Based Analytical Decision Maker for Routine Controls. Food Chem. 2021, 336, 127691. [Google Scholar] [CrossRef]
- Pan, Y.-Y.; Chen, Y.-C.; Chang, W.C.-W.; Ma, M.-C.; Liao, P.-C. Visualization of Statistically Processed LC-MS-Based Metabolomics Data for Identifying Significant Features in a Multiple-Group Comparison. Chemom. Intell. Lab. Sys. 2021, 210, 104271. [Google Scholar] [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the Aromatic Profile of Purple Passion Fruit (Passiflora edulis Sims) during Ripening by HS-SPME-GC/MS and RNA Sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Wang, B.; Song, H.; Zou, T. Screening of the Volatile Compounds in Fresh and Thermally Treated Watermelon Juice via Headspace-Gas Chromatography-Ion Mobility Spectrometry and Comprehensive Two-Dimensional Gas Chromatography-Olfactory-Mass Spectrometry Analysis. LWT 2021, 137, 110478. [Google Scholar] [CrossRef]
- Shi, J.; Fei, X.; Hu, Y.; Liu, Y.; Wei, A. Identification of Key Genes in the Synthesis Pathway of Volatile Terpenoids in Fruit of Zanthoxylum bungeanum Maxim. Forests 2019, 10, 328. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Pu, D.; Shan, Y.; Duan, W.; Huang, Y.; Liang, L.; Yan, Y.; Zhang, Y.; Sun, B.; Hu, G. Characterization of the Key Aroma Compounds in the Fruit of Litsea pungens Hemsl. (LPH) by GC-MS/O, OAV, and Sensory Techniques. J. Food Qual. 2021, 2021, 6668606. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Yang, W.; Sun, B.; Zhou, Y.; Zheng, Y.; Huang, M.; Yang, W. Characterization of the Potent Odorants in Zanthoxylum armatum DC Prodr. Pericarp Oil by Application of Gas Chromatography–Mass Spectrometry–Olfactometry and Odor Activity Value. Food Chem. 2020, 319, 126564. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.K.; Asrey, R. Tree Age Affects Postharvest Attributes and Mineral Content in Amrapali Mango (Mangifera indica) Fruits. Hort. Plant J. 2018, 4, 55–61. [Google Scholar] [CrossRef]
- Bergman, M.E.; Huang, X.-Q.; Baudino, S.; Caissard, J.-C.; Dudareva, N. Plant Volatile Organic Compounds: Emission and Perception in a Changing World. Curr. Opin. Plant Biol. 2025, 85, 102706. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, L. Tea Quality Evaluation by Applying E-Nose Combined with Chemometrics Methods. J. Food Sci. Technol. 2021, 58, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Ewais, O.; Abdel-Tawab, H.; El-Fayoumi, H.; Aboelhadid, S.M.; Al-Quraishy, S.; Falkowski, P.; Abdel-Baki, A.-A.S. Antioxidant Properties of D-Limonene and Its Nanoemulsion Form Enhance Its Anticoccidial Efficiency in Experimentally Infected Broilers with Eimeria tenella: An In Vitro and In Vivo Study. Vet. Res. Commun. 2024, 48, 3711–3725. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Vlaisavljevic, S.; Popovic, M.; Vastag, D.; Djurendic-Brenesel, M. Antioxidant Properties of Marrubium peregrinum L. (Lamiaceae) Essential Oil. Molecules 2010, 15, 5943–5955. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model Studies on the Antioxidant Activity of Common Terpenoid Constituents of Essential Oils by Means of the 2,2-Diphenyl-1-Picrylhydrazyl Method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A Review on Antioxidants, Prooxidants and Related Controversy: Natural and Synthetic Compounds, Screening and Analysis Methodologies and Future Perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In Vivo Antioxidant Activity of Phenolic Compounds: Facts and Gaps. Trends Food Sci Tech. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and Isoprenoids: A Wealth of Compounds for Global Use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-M.; Li, J.-X.; Zhang, T.-Q.; Xu, Z.-G.; Ma, M.-L.; Zhang, P.; Wang, J.-W. The Structure of B-ARR Reveals the Molecular Basis of Transcriptional Activation by Cytokinin. Proc. Natl. Acad. Sci. USA 2024, 121, e2319335121. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Nara, U.; Rani, N.; Pathak, D.; Sangha, M.K.; Kaur, K. Mineral Content Variation in Leaves, Stalks, and Seeds of Celery (Apium graveolens L.) Genotypes. Biol. Trace Elem. Res. 2023, 201, 2665–2673. [Google Scholar] [CrossRef]
- Sourati, R.; Sharifi, P.; Poorghasemi, M.; Alves Vieira, E.; Seidavi, A.; Anjum, N.A.; Sehar, Z.; Sofo, A. Effects of Naphthaleneacetic Acid, Indole-3-Butyric Acid and Zinc Sulfate on the Rooting and Growth of Mulberry Cuttings. J. Integr. Plant Biol. 2022, 13, 245–256. [Google Scholar] [CrossRef]
- Mom, R.; Réty, S.; Mocquet, V.; Auguin, D. Deciphering Molecular Mechanisms Involved in the Modulation of Human Aquaporins’ Water Permeability by Zinc Cations: A Molecular Dynamics Approach. Int. J. Mol. Sci. 2024, 25, 2267. [Google Scholar] [CrossRef]
| No. | Volatile Components | Ret Time | RI | CAS |
|---|---|---|---|---|
| 1 | (-)-β-Pinene | 11.37 | 1081 | 18172-67-3 |
| 2 | Sabinene | 11.65 | 1089 | 3387-41-5 |
| 3 | β-Myrcene | 12.49 | 1116 | 123-35-3 |
| 4 | D-Limonene | 13.43 | 1146 | 5989-27-5 |
| 5 | γ-Terpinene | 14.61 | 1184 | 99-85-4 |
| 6 | o-Cymene | 15.26 | 1205 | 527-84-4 |
| 7 | 4-Carene | 15.67 | 1218 | 29050-33-7 |
| 8 | Nonanal | 18.80 | 1379 | 124-19-6 |
| 9 | Tetradecane | 19.23 | 1400 | 629-59-4 |
| 10 | trans-Thujone | 20.09 | 1439 | 471-15-8 |
| 11 | cis-Linalool Oxide | 20.56 | 1443 | 5989-33-3 |
| 12 | 2-Methyltetradecane | 20.95 | 1469 | 1560-95-8 |
| 13 | 4-Thujanol | 21.21 | 1472 | 17699-16-0 |
| 14 | trans-Furan linalool oxide | 21.50 | 1483 | 34995-77-2 |
| 15 | δ-EIemene | 21.83 | 1489 | 20307-84-0 |
| 16 | Decanal | 22.58 | 1498 | 112-31-2 |
| 17 | Pentadecane | 22.90 | 1500 | 629-62-9 |
| 18 | Linalool | 24.39 | 1502 | 78-70-6 |
| 19 | Sabinene hydrate | 24.56 | 1512 | 546-79-2 |
| 20 | 2-p-Menthen-1-ol | 24.98 | 1515 | 619-62-5 |
| 21 | β-copaene | 25.76 | 1576 | 18252-44-3 |
| 22 | (-)-cis-β-Elemene | 26.22 | 1587 | 33880-83-0 |
| 23 | Caryophyllene | 26.84 | 1600 | 515-13-9 |
| 24 | Alloaromadendrene | 27.04 | 1605 | 25246-27-9 |
| 25 | cis-2-Menthenol | 27.38 | 1607 | 29803-82-5 |
| 26 | Myrtenal | 27.82 | 1612 | 564-94-3 |
| 27 | β-Caryophyllene | 27.93 | 1625 | 87-44-5 |
| 28 | γ-Muurolene | 28.28 | 1633 | 30021-74-0 |
| 29 | trans-Pinocarvyl acetate | 28.42 | 1639 | 1686-15-3 |
| 30 | (E)-β-Farnesene | 28.78 | 1644 | 18794-84-8 |
| 31 | (-)-γ-cadinene | 29.27 | 1655 | 39029-41-9 |
| 32 | 1,5,9,9-tetramethyl-1,4,7-Cycloundecatriene | 29.60 | 1662 | 515812-15-4 |
| 33 | L-α-Terpineol | 30.29 | 1685 | 10482-56-1 |
| 34 | Viridiflorene | 30.55 | 1683 | 21747-46-6 |
| 35 | cis-β-Copaene | 31.29 | 1700 | 18252-44-3 |
| 36 | β-Selinene | 31.71 | 1709 | 17066-67-0 |
| 37 | α-Selinene | 31.91 | 1714 | 473-13-2 |
| 38 | (+)-Bi-cyclo-germacrene | 32.20 | 1720 | 24703-35-3 |
| 39 | Geranyl acetate | 32.61 | 1732 | 105-87-3 |
| 40 | β-Cadinene | 32.97 | 1737 | 523-47-7 |
| 41 | 7-epi-α-selinene | 33.40 | 1747 | 123123-37-5 |
| 42 | Cumin-aldehyde | 33.87 | 1799 | 122-3-2 |
| 43 | Phenethyl acetate | 35.10 | 1808 | 103-45-7 |
| 44 | cis-Calamenene | 36.00 | 1835 | 72937-55-4 |
| 45 | Geraniol | 36.27 | 1876 | 106-24-1 |
| 46 | Geranyl-acetone | 36.64 | 1892 | 3796-70-1 |
| 47 | Carveol | 37.11 | 1893 | 99-48-9 |
| 48 | Iso-ascaridol | 37.26 | 1898 | 17948-59-3 |
| 49 | Benzyl alcohol | 37.43 | 1901 | 100-51-6 |
| 50 | 1,5-Epoxy-4(14)-salvialene | 39.91 | 1962 | 88395-47-5 |
| 51 | 2,6-Dimethyl-3,7-octadiene-2,6-diol | 40.36 | 1966 | 51276-34-7 |
| 52 | Phenethyl iso-butyrate | 41.26 | 1963 | 103-48-0 |
| 53 | Caryophyllene oxide | 42.27 | 1975 | 1139-30-6 |
| 54 | Mintketone | 43.00 | 1981 | 73809-82-2 |
| 55 | 8-Hydroxylinalool | 43.28 | 1990 | 64142-78-5 |
| 56 | trans-Nerolidol | 43.51 | 1993 | 40716-66-3 |
| 57 | Humulene epoxide II | 43.86 | 1995 | 19888-34-7 |
| 58 | Elemol | 44.63 | 1997 | 639-99-6 |
| 59 | γ-Eudesmol | 45.30 | 2012 | 1209-71-8 |
| 60 | 2,6-dimethyl-1,7-Octadiene-3,6-diol | 45.49 | 2017 | 51276-33-6 |
| 61 | Spathulenol | 45.66 | 2020 | 6750-60-3 |
| 62 | Neo-intermedeol | 45.94 | 2027 | 5945-72-2 |
| 63 | Cedrelanol | 46.55 | 2040 | 5937-11-1 |
| 64 | Agarospirol | 46.76 | 2045 | 1460-73-7 |
| Sample | ABTS (mg/g) | DPPH (mg/g) | RC (mg/g) |
|---|---|---|---|
| 1T5 | 53.94 ± 1.38 bc | 44.59 ± 1.49 bc | 35.29 ± 0.23 ef |
| 1M5 | 47.10 ± 0.79 def | 33.62 ± 4.74 ef | 31.63 ± 0.27 f |
| 1B5 | 51.84 ± 2.00 bcd | 40.26 ± 2.22 cd | 35.23 ± 1.75 ef |
| 1T3 | 46.21 ± 1.31 def | 36.07 ± 1.72 de | 27.51 ± 1.33 g |
| 1M3 | 47.40 ± 1.11 def | 35.50 ± 2.79 de | 50.01 ± 0.58 b |
| 1B3 | 46.67 ± 1.66 def | 32.00 ± 1.01 ef | 45.22 ± 0.96 c |
| 2T5 | 60.61 ± 2.026 a | 51.96 ± 3.38 a | 54.17 ± 2.71 a |
| 2M5 | 55.48 ± 3.56 ab | 43.44 ± 5.87 bc | 51.29 ± 4.00 ab |
| 2B5 | 56.45 ± 4.45 ab | 46.95 ± 4.76 ab | 50.86 ± 1.26 ab |
| 2T3 | 42.53 ± 3.82 f | 33.31 ± 2.91 ef | 40.79 ± 0.70 d |
| 2M3 | 36.81 ± 1.35 g | 25.95 ± 2.16 gh | 38.95 ± 0.12 de |
| 2B3 | 43.55 ± 4.02 f | 24.93 ± 3.88 h | 37.25 ± 1.108 de |
| 3T5 | 50.04 ± 5.04 cde | 35.73 ± 1.53 de | 41.31 ± 1.88 d |
| 3M5 | 46.88 ± 1.00 def | 34.31 ± 2.44 e | 38.75 ± 0.17 de |
| 3B5 | 46.71 ± 6.34 def | 35.18 ± 3.37 de | 39.63 ± 6.66 d |
| 3T3 | 35.69 ± 1.50 g | 23.68 ± 2.97 h | 31.86 ± 1.02 f |
| 3M3 | 44.44 ± 0.83 ef | 28.28 ± 1.07 fgh | 39.96 ± 0.75 d |
| 3B3 | 43.59 ± 3.03 f | 30.84 ± 2.40 efg | 34.88 ± 3.40 ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Gu, T.; Hu, S.; Kong, Y.; Huang, J.; Sun, Y.; Yu, T.; Zhuang, G.; Gao, S. Assessment of Nutritional Components, Mineral Profiles, and Aroma Compounds in Zanthoxylum armatum Fruit from Different Harvest Times, Tree Age and Fruiting Position. Horticulturae 2025, 11, 1028. https://doi.org/10.3390/horticulturae11091028
Xiao Y, Gu T, Hu S, Kong Y, Huang J, Sun Y, Yu T, Zhuang G, Gao S. Assessment of Nutritional Components, Mineral Profiles, and Aroma Compounds in Zanthoxylum armatum Fruit from Different Harvest Times, Tree Age and Fruiting Position. Horticulturae. 2025; 11(9):1028. https://doi.org/10.3390/horticulturae11091028
Chicago/Turabian StyleXiao, Yixiao, Tao Gu, Shiyao Hu, Yiming Kong, Jingwen Huang, Yaxuan Sun, Ting Yu, Guoqing Zhuang, and Shun Gao. 2025. "Assessment of Nutritional Components, Mineral Profiles, and Aroma Compounds in Zanthoxylum armatum Fruit from Different Harvest Times, Tree Age and Fruiting Position" Horticulturae 11, no. 9: 1028. https://doi.org/10.3390/horticulturae11091028
APA StyleXiao, Y., Gu, T., Hu, S., Kong, Y., Huang, J., Sun, Y., Yu, T., Zhuang, G., & Gao, S. (2025). Assessment of Nutritional Components, Mineral Profiles, and Aroma Compounds in Zanthoxylum armatum Fruit from Different Harvest Times, Tree Age and Fruiting Position. Horticulturae, 11(9), 1028. https://doi.org/10.3390/horticulturae11091028







