Abstract
Phytophthora fruit rot caused by Phytophthora capsici is a devastating disease in many solanaceous vegetables, resulting in tremendous yield and economic losses. However, the underlying resistance or susceptibility to P. capsici in eggplant remains obscure. In this study, the transcriptomic analysis was performed between the resistant (G42) and susceptible (EP28) eggplant genotypes at 0, 1, 3 and 5 days post-inoculation (dpi). Taking 0 dpi as the control, a total of 4111, 7496 and 7325 DEGs were expressed at 1, 3 and 5 dpi, respectively, in G42 and 5316, 12675 and 12048 DEGs were identified at 1, 3 and 5 dpi, respectively, in EP28. P. capsici infection induced substantial transcriptional changes in the inoculated fruits. The analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) identified defense-related pathways including ‘plant-pathogen interactions’, ‘mitogen-activated protein kinase (MAPK)’ and ‘hormone biosynthesis and signal transduction’. The hormone-related genes encompassing ethylene, abscisic acid, auxins and gibberellins showed differential expression between G42 and EP28 eggplant genotypes, signifying their important roles in plant disease resistance. P. capsici infection induced the expression of major transcription factors such as MYB, NAC/NAM, bHLH, WRK, HSF, HD-ZIPAP2/ERF and Mad-box. qRT-PCR validation of the selected genes corroborates with RNA-seq, depicting the precision and consistency of the transcriptomic data. According to qRT-PCR and RNA-seq analyses, the expression of the pathogenesis-related gene transcriptional activator, SmPTI6 (Smechr0603020), is upregulated in G42 and downregulated in EP28. This differential expression suggests a potential role in the resistance to P. capsici. Functional analysis via a virus-induced gene silencing (VIGS) system found that silencing SmPTI6 in G42 enhanced infection by P. capsici, indicating that SmPTI6 performs a critical role in response to pathogen attack. The comprehensive results obtained in this study provide a valuable resource for understanding the molecular mechanisms underlying eggplant resistance to P. capsici and for establishing breeding resistant eggplant genotypes to P. capsici.
1. Introduction
Eggplant (Solanum melongena L.) is an important solanaceous vegetable crop cultivated worldwide [1]. China alone produces over 60 percent of the total eggplant produced globally annually [2]. There is a continuous increase each year in planting hectarage and yield, and various commercial gains are experiencing steady growth. Eggplant fruit contains multiple nutrients, such as vitamins, phenolics and antioxidants, which benefit human health [3]. However, its potential yield is affected by environmental stresses and biotic factors, including Phytophthora fruit rot [4,5]. Phytophthora fruit rot is a disease caused by the pathogenic oomycete Phytophthora capsici, which is found worldwide [6,7]. P. capsici can occur and infect eggplant at any growth and developmental period and affect different parts of the plant, causing damping off at the seedling stage, root rot, stem rot, collar rot, fruit rot and blight on the foliage [8,9]. P. capsici fruit rot is the most common and devastating disease in eggplant under suitable environmental conditions, comprising temperatures of 25–28 °C and 90% relative humidity [10,11]. By studying plant responses to pathogen infection, it is possible to gain insights into the mechanisms by which plants interact with pathogens, thereby elucidating the functional dynamics of plant immune systems, which are crucial for advancing disease-resistance breeding efforts and variety improvement. The immune response in plants is typically divided into two fundamental stages [12]. The primary layer of defense in plants is characterized by the immune response elicited by pathogen-associated molecular patterns (PAMPs), known as PAMP-triggered immunity (PTI). This phase consists of a range of immune responses initiated by pattern recognition receptors (PRRs) located on the surface of plant cells, which can detect PAMPs. In turn, pathogens utilize various tactics to undermine PTI, including releasing harmful effectors. To counteract these strategies, plants have developed nucleotide-binding leucine-rich repeat receptor (NLR) proteins that detect these effectors and inhibit their function, thereby bolstering their resistance. Consequently, this aspect of immunity is referred to as effector-triggered immunity (ETI) [12,13]. In plants, proteins associated with disease resistance operate as key immune receptors, tasked with detecting pathogens and activating strong defense responses [13]. For example, pathogenesis-related (PR) proteins (such as chitinases and glucanases) degrade microbial cell walls [14]. Nucleotide-binding leucine-rich repeat (NLR) proteins act as intracellular immune receptors [15]. Antimicrobial peptides (AMPs) like defensins disrupt pathogen membranes [16].
Significant yield losses occur in field production where conditions are suitable for P. capsici to thrive [17]. Some cultivated germplasms of eggplant are susceptible to Phytophthora fruit rot, highlighting the necessity for future breeding programs to develop eggplant varieties that can resist this affliction. While there are cultivars susceptible to P. capsici, there are also resistant cultivars, which need to be studied to understand their defense mechanisms. Analyzing the complete gene expression can yield important information regarding the molecular basis of the interactions between host plants and Phytophthora pathogens [18,19]. This approach provides valuable insights into the complex mechanisms that control resistance and basal defense responses, elucidating the complex interactions between these two entities. Previous studies on transcriptomics have yielded significant genes that have allowed the elucidation of plant–pathogen interactions. For example, transcriptomic responses of two wild tomato germplasms to Phytophthora parasitica infection revealed that certain genes associated with protease inhibitors, chitinases, defensins and PR-1 exhibited significant upregulation in the resistant germplasms [20]. In cucumber, the genetic factors that contribute to age-related resistance against P. capsici were investigated. The findings indicated that a significant number of genes associated with flavonoid and terpenoid biosynthesis were upregulated in the peels of resistant fruit at 16 days post-pollination [21]. Tobacco leaves infected with P. parasitica identified genes related to jasmonic acid and ethylene signaling pathways, as well as receptor-like kinases, pathogenesis-related (PR) genes, and various transcription factors [22]. In pepper, transcriptomic analysis revealed a greater number of genes linked to the phenylpropanoid biosynthesis pathway in the resistant Piper flaviflorum when compared to the susceptible Piper nigrum cv. Reyin-1 during P. capsici infections [23]. The comparative transcriptome and metabolome analysis of P. flaviflorum and P. nigrum in response to P. capsici infection demonstrated that the altered genes showed significant enrichment in pathways related to plant–pathogen interactions, phytohormone signal transduction, and secondary metabolic pathways, notably phenylpropanoid biosynthesis [24]. Therefore, it is crucial to analyze the responses of both resistant and susceptible plant lines to P. capsici infection to understand the mechanisms of defense.
In this study, two eggplant genotypes, G42 resistant and EP28 susceptible to P. capsici, were used. Phenotypic and transcriptomic analyses were conducted on the fruits of G42 and EP28 in response to P. capsici at 0, 1, 3 and 5 days post-inoculation (dpi). Our results unveiled the key Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway that could contribute to the resistance mechanism to P. capsici infections. Identifying pathways of differentially expressed genes, expression patterns and functional analysis via VIGs of the SmPTI6 gene involved in defense response will contribute to a deeper understanding of the molecular mechanism underlying resistance to P. capsici in eggplant and contribute to eggplant genotype improvement.
2. Materials and Methods
2.1. Plant Materials
The experimental plant materials comprised two contrasting eggplant genotypes involving G42, which is a resistant, and EP28, a type susceptible to P. capsici. The resistant genotype (G42) maintains green fruit coloration at all developmental stages, while the susceptible genotype (EP28) develops dark purple pigmentation. These eggplant germplasm materials were obtained from the Vegetable Institute, Jiangsu Academy of Agricultural Sciences, Nanjing, China. The plants were cultivated in a greenhouse during the spring–summer period, with air temperatures of 28–33 °C during the day and 14–20 °C at night. The fruits were collected at the commercial stage (30 days after anthesis). Eggplant fruits without skin blemishes were collected for in vitro inoculation with P. capsici.
The G42 and EP28 fruits were inoculated following the previous protocols [25,26,27]. Briefly, the collected fruits were subjected to surface disinfection using a 10% bleach solution for 5 min, followed by rinsing with distilled water. The fruits were arranged in plastic boxes and then inoculated with a mycelium V8 agar plug (6 mm in diameter) containing actively growing colonies of P. capsici on the fruit surface derived. The V8 Agar plug was placed was upside down on the eggplant fruit surface to ensure the part with active P. capsici mycelium was in direct contact with the fruit. The boxes were covered with polythene film and additionally with the box cover. The eggplant fruits were maintained in the humidity chambers under continuous light at room temperature (25 °C) (Figure S1). Evaluations of the fruits were conducted at 0, 1, 3 and 5 days post-inoculation (dpi). The evaluation time, 0–5 days, was based on the preliminary disease progression time of P. capsici and a previous study protocol [25]. The samples were obtained from the inoculated fruit parts for sequencing at each time point. Samples were referred herein as G42_0, G42_1, G42_3 and G42_5 for the resistant line, and EP28_0, EP28_1, EP28_3 and EP28_5 for the susceptible line. The sampled fruits were wrapped in tinfoil, immediately snap-frozen in liquid nitrogen, and stored at −80 °C. Three biological replications, three fruits for each replicate, were sampled and pooled for RNA extraction.
2.2. Total RNA Isolation and Illumina Sequencing
RNA was isolated from a total of 24 samples, with three replicates for each treatment, using Trizol reagent (Invitrogen, Waltham, MA, USA). RNA quantification was carried out using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA) and evaluated with the RNA Assay Kit (Nano 6000) on the Agilent Bioanalyzer 2100 System (Agilent Technologies, Santa Clara, CA, USA). A sequencing library was then constructed using the NEBNext Ultra-TM-RNA Library Preparation Kit (NEB, Ipswich, MA, USA). After completing quality checks and inspections, sequencing was performed on an Illumina sequencing platform (Beijing Baimaike Biotechnology Co., Ltd., Beijing, China).
2.3. Mapping of RNA Sequencing Reads and Differentially Expressed Genes
To obtain high-quality reads, raw fastQ data files were subjected to a cross-check for quality, with a threshold set at ≤20. Inadequate raw reads were trimmed using Trimmomatic-0.30 [28]. The resultant filtered reads were aligned to the eggplant genome (http://www.eggplant-hq.cn/, accessed on 7 July 2024) utilizing HISAT2 software (V2.2.1), and gene expression levels were quantified using FPKM (fragments per kb per million fragments) [29]. DEGs were identified through the DESeq2 method [30]. The p-value threshold was determined via the false discovery rate (FDR) control approach, with significance criteria for DEGs established at an FDR < 0.01 and an absolute log2 ratio ≥ 1. Gene ontology (GO) and KEGG pathway enrichment analyses were conducted using the topGO method and KOBAS 2.0 (http://www.biostars.org/p/200126, accessed on 7 July 2024), respectively.
2.4. qRT-PCR Validation of DEGs Associated with P. capsici Infection
To validate the findings from the RNA-seq analysis, quantitative real-time PCR (qRT-PCR) was performed on 12 DEGs potentially associated with resistance to P. capsici. First-strand cDNA was synthesized from total RNA extracted from fruit samples of G42 and EP28 at four distinct time points (0, 1, 3 and 5 days post-inoculation), utilizing the ReverTran Ace qPCR RT kit (Toyobo, Shanghai Biotech Co., Ltd., Shanghai, China). Specific primers were designed with the aid of Primer 3 (https://www.primer3plus.com/, accessed on 23 August 2024. The actin gene of eggplant served as an internal control. The qRT-PCR was executed on the Roche LightCycler 960 II system employing a SYBR Green-based PCR assay. Each sample underwent three independent biological replicates, with three technical replicates for each biological sample included in the qRT-PCR analysis. The reaction mixture comprised 10 μL of diluted cDNAs (20 ng/reaction of cDNA) and 0.4 μL of each primer, adjusted to a final volume of 20 μL. The PCR protocol involved an initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s and PCR elongation at 72 °C for 10 min. Relative gene expression levels were determined using the 2−ΔΔCt method [31]. The primer information of the genes used for qRT-PCR is listed in Table S1.
2.5. Virus-Induced Gene Silencing (VIGS)
The VIGS procedure was executed following the previously outlined protocols [32,33], albeit with some alterations. In essence, a 300 bp cDNA fragment of SmPTI6 was inserted into the pTRV2 vector and transformed into the Agrobacterium strain GV3101. Following this, the Agrobacterium containing the pTRV2–SmPTI6 construct was mixed in equal volumes with Agrobacterium that contained pTRV1. pTRV1 produces viral RNA1, which contains genes for viral replication and movement, while pTRV2 encodes viral RNA2, which carries the gene of interest (or a fragment of it) that will be silenced. The resulting bacterial mixture was resuspended in an infiltration buffer consisting of 10 mM MgCl2, 200 mM acetosyringone and 10 mM MES (2-(N-morpholino) ethanesulfonic acid) at a pH of 5.6, and it was subsequently employed for VIGS. Eggplant fruits of G42 were collected for VIGS analysis 25 days post-anthesis. After the fruits were surface sterilized with 75% ethanol, they were injected with the previously described agrobacterial mixture. The fruits were then placed in a dark chamber at 21 °C with 90% humidity for 24 h. This was followed by 5 days of photoperiod (16/8 h), during which the temperature and humidity were kept stable. A control experiment was executed using the pTRV2 empty vector instead of pTRV2–SmPTI6. P. capsici inoculation was performed by applying a mycelium plug to the fruit’s surface 7 days post-VIGS silencing, and the inoculated fruits were incubated at 25 °C under dark conditions with 90% humidity. Using qRT-PCR, the expression of SmPTI6 was measured at 0, 3 and 5 dpi.
2.6. Data Analysis
The quantitative experimental data were conducted in triplicates to ensure both accuracy and accountability. One-way ANOVA was used for data comparison, and the least significant difference (LSD) test at p < 0.05 and 0.01 was used for multiple comparisons.
Figures were drawn using Excel, Origin 8.0 and SRplot (http://www.bioinformatics.com.cn/en, accessed on 15 September 2024), and heatmaps were generated using TBtools-II (Ver 2.326) [34].
3. Results
3.1. Phenotypic Observations of P. capsici-Infected Eggplant Plants
We initially conducted disease assays to confirm the response of the two eggplant breeding lines (G42 and EP28) to P. capsici infection. The progression of the infection was observed over a period of five days in both genotypes. Symptoms indicative of P. capsici infection first manifested on the fruit surface at three days post-inoculation (dpi), presenting as light brown lesions around the inoculation site, which expanded as the disease advanced (Figure 1). By the fifth dpi, significant tissue colonization was evident on the fruit surface of the EP28 and showed extensive girdling due to P. capsici infection. Conversely, the resistant line (G42) exhibited only small, localized lesions with a reddish hue at the inoculation point (Figure 1). By5 dpi, it was clear that the G42 line had effectively limited fungal proliferation on the fruit surface (EP28) (Figure 1).

Figure 1.
Disease symptoms in the genotypes G42 and EP28 at different times post-inoculation by Phytophthora capsici. In G42, no symptoms were observed, while in EP28, brown necrosis enlarged forming water-soaked lesions.
3.2. Transcriptome Analysis of Eggplant Fruits Inoculated with P. capsici
Twenty-four cDNA libraries were constructed in our study, generating more than 976 million raw reads (approximately 146 GB of data), with each RNA-seq library producing an average of 40 million raw reads (Table 1). The clean reads exhibited a proportion that spanned from 98.05% to 98.72% and a total of 144 GB clean bases. The average data size for each sample was up to 6.00 GB. The quality scores (Q30 were above 97%, and GC contents ranged from 42 to 47% (Table 1). The total reads that were mapped to the reference genome of the eggplant exhibited a percentage between 60.6% and 96.43%; uniquely mapped reads to the reference genome ranged from 58.33% to 93.28% and 2.34% to 5.17% and were aligned to multiple locations (Table S2). The proportion of reads aligned to exons ranged from 89.37% to 92.03% (Table S3). Utilizing the FPKM values, the computed correlation coefficients for each sample, derived from the three biological replicates of samples were determined. The correlation coefficients for all comparisons exceeded 0.94, demonstrating a high level of reproducibility (Figure S2). These findings suggest that the RNA sequencing data is highly dependable and appropriate for subsequent analyses.

Table 1.
Summary of RNA sequencing from resistant (G42) and susceptible (EP28) eggplant genotypes inoculated with P. capsici.
3.3. Identification of DEGs in Response to Phytophthora capsici Infection
The gene expression profiles of healthy eggplant fruits of G42 and EP28 were utilized as the control group. Any gene that exhibited a two-fold or higher change in expression levels in infected fruits compared to the control group (p < 0.001) was identified as a differentially expressed gene (DEG). For G42 inoculated with P. capsici, there were a total of 4111 DEGs (1761 upregulated and 2350 downregulated) at G42_1, and the number of DEGs increased to 7496 (2701 upregulated and 4795 downregulated) at G42_3, then slightly decreased to 7325 (2924 upregulated and 4401 downregulated) at G42_5. In comparison to EP28, there were a total of 5316 DEGs (2241 upregulated and 3075 downregulated) at EP28_1, and the number of DEGs increased to 12,675 (3866 upregulated and 8809 downregulated) at EP28_3, then slightly decreased to 12,048 (3659 upregulated and 8389 downregulated) at EP28_5 (Figure 2A). Throughout all time points, a fewer number of genes displayed altered expression levels in resistant genotypes compared to susceptible genotypes. The results imply the stress caused by the infection is more severe in the susceptible genotype compared to the resistant genotype, and that this level of stress is accompanied by a greater change in gene expression. The results indicated that 3 dpi corresponded to the peak number of DEGs observed in the two genotypes. This suggests that the infection by P. capsici at this specific time point is pivotal for examining the defense response mechanisms.
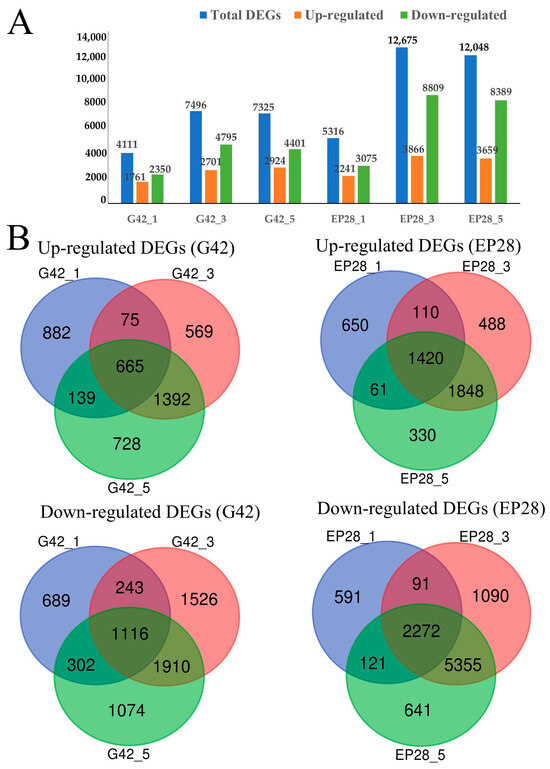
Figure 2.
DEGs involved in P. capsici inoculated eggplant materials G42 and EP28 at different post-inoculation times. (A) Distribution of identified DEGS in all time points. (B). Venn diagrams of DEGs in pairwise comparison with 0 dpi as the control.
The Venn diagram representing the DEGs following the inoculation of P. capsici at distinct point times effectively conveyed the unique and co-expressed DEGs among the samples (Figure 2B). A cumulative count of 665 upregulated genes and 1,116 downregulated genes was identified in the (G42) samples at the three time intervals relative to the control. A total of 1420 genes exhibited upregulation, while 2272 genes demonstrated downregulation, with co-expression observed at all time points in EP28. Intersection analysis of DEGs across various time points revealed a greater number of DEGs at 3 days post-inoculation (dpi) compared to 5 dpi in both genotypes. This finding aids in comprehending the persistent DEGs resulting from pathogen infection over time, thereby facilitating a deeper analysis of the dynamic responses associated with plant–pathogen interactions in eggplant. To illustrate the evolving trends among the sample groups, all DEGs were categorized into nine clusters utilizing the K-means method in MeV software (Figure 3). Genes classified within clusters 1, 5 and 7 exhibited the highest expression levels in the G42 group across various time points. Conversely, genes from clusters 2, 3, 4, 6 and 9 demonstrated peak expression levels in the EP28 group at designated time intervals. The expression profiles of genes in cluster 4, comprising 2304 DEGs, remained relatively stable before and after the inoculation with P. capsici for both genotypes, with the exception of EP28_5. In cluster 5, which includes 2452 genes, most exhibited stability throughout all time points in both genotypes, with the notable exception of G42-1. Cluster 7 maintained stability across all time points in the EP28 genotype, while variations were observed in G42. Cluster 8, consisting of 2388 DEGs, showed stability in both cultivars, except at G42-0. Lastly, cluster 9, containing 1689 DEGs, remained stable across all time points, although fluctuations were noted among different time points, including both upregulated and downregulated genes in EP28.
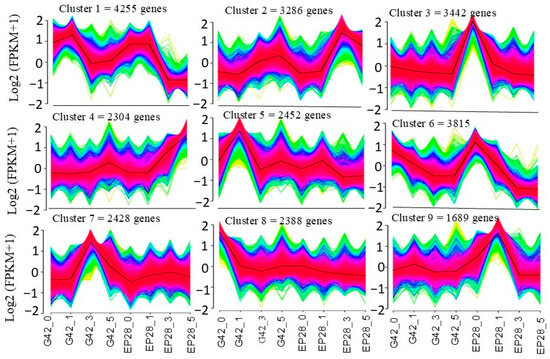
Figure 3.
Cluster analysis of DEGs between the G42 and EP28 genotypes at time points based on the K-means method or hierarchical clustering. G42_0 and EP28_0 are control samples. In all panels, grey lines indicate the expression levels of individual genes, while blue and green lines indicate a consensus of all the DEGs within a specific cluster.
Because exposure to pathogens is known to involve the induction of defense-response genes [25], emphasis was placed on identifying DEGs that are expressed to a higher degree in the G42 and determining their expression in the EP28. A list of the top 25 of the highest-scoring genes with increased expression in G42 and the relative expression, in EP28, at the three time points in comparison to 0 dpi are shown in Table S4. Among the top DEGS are transcription factor MYB92, protein PELKI, Cysteine-rich receptor-like protein kinase, ethylene-responsive transcription factor_ERF098, probable glutathione S-transferase parA and homeobox-leucine zipper protein, among others. Moreover, in this study, two E3 ubiquitin ligases were also found to be continuously expressed differentially in the two cultivars after pathogen infection.
3.4. Gene Ontology Enrichment Analysis of Differentially Expressed Genes
The Venn diagram established that 1190 DEGs were specifically expressed in G42, while 4371 DEGs were expressed in EP28. A total of 13,649 DEGs were constitutively co-expressed between G42 and EP28 at all time points (Figure 4A). The results demonstrate that the inoculation of P. capsici induces significant changes in gene expression in eggplant fruits, indicating considerable differences in the responses of the resistant and susceptible eggplant lines utilized in this investigation. The DEGs at various time points for G42 and EP28 were analyzed using GOseq, with a corrected p-value cut-off of 10 (−log10 p-value). This analysis was conducted to discern the differences in gene ontology (GO) term enrichment between the two genotypes post-inoculation. In total, 29 GO terms were identified for G42, while EP28 revealed 34 GO terms (Figure 4B), which were categorized into biological processes (BP), cellular components (CC) and molecular functions (MF).
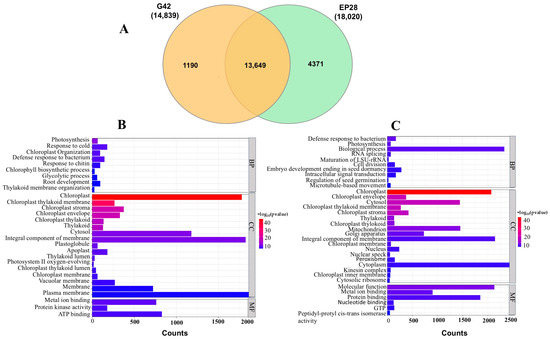
Figure 4.
Gene distribution and functional analysis of specifically regulated genes after inoculation with P. capsici. (A) Venn diagram of DEGS in G42 and EP28. The figures in parentheses are the total DEGs in each genotype, while shared DEGs are shown within the Venn diagram. (B) Enriched GO terms associated with specifically regulated genes in G42 after P. capsici inoculation. (C) Enriched GO terms associated with specifically regulated genes in EP28 after P. capsici inoculation. The GO terms are categorized into biological processes (BP), cellular components (CC) and molecular functions (MF). The abscissa represents the number of genes associated with each process.
In BP terms, expression of genes related to ‘response to chitin’, ‘glycolytic process’ and ‘response to cold’ were remarkably more significant in G42, while the expression of ‘intracellular signal transductions’, ‘microtubule-based movement’, ‘RNA splicing’ and ‘maturation of LSU-Rrna’ were prominent in EP28. ‘Photosynthesis’ and ‘defense response to bacterium processes’ were present in both genotypes. In CC terms, ‘chloroplast’, ‘chloroplast thylakoid membrane’ and ‘chloroplast envelope’, were present in both genotypes. EP28 expressed GO terms related to ‘kinesin complex’, ‘Golgi body’, ‘mitochondria’ and ‘peroxisomes’. In MF terms, ‘protein kinase activity’ and ‘ATP binding’ were remarkably more significant in G42 than in EP28. In contrast, ‘molecular function’, ‘nucleotide binding’, ‘peptidyl-protyl-cis trans isomerase’, and ‘guanosine triphosphate (GTP)’ were more significant in G42 than in EP28.
3.5. Kyoto Encyclopedia of Genes and Genomes Enrichment (KEGG) Analysis of DEGS
We performed a KEGG pathway enrichment analysis of the DEGs to investigate the response and resistance mechanism of eggplant in response to P. capsici inoculation. The top 20 enriched KEGG pathways were used to predict the biochemical pathways of eggplant in reactions induced by P. capsici infections. In G42, we found that the DEGs that appeared at G42_0 vs. _1 were significantly enriched in the photosynthetic antenna proteins, MAPK signaling pathway–plant, porphyrin metabolism, alpha-linolenic acid metabolism, plant–pathogen interactions and glycerophospholipid metabolism (Figure 5A). At 3 dpi (Figure 5B), the eggplant exhibited DEGs significantly enriched in plant hormone signal transduction, starch and sucrose metabolism, MAPK signaling pathway–plant and phenylpropanoid metabolism, among others. At the late stage, 5 dpi after P. capsici inoculation, most DEGs were significantly enriched in phenylpropanoid biosynthesis, phenylalanine biosynthesis, starch and sucrose metabolism, plant hormone signal transduction, ABC transporters, isoquinoline and glutathione metabolism, among others (Figure 5C). Interestingly, most of these pathways occur in all time points with varying intensity, thus signifying their importance in disease infection responses.
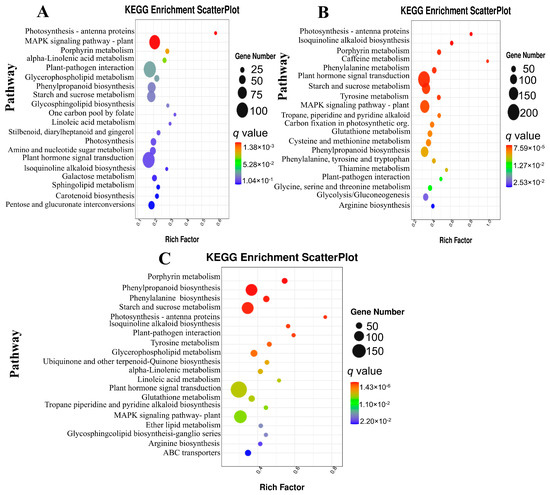
Figure 5.
Scatterplot of KEGG pathways enrichment analysis for DEGs in genotype G42. (A) G42_0 vs. G42-1. (B) G42_0 vs. G42-3. (C) G42_0 vs. _5. The rich factor represents the proportion of differentially expressed genes (DEGs) that are associated with a specific pathway term relative to the total number of genes annotated within that pathway. An elevated rich factor signifies a higher level of enrichment. The q value, which is the adjusted p value, varies between 0 and 1, where a smaller q value reflects a higher degree of significance. The diameter of the circles corresponds to the quantity of genes involved. The twenty most-enriched pathway terms from the KEGG database are presented.
In the susceptible genotype (EP28), the results showed that the DEGs during the early stage of P. capsici inoculation (1 dpi) were significantly enriched in plant hormone signaling transductions, phenylpropanoid biosynthesis, starch and sucrose metabolism, MAPK signaling pathway and plant–pathogen interaction, among others (Figure 6A). At 3 dpi, DEGs were significantly enriched in glutathione metabolism, Arachidonic acid metabolism, glycosphingolipid metabolism, starch, sucrose metabolism and plant hormone signal transductions, among others (Figure 6B). At 5 dpi in EP28, the DEGs were enriched in glycosphingolipid biosynthesis, photosynthesis antenna proteins, porphyrin metabolism, plant hormone signal transduction, sphingolipid metabolism, tyrosine metabolism, starch and sucrose metabolism, among others (Figure 6C). In both genotypes, the rich factor of the metabolic pathways varied considerably and was highest at late stage 5 dpi.
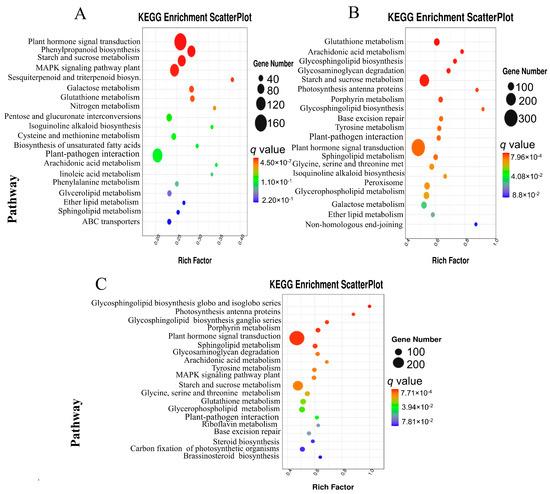
Figure 6.
Scatterplot of KEGG pathways enrichment analysis for DEGs in genotype EP28. (A) EP28_0 vs. _1 G42-1. (B) EP28_0 vs. _3. (C) EP28_0 vs. _5. The rich factor represents the proportion of DEGs that are associated with a specific pathway term relative to the total number of genes annotated within that pathway. An elevated rich factor signifies a higher level of enrichment. The q value, which is the adjusted p value, varies between 0 and 1, where a smaller q value reflects a higher degree of significance. The diameter of the circles corresponds to the quantity of genes involved. The twenty most-enriched pathway terms from the KEGG database are presented.
3.6. Identification of DEGs Involved in Defense Response to P. capsici Infections
The integration of KEGG pathway and GO enrichment analyses revealed a varied array of pathways associated with defense mechanisms following eggplant P. capsici infections in G42 and susceptible EP28 eggplant genotypes. The plant–pathogen interaction pathway plays a significant role in plant disease resistance. In this study, the plant–pathogen interaction showed the expression of DEGs related to autophagy-related proteins, 18 calcium-binding proteins, 16 calcium-dependent protein kinase (CDPK) 3 calmodulin-like proteins, 3 CBS-domain-containing proteins, 10 cyclic nucleotide-gated ion channels (CNGC), 5 disease-resistance proteins, 8 ethylene-responsive transcription factor, 9 late blight-resistance protein homolog, 2 LRR-receptor like serine, 10 WRKY transcription factor, 4 respiratory burst oxidase homolog (Rboh) and pathogenesis-related genes transcriptional activator (PTI6) (Figure 7; Table S5). In the plant–pathogen interaction pathway, one disease-resistance protein (Smechr0402283) was upregulated in EP28 compared to G42 (Table S5). In this pathway, some calcium-binding proteins such as CML15 (Smechr0103759), CML49 (Smechr1002778), CML19 (Smechr1201626) and CML21 (Smechr0400107) were upregulated in EP28 at 3 and 5 dpi compared to G42. In G42, the calcium-binding proteins CML16 (Smechr1102012), CML48 (Smechr1201717), CML50 (Smechr1102453) and CML41 (Smechr300575) were upregulated, while CML18 (Smechr0200838) and CML19 (Smechr1201626) were downregulated at 3 and 5 dpi (Figure 7).
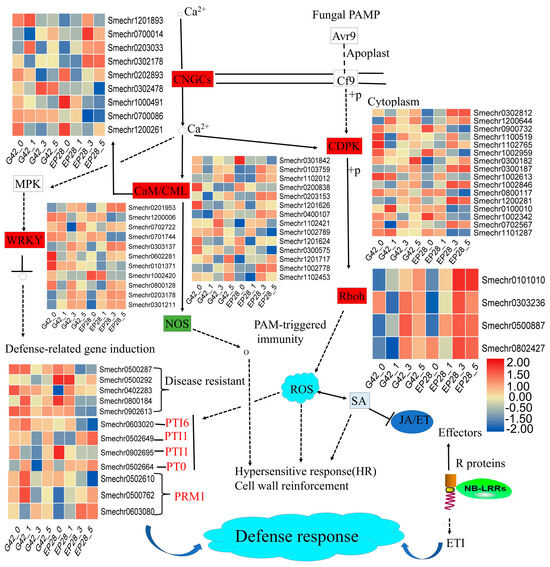
Figure 7.
Differentially expressed genes (DEGs) linked to the plant–pathogen interaction pathway were analyzed. A threshold for log2 FPKM values was established at ±2.0, with red indicating upregulation and blue indicating downregulation. The gene IDs are displayed in horizontal rows, while the vertical columns correspond to various time points.
The MAPK signaling pathway plays a crucial role in plant signal transduction networks, through a tertiary kinase manner involving MAPKKK, MAPKK and MAPK signaling cascades. Through transcription factors such as WRKY or directly, MAPK signaling cascade induces pathogenesis-related protein 1 (PR1) and protein kinase-C-related kinases (PRK). The analysis of DEGs identified 38 DEGs associated with the MAPK signaling pathway (Table S5). The expression profiles of differential genes involved in the MAPK signaling pathway post-pathogen infection were assessed (Figure 8A; Table S5), highlighting significant upregulation of Smechr0101057 in G42 and downregulation in EP28. Eight MAPK genes (Smechr0303698, Smechr0100663, Smechr0800498, Smechr1000008, Smechr0101444, Smechr0500071, Smechr0302543 and Smechr0200932) were distinctively upregulated in EP28 at 3 and 5 dpi, hence depicting their significant role in eggplant susceptibility to P. capsici infection.
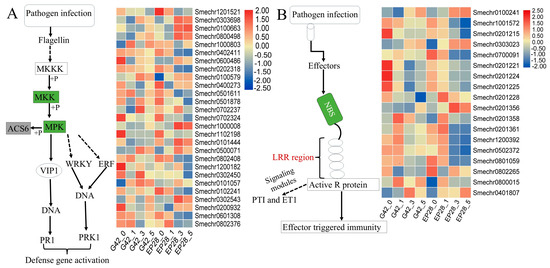
Figure 8.
Expression of MAPK signaling pathway and NBS-LRR genes in response to P.capsici infection using a heatmap. Colors represent the log2 fold change values. (A) MAPK signaling pathway genes. (B) NBS-LRR related genes.
Calcium-dependent protein kinase (CDPK) plays a significant role in plant defense. CDPK DEGs showed significant differential expressions between G42 and EP28 at different time points. In G42, the expression of CDPK genes such as Smechr1200644 and Smechr0300182 were upregulated, while Smechr1102765 showed downregulation upon P. capsici infection. In (EP28), the expressions of Smechr0302812, Smechr1200644, Smechr0300187, Smechr1002846 and Smechr1200281 were upregulated, while Smechr0900732, Smechr0800117, Smechr0100010 and Smechr1002342 were downregulated at 3 and 5 dpi. The cyclic nucleotide-gated ion channels CNGCs such as Smechr0700086 and Smechr03024478 were upregulated in (G42) but showed downregulation in EP28 at 3 and 5 dpi. In EP28, Smechr0700014, Smechr0203033 and Smechr0302178 and Smechr0202893 were upregulated at 3 and 5 dpi, thus suggesting the role in P. capsici defense. Rboh (respiratory burst oxidase homologs, Smechr0101010, Smechr0303236 and Smechr0500887) were significantly upregulated in the reactive oxygen species (ROS)-related pathway during the P. capsici infection, especially at 3 and 5 dpi stages in EP28 more than G42 (Figure 7).
The plant–pathogen interaction pathway also presents WRKY and ERF transcription factors. WRKY40 (Smechr0602281) was upregulated in EP28, while it was downregulated in G42. WRKY6 (Smechr0201953) and WRKY75 (Smechr0301211) were upregulated in both G42 and EP28 after P. capsici inoculation. WRKY20/1/41 and 44 (Smechr1200006, Smechr0701744, Smechr0101371 and Smechr1002420) were specifically downregulated in EP28 at 3 and 5 dpi (Figure 7). The ERF1 (Smechr0500241) and ERF119 (Smechr1000134) are upregulated in both genotypes, but ERF1 is more upregulated in EP28. The ERF118 (Smechr1000134) is downregulated in G42, while it shows upregulation at 1 dpi and downregulation at 3 and 5 dpi in EP28 (Table S5). The expression of the WRKY transcription factors induced the downstream defense-related R genes, PTI (pattern-triggered immunity) and RPM (plant resistance modulators)-interacting proteins. The pathogen-related gene PTI6 (Smechr0603020) was highly expressed in the G42-resistant genotype but downregulated in EP28 at 3 and 5 dpi (Figure 7). Two of the RPM1-interacting protein 4 (Smechr0500762, and Smechr063080) were significantly upregulated in EP28 at 3 and 5 dpi compared to G42, signifying their importance in response to P. capsici infections in eggplant fruit.
The nucleotide-binding site (NBS) and leucine-rich repeat (LRR) domains form the most important R genes in response to pathogen infection. The active R protein signaling induces pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). The analysis of NBS-LRR yielded a total of 202 related genes in our study (Table S5). The expression of the selected 18 NBS-LRR genes (Figure 8B) shows that 13 genes were downregulated at 3 and 5 dpi in EP28, while 5 were upregulated at later stages. In G42, NBS-LRR-related genes (Smechr0800015) showed upregulation at 3 and 5 dpi in response to P. capsici infections but had a downregulation in EP28, hence offering resistance by recognizing receptors within pathogens.
Plant hormone signal transduction plays an important role in plant growth, development and defense systems. In this study, plant hormones including ethylene, abscisic acid, auxin (Aux), cytokinin (CK) and gibberellins (GA) were the majority and showed differential expression between G42 and EP28 at different time points upon P. capsici infection (Figure 9, Table S5). Auxin, in the tryptophan metabolic pathway, plays a significant role in pathogen defense systems. Thirty-seven auxin-related genes comprising AUX, SAUR and IAA showed differential expression in the two genotypes upon P. capsici infection. Most of the auxin-related genes were highly downregulated in EP28 than in G42 at 3 and 5 dpi (Figure 9A), while only six genes (Smechr0901825, Smechr091897, Smechr0302266, Smechr0302267, Smechr032402 and Smechr061419) showed upregulation in EP28. One auxin gene (Smechr062826) showed significant upregulation at 5 dpi in G42. Gibberellin, synthesized via the diterpenoid pathway, plays a crucial role in regulating plant disease resistance by promoting the degradation of DELLAs, which are a group of nuclear proteins that inhibit growth and serve as key suppressors of gibberellin signaling. In response to pathogen attacks, plants develop a diverse array of defensive mechanisms. Here, 31 GA-related genes were identified (Figure 9B), with 11 and 17 upregulated while 10 and 14 downregulated in G42 and EP28, respectively, at 3 and 5 dpi. The ABA signaling pathway, derived downstream of the carotenoid biosynthetic pathway, influences the synthesis of various defense-related secondary metabolites to enhance plant disease resistance during pathogen invasion. A total of 15 genes associated with the ABA signal transduction pathway were identified, including PYL protein-related genes, abscisic acid-insensitive 5-like proteins and abscisic acid 8′-hydroxylase 4-like (Table S5). The selected nine DEGs (Figure 9C) showed two ABA-related genes (Smechr0103889 and Smechr0103890) were specifically upregulated in EP28 at 3 and 5 dpi, signifying their response to P. capsici infections. The ethylene-related genes, synthesized through the cysteine and methionine, were found to be present in the plant–pathogen interaction pathway and the MAPK signaling cascade pathway, thus signifying their critical role in response to pathogen defense. Most ethylene-related genes were upregulated in G42, whereas many were downregulated in EP28 at the 3 and 5 dpi.
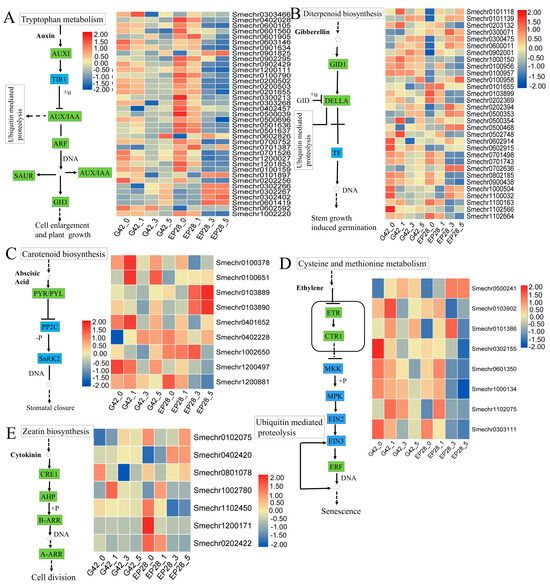
Figure 9.
Differentially expressed genes (DEGs) that participate in phytohormone signaling pathways include those related to (A) Auxin, (B) Gibberellin, (C) Abscisic acid (ABA), (D) Ethylene (ETH) and (E) Cytokinin (CK). A heat map illustrates the expression levels of these DEGs, with red indicating upregulation and blue indicating downregulation of hormone-related genes. The blue boxes represent divergent regulation, while the green boxes represent regulation of interest in this study.
3.7. Transcription Factors Involved in Eggplant Fruit P. capsici Infection
Transcription factors play a crucial role in regulating the plant immune defense system in response to biotic and abiotic stresses, facilitating the activation or deactivation of these protective mechanisms. A comprehensive analysis has identified 703 transcription factors across 37 different families (Figure 10A, Table S6). The top 15 abundant transcription factor families included ERF/ETH (Ethylene; 121), MYB (myeloblastosis-related proteins; 93), NAM/NAC 64, bHLH (Basic helix–loop–helix; 58), HSF (Heat shock TFs; 49, WRKY; 48), NTFY (nuclear transcription factor Y subunit; 34), HD-ZIP (homeobox-leucine zipper protein; 32), GATA 30, bZIP (basic leucine zipper; 29), Mad-box (16), ASIL (Arabidopsis 6b-interacting protein 1-like; 15), Dof (Dof domain, zinc finger family protein; 13) and TCP (Teosinte branched1/Cincinnata/proliferating cell factor; 9) (Figure 10A). After ethylene, MYB genes form the second largest number of genes with 93 DEGs. In EP28, most of the MYB were downregulated at 3 and 5 dpi compared to their expression in G42 (Figure 10B). This result indicated that MYB TFs significantly mediate plant defense responses against P. capsici infection. A similar trend was observed in bHLH (Figure 10D) and HDZIP (Figure 10F).
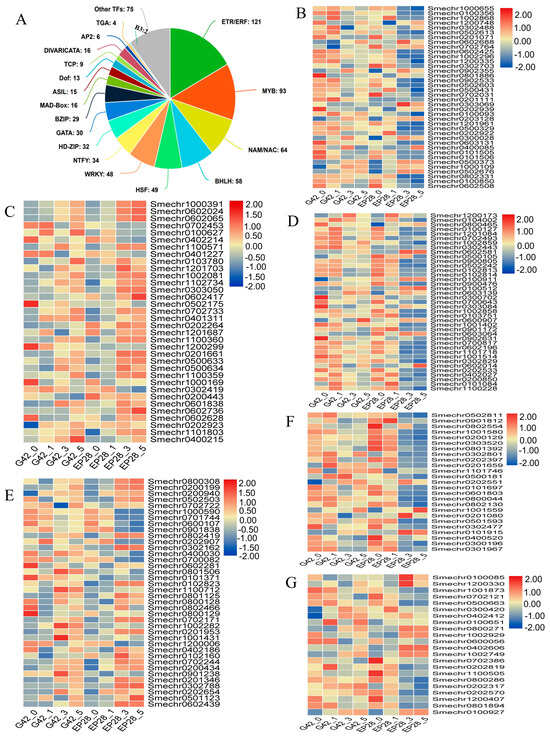
Figure 10.
Comparison of transcription factor-related genes expressed in the G42 and EP28 eggplant genotypes in response to P. capsici infection. (A) Number of DEGs belonging to different transcription factor (TF) families. (B) MYB. (C) NAM/NAC. (D) Basic helix-loop-helix (bHLH). (E) WRKY. (F) HD-ZIP. (G) bZIP. Colors represent the log2 fold change values.
NAM/NAC plays a significant role in plant immunity in response to pathogen attack. Here, a significant number of NAC TFs were induced in EP28 compared to the G42 at 3 and 5 dpi (Figure 10C). Of the selected 34 NAM/NAC TFs, 26 genes were upregulated, while 8 were downregulated in EP28 at 3 and 5 dpi. The result depicts the importance of NAM/NAC transcription factors in response to P. capsici infection in eggplant fruits. WRKY transcription factors are involved in abiotic and biotic stress responses. The WRKY TFs were found to be present in the “plant-pathogen interaction pathway” and “MAPK signaling cascade pathway”. Thirty-eight selected WRKY genes showed varied expression patterns between G42 and EP28 eggplant genotypes (Figure 10E), with 21 and 23 upregulated while 15 and 17 WRKY genes were downregulated in G42 and EP28, respectively. Specifically, 4 WRKY genes (Smechr0801506, Smechr1100712, Smechr1002282 and Smechr0901238) showed distinctive upregulation in G42 and downregulation in EP28 at 3 and 5 dpi. These suggest their potential role in resistance to P. capsici infection.
3.8. qRT-PCR Validation of Differentially Expressed Genes
Twelve genes across the four stages of P. capsici infection were selected from the RNA-Seq data for qRT-PCR analysis to verify the expression patterns. Among these, expression levels of DEGs involved in LRR (Smechr0202100, Smechr0502372, Smechr0201272, Smechr01358, Smechr0801732, Smechro1102377 and Smechr0100358), WRKY7/29 (Smechr0402186 and Smechr0802419), pathogenesis-related genes transcriptional activator (PTI6, Smechr0603020), membrane kinase regulator (MAKR1, Smechr0902144) and ethylene-responsive TF (Smechr0802118) were analyzed. The results obtained from the qRT-PCR analysis aligned with the RNA-Seq data, revealing similar patterns of gene expression, whether upregulated or downregulated (Figure 11). This alignment suggests that both analytical approaches, RNA-Seq and qRT-PCR, can provide consistent and trustworthy insights into the molecular mechanisms underlying resistance to Phytophthora fruit rot.
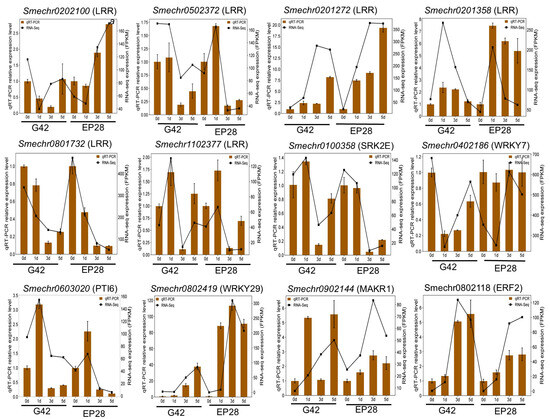
Figure 11.
qRT-PCR validation of DEGs related to disease resistance. The twelve genes included members of LRR, WRKY, pathogenesis-related genes transcriptional activator (PTI6), membrane kinase regulator (MAKR1) and ethylene-responsive TF. The error bars represent ± SE of three replicates (p ≤ 0.05).
3.9. VIGS-Mediated Silencing of SmPTI6 Enhances Eggplant Fruit Susceptibility to P. capsici Infestation
Several upregulated genes following P. capsici inoculation are promising candidates for studying the resistance mechanism against this oomycete in eggplant fruit. Consequently, we selected pathogenesis-related gene transcriptional activator (SmPTI6) for functional analysis using VIGS following its upregulation in the resistant eggplant genotype and downregulation in the susceptible genotype. Further, to justify the reason for choosing SmPTI6 as our gene for VIGS analysis is the basis that Pti6 from tomato, when expressed in Arabidopsis thaliana, induced the expression of a diverse range of pathogenesis-related (PR) genes and served significant and unique functions in the defense mechanisms against Pseudomonas syringae pv. tomato [35]. However, no reports of the role of PTI6 regarding pathogen resistance have been reported in eggplant. Therefore, we conducted VIGs to determine the feasibility of SmPTI6 involvement in P. capsici resistance. Seven days post-silencing, the fruits were inoculated with a mycelium agar plug of actively growing P. capsici colonies, and lesions were evaluated at 0, 3 and 5 dpi. The findings indicated that the SmPTI6-silenced fruits of G42 eggplant genotype exhibited disease symptoms following inoculation with P. capsici in contrast to the TRV2 empty vector plants (Figure 12A). In the fruits of the SmPTI6-silenced plants, the lesions became more pronounced and severe by 5 dpi. Conversely, the TRV2 empty vector plants showed no signs of disease. These observations suggest that the silencing of the SmPTI6 gene in resistant eggplant fruit may compromise its resistance to P. capsici. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis demonstrated a significant downregulation of SmPTI6 in the silenced fruits when compared to the TRV2:00 empty vector, with notable reductions in SmPTI6 expression at both 3 and 5 dpi in the TRV:SmPTI6 samples (Figure 12B).
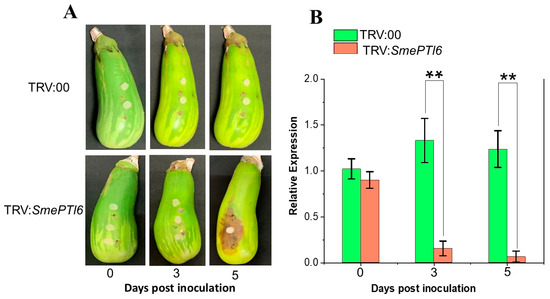
Figure 12.
Effect of virus-induced gene silencing of SmPTI6 on the resistance of G42 eggplant fruits to Phytophthora capsici. (A) Eggplant fruits were inoculated with P. capsici 7 days after agrobacterial infection, and infection progression was observed from 0 to 5 days dpi. TRV:00 is the control, injected with a mixture of agrobacteria containing empty vectors. TRV:SmPTI6 are the VIGS fruits injected with a mix of agrobacteria harboring pTRV1 or pTRV2-SmPTI6. (B) VIGS reduced SmPTI6 expression. The data are presented as the mean ± SD (n = 3). Statistical significance was determined by one-way ANOVA followed by Tukey’s test. Asterisks denote significant differences (** p < 0.01).
4. Discussion
Phytophthora fruit rot (Phytophthora capsici L.) is a disease that poses a significant threat to various members of the solanaceous group, specifically crops like peppers, tomatoes and eggplants [6]. A comprehensive transcriptomic analysis of eggplant fruits inoculated with P. capsici generated a considerable dataset, which allowed for a detailed analysis of the differences between the resistant variety (G42) and the susceptible variety (EP28). This investigation focused on pinpointing the genes that contribute to resistance against P. capsici infections. In both genotypes, a high number of DEGs were exhibited at 3 dpi and then slightly reduced at 5 dpi, with EP28 showing the highest number of DEGs across all time points (Figure 2A). The KEGG analysis revealed the expression of several pathways, including the signal transduction pathways for plant hormones, the phenylpropanoid synthesis and phenylalanine metabolic pathways, the pathways governing plant–pathogen interactions and the mitogen-activated protein kinase (MAPK) pathway. Furthermore, we discovered that disease-resistance genes (R genes) such as Smechr0500287, Smechr0500297 and Smechr0402283, among others, are linked to proteins related to specific courses such as pathogen recognition, immune response activation transcription factors and protein kinases. This suggests that these pathways contain genes contributing to disease resistance during pathogen attacks, enhancing the plant’s ability to resist diseases. Plant disease resistance is a multifaceted systemic response, characterized by varying reactions from different resistance genes [36].
In response to pathogen stress, plants engage a sophisticated immune system characterized by two primary layers: pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). These immune mechanisms can trigger the expression of genes involved in defensive responses, the hypersensitive response (HR) and programmed cell death (PCD) [37,38]. The combined action of PTI and ETI fosters a robust resistance against a variety of pathogens, mediated by calcium ion signaling, the production of reactive oxygen species (ROS), the stimulation of mitogen-activated protein kinases (MAPKs) and the reactivation of specific genes that respond to pathogen attacks. The infection induced by P. capsici initiates the propagation of calcium ion signals downstream via the cyclic nucleotide-gated channels known as CNGCs. Notably, there is a continuous upregulation of certain CNGC members, including Smechr0700086 and Smechr03024478, particularly in the G42 context, which enhances the transmission of calcium signals. In response to this signaling, Rboh-related genes such as Smechr0101010, Smechr0303236 and Smechr0500887 were upregulated. This cascade of events results in alterations in ROS levels and ultimately triggers the hypersensitive response (HR). These findings suggest that the genes exhibiting marked upregulation could participate in mediating responses induced by P. capsici infections in plants (Figure 7).
Calcium-binding proteins are integral to the plant’s response to pathogenic invasions. In stressful environments, numerous Ca2+ sensors or binding proteins can perceive changes in the cytoplasmic Ca2+ concentration, which regulates downstream gene expression to enhance plant resistance [39]. This study revealed that certain calcium-binding proteins, such as Smechr1001819, CML15 (Smechr0103759), CML49 (Smechr1002778), CML19 (Smechr1201626) and CML21 (Smechr0400107), were significantly upregulated in EP28 at 3 and 5 days post-inoculation (dpi) compared to G42. In G42, the calcium-binding proteins CML16 (Smechr1102012), CML48 (Smechr1201717), CML50 (Smechr1102453) and CML41 (Smechr0300575) were upregulated, while CML18 (Smechr0200838) and CML19 (Smechr1201626) were downregulated 3 and 5 dpi. The difference in expression pattern of calcium-binding protein between susceptible and resistant genotypes is likely from the fact that, in the susceptible genotype, some genes may be overexpressed to suppress or modulate immune responses, possibly to prevent excessive cell death or stress, while the overexpressed in resistant genotype is to enhance pathogen recognition, defense gene activation and cell wall reinforcement [40]. Previous studies demonstrated that in Irish potatoes, the calcium-independent protein kinase StCDPK5 directly influences the formation of reactive oxygen species (ROS) by promoting the phosphorylation of respiratory burst oxidase homologs (Rboh) [41]. In tea plants, however, it was observed that the upstream cyclic nucleotide-gated channel (CNGC) experiences a significant downregulation during calcium transfer. At the same time, the majority of downstream Crassulacean acid metabolism/Calmodulin-like proteins (CaM/CMLs) genes exhibit a pronounced upregulation in expression during both the initial and later stages of the response to blister blight disease caused by Exobasidium vexans Massee [42]. In our study, CDPK genes showed significant differential expression between G42 and EP28 at different times, signifying their important role in response to P. capsici infections. For instance, six CDPK genes (Smechr0900732, Smechr1002959, Smechr0800117, Smechr0100010, Smchr10002342 and Smechr1101287) were downregulated in EP28 compared to G42, signifying their role in defense against P. capsici in the resistant genotype.
The synthesis and transmission of ROS signals are vital components of the plant’s defensive response to pathogenic threats. The homologous protein Rboh, responsible for modulating ROS levels, is regulated by calcium ions and the action of fungal effectors. The expression of Rboh-related genes, Smechr0101010, Smechr0303236 and Smechr0500887, were markedly increased in the ROS pathway during the infection caused by P. capsici, particularly at the 3 and 5 dpi stages in EP28 compared to G42. The elevated synthesis and accumulation of ROS through the action of Rboh enable plant tissues to trigger their defense responses when faced with pathogenic threats [43,44]. Alternatively, genes associated with increased ROS are overexpressed in the susceptible genotype, which could suggest that an accumulation of ROS favors the development of the pathogen. This is likely because ROS can act as a signing molecule to pathogen growth by causing oxidative damage to host cells, suppressing immune cell function, or ROS-induced host cell lysis can release nutrients that pathogens scavenge for growth [45]. RPM1-induced protein kinase RIPK is instrumental in regulating RbohD-mediated reactive oxygen species ROS signaling across a range of immune responses, such as pathogen-triggered immunity (PTI), effector-triggered immunity (ETI), disease resistance (DTI, and systemic acquired resistance (SAR) [46]. Classic genetic analyses have frequently indicated that resistance (R) genes are predominantly found at single loci that confer resistance to various pathogens. The predominant category of identified R proteins comprises those featuring a nucleotide binding site (NBS) alongside leucine-rich repeat domains (LRR proteins) [47,48]. Proteins encoded by NBS-LRR play a crucial role in plant resistance by identifying receptors present in the pathogens. Certain R genes associated with specific diseases are capable of detecting the effectors linked to pathogen-associated molecular patterns (PTI) and effector-triggered immunity (ETI) [37,49]. The functional role of NBS-LRR-related genes has been previously elucidated in some vegetable species in response to fungal pathogenic attacks. The expression of RsTNL03 and RsTNL09 enhances the resistance of radish to Fusarium oxysporum, whereas RsTNL06 plays a negative regulatory role [50]. Additionally, the transcriptional silencing of MaNBS89 resulted in more severe leaf damage in banana plants (Musa acuminata) compared to the control group [51]. In our study, the observed upregulated NBS-LRR genes in the resistant genotype signify their potential roles in response to P. capsici infections. Their overexpression suggests an attempt to recognize P. capsici effectors and trigger resistance mechanisms in the resistant genotype compared to the susceptible type. The expression of plant-resistance genes serves as a negative regulator of effector-triggered immunity (ETI) while simultaneously having the capacity to genetically activate pattern-triggered immunity (PTI) through mediator proteins [52]. In this study, PTI-related genes, Smechr0603020 (PTI6), were triggered in reaction to P. capsici infection showing its significance in response to pathogenic attack (Figure 7).
The role of mitogen-activated protein kinases (MAPKs) is significant in numerous (R)-mediated defense responses to pathogens affecting plants [53]. MAPK cascades are integral to various signaling pathways that operate downstream of receptor kinases, particularly in the biosynthesis of phytoalexins within plant systems [54]. The StMKK5-StSIPK module in potato (Solanum tuberosum) significantly contributes to the enhancement of resistance against phytophthora pathogens by activating salicylic acid and ethylene signaling pathways [55]. In rice (Oryza sativa), the regulatory mechanisms involving OsMAPKKK16, OsMAPKKK18 and OsMAPKKK19 and the OsBSK1-2-OsMAPKKK16-18-19-OsMKK4-5 module have been identified as critical for the plant’s response to rice blast [56]. Furthermore, research indicates that HvMPK4 phosphorylates HvWRKY1, amplifying the suppression of barley’s immune response to the powdery mildew pathogen, Blumeria graminis f. sp. hordei. Additionally, the activation of the MAPK signaling pathway in maize in response to Fusarium verticillioides has been documented [57]. It has been observed that OsMPK12 regulates plant defense mechanisms against rice blast [58]. OsMPK6 regulates bacterial blight resistance in rice [59]. Additionally, the MAPK signaling cascades (MsDef1 and MtDef4) have been identified as regulators of host plant resistance to Fusarium graminearum [60]. In soybeans, the GmMKK4–GmMPK6 and GmERF113 cascade has been shown to promote the expression of genes associated with defense, thereby enhancing resistance to Phytophthora sojae [61]. In our study, most of the MAPK DEGs identified were upregulated in G42. Moreover, this study revealed that the MAPK cascade reaction led to the expression of downstream genes, resulting in the activation of PR proteins such as PRM1 (plant-resistant modulators) (Smechr0502610, Smechr0500762 and Smechr0603080), PT0 (Smechr0502664), PT1 (Smechr0502649 and Smechr0902695) and PTI6 (Smechr0603020) that could ultimately contribute to the enhancement of resistance to pathogens.
Hormonal interactions are fundamental to the ability of plants to defend themselves against various pathogens [62]. Several hormones signaling pathways, including ethylene, abscisic, auxins and gibberellins expressed differently in both G42 and EP28 eggplant genotypes after P. capsici infections. The role of auxin in the stress response of plants has been recognized and displays complex plant–pathogen interaction patterns [63,64]. In this study, three auxin-related genes (Smechr0602219, Smechr1201844 and Smechr0100147) were significantly upregulated in G42 compared to EP28. The results imply that signaling pathways linked to auxin mediate significant responses to P. capsici infections. The role of abscisic acid (ABA) in plant pathology is characterized as a negative regulator of disease resistance, where elevated levels of its expression are linked to an increased sensitivity to various diseases [65]. In this study, the expression of ABA-related genes changed after inoculation, showing upregulation in EP28, suggesting the relevance of ABA signaling pathway genes involvement in eggplant susceptibility response to P. capsici pathogen. Maize J1259 was found to activate two ABA signal transduction pathways in response to Fusarium verticillioides infection [57]. ERF, an ethylene response factor, contributes positively to the regulation of resistance gene expression through binding to the promoters of genes related to ethylene resistance [66], thus regulating plant immune responses. Ethylene-responsive transcription factors such as Smechr092335, Smechr0300032 and Smechr0500152 were upregulated in G42, while Smechr010171 and Smechr0300030 were upregulated in EP28, hence implying that ethylene-mediated defense response was involved in the response of eggplant to P. capsici. Ethylene is also associated with plant–pathogen interactions and transcription factors, thus displaying its diverse role in response to pathogen attack.
The regulation of the plant immune defense system in response to biotic and abiotic stresses is significantly influenced by transcription factors, which can either activate or inhibit these protective mechanisms [67]. In total, 703 transcription factors were recognized, categorized into 37 unique families (Figure 10A, Table S6). The top 15 abundant transcription factor families included ERF/ETH (Ethylene;121), MYB (myeloblastosis-related proteins; 93), NAM/NAC 64, bHLH (Basic helix–loop–helix; 58), HSF (Heat shock TFs; 49, WRKY; 48), NTFY (nuclear transcription factor Y subunit; 34), HD-ZIP (homeobox-leucine zipper protein; 32), GATA 30, bZIP (basic leucine zipper; 29), Mad-box 16, ASIL (Arabidopsis 6b-interacting protein 1-like; 15), Dof (Dof domain, zinc finger family protein; 13) and TCP (Teosinte branched1/Cincinnata/proliferating cell factor; 9) (Figure 10A). Additionally, transcription factors were found to be enriched in plant hormone signal transduction, MAPK signaling pathway, and plant–pathogen interaction. The results indicated that transcription factors from different families continue to be significantly expressed in response to P. capsici infections, suggesting a response to the pathogen and a crucial regulatory role in the process of disease resistance. The MYB gene family is characterized as the largest group of transcription factors found in plants. MYB-associated genes are significantly involved in regulating the flavonoid metabolism pathway, hormone signaling mechanisms, and stress response processes [68]. Furthermore, MYB genes play pivotal functions in plant disease resistance [69]. Importantly, one of the most represented TFs in our study was observed in the MYB family with 93 DEGs. MYB TFs recorded differential expressions between the two eggplant genotypes and different stages, with the majority showing upregulation in G42 at 3 and 5 dpi (Figure 10B). The findings suggest that MYB transcription factors mediate plant defense mechanisms in response to P. capsici infection. This aligns with previous research demonstrating their involvement in apple resistance to Alternaria alternata [70]. Additionally, among the various families of transcription factors in plants, bHLH transcription factors are distinguished as the second largest group [70]. In this study, 58 differentially expressed genes associated with bHLH transcription were obtained. It has been established that bHLH genes play a significant role in the defense response of potato plants to common scab, which is induced by Streptomyces scabies [71]. Therefore, the considerable accumulation of bHLH observed in this study may be indicative of the plant’s resistance mechanisms against P. capsici.
WRKY proteins interact with the conserved W-box motif, which is present in the promoters of numerous genes associated with plant defense mechanisms [72]. In addition, the pronounced expression of WRKY-dependent components is of considerable interest in facilitating the generation of proteins that enhance plant disease resistance [67]. The enhancement of resistance to bacterial and fungal infections in wild tobacco (Nicotiana benthamiana) has been attributed to the overexpression of the cotton gene GhWRKY39 [73]. In rice (Oryza sativa), the WRKY45 gene functions as a positive regulator of resistance against the hemibiotrophic fungus Magnaporthe grisea, which causes rice blast [74]. In addition, in pepper (Capsicum annum), the upregulation of CaWRKY01-10 and CaWRKY08-4 genes is instrumental in providing resistance to Phytophthora capsici by directly activating a cluster of genes involved in defense responses [75]. In this study, WRKY TFs were significantly upregulated in G42 and downregulated in EP28. Additionally, two WRKY TFs (WRKY7 and WRKY24) identified in the “plant-pathogen interaction pathway” (Figure 10E) showed contrasting expressions, suggesting that WRKY TFs have an influence on the expression of disease-resistance-related genes in eggplant to P. capsici infections. The difference in the expression pattern of WRKY TFs suggests that each WRKY gene plays a significant role in either suppressing or enhancing disease resistance, depending on the genotype.
We further conducted VIGs to determine the function of SmPTI6 in eggplant response to P. capsici infection. Our data showed that silencing of SmPTI6 significantly enhanced infection of eggplant plants to P. capsici (Figure 12A). The decreased expression of SmPTI6 in silenced resistant fruits compared to the control empty vector samples signifies a successful VIGS process (Figure 12B). These data demonstrate that SmPTI6 positively regulates eggplant response to P. capsici. A previous report showed that overexpression of Pti6 in tomato promotes plant defense [35]. PTI-related proteins (Pti4, Pti5 and Pti6) from tomato were characterized by their interaction with the PTO disease-resistance gene product, a serine-threonine protein kinase. These proteins are part of the ethylene-response factor (ERF) family, which consists of transcription factors exclusive to plants, and they specifically bind to the GCC-box cis-element located in the promoters of various pathogenesis-related (PR) genes [35], hence enhancing disease resistance.
Finally, we have provided a comprehensive summary of the molecular defense responses in G42 and EP28 eggplant genotypes against P. capsici infection, integrating gene expression dynamics, pathway interactions and regulatory networks (Figure 13). The data is structured to highlight not only the differences between the two varieties but also the functional relationships between key components, as indicated by directional arrows. The figure illustrates that the resistant eggplant mounts a robust, multi-layered defense against P. capsici, involving rapid gene expression changes, immune signaling (MAPK), hormone modulation and TF-mediated reprogramming, all dynamically interacting to mount an effective defense. In contrast, the susceptible genotype may lack this coordinated response, or disrupted interactions may lead to disease progression. Further, the upregulation of disease-resistance genes in G42 justifies their resistant capability to P. capsici, while the opposite is manifested in EP28. These insights could guide breeding strategies to enhance P. capsici in eggplants.
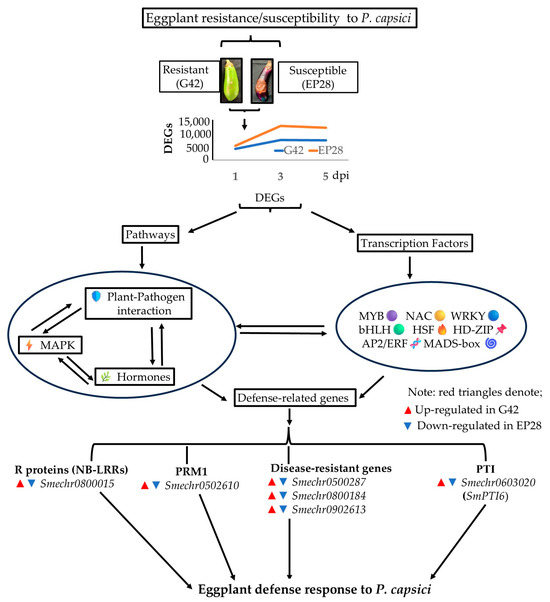
Figure 13.
Summary of molecular defense responses in resistant (G42) and susceptible (EP28) eggplant varieties against P. capsici infection. The directional arrows show functional relationships or interactions between key components.
5. Conclusions
The comparative analysis of transcriptomes and gene expression patterns between G42 and EP28 genotypes infected with P. capsici has provided a thorough theoretical background for elucidating the defense mechanisms engaged in the response of eggplant to P. capsici infection. The infection by P. capsici triggered the activation of various resistance pathways, with distinct genes contributing to the defense response and the associated signal transduction processes. VIGS silencing of SmPTI6 significantly increased susceptibility to P. capsici infections, thus providing a candidate gene for studying the resistance mechanism to P. capsici in eggplant fruits. Analyzing the transcriptome contributes to a more profound understanding of the molecular mechanisms that are fundamental to biological functions regarding the resistance of eggplant to Phytophthora fruit rot. The findings from this research may also facilitate the identification of potential resistance genes that could be utilized for the genetic enhancement of germplasm resources.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11091026/s1, Figure S1: Fruit treatments in the humid chamber; Figure S2: Pearson correlation coefficients between samples. A0–A5 are different time points for G42, while C0–C5 are different time points for EP28; Table S1: Primers used in this study for qRT-PCR analysis; Table S2: Summary of mapped reads; Table S3: Mapping region statistics; Table S4: Top 25 DEGs upregulated in the resistant genotype G42 by Phytophthora capsici inoculation and relative up- or downregulation in EP28; Table S5: Differentially expressed genes involved in different pathways; Table S6: Transcription factors related genes involved in P. capsici infections.
Author Contributions
Conceptualization, Y.Z., X.Z. and H.O.O.; data curation, H.O.O. and X.Z.; formal analysis, H.O.O., X.Z., Y.Y. and J.L.; funding acquisition, Y.Z.; investigation, H.O.O., X.Z., S.L. and J.L.; methodology, H.O.O., X.Z. and S.L.; project administration, J.L. and Y.Z.; resources, X.Z., Y.Y., J.L. and Y.Z.; software, H.O.O., X.Z., S.L. and Y.Y.; supervision, Y.Z.; validation, X.Z. and Y.Y.; visualization, H.O.O. and X.Z.; writing—original draft, H.O.O.; writing—review and editing, X.Z., S.L., Y.Y., J.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the funding support provided by the Key Research and Development Program of Jiangsu Province of China (funding number: BE2023349) and the Independent Innovation Foundation of Agricultural Sciences of Jiangsu Province, China (funding number: CX (24)3015).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We appreciate the technical support, access to and operation of laboratory equipment by the technical staff at the Vegetable Institute of the Jiangsu Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fallahi, F.; Abdossi, V.; Bagheri, M.; Ghanbari Jahromi, M.; Mozafari, H. Genetic diversity analysis of Eggplant Germplasm from Iran: Assessments by morphological and SSR markers. Mol. Biol. Rep. 2022, 49, 11705–11714. [Google Scholar] [CrossRef]
- FAOSTAT. Statistical Database of the Food and Agriculture Organization of the United Nations; Food and Agriculture Organization of the United Nations, Ed.; Online database; FAO: Rome, Italy, 2022; Available online: http://www.fao.org/faostat/en/#data (accessed on 10 September 2024).
- Sharma, M.; Kaushik, P. Biochemical composition of eggplant fruits: A review. Appl. Sci. 2021, 11, 7078. [Google Scholar] [CrossRef]
- Garcia-Estrada, R.S.; Cruz-Lachica, I.; Osuna-García, L.A.; Márquez-Zequera, I. First report of eggplant fruit rot caused by Phytophthora nicotianae in Mexico. Plant Dis. 2021, 105, 513. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Gupta, M.; Bist, C.M.S.; Gupta, B.; Sharma, S. Isolation, identification and multi-locus sequence typing of Phytophthora capsici from capsicum fields and its cross-infectivity in different crop species. Physiol. Mol. Plant Pathol. 2024, 134, 102413. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.M.; Parada-Rojas, C.H.; Hansen, Z.; Vogel, G.; Smart, C.; Hausbeck, M.K.; Lamour, K. Phytophthora capsici: Recent progress on fundamental biology and disease management 100 years after its description. Annu. Rev. Phytopathol. 2023, 61, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Arkhipov, A.; Carvalhais, L.C.; Schenk, P.M. PGPR control Phytophthora capsici in tomato through induced systemic resistance, early hypersensitive response and direct antagonism in a cultivar-specific manner. Eur. J. Plant Pathol. 2023, 167, 811–832. [Google Scholar] [CrossRef]
- Ghaderi, F.; Askari, S.; Abdollahi, M. Response of eggplant genotypes to Phytophthora capsici the causal agent of black stem disease in Kohgiluyeh va Boyer Ahmad, Iran. Seed Plant J. 2012, 28, 215–226. [Google Scholar] [CrossRef]
- Kaniyassery, A.; Thorat, S.A.; Kiran, K.R.; Murali, T.S.; Muthusamy, A. Fungal diseases of eggplant (Solanum melongena L.) and components of the disease triangle: A review. J. Crop Improvement. 2023, 37, 543–594. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.M.; Hausbeck, M.K. Resistance in tomato and wild relatives to crown and root rot caused by Phytophthora capsici. Phytopathology 2010, 100, 619–627. [Google Scholar] [CrossRef]
- Mohammadbagheri, L.; Nasr-Esfahani, M.; Al-Sadi, A.M.; Khankahdani, H.H.; Ghadirzadeh, E. Screening for resistance and genetic population structure associated with Phytophthora capsici-pepper root and crown rot. Physiol. Mol. Plant Pathol. 2022, 122, 101924. [Google Scholar] [CrossRef]
- Yu, X.Q.; Niu, H.Q.; Liu, C.; Wang, H.L.; Yin, W.; Xia, X. PTI-ETI synergistic signal mechanisms in plant immunity. Plant Biotechnol. J. 2024, 22, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Pandey, V.K.; Jha, A.K.; Srivastava, S.; Jakhar, S.; Singh, G.; Choudhary, P. Intricacies of plants’ innate immune responses and their dynamic relationship with Fungi: A Review. Microbiol. Res. 2024, 285, 127758. [Google Scholar] [CrossRef]
- Han, Z.; Schneiter, R. Dual functionality of pathogenesis-related proteins: Defensive role in plants versus immunosuppressive role in pathogens. Front. Plant Sci. 2024, 15, 1368467. [Google Scholar] [CrossRef]
- Qingshuo, G.; Shasha, L.; Zuhua, H.; Xiangzong, M.; Yiwen, D. NLRs in plant immunity: Structural insights and molecular mechanisms. Crop Design 2025, 4, 100103. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, J.; Latif, M.Z.; Yue, Y.; Li, Y.; Lu, C.; Li, Y.; Yin, Z.; Ding, X. Antimicrobial peptides: An important link in the game theory between plants and pathogens. J. Adv. Res. 2025, S2090–S1232, 00492–00498. [Google Scholar] [CrossRef]
- Saltos, L.A.; Monteros-Altamirano, Á.; Reis, A.; Garcés-Fiallos, F.R. Phytophthora capsici: The diseases it causes and management strategies to produce healthier vegetable crops. Hortic. Bras. 2022, 40, 5–17. [Google Scholar] [CrossRef]
- Gaikwad, P.N.; Sharma, V.; Singh, J.; Sidhu, G.S.; Singh, H.; Omar, A.A. Biotechnological advancements in Phytophthora disease diagnosis, interaction and management in citrus. Sci. Hortic. 2023, 310, 111739. [Google Scholar] [CrossRef]
- Shands, A.C.; Xu, G.; Belisle, R.J.; Seifbarghi, S.; Jackson, N.; Bombarely, A.; Manosalva, P.M. Genomic and transcriptomic analyses of Phytophthora cinnamomi reveal complex genome architecture, expansion of pathogenicity factors, and host-dependent gene expression profiles. Front. Microbiol. 2024, 15, 1341803. [Google Scholar] [CrossRef]
- Naveed, Z.A.; Ali, G.S. Comparative transcriptome analysis between a resistant and a susceptible wild tomato accession in response to Phytophthora parasitica. Int. J. Mol. Sci. 2018, 19, 3735. [Google Scholar] [CrossRef]
- Mansfeld, B.N.; Colle, M.; Zhang, C.; Lin, Y.C.; Grumet, R. Developmentally regulated activation of defense allows for rapid inhibition of infection in age-related resistance to Phytophthora capsici in cucumber fruit. BMC Genom. 2020, 21, 628. [Google Scholar] [CrossRef]
- Shen, D.; Chai, C.; Ma, L.; Zhang, M.; Dou, D. Comparative RNA-Seq analysis of Nicotiana benthamiana in response to Phytophthora parasitica infection. Plant Growth Regul. 2016, 80, 59–67. [Google Scholar] [CrossRef]
- Hao, C.; Xia, Z.; Fan, R.; Tan, L.; Hu, L.; Wu, B.; Wu, H. De novo transcriptome sequencing of black pepper (Piper nigrum L.) and an analysis of genes involved in phenylpropanoid metabolism in response to Phytophthora capsici. BMC Genom. 2016, 17, 822. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Tao, X.Y.; Xia, Z.Q.; Sim, S.; Hu, L.S.; Wu, B.D.; Hao, C.Y. Comparative transcriptome and metabolome analysis of resistant and susceptible piper species upon infection by the oomycete Phytophthora capsici. Front. Plant Sci. 2022, 13, 864927. [Google Scholar] [CrossRef] [PubMed]
- Naegele, R.; Hill, T.A.; Ashrafi, H.; Reyes Chin-Wo, S.; Van Deynze, A.; Hausbeck, M.K. QTL mapping of fruit rot resistance to the plant pathogen Phytophthora capsici Leonian in a recombinant inbred line Capsicum annuum L. population. Phytopathology 2014, 104, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Naegele, R.P.; Boyle, S.; Quesada-Ocampo, L.M.; Hausbeck, M.K. Genetic diversity, population structure, and resistance to Phytophthora capsici of a worldwide collection of eggplant germplasm. PLoS ONE 2014, 9, e95930. [Google Scholar] [CrossRef]
- Naegele, R.P.; Hausbeck, M.K. Phytophthora root rot resistance and its correlation with fruit rot resistance in Capsicum annuum. HortScience 2020, 55, 1931–1937. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Florea, L.; Salzberg, S.L. Thousands of exons skipping events differentiate among splicing patterns in sixteen human tissues. F1000Research 2013, 2, 188. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gao, X.Q.; Shan, L.B. Functional genomic analysis of cotton genes with Agrobacterium-mediated virus-induced gene silencing. Methods Mol. Biol. 2013, 975, 157–165. [Google Scholar] [CrossRef]
- Zhou, H.; Bai, S.; Wang, N.; Sun, X.; Zhang, Y.; Zhu, J.; Dong, C. CRISPR/Cas9-Mediated Mutagenesis of MdCNGC2 in Apple Callus and VIGS-Mediated silencing of MdCNGC2 in fruits improve resistance to Botryosphaeria dothidea. Front. Plant Sci. 2020, 11, 575477. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, G.; Zhang, Z.; Liu, Y.; Ma, Y.; Wang, Y.; Niu, X. Overexpression of Pti4, Pti5, and Pti6 in tomato promote plant defense and fruit ripening. Plant Sci. 2021, 302, 110702. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Balint-Kurti, P.; Xu, M.; Yang, Q. Quantitative disease resistance: Multifaceted players in plant defense. J. Integr. Plant Biol. 2023, 65, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Qari, S.H.; Al Surhanee, A.A.; Yasin, G.; Alamri, S.; Al-Saadi, A.M. Plant hypersensitive response vs pathogen ingression: Death of few gives’ life to others. Microb. Pathog. 2020, 145, 104224. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.P.; Sheen, J.; Séguin, A. Ancient signals: Comparative genomics of green plant CDPKs. Trends Plant Sci. 2014, 19, 79–89. [Google Scholar] [CrossRef]
- Sampaio, J.R.; Oliveira, W.D.d.S.; Nascimento, F.d.S.; Junior, L.C.d.S.; Rebouças, T.A.; Moreira, R.F.C.; Ramos, A.P.D.S.; Santos-Serejo, J.A.d.; Amorim, E.P.; Ferreira, C.F. Calcium-Binding Protein and Polymorphism in Musa spp. Somaclones Resistant to Fusarium oxysporum. Curr. Issues Mol. Biol. 2024, 46, 12119–12132. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Yoshioka, H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef]
- Zhou, X.L.; Hoang, N.H.; Tao, F.; Fu, T.T.; Guo, S.J.; Guo, C.M.; Buensanteai, K. Transcriptomics and phytohormone metabolomics provide comprehensive insights into the response mechanism of tea against blister blight disease. Sci. Hortic. 2024, 324, 112611. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Biochem. 2024, 207, 108398. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Liang, Y. Role of ROS signaling in the plant defense against vascular pathogens. Curr. Opin. Plant Biol. 2024, 81, 102617. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, L.; Qi, F.; Htwe, N.M.P.S.; Li, Q.; Zhang, D.; Liang, Y. The receptor-like cytoplasmic kinase RIPK regulates broad-spectrum ROS signaling in multiple layers of plant immune system. Mol. Plant 2021, 14, 1652–1667. [Google Scholar] [CrossRef]
- Lin, L.; Chen, Q.; Yuan, K.; Xing, C.; Qiao, Q.; Huang, X.; Zhang, S. E3 ubiquitin ligase PbrATL18 is a positive factor in pear resistance to drought and Colletotrichum fructicola infection. Hortic. Plant J. 2024, 10, 698–712. [Google Scholar] [CrossRef]
- Wei, W.; Wu, X.; Garcia, A.; McCoppin, N.; Viana, J.P.G.; Murad, P.S.; Clough, S.J. An NBS-LRR protein in the Rpp1 locus negates the dominance of Rpp1-mediated resistance against Phakopsora pachyrhizi in soybean. Plant J. 2023, 113, 915–933. [Google Scholar] [CrossRef]
- Liu, R.; Tan, X.; Wang, Y.; Lin, F.; Li, P.; Rahman, F.U.; Zhang, Y. The cysteine-rich receptor-like kinase CRK10 targeted by Coniella diplodiella effector CdE1 contributes to white rot resistance in grapevine. J. Exp. Bot. 2024, 75, 3026–3039. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chhapekar, S.S.; Lu, L.; Oh, S.; Singh, S.; Kim, C.S.; Choi, S.R. Genome-wide identification and characterization of NBS-encoding genes in Raphanus sativus L. and their roles related to Fusarium oxysporum resistance. BMC Plant Biol. 2021, 21, 47. [Google Scholar] [CrossRef]
- Liu, S.W.; Huang, H.Q.; Li, Z.Y.; Ahmad, M.; Zhuo, M.X.; Li, C.Y.; Li, Y.D. NBS-LRR genes of Musa acuminata is involved in disease resistance to Fusarium wilt. Sci. Hortic. 2024, 336, 113361. [Google Scholar] [CrossRef]
- Ma, M.; Li, M.; Zhou, R.; Yu, J.B.; Wu, Y.; Zhang, X.; Liang, X. CPR5 positively regulates pattern-triggered immunity via a mediator protein. J. Integr. Plant Biol. 2023, 65, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, S.; Zhong, G.; Gao, C.; Zhang, Q.; Tang, D. Mitogen-activated protein kinase3 enhances disease resistance of edr1 mutants by phosphorylating MAPKKK5. Plant Physiol. 2024, 194, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45 Pt A, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, X.; Yang, R.; Cheng, J.; Chen, Y.; Joosten, M.H.A.J.; Du, Y. The potato StMKK5-StSIPK module enhances resistance to Phytophthora pathogens through activating the salicylic acid and ethylene signalling pathways. Mol. Plant Pathol. 2023, 24, 399–412. [Google Scholar] [CrossRef]
- Li, S.; Xiang, X.; Diao, Z.; Xia, N.; Lu, L.; Zhang, J.; Tang, D. The OsBSK1-2-MAPK module regulates blast resistance in rice. Crop J. 2024, 12, 110–120. [Google Scholar] [CrossRef]
- Zeng, H.; Cai, H.; Bai, T.; Ren, X.; Ci, J.; Yang, W. Transcriptome analysis of maize resistance to Fusarium verticillioides. J. Plant Interact. 2024, 19, 2393803. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Zheng, X.; Xia, N.; Diao, Z.; Wang, X.; Li, S. OsMPK12 positively regulates rice blast resistance via OsEDC4-mediated transcriptional regulation of immune-related genes. Plant Cell Environ. 2024, 47, 3712–3731. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Liu, Q.; Liu, Y.; Wang, Y.; Qin, R.; Zhou, H.; Xu, X. Reduction of OsMPK6 Activity by a R89K Mutation Induces Cell Death and Bacterial Blight Resistance in Rice. Plant Cell Rep. 2021, 40, 835–850. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Zhao, X.; Snyder, A.K.; Xu, J.R.; Shah, D.M. Two mitogen-activated protein kinase signaling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell. Microbiol. 2007, 9, 1491–1506. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, L.; Du, B.; Ning, B.; Ding, X.; Zhang, C.; Zhang, S. GmMKK4-activated GmMPK6 stimulates GmERF113 to trigger resistance to Phytophthora sojae in soybean. Plant J. 2022, 111, 473–495. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Radouane, N.; Goura, K.; Lahmamsi, H.; Kenfaoui, J.; Farhaoui, A.; Belabess, Z.; Lahlali, R. Phytohormone signaling and plant–Pathogen interaction. In Plant Pathogen Interaction; Springer Nature: Singapore, 2024; pp. 185–220. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Hada, A.; Biswas, S.; Mishra, S.; Prusty, M.R.; Das, S.P.; Sarker, U. Jasmonic acid (JA) in plant immune response: Unravelling complex molecular mechanisms and networking of Defense signaling against pathogens. J. Plant Growth Regul. 2024, 44, 89–114. [Google Scholar] [CrossRef]
- Jiang, Y.; Yue, Y.; Wang, Z.; Lu, C.; Wang, Z.; Yin, Z.; Ding, X. A novel ABA structural analogue enhanced plant resistance by inducing plant immunity and inactivating ABA signaling pathway. Adv. Agrochem 2024, 3, 64–73. [Google Scholar] [CrossRef]
- Tang, Q.; Wei, S.; Zheng, X.; Tu, P.; Tao, F. APETALA2/ethylene-responsive factors in higher plant and their roles in the regulation of plant stress response. Crit. Rev. Biotechnol. 2024, 44, 1533–1551. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Pratyusha, D.S.; Sarada, D.V.L. MYB transcription factors—Master regulators of phenylpropanoid biosynthesis and diverse developmental and stress responses. Plant Cell Rep. 2022, 41, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Yu, Y.; Cui, N.; Yu, G.; Zhao, H.; Fan, H. The pivotal role of MYB transcription factors in plant disease resistance. Planta 2023, 258, 16. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, C.; Zhang, L.; Lan, L.; Fu, W.; Wang, S. Molecular mechanism of resistance to Alternaria alternata apple pathotype in apple by alternative splicing of transcription factor MdMYB6-like. Int. J. Mol. Sci. 2024, 25, 4353. [Google Scholar] [CrossRef]
- Tao, H.; Wang, S.; Li, X.; Li, X.; Cai, J.; Zhao, L.; Cai, Y. Biological control of potato common scab and growth promotion of potato by Bacillus velezensis Y6. Front. Microbiol. 2023, 14, 1295107. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Nayak, J.; Srivastava, R.; Samal, S.; Kumar, D.; Chanwala, J.; Giri, M.K. Unraveling the involvement of WRKY TFs in regulating plant disease defense signaling. Planta 2024, 259, 7. [Google Scholar] [CrossRef]
- Shi, W.; Liu, D.; Hao, L.; Wu, C.A.; Guo, X.; Li, H. GhWRKY39, a member of the WRKY transcription factor family in cotton, has a positive role in disease resistance and salt stress tolerance. Plant Cell Tissue Organ Cult. 2014, 118, 17–32. [Google Scholar] [CrossRef]
- Nakayama, A.; Fukushima, S.; Goto, S.; Matsushita, A.; Shimono, M.; Sugano, S.; Jiang, C.J.; Akagi, A.; Yamazaki, M.; Inoue, H.; et al. Genome-Wide Identification of WRKY45-Regulated Genes That Mediate Benzothiadiazole-Induced Defense Responses in Rice. BMC Plant Biol. 2013, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wang, N.; Li, Y.; Zhou, X.; Bai, X.; Liu, L.; Chu, M. CaWRKY01-10 and CaWRKY08-4 confer pepper’s resistance to Phytophthora capsici Infection by Directly Activating a Cluster of Defense-Related Genes. J. Agric. Food Chem. 2024, 72, 11682–11693. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).