Exploring Polyploidization in Nigella sativa L.: An Applicable Strategy Towards Crop Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cytological and Flow Cytometry Analysis
2.3. Establishment of Seedlings
2.4. Soil Analysis and Cultivation of Black Cumin Seedlings
2.5. Assessment of Plant Morphology, Flowering, and Yield Traits Under Field Conditions
2.6. Analysis of Thymoquinone Content
2.7. Statistical Analyses
3. Results
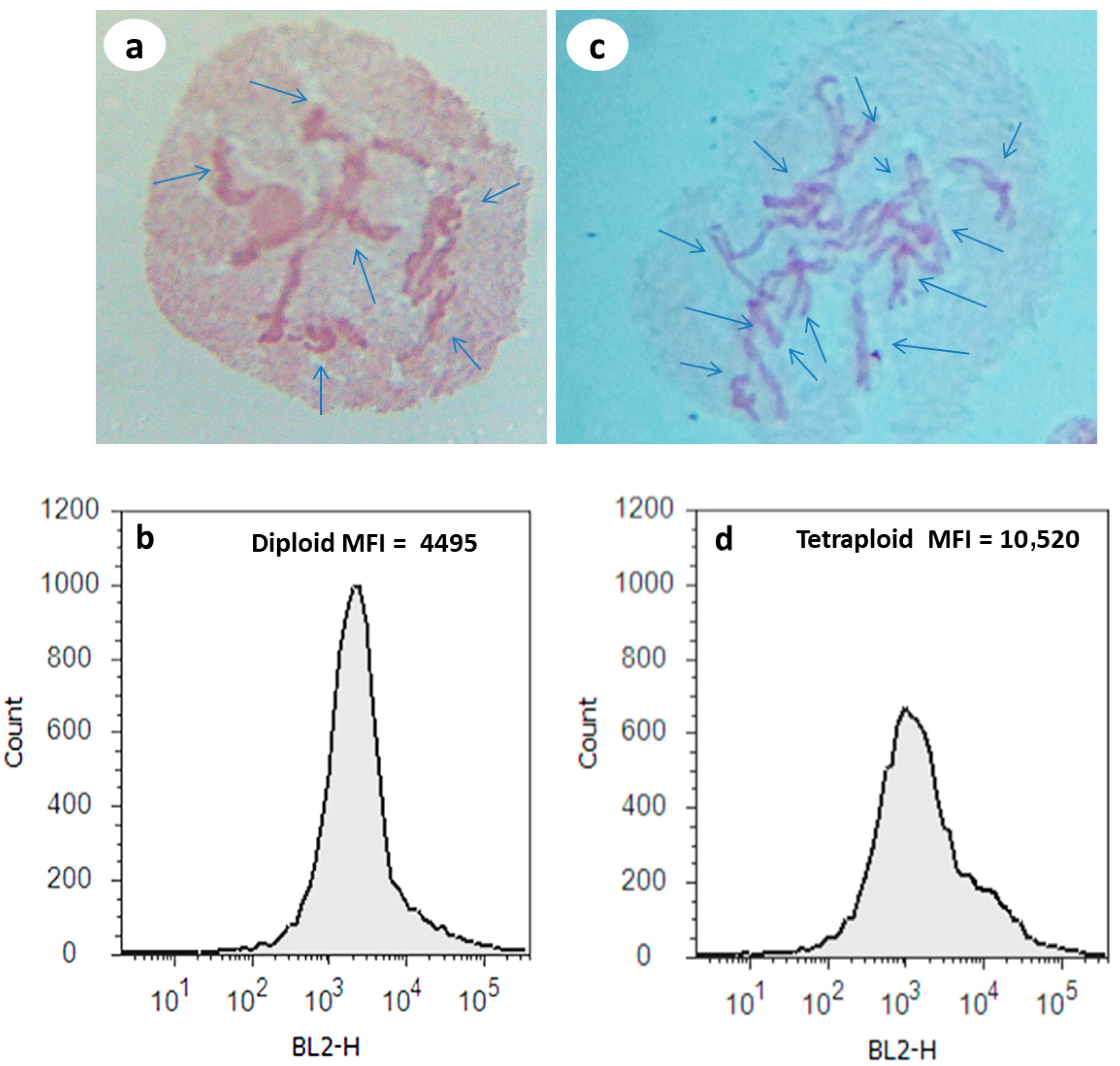
3.1. Cytological and Flow Cytometry Analysis of Diploid vs. Tetraploid Plants
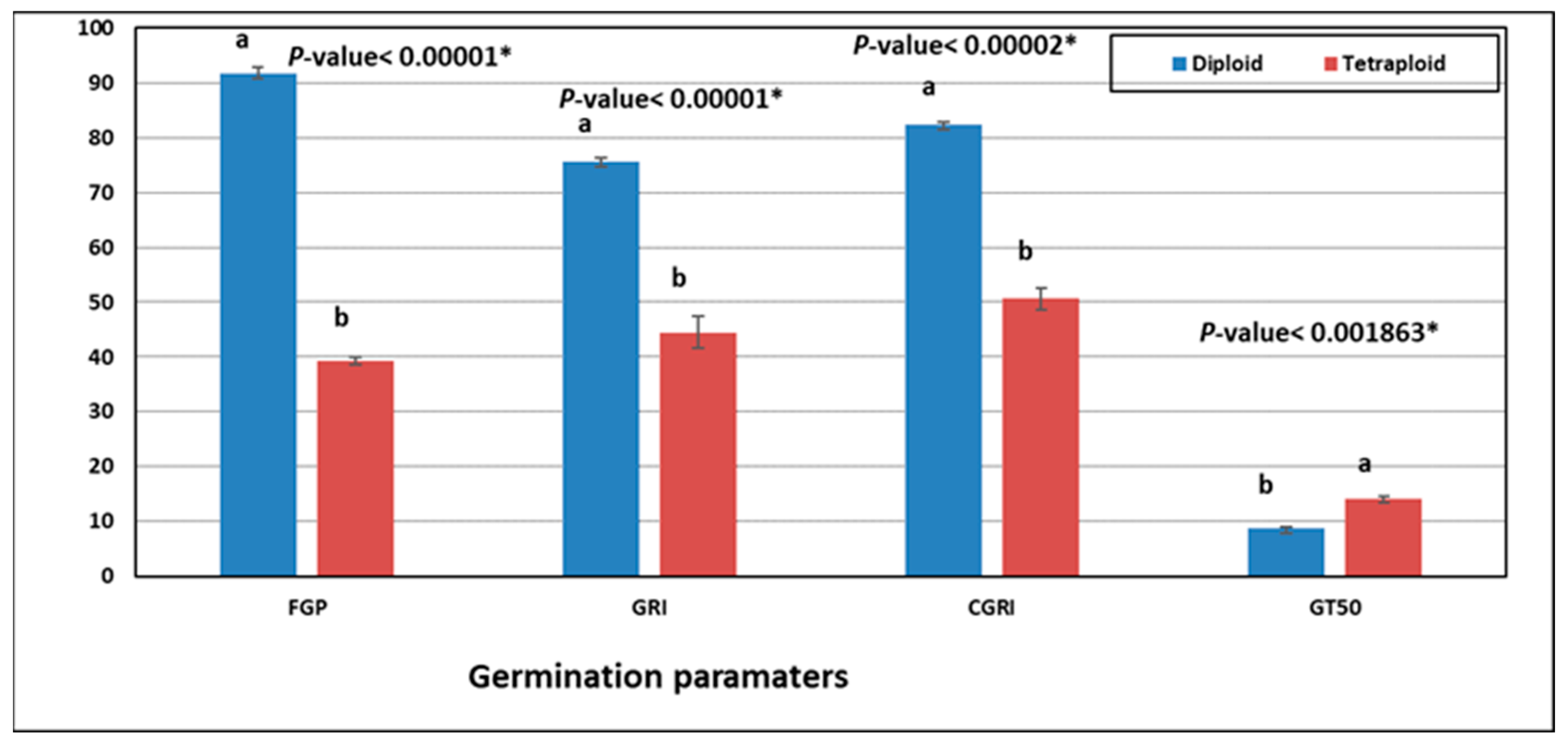
3.2. Germination of Diploid and Tetraploid Black Cumin
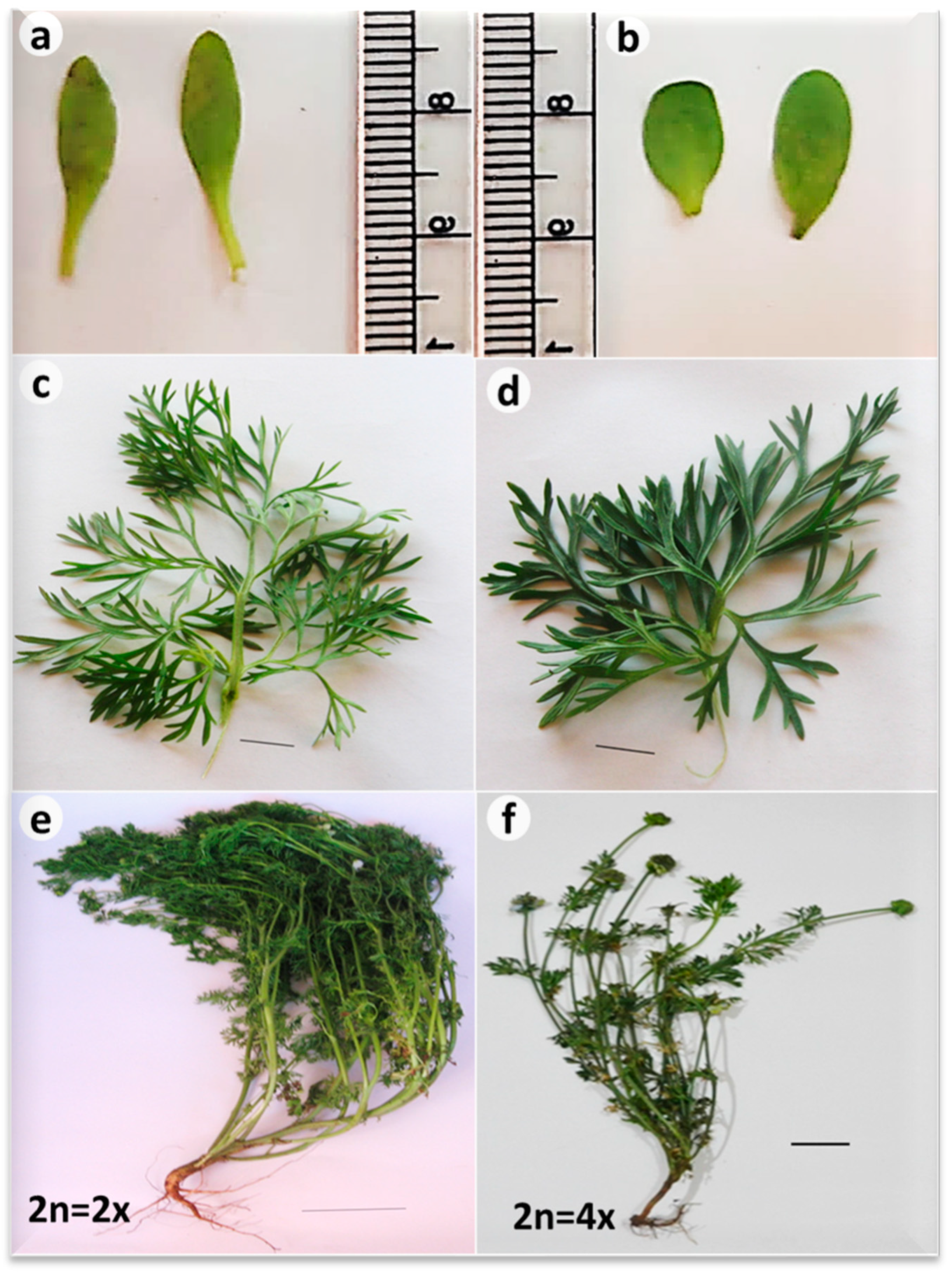
3.3. Morphology of Black Cumin Seedlings in Diploid Versus Tetraploid Plants
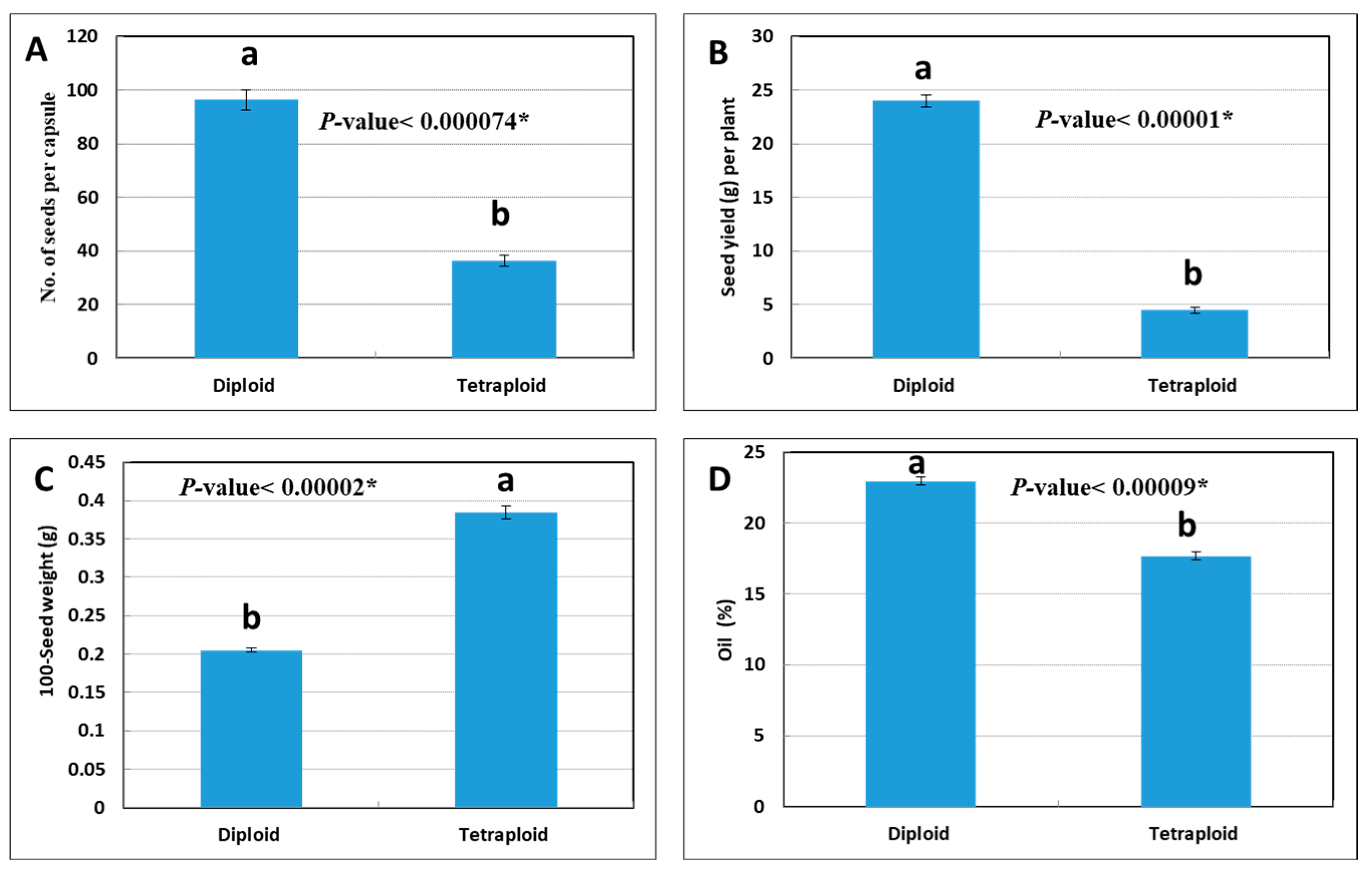
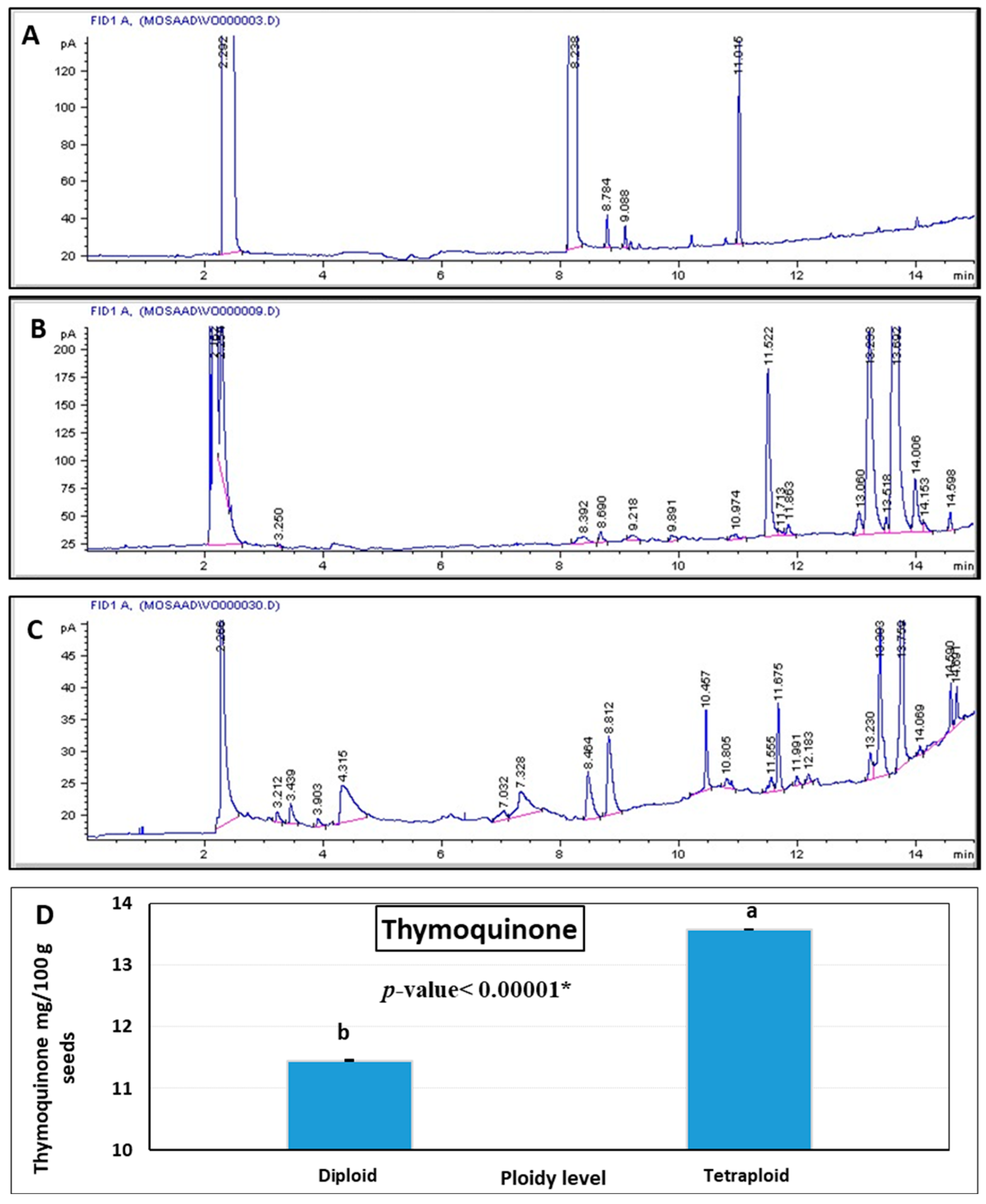
3.4. Yield Traits and Thymoquinone Content in Diploid vs. Tetraploid Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.; Siddique, N.A.; Damanhouri, Z.A.; Anwa, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Adam, S.H.; Abu, I.F.; Kamal, D.A.M.; Febriza, A.; Kashim, M.I.A.M.; Mokhtar, M.H. A Review of the Potential Health Benefits of Nigella sativa on Obesity and Its Associated Complications. Plants 2023, 12, 3210. [Google Scholar] [CrossRef]
- Hussain, S.; Rukhsar, A.; Iqbal, M.; Ain, Q.; Fiaz, J.; Akhtar, N.; Afzal, M.; Ahmad, N.; Ahmad, I.; Mnif, W.; et al. Phytochemical profile, nutritional and medicinal value of Nigella sativa. Biocatal. Agric. Biotechnol. 2024, 60, 103324. [Google Scholar] [CrossRef]
- Verma, S.; Hariwal, M.; Kumar, S. Exploratory analysis of agro-morphological characteristics in Nigella sativa L. plant genotypes to determine mutagen colchicine ameliorative/non-ameliorative impacts. Sci. Rep. 2024, 14, 24521. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Oubannin, S.; Jadouali, S.M.; Atifi, H.; Bijla, L.; Ibourki, M.; Gagour, J.; Bouzid, H.A.; Aabd, N.A.; Bouyahya, A.; Harhar, H.; et al. Antioxidant activity, physico-chemical properties, and bioactive compounds of Nigella sativa seeds and oil impacted by microwave processing technique. Heliyon 2024, 10, e37603. [Google Scholar] [CrossRef]
- Higazy, A.E.; El-Mahrouk, M.E.; El-Banna, A.N.; Maamoun, M.K.; El-Ramady, H.; Abdalla, N.; Dobránszki, J. Production of Black Cumin via Somatic Embryogenesis, Chemical Profile of Active Compounds in Callus Cultures and Somatic Embryos at Different Auxin Supplementations. Agronomy 2023, 13, 2633. [Google Scholar] [CrossRef]
- Dubey, P.N.; Singh, B.; Mishra, B.K.; Kant, K.; Solanki, R. Nigella (Nigella sativa): A high value seed spice with immense medicinal potential. Indian J. Agric. Res. 2016, 86, 967–979. [Google Scholar] [CrossRef]
- Lal, G.; Meena, S.S.; Lal, S. Nigella (Nigella sativa L.), a novel herb can cure many diseases: A review. Int. J. Seed Spices 2020, 10, 1–10. [Google Scholar]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef]
- Li, H.; Da Silva, N.A.; Liu, W.; Xu, J.; Dombi, G.W.; Dain, J.A.; Li, D.; Chamcheu, J.C.; Seeram, N.P.; Ma, H. Thymocid®, a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells. Nutrients 2020, 12, 2146. [Google Scholar] [CrossRef]
- Datta, A.K.; Saha, A.; Bhattacharya, A.; Mandal, A.; Paul, R.; Sengupta, S. Black Cumin (Nigella sativa L.)—A Review. J. Plant Dev. Sci. 2012, 4, 1–43. [Google Scholar]
- Heslop-Harrison, J.S.; Schwarzacher, T.; Liu, Q. Polyploidy: Its consequences and enabling role in plant diversification and evolution. Ann. Bot. 2023, 131, 1–10. [Google Scholar] [CrossRef]
- Morris, J.P.; Baslan, T.; Soltis, D.E.; Soltis, P.S.; Fox, D.T. Integrating the Study of Polyploidy Across Organisms, Tissues, and Disease. Annu. Rev. Genet. 2024, 58, 297–318. [Google Scholar] [CrossRef]
- Blakeslee, A.F.; Avery, A.G. Methods of inducing doubling of chromosomes in plants: By treatment with colchicine. J. Hered. 1937, 28, 393–411. [Google Scholar] [CrossRef]
- Biswas, A.K.; Chatterjee, A.K. Studies on the induction of ploidy in some species. Bull. Bot. Soc. Bengal 1971, 25, 19–21. [Google Scholar]
- Biswas, A.K.; Datta, A.K. Studies on induced autotetraploids in Nigella sativa L. Cell Chromosome Res. 1982, 5, 81–83. [Google Scholar]
- Saha, A.; Datta, A.K. Induced autotetraploidy in black cumin (Nigella sativa L). Indian J. Genet. Plant Breed. 2002, 62, 275–276. [Google Scholar]
- El-Mahrouk, M.E.; Maamoun, M.K.; Dewir, Y.H.; Omran, S.A.; EL-Banna, A.N. Morphological and molecular characterization of induced mutants in Nigella sativa L. using irradiation and chemical mutagens. Egypt. J. Plant Breed. 2015, 19, 257–272. [Google Scholar]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- El-Mahrouk, M.E.; Maamoun, M.K.; Abu El-Leel, O.F.; Dewir, Y.H.; El-Banna, A.N.; Naidoo, Y.; Datta, S.K. Morpho-agronomical and Biochemical Traits Screening and Genetic Variability in Selected Black Cumin (Nigella sativa) Mutant Lines. Sains Malays. 2020, 49, 503–515. [Google Scholar] [CrossRef]
- Wu, J.; Ferguson, R.A.; Murray, B.G.; Jia, Y.; Datson, P.M.; Zhang, J. Induced polyploidy dramatically increases the size and alters the shape of fruit in Actinidia chinensis. Ann. Bot. 2012, 109, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Zahumenická, P.; Fernández, E.; Šedivá, J.; Žiarovská, J.; Ros-Santaella, J.L.; Martínez-Fernández, D.; Russo, D.; Milella, L. Morphological, physiological and genomic comparisons between diploids and induced tetraploids in Anemone sylvestris L. Plant Cell Tissue Organ Cult. 2018, 132, 317–327. [Google Scholar] [CrossRef]
- Dhawan, O.P.; Lavania, U.C. Enhancing the productivity of secondary metabolites via induced polyploidy: A review. Euphytica 1996, 87, 81–89. [Google Scholar] [CrossRef]
- De Jesus-Gonzalez, L.; Weathers, P.J. Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep. 2003, 21, 809–813. [Google Scholar] [CrossRef]
- Pei, Y.; Yao, N.; He, L.; Deng, D.; Li, W.; Zhang, W. Comparative study of the morphological, physiological and molecular characteristics between diploid and tetraploid radish (Raphunas sativus L.). Sci. Hortic. 2019, 257, 108739. [Google Scholar] [CrossRef]
- Hancock, J.F. The colchicine story. Hortic. Sci. 1997, 32, 1011–1012. [Google Scholar] [CrossRef]
- Hias, N.; De Dauw, K.; Davey, M.W.; Leus, L.; Van Labeke, M.C.; Van Huylenbroeck, J.; Keulemans, J. Influence of ploidy level on the physiological response of apple to water deficit. Acta Hortic. 2017, 1177, 333–338. [Google Scholar] [CrossRef]
- Masterson, J. Stomatal size in fossil plants: Evidence for polyploidy in majority of angiosperms. Science 1994, 264, 421–423. [Google Scholar] [CrossRef]
- Guerra, D.; Wittmann, M.T.S.; Schwarz, S.F.; de Souza, P.V.D.; Mateus Pereira Gonzatto, M.P.; Weiler, R.L. Comparison between diploid and tetraploid citrus rootstocks: Morphological characterization and growth evaluation. Bragantia 2014, 73, 1–7. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and it skeyrolein plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef]
- Rao, S.; Kang, X.; Li, J.; Chen, J. Induction, identification and characterization of tetraploidy in Lycium ruthenicum. Breed. Sci. 2019, 69, 160–168. [Google Scholar] [CrossRef]
- El-Mahrouk, M.E.; Maamoun, M.K.; EL-Banna, A.N.; Omran, S.A.; Dewir, Y.H.; El-Hendawy, S. In Vitro Gynogenesis and Flow Cytometry Analysis of the Regenerated Haploids of Black Cumin (Nigella sativa). Hortic. Sci. 2018, 53, 681–686. [Google Scholar] [CrossRef]
- Abdoli, M.; Moieni, A.; Badi, H.N. Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol. Plant. 2013, 35, 2075–2083. [Google Scholar] [CrossRef]
- Tomaszewska, P.; Pellny, T.K.; Hernández, L.M.; Mitchell, R.A.C.; Castiblanco, V.; de Vega, J.J.; Schwarzacher, T.; Heslop-Harrison, P. Flow Cytometry-Based Determination of Ploidy from Dried Leaf Specimens in Genomically Complex Collections of the Tropical Forage Grass Urochloa s. l. Genes 2021, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Anamthawat, J.K. Preparation of chromosomes from plant leaf meristems karyotype analysis and in situ hybridization. Methods Cell Sci. 2003, 25, 91–95. [Google Scholar] [CrossRef]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Esechie, H. Interaction of salinity and temperature on the germination of sorghum. J. Agron. Crop Sci. 1994, 172, 194–199. [Google Scholar] [CrossRef]
- Hsu, F.H.; Nelson, C.J.; Matches, A.G. Temperature effects on germination of perennial warm-season forage grasses. Crop Sci. 1985, 25, 212–220. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India, Private Limited: New Delhi, India, 1973. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methodsofsoil Analysis. Part 3. Chemical Methods; Black, C.A., Ed.; Soil Science of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis. Agron. No. 9 Part II; Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Agron. No. 9, Part 2; Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Black, C.A. Methods of Soil Analysis Part I No. 9; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Rajeswara, R.B.R.; Singh, K.; Kaul, P.N.; Bhattacharya, A.K. The effect of plant spacing and application of N and P fertilizers on the productivity and nutrient uptake of davana (Artemisia pallens Wall.). Int. J. Trop. Agric. 1989, 7, 229–236. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Gatarira, C.; Sládeková, L.; Němečková, A.; Šimoníková, D.; Paliwal, R.; Asfaw, A.; Abberton, M.; Badara, G.; Asiedu, R.; Čížková, J.; et al. Cytological and Molecular Characterization for Ploidy Determination in Yams (Dioscorea spp.). Agronomy 2021, 11, 1897. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santo, C. Comparison of four nuclear isolation buffers for plant DNA flow cytometry. Ann. Bot. 2006, 98, 679–689. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Couto, E.G.; Davide, L.M.; Bustamante, F.; VonPinho, R.G.; Silva, T.N. Identification of haploid maize by flow cytometry morphological and molecular markers. Cienc. Agrotecnol. 2013, 37, 25–31. [Google Scholar] [CrossRef]
- Kie1kowska, A.; Adamus, A. In vitro culture of unfertilized ovules in carrot (Daucus carota L.). Plant Cell Tissue Organ Cult. 2010, 102, 309–319. [Google Scholar] [CrossRef]
- Chan, J.C.S.; Ooi, M.K.J.; Guja, L.K. Polyploidy but Not Range Size Is Associated with Seed and Seedling Traits That Affect Performance of Pomaderris Species. Front. Plant Sci. 2022, 12, 779651. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.V.; Nicotra, A.B.; Godfree, R.C.; Guja, L.K. Polyploidy affects the seed, dormancy and seedling characteristics of a perennial grass, conferring an advantage in stressful climates. Plant Biol. 2020, 22, 500–513. [Google Scholar] [CrossRef]
- Hacker, J.B. Polyploid distribution and seed dormancy in relation to provenance rainfall in the Digitaria milanjiana complex. Aust. J. Bot. 1988, 36, 693–700. [Google Scholar] [CrossRef]
- Dixit, V.; Verma, S.; Chaudhary, B.R. Changes in ploidy and its effect on thymoquinone concentrations in Nigella sativa L. seeds. J. Hortic. Sci. Biotechnol. 2015, 90, 537–542. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Nadjafi, F.; Asareh, M.H.; Rezadoost, H. Morphological and yield related traits, essential oil and oil production of different landraces of black cumin (Nigella sativa) in Iran. Sci. Hortic. 2018, 233, 1–8. [Google Scholar] [CrossRef]
- Telci, İ.; Özek, T.; Gül, F.; Yur, S.; Özek, G.; Demirtaş, İ.; Günay, E.; Aslancan, H.; Kacar, O. Diversity of black cumin genotypes and their classification based on functional properties. Biochem. Syst. Ecol. 2024, 113, 104802. [Google Scholar] [CrossRef]
- Niu, L.; Tao, Y.; Chen, M.; Fu, Q.; Dong, Y.; He, H.; Xu, Z. Identification and characterization of tetraploid and octoploid Jatropha curcas induced by colchicine. Caryologia 2016, 69, 58–66. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Mirzaeea, M.; Hassani, M.E.; Sedghi-Moghadam, M. Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int. J. Plant Prod. 2010, 4, 87–98. [Google Scholar]
- Fakhrzad, F.; Jowkar, A.; Shekafandeh, A.; Kermani, M.J.; Moghadam, A. Tetraploidy induction enhances morphological, physiological and biochemical characteristics of wallflower (Erysimum cheiri (L.) Crantz). Sci. Hortic. 2023, 308, 111596. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Han, Q.; Kang, X. Molecular Mechanism of Slow Vegetative Growth in Populus Tetraploid. Genes 2020, 11, 1417. [Google Scholar] [CrossRef]
- Ranney, T.G. Polyploidy: From Evolution to New Plant Development. Proc. Int. Plant Propagators Soc. 2006, 56, 604–607. [Google Scholar]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Sanaei-Hoveida, Z.; Mortazavian, S.M.M.; Norouzi, M.; Sadat-Noori, S.A. Elevating morphology and essential oil in cumin genotypes through polyploidy induction. Sci. Hortic. 2024, 329, 113031. [Google Scholar] [CrossRef]
- Muthoni, J.; Shimelis, H.; Melis, R. Production of hybrid potatoes: Are heterozygosity and ploidy levels important? Aust. J. Crop Sci. 2019, 13, 687–694. [Google Scholar] [CrossRef]
- Pandey, J.; Scheuring, D.C.; Koym, J.W.; Vales, M.I. Genomic regions associated with tuber traits in tetraploid potatoes and identification of superior clones for breeding purposes. Front. Plant Sci. 2022, 13, 952263. [Google Scholar] [CrossRef]
- Kumar, S.; Dobos, G.J.; Rampp, T. The significance of ayurvedic medicinal plants. Evid.-Based Complement. Altern. Med. 2017, 22, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Ravi, Y.; Vethamoni, P.I.; Saxena, S.N.; Raveendran, M.; Velmurugan, S.; Santhanakrishnan, P. Extraction and estimation of thymoquinone (a highly valued metabolite) from Nigella sativa L. Med. Plants-Int. J. Phytomed. Relat. Ind. 2022, 14, 492–498. [Google Scholar]
- Ravi, Y.; Vethamoni, I.P.; Saxena, S.N.; Velmurugan, S.; Santanakrishnan, V.P.; Raveendran, M.; Bariya, H.; Harsh, M. Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique. Open Life Sci. 2023, 18, 20220536. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Yadav, R.; Yadav, N.; Bansal, S.; Sangwan, N.S. Influence of Genetics on the Secondary Metabolites of Plants. In Natural Secondary Metabolites: From Nature, Through Science, to Industry; Springer International Publishing AG: Cham, Switzerland, 2023; pp. 403–433. [Google Scholar]
- Jmii, G.; Gharsallaoui, S.; Mars, M.; Haouala, R. Polyploidization of Trigonella foenum-graecum L. enhances its phytotoxic activity against Cyperus rotundus L. S. Afr. J. Bot. 2023, 153, 336–345. [Google Scholar] [CrossRef]
- Gupta, N.; Bhattacharya, S.; Dutta, A.; Tauchen, J.; Landa, P.; Urbanová, K.; Houdková, M.; Fernández-Cusimamani, E.; Leuner, O. Synthetic polyploidization induces enhanced phytochemical profile and biological activities in Thymus vulgaris L. essential oil. Sci. Rep. 2024, 14, 5608. [Google Scholar] [CrossRef] [PubMed]

| EC (dS m−1) (1:5) | pH (1:2.5) | Available Nutrients (mg kg−1) | Soluble Cations (mmolc L−1) | Soluble Anions (mmolc L−1) | OM (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Mg2+ | Ca2+ | K+ | Na+ | CO32− | HCO3− | Cl− | SO42− | |||
| 3.96 | 8.13 | 32.65 | 10.9 | 380 | 7.1 | 12.47 | 0.24 | 19.77 | 0.0 | 3.33 | 18.4 | 17.9 | 1.45 |
| Trait (Unit) | Diploid | Tetraploid | p-Value |
|---|---|---|---|
| Plant height (cm) | 116.50 ± 0.866 | 95.00 ± 2.887 | 0.001021 * |
| The main number of branches | 10.50 ± 0.289 | 8.75 ± 0.144 | 0.002804 * |
| Number of secondary branches per plant | 112.00 ± 2.309 | 22.00 ± 1.155 | 0.00001 * |
| First days to flowering (day) | 132.70 ± 1.443 | 97.09 ± 3.453 | 0.00001 * |
| Number of flowers per plant | 111.70 ± 1.732 | 24.75 ± 0.289 | 0.00001 * |
| Flower diameter (cm) | 3.70 ± 0.058 | 3.27 ± 0.010 | 0.000918 * |
| Converted flowering parts (petaloid stamen) | 20.34 ± 0.510 | 188.00 ± 1.020 | 0.00001 * |
| Stamen number | 43.34 ± 0.820 | 9.44 ± 0.290 | 0.00021 * |
| Capsule diameter (cm) | 1.49 ± 0.078 | 0.83 ± 0.880 | 0.002601 * |
| Shoot fresh weight (g) | 610.00 ± 5.774 | 147.50 ± 1.443 | 0.00001 * |
| Shoot dry weight (g) | 110.50 ± 2.598 | 37.00 ± 1.732 | 0.00001 * |
| Crown diameter (cm) | 1.91 ± 0.052 | 1.16 ± 0.118 | 0.002142 * |
| Leaf area (mm) | 17.50 ± 0.058 | 22.50 ± 0.289 | 0.000035 * |
| Root length (cm) | 21.65 ± 0.202 | 19.00 ± 0.577 | 0.006164 * |
| Root fresh weight (g) | 8.00 ± 0.577 | 5.95 ± 0.029 | 0.011939 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mahrouk, M.E.; Maamoun, M.K.; Saifan, S.; Bayoumi, Y.A.; El-Ramady, H.; Abdalla, N. Exploring Polyploidization in Nigella sativa L.: An Applicable Strategy Towards Crop Improvement. Horticulturae 2025, 11, 1122. https://doi.org/10.3390/horticulturae11091122
El-Mahrouk ME, Maamoun MK, Saifan S, Bayoumi YA, El-Ramady H, Abdalla N. Exploring Polyploidization in Nigella sativa L.: An Applicable Strategy Towards Crop Improvement. Horticulturae. 2025; 11(9):1122. https://doi.org/10.3390/horticulturae11091122
Chicago/Turabian StyleEl-Mahrouk, Mohammed E., Mossad K. Maamoun, Sobhia Saifan, Yousry A. Bayoumi, Hassan El-Ramady, and Neama Abdalla. 2025. "Exploring Polyploidization in Nigella sativa L.: An Applicable Strategy Towards Crop Improvement" Horticulturae 11, no. 9: 1122. https://doi.org/10.3390/horticulturae11091122
APA StyleEl-Mahrouk, M. E., Maamoun, M. K., Saifan, S., Bayoumi, Y. A., El-Ramady, H., & Abdalla, N. (2025). Exploring Polyploidization in Nigella sativa L.: An Applicable Strategy Towards Crop Improvement. Horticulturae, 11(9), 1122. https://doi.org/10.3390/horticulturae11091122








