Phytochemical Composition, Sensory Acceptance, and Cultivation Potential of Sanguisorba verrucosa, Eruca vesicaria, and Scorzonera laciniata
Abstract
1. Introduction
2. Materials and Methods
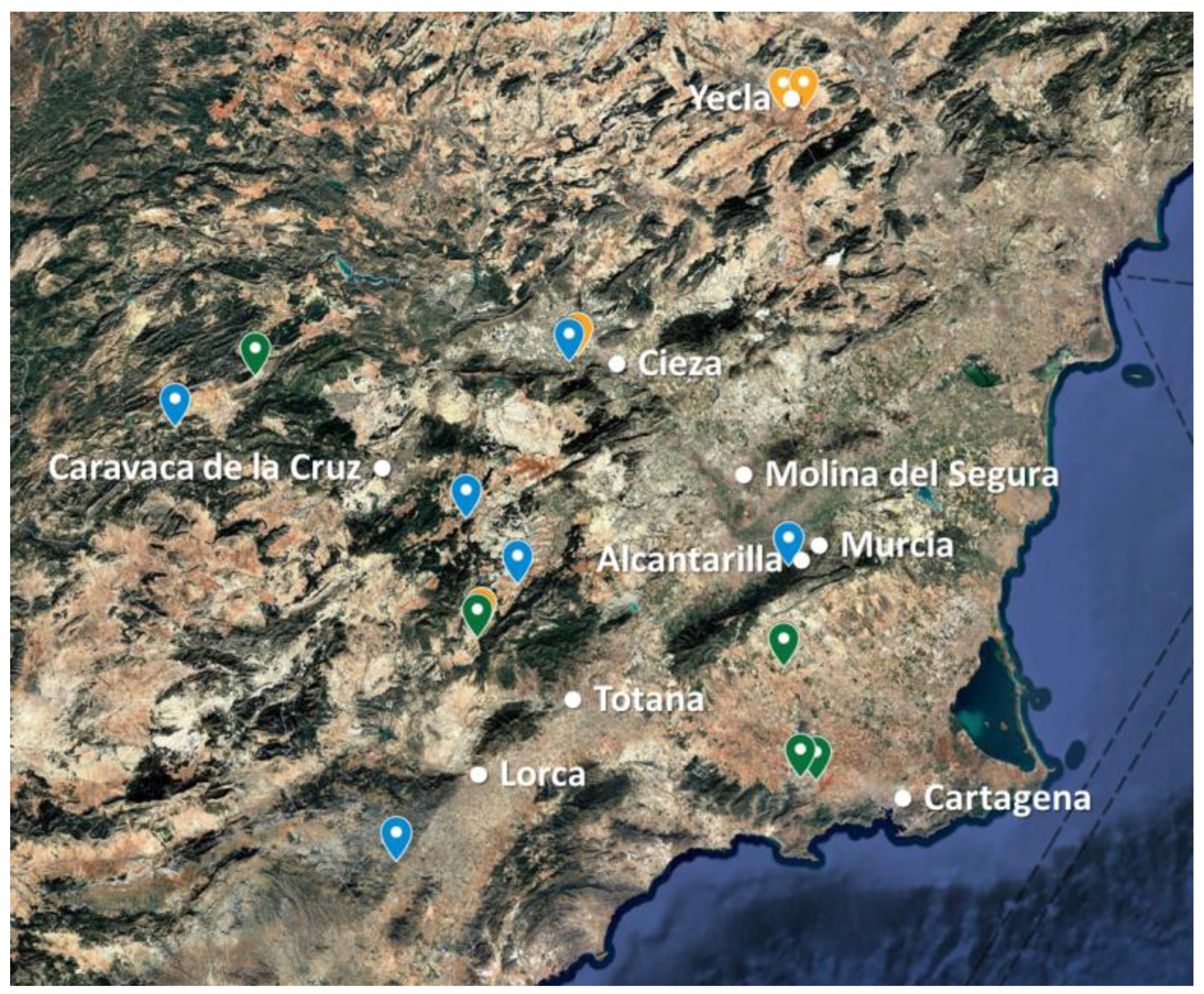
2.1. Gathering of Wild Plants
2.2. Germination Assay in Petri Dishes
2.3. Field Germination Assays
2.4. Cultivation Trial
2.5. Metabolite Analysis
2.5.1. Metabolite Extraction
2.5.2. Determination of Sugar and Organic Acids
2.5.3. Chlorophyll and Carotenoid Analysis
2.5.4. Ascorbic Acid Analysis
2.5.5. Total Phenolic Content Analysis
2.6. Sensory Assessment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Germination and Cultivation
3.2. Soluble Sugars
3.3. Organic Acids
3.4. Carotenoids
3.5. Chlorophylls
3.6. Vitamin C
3.7. Total Phenolic Content
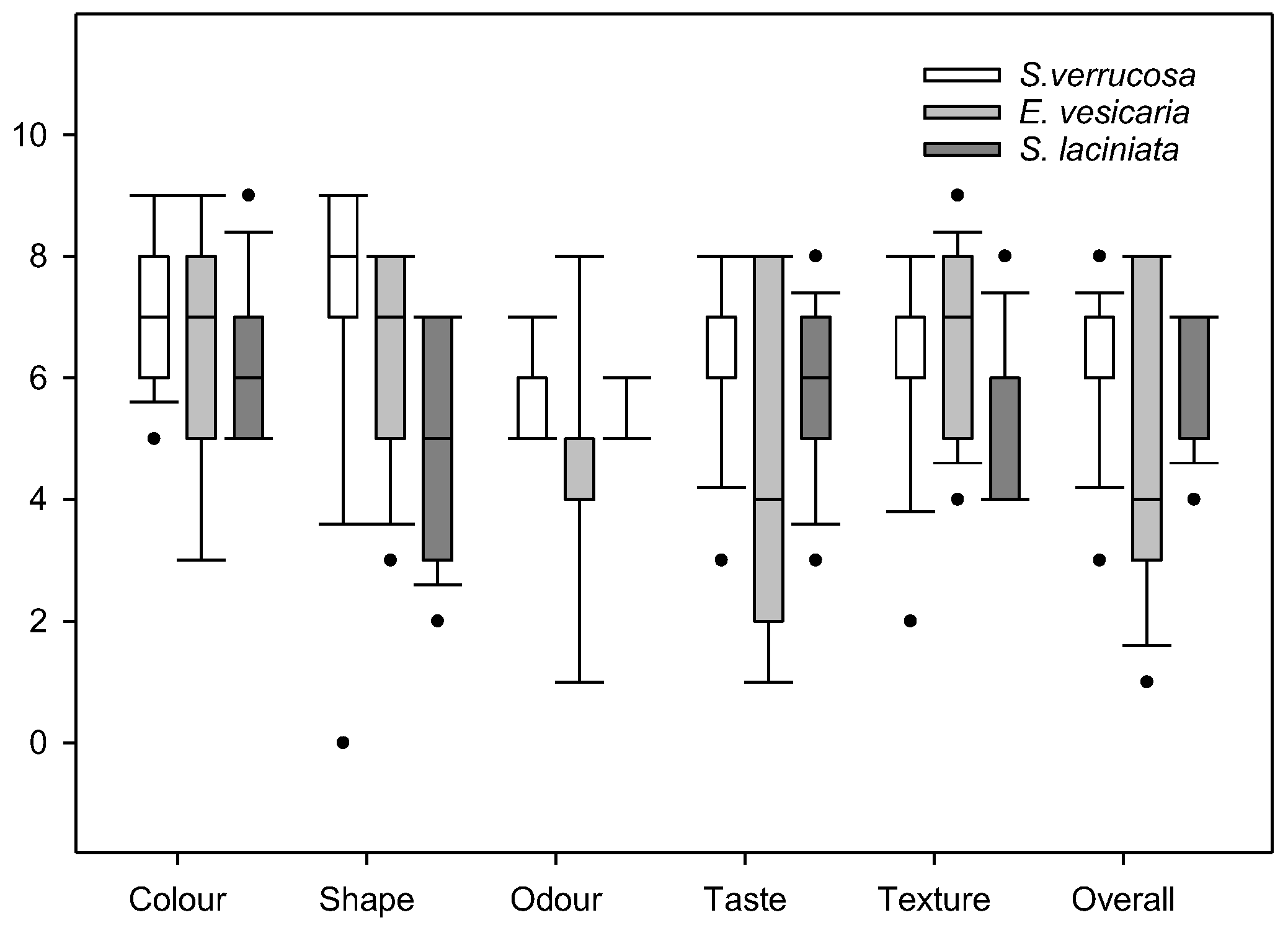
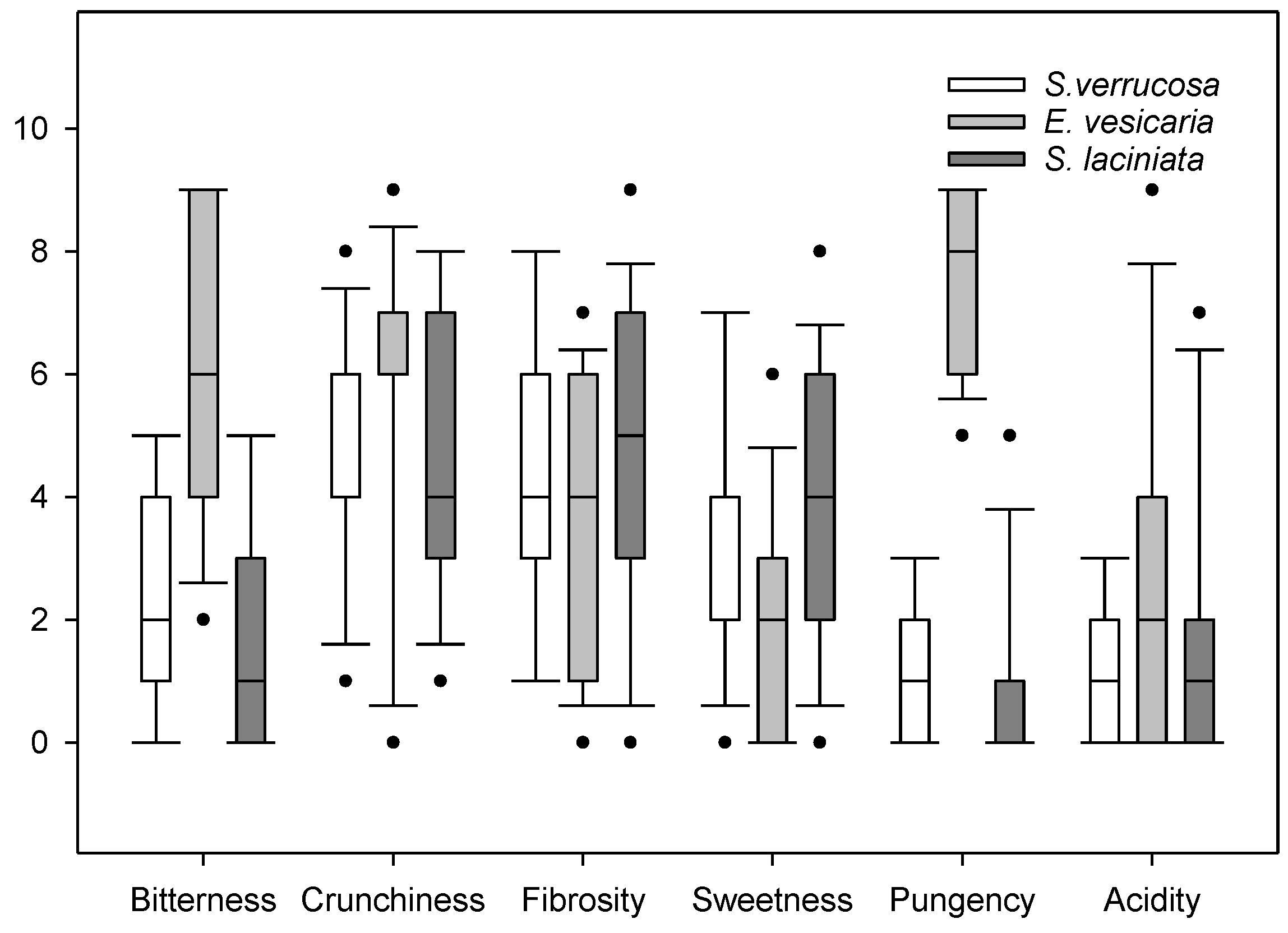
3.8. Sensory Attributes
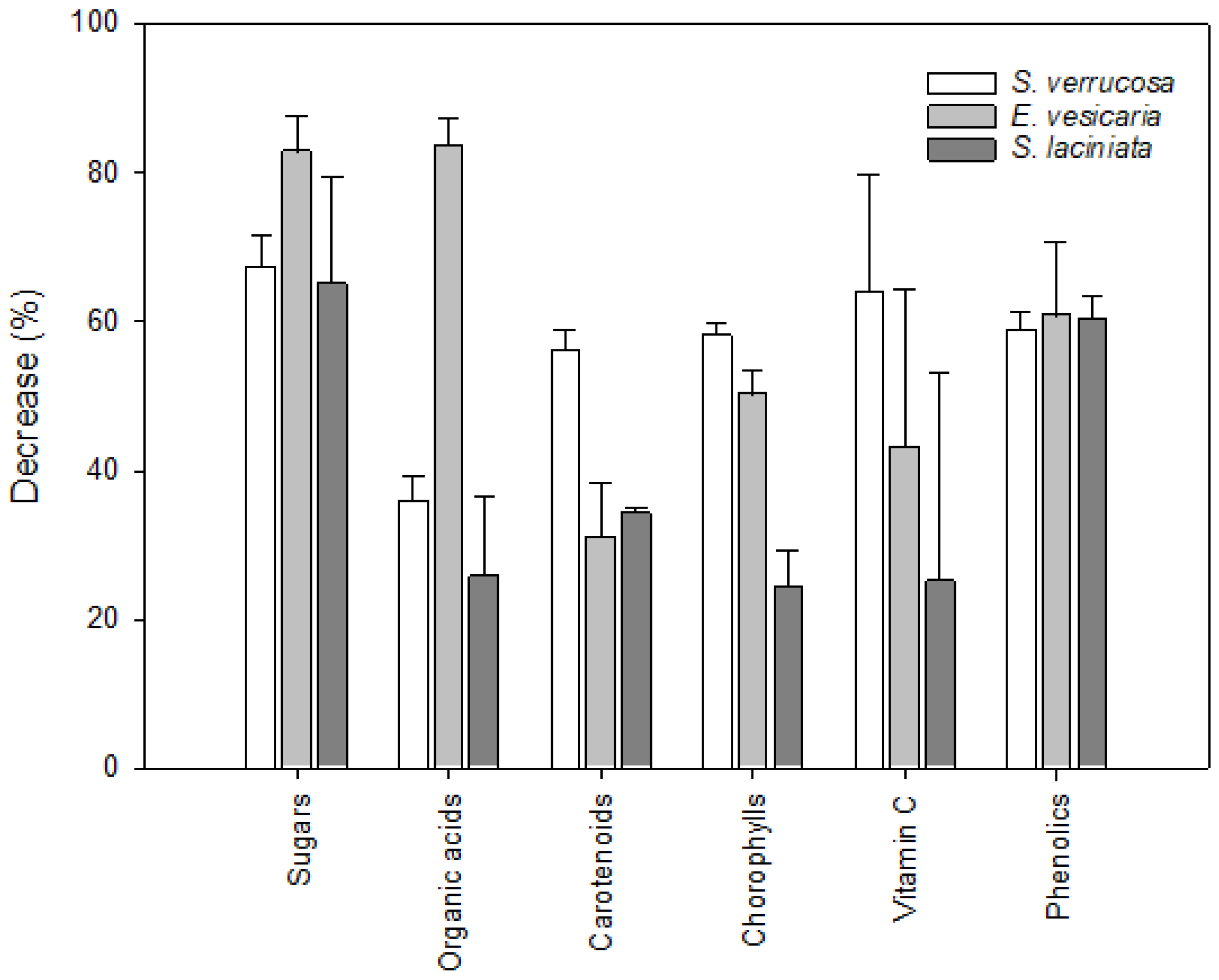
3.9. Wild-Gathered vs. Cultivated Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacchetta, L.; Visioli, F.; Cappelli, G.; Caruso, E.; Martin, G.; Nemeth, E.; Bacchetta, G.; Bedini, G.; Wezel, A.; van Asseldonk, T.; et al. A manifesto for the valorization of wild edible plants. J. Ethnopharmacol. 2016, 191, 180–187. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Tardio, J. (Eds.) Mediterranean Wild Edible Plants. Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Nirmala, C.; Shahar, B.; Dolma, N.; Santosh, O. Promising underutilized wild plants of cold desert Ladakh, India for nutritional security and health benefits. Appl. Food Res. 2022, 2, 100145. [Google Scholar] [CrossRef]
- Saha, S.; Saha, S.; Mandal, S.; Rahaman, C. Unco nventional but valuable phytoresources: Exploring the nutritional benefits of 18 wild edible Asteraceae from West Bengal, India. Genet. Resour. Crop Evol. 2023, 70, 2161–2192. [Google Scholar] [CrossRef]
- FAO. Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries. In Proceedings of the Ninth Regular Session of the Commission on Genetic Resources for Food and Agriculture, Rome, Italy, 12–13 October 2002; Inter-Departmental Working Group on Biological Diversity for Food and Agriculture: Rome, Italy, 2002. [Google Scholar]
- Luczaj, L.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Soukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Schulp, C.; Thuiller, W.; Verburg, P. Wild food in Europe: A synthesis of knowledge and data of terrestrial wild food as an ecosystem service. Ecol. Econ. 2014, 105, 292–305. [Google Scholar] [CrossRef]
- Romojaro, A.; Botella, M.; Obón, C.; Pretel, M. Nutritional and antioxidant properties of wild edible plants and their use as potential ingredients in the modern diet. Int. J. Food Sci. Nutr. 2013, 64, 944–952. [Google Scholar] [CrossRef]
- de Medeiros, P.; Figueiredo, K.; Gonçalves, P.; Caetano, R.; Santos, É.; dos Santos, G.; Barbosa, D.; de Paula, M.; Mapeli, A. Wild plants and the food-medicine continuum-an ethnobotanical survey in Chapada Diamantina (Northeastern Brazil). J. Ethnobiol. Ethnomed. 2021, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Villalba, J.; Burló, F.; Hernández, F.; Carbonell-Barrachina, A. Valorization of wild edible plants as food ingredients and their economic value. Foods 2023, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Alu’datt, M.; Rababah, T.; Alhamad, M.; Gammoh, S.; Al-Mahasneh, M.; Tranchant, C.; Rawshdeh, M. Pharmaceutical, nutraceutical and therapeutic properties of selected wild medicinal plants: Thyme, spearmint, and rosemary. In Therapeutic, Probiotic, and Unconventional Foods; Mihai, A., Holban, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 275–290. [Google Scholar]
- Fukalova, T.; Martínez, M.; Raigón, M. Five undervalued edible species inherent to autumn-winter season: Nutritional composition, bioactive constituents and volatiles profile. PeerJ 2021, 9, e12488. [Google Scholar] [CrossRef] [PubMed]
- Huyan, T.; Li, Q.; Wang, Y.; Li, J.; Zhang, J.; Liu, Y.; Shahid, M.; Yang, H.; Li, H. Anti-tumor effect of hot aqueous extracts from Sonchus oleraceus (L.) L. and Juniperus sabina L—Two traditional medicinal plants in China. J. Ethnopharmacol. 2016, 185, 289–299. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E.; Adewumi, O.M.; Okeleji, L.O.; Mujaidu, K.B.; Olaleye, S.B. Effect of ethanolic extract of Cryptolepis sanguinolenta stem on in vivo and in vitro glucose absorption and transport: Mechanism of its antidiabetic activity. Indian J. Endocrinol. Metab. 2012, 16, S91–S96. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj, Ł.; Pieroni, A. Nutritional ethnobotany in Europe: From emergency foods to healthy folk cuisines and contemporary foraging trends. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Sánchez-Mata, M.C., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 75–90. [Google Scholar] [CrossRef]
- Pereira, A.; Fraga-Corral, M.; García-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Chen, Q.; Wang, L.; Yang, J.; Wu, A.; Jiang, N.; Liu, Y.; Chen, J.; Zou, W.; et al. A comprehensive review of genus sanguisorba: Traditional uses, chemical constituents and medical applications. Front. Pharmacol. 2021, 12, 750165. [Google Scholar] [CrossRef] [PubMed]
- Tocai, A.; Kokeric, T.; Tripon, S.; Barbu-Tudoran, L.; Barjaktarevic, A.; Cupara, S.; Vicas, S. Sanguisorba minor Scop.: An overview of its phytochemistry and biological effects. Plants 2023, 12, 2128. [Google Scholar] [CrossRef]
- Tardio, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Rivera, D.; Alcaraz, F.J.; Verde, A.; Fajardo, J.; Obón, C. Las Plantas en la Cultura Popular. Enciclopedia Divulgativa de la Historia Natural de Jumilla-Yecla; Fundación Cajamurcia: Murcia, Spain, 2008. [Google Scholar]
- Schaffer, S.; Schmitt-Schillig, S.; Muller, W.E.; Eckert, G.P. Antioxidant properties of Mediterranean food plant extracts: Geographical differences. J. Physiol. Pharmacol. 2005, 56 (Suppl. S1), 115–124. [Google Scholar]
- Bogani, P.; Visioli, F. Antioxidants in the Mediterranean diets: An update. World Rev. Nutr. Diet. 2007, 97, 162–179. [Google Scholar] [CrossRef]
- Perry, L.M.; Metzger, J. Medicinal Plants of East and Southeast Asia: Attributed Properties and Uses; MIT Press: Cambridge, MA, USA, 1978. [Google Scholar]
- Yaniv, Z.; Schafferman, D.; Amar, Z. Tradition, uses and biodiversity of rocket (Eruca sativa, Brassicaceae) in Israel. Econ. Bot. 1998, 52, 394–400. [Google Scholar] [CrossRef]
- Zidorn, C.; Ellmerer-Müller, E.; Stuppner, H. Sesquiterpenoids from Scorzonera hispanica L. Pharmazie 2000, 55, 550–551. [Google Scholar]
- Gong, Y.; Shi, Z.; Yu, J.; He, X.; Meng, X.; Wu, Q.; Zhu, Y. The genus Scorzonera L. (Asteraceae): A comprehensive review on traditional uses, phytochemistry, pharmacology, toxicology, chemotaxonomy, and other applications. J. Ethnopharmacol. 2024, 320, 116787. [Google Scholar] [CrossRef] [PubMed]
- Erden, Y.; Kırbağ, S. Chemical and biological activities of some Scorzonera species: An in vitro study. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 319–326. [Google Scholar] [CrossRef]
- Puccinelli, M.; Carmassi, G.; Pardossi, A.; Incrocci, L. Wild edible plant species grown hydroponically with crop drainage water in a Mediterranean climate: Crop yield, leaf quality, and use of water and nutrients. Agric. Water Manag. 2023, 282, 108275. [Google Scholar] [CrossRef]
- Misra, S. Survey of edible plants for human consumption in South Odisha, India. Int. J. Emerg. Technol. Innov. Res. 2020, 7, 277–296. Available online: http://www.jetir.org/papers/JETIR2012040.pdf (accessed on 25 June 2025).
- Ceccanti, C.; Finimundy, T.; Barros, L. Nutritional value of wild and domesticated Sanguisorba minor Scop. Plant. Hortic. 2023, 9, 560. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Baldi, A.; Lenzi, A.; Bulgari, R. Wild plant species as potential horticultural crops: An opportunity for farmers and consumers. Horticulturae 2023, 9, 1193. [Google Scholar] [CrossRef]
- Lenzi, A.; Orlandini, A.; Bulgari, R.; Ferrante, A.; Bruschi, P. Antioxidant and mineral composition of three wild leafy species: A comparison between microgreens and baby greens. Foods 2019, 8, 487. [Google Scholar] [CrossRef]
- Botella, M.; Hellín, P.; Hernández, V.; Dabauza, M.; Robledo, A.; Sánchez, A.; Fenoll, J.; Flores, P. Chemical composition of wild collected and cultivated edible plants (Sonchus oleraceus L. and Sonchus tenerrimus L.). Plants 2024, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Javier, G.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Hernández, V.; Botella, M.; Hellín, P.; Cava, J.; Fenoll, J.; Mestre, T.; Martínez, V.; Flores, P. Phenolic and carotenoid profile of lamb’s lettuce and improvement of the bioactive content by preharvest conditions. Foods 2021, 10, 188. [Google Scholar] [CrossRef]
- Flores, P.; Hellín, P.; Fenoll, J. Determination of organic acids in fruits and vegetables by liquid chromatography with tandem-mass spectrometry. Food Chem. 2012, 132, 1049–1054. [Google Scholar] [CrossRef]
- Fenoll, J.; Martinez, A.; Hellín, P.; Flores, P. Simultaneous determination of ascorbic and dehydroascorbic acids in vegetables and fruits by liquid chromatography with tandem-mass spectrometry. Food Chem. 2011, 127, 340–344. [Google Scholar] [CrossRef]
- Sancho, J.; Bota, E.; Castro, J. Introducción al Análisis Sensorial de los Alimentos; Editorial Alfaomega: Barcelona, Spain, 1999. [Google Scholar]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Maleki, K.; Soltani, E.; Seal, C.E.; Pritchard, H.W.; Lamichhane, J.R. The seed germination spectrum of 528 plant species: A global meta-regression in relation to temperature and water potential. bioRxiv 2022. [Google Scholar] [CrossRef]
- Karkanis, A.; Fernandes, Å.; Vaz, J.; Petropoulos, S.; Georgiou, E.; Ciric, A.; Sokovic, M.; Oludemi, T.; Barros, L.; Ferreira, I. Chemical composition and bioactive properties of Sanguisorba minor Scop. under Mediterranean growing conditions. Food Funct. 2019, 10, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.; López, A.; Fenoll, J.; Hellín, P.; Kelly, S. Classification of organic and conventional sweet peppers and lettuce using a combination of isotopic and bio-markers with multivariate analysis. J. Food Compos. Anal. 2013, 31, 217–225. [Google Scholar] [CrossRef]
- Schmitzer, V.; Senica, M.; Slatnar, A.; Stampar, F.; Jakopic, J. Changes in metabolite patterns during refrigerated storage of lamb’s lettuce (Valerianella locusta L. Betcke). Front. Nutr. 2021, 8, 731869. [Google Scholar] [CrossRef]
- Spinardi, A.; Ferrante, A. Effect of storage temperature on quality changes of minimally processed baby lettuce. J. Food Agric. Environ. 2012, 10, 38–42. [Google Scholar]
- Yadav, P.; Chauhan, A.K.; Singh, R.B.; Khan, S.; Halabi, G. Organic acids: Microbial sources, production, and applications. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 325–337. [Google Scholar] [CrossRef]
- Gold, R.; Linker, R.; Stangel, M. Fumaric acid and its esters: An emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clin. Immunol. 2012, 142, 44–48. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaldate, R.; Bisht, A. Citric acid, antioxidant effects in health. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar] [CrossRef]
- Gordon, M.H. The mechanism of antioxidant action in vitro. In Food Antioxidants; Hudson, B.J.F., Ed.; Elsevier: New York, NY, USA, 1990; pp. 1–18. [Google Scholar]
- Fabian, F.; Blum, H. Relative taste potency of some basic food constituents and their competitive and compensatory action. J. Food Sci. 2006, 8, 179–193. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2024, 40, 3408–3437. [Google Scholar] [CrossRef]
- Kim, D.; Shang, X.; Assefa, A.; Keum, Y.; Saini, R. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef]
- Komeroski, M.; Portal, K.; Comiotto, J.; Klug, T.; Flores, S.; Rios, A. Nutritional quality and bioactive compounds of arugula (Eruca sativa L.)sprouts and microgreens. Int. J. Food Sci. Technol. 2023, 58, 5089–5096. [Google Scholar] [CrossRef]
- Znidarcic, D.; Ban, D.; Sircelj, H. Carotenoid and chlorophyll composition of commonly consumed leafy vegetables in Mediterranean countries. Food Chem. 2011, 129, 1164–1168. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.; Biesalski, H. β-Carotene is an important vitamin a source for human. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene—A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Schieber, A.; Carle, R. Occurrence of carotenoid cis-isomers in food: Technological, analytical, and nutritional implications. Trends Food Sci. Technol. 2005, 16, 416–422. [Google Scholar] [CrossRef]
- Tao, W.; Ye, X.; Cao, Y. Isomerization and degradation of all-trans-β-carotene during in-vitro digestion. Food Sci. Hum. Wellness 2021, 10, 370–374. [Google Scholar] [CrossRef]
- Deming, D.; Teixeira, S.; Erdman, J. All-trans β-carotene appears to be more bioavailable than 9-cis or 13-cis β-carotene in gerbils given single oral doses of each isomer. J. Nutr. 2002, 132, 2700–2708. [Google Scholar] [CrossRef]
- Erdman, J.; Thatcher, A.; Hofmann, N.; Lederman, J.; Block, S.; Lee, C.; Mokady, S. All-trans β-carotene is absorbed preferentially to 9-cis β-carotene, but the latter accumulates in the tissues of domestic ferrets (Mustela putorius puro). J. Nutr. 1998, 128, 2009–2013. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Comp. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Maiani, G.; Castón, M.; Catasta, G.; Toti, E.; Cambrodon, I.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Molnár, P.; Deli, J.; Tanaka, T.; Kann, Y.; Tani, S.; Gyémánt, N.; Molnár, J.; Kawase, M. Carotenoids with anti-Helicobacter pylori activity from Golden Delicious apple. Phytother. Res. 2010, 24, 644–648. [Google Scholar] [CrossRef]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar] [CrossRef]
- Upreti, S.; Prusty, J.; Pandey, S.; Kumar, A.; Samant, M. Identification of novel inhibitors of angiotensin-converting enzyme 2 (ACE-2) receptor from Urtica dioica to combat coronavirus disease 2019 (COVID-19). Mol. Divers. 2021, 25, 1795–1809. [Google Scholar] [CrossRef]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sola, M.A.; Rodríguez-Concepción, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arab. Book 2012, 10, e0158. [Google Scholar] [CrossRef]
- Rumengan, A.P.; Mandiangan, E.S.; Tanod, W.A. Identification of pigment profiles and antioxidant activity of Rhizophora mucronata mangrove leaves originating from Lembeh, North Sulawesi, Indonesia. Biodiversitas 2021, 22, 2805–2816. [Google Scholar] [CrossRef]
- Chassagne, F.; Huang, X.; Lyles, J.; Quave, C. Validation of a 16th century traditional Chinese medicine use of Ginkgo biloba as a topical antimicrobial. Front. Microbiol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Ibrahim, M.; Jaafar, H.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian herb Kacip Fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Chen, K.; Pérez-Gálvez, A. 6-Chlorophylls. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 125–158. [Google Scholar]
- Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.; Rosa, E.; Antunes, L. Enhancing health benefits through chlorophylls and chlorophyll-rich agro-food: A comprehensive review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef] [PubMed]
- Sandrika, A.; Munir, M.A.; Aprilia, V.; Emelda, E. Determination of vitamin C in spinach (Amaranthus sp.) using titration method. J. Pena Sains 2023, 10, 73–79. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Oluchukwu, N.V.; Salisu, A.; Nkemakonam, O.M. Nutritional and phytochemical analysis of spinach leaf aqueous extract: A comprehensive study on proximate composition, minerals, vitamins, and antioxidant activity. Eurasian J. Sci. Technol. 2025, 5, 302–311. [Google Scholar] [CrossRef]
- Hasanah, U. Penentuan kadar vitamin menggunakan metode iodometri. J. Kel. Sehat Sejah. 2018, 16, 90–95. [Google Scholar] [CrossRef]
- Evana, E.; Barek, M.S. Determination of vitamin C (ascorbic acid) contents in two varieties of melon fruits (Cucumis melo L.) by iodometric titration. Fuller. J. Chem. 2021, 6, 143–147. [Google Scholar]
- Rahayuningsih, J.; Sisca, V.; Angasa, E.E. Analisis vitamin C pada buah jeruk Pasaman untuk meningkatkan kekebalan tubuh pada masa pandemi COVID-19. J. Rekayasa Chem. 2022, 4, 29–33. [Google Scholar] [CrossRef]
- Mohankumar, J.B.; Uthira, L.; Maheswari, S.U. Total phenolic content of organic and conventional green leafy vegetables. J. Nutr. Hum. Health 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, J.; Wu, X.; Liu, R. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhe, R.; Van Camp, J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bayili, R.; Abdoul-Latif, F.; Kone, O.; Diao, M.; Bassole, I.; Dicko, M. Phenolic compounds and antioxidant activities in some fruits and vegetables from Burkina Faso. Afr. J. Biotechnol. 2011, 10, 13543–13547. [Google Scholar] [CrossRef]
- Mazzucotelli, C.; González-Aguilar, G.; Villegas-Ochoa, M.; Domínguez-Avila, A.; Ansorena, M.; Di Scala, K. Chemical characterization and functional properties of selected leafy vegetables for innovative mixed salads. J. Food Biochem. 2017, 42, e12461. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.M.A. Plant growth, phytochemical accumulation and antioxidant activity of substrate-grown spinach. Heliyon 2018, 4, e00751. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Davis, D. Declining fruit and vegetable nutrient composition: What is the evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Di Gioia, F.; Avato, P.; Serio, F.; Argentieri, M. Glucosinolate profile of Eruca sativa, Diplotaxis tenuifolia and Diplotaxis erucoides grown in soil and soilless systems. J. Food Compos. Anal. 2018, 69, 197–204. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Venturi, F.; Taglieri, I.; Ferroni, G.; Guidi, L. Comparison of three domestications and wild-harvested plants for nutraceutical properties and sensory profiles in five wild edible herbs: Is. domestication possible? Foods 2020, 9, 1065. [Google Scholar] [CrossRef]
- Fukalova, T.; Garcia-Martinez, M.; Raigon, M. Nutritional composition, bioactive compounds, and volatiles profile characterization of two edible undervalued plants: Portulaca oleracea L. and Porophyllum ruderale (Jacq.) Cass. Plants 2022, 11, 377. [Google Scholar] [CrossRef]
- Riquelme, J.; Olaeta, J.; Gálvez, L.; Undurraga, P.; Fuentealba, C.; Osses, A.; Orellana, J.; Gallardo, J.; Pedreschi, R. Nutritional and functional characterization of wild and cultivated Sarcocornia neei grown in Chile. Cienc. Investig. Agrar. 2016, 43, 283–293. [Google Scholar] [CrossRef]
- Paschoalinotto, B.; Polyzos, N.; Compocholi, M.; Rouphael, Y.; Alexopoulos, A.; Dias, M.; Barros, L.; Petropoulos, S. Domestication of wild edible species: The response of Scolymus hispanicus plants to different fertigation regimes. Horticulturae 2023, 9, 103. [Google Scholar] [CrossRef]
- Yamada, K.; Osakabe, Y. Sugar compartmentation as an environmental stress adaptation strategy in plants. Semin. Cell Dev. Biol. 2018, 83, 106–114. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- de Oliveira, H.; Siqueira, J.; Medeiros, D.; Fernie, A.; Nunes-Nesi, A.; Araújo, W. Harnessing the dynamics of plant organic acids metabolism following abiotic stresses. Plant Physiol. Biochem. 2025, 220, 109465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; De Pinto, M. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | S. verrucosa | E. vesicaria | S. laciniata |
|---|---|---|---|
| Glucose | 5275.0 ± 303.7 | 1877.3 ± 182.5 | 2527.3 ± 630.7 |
| Sucrose | 4699.7 ± 197.6 | 560.0 ± 29.2 | 1953.2 ± 217.8 |
| Fructose | 3837.1 ± 384.7 | 406.0 ± 21.1 | 2324.0 ± 541.5 |
| Citric | 762.4 ± 1.3 | 1235.9 ± 56.9 | 528.4 ± 80.7 |
| Malic | 445.4 ± 8.1 | 428.6 ± 18.6 | 1759.5 ± 72.3 |
| Tartaric | 2.3 ± 0.3 | 5.3 ± 0.2 | 7.8 ± 0.9 |
| Fumaric | 802.1 ± 38.7 | 54.2 ± 1.6 | 196.8 ± 34.8 |
| Succinic | 60.0 ± 4.6 | 30.8 ± 2.7 | 79.4 ± 9.0 |
| Quinic | 13.9 ± 0.7 | 9.6 ± 0.5 | 285.0 ± 5.3 |
| Malonic | 12.5 ± 1.3 | 9.9 ± 0.4 | 6.5 ± 1.0 |
| Isocitric | 21.2 ± 0.7 | 50.7 ± 3.1 | 11.7 ± 3.3 |
| Ketoglutaric | 2.8 ± 0.2 | 9.1 ± 0.6 | 16.7 ± 1.7 |
| Glutamic | 7.1 ± 0.3 | 7.4 ± 0.4 | 4.2 ± 0.4 |
| Skimic | 6.5 ± 0.0 | 0.9 ± 0.0 | 3.0 ± 0.3 |
| Chlorophyll a | 64.3 ± 1.2 | 96.4 ± 3.4 | 108.4 ± 3.4 |
| Chlorophyll b | 31.8 ± 0.9 | 45.4 ± 1.0 | 45.0 ± 1.5 |
| All-trans-β-carotene | 30.0 ± 1.0 | 16.0 ± 0.4 | 36.6 ± 0.0 |
| Lutein | 10.7 ± 0.3 | 13.0 ± 0.3 | 13.8 ± 0.2 |
| All-trans violaxanthin | 2.9 ± 0.0 | 3.6 ± 0.1 | 5.5 ± 0.2 |
| 9-cis-Neoxanthin | 1.8 ± 0.1 | 2.6 ± 0.1 | 2.3 ± 0.0 |
| 9-cis-β-carotene | 2.9 ± 0.1 | 18.3 ± 0.0 | 3.5 ± 0.0 |
| Luteoxanthin | 3.1 ± 0.2 | 6.4 ± 0.1 | 2.6 ± 0.1 |
| 13-cis-β-carotene | 1.0 ± 0.1 | 1.7 ± 0.0 | 1.3 ± 0.0 |
| Vitamin C | 933.4 ± 204.3 | 1012.9 ± 128.5 | 530.8 ± 99.3 |
| Total phenolics | 3098.3 ± 93.6 | 246.7 ± 19.2 | 286.6 ± 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botella, M.Á.; Hellín, P.; Hernández, V.; Dabauza, M.; Robledo, A.; Sánchez, A.; Fenoll, J.; Flores, P. Phytochemical Composition, Sensory Acceptance, and Cultivation Potential of Sanguisorba verrucosa, Eruca vesicaria, and Scorzonera laciniata. Horticulturae 2025, 11, 1021. https://doi.org/10.3390/horticulturae11091021
Botella MÁ, Hellín P, Hernández V, Dabauza M, Robledo A, Sánchez A, Fenoll J, Flores P. Phytochemical Composition, Sensory Acceptance, and Cultivation Potential of Sanguisorba verrucosa, Eruca vesicaria, and Scorzonera laciniata. Horticulturae. 2025; 11(9):1021. https://doi.org/10.3390/horticulturae11091021
Chicago/Turabian StyleBotella, María Ángeles, Pilar Hellín, Virginia Hernández, Mercedes Dabauza, Antonio Robledo, Alicia Sánchez, José Fenoll, and Pilar Flores. 2025. "Phytochemical Composition, Sensory Acceptance, and Cultivation Potential of Sanguisorba verrucosa, Eruca vesicaria, and Scorzonera laciniata" Horticulturae 11, no. 9: 1021. https://doi.org/10.3390/horticulturae11091021
APA StyleBotella, M. Á., Hellín, P., Hernández, V., Dabauza, M., Robledo, A., Sánchez, A., Fenoll, J., & Flores, P. (2025). Phytochemical Composition, Sensory Acceptance, and Cultivation Potential of Sanguisorba verrucosa, Eruca vesicaria, and Scorzonera laciniata. Horticulturae, 11(9), 1021. https://doi.org/10.3390/horticulturae11091021








