Foliar Application of Iron and Zinc Affected Aromatic Plants Grown Under Conventional and Organic Agriculture Differently
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Species, Soil Characteristics, and Experimental Setup
2.2. Plant Biomass and Photochemistry
2.3. Total Phenols, Total Flavonoids, and Antioxidant Capacity
2.4. Lipid Peroxidation and Hydrogen Peroxide Content
2.5. Nutrient Content in Plants
2.6. Statistical Analysis
3. Results
3.1. Soil Analysis
3.2. Two-Way Anova Analysis
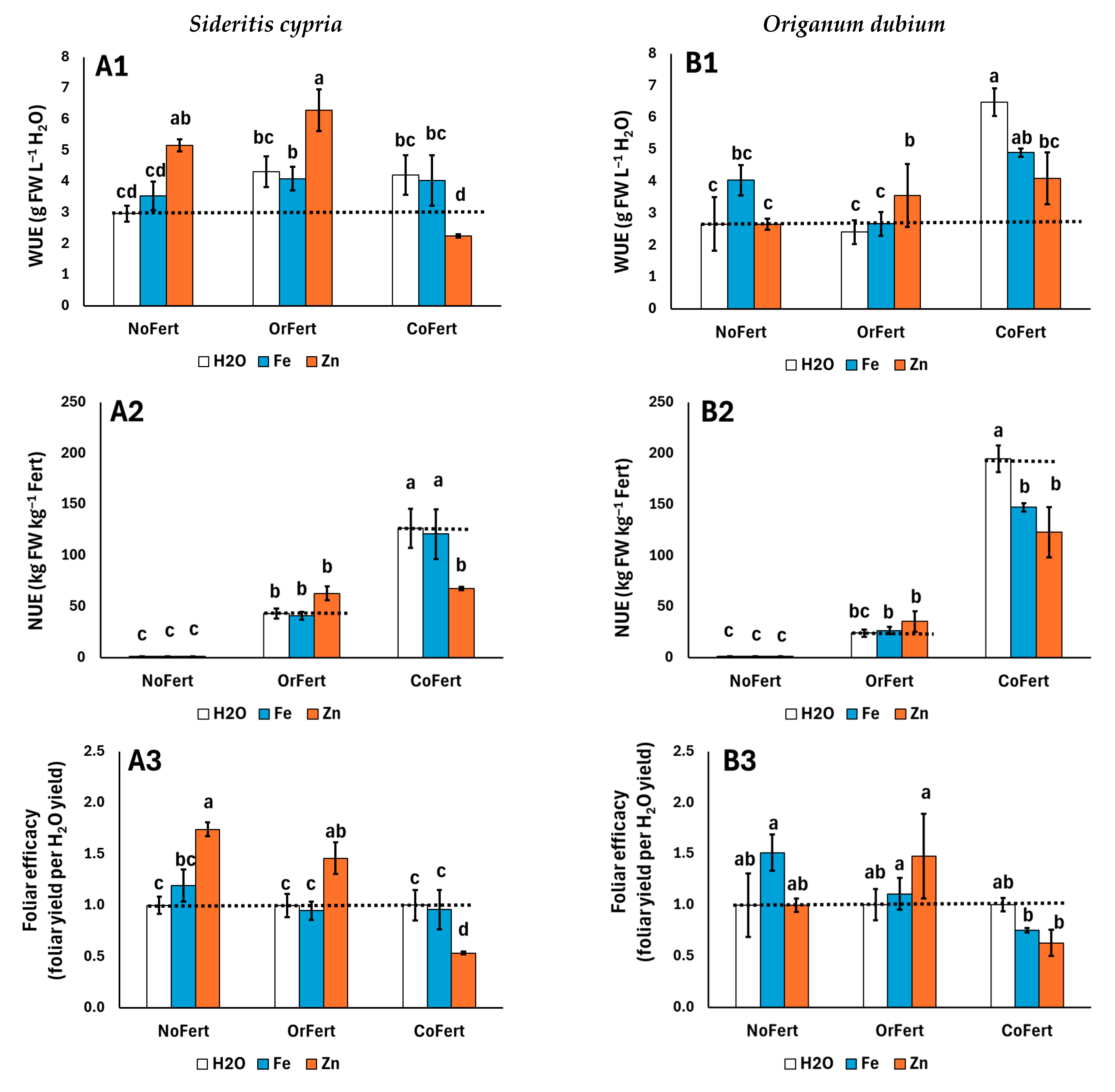
3.3. Plant Biomass, Photochemistry, and Mineral Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-De-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Feng, T.; Xiong, R.; Huan, P. Productive use of natural resources in agriculture: The main policy lessons. Resour. Policy 2023, 85, 103793. [Google Scholar] [CrossRef]
- Rostaei, M.; Fallah, S.; Carrubba, A.; Lorigooini, Z. Organic manures enhance biomass and improve content, chemical compounds of essential oil and antioxidant capacity of medicinal plants: A review. Heliyon 2024, 10, e36693. [Google Scholar] [CrossRef]
- Seppelt, R.; Klotz, S.; Peiter, E.; Volk, M. Agriculture and food security under a changing climate: An underestimated challenge. iScience 2022, 25, 105551. [Google Scholar] [CrossRef] [PubMed]
- Mardanluo, S.; Souri, M.K.; Ahmadi, M. Plant growth and fruit quality of two pepper cultivars under different potassium levels of nutrient solutions. J. Plant Nutr. 2018, 41, 1604–1614. [Google Scholar] [CrossRef]
- Du, J.; Yu, Y.; Tang, C.; Zong, K.; Zhang, S.; Zhang, Q.; Fang, L.; Li, Y. Organic fertilizers increase the proportion of saprotrophs favoring soil nitrification under medicinal plants Fritillaria thunbergii. Ind. Crops Prod. 2024, 219, 119129. [Google Scholar] [CrossRef]
- Järvan, M.; Edesi, L. The effect of cultivation methods on the yield and biological quality of potato. Agron. Res. 2009, 7, 289–299. [Google Scholar]
- Chrysargyris, A.; Kloukina, C.; Vassiliou, R.; Tomou, E.-M.; Skaltsa, H.; Tzortzakis, N. Cultivation strategy to improve chemical profile and anti-oxidant activity of Sideritis perfoliata L. subsp. perfoliata. Ind. Crops Prod. 2019, 140, 111694. [Google Scholar] [CrossRef]
- Manik, S.M.N.; Pengilley, G.; Dean, G.; Field, B.; Shabala, S.; Zhou, M. Soil and crop management practices to minimize the impact of waterlogging on crop productivity. Front. Plant Sci. 2019, 10, 140. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Dekemati, I.; Simon, B.; Vinogradov, S.; Birkás, M. The effects of various tillage treatments on soil physical properties, earthworm abundance and crop yield in Hungary. Soil Tillage Res. 2019, 194, 104334. [Google Scholar] [CrossRef]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond organic farming–harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef]
- Fernández, J.A.; Ayastuy, M.E.; Belladonna, D.P.; Comezaña, M.M.; Contreras, J.; de Maria Mourão, I.; Orden, L.; Rodríguez, R.A. Current trends in organic vegetable crop production: Practices and techniques. Horticulturae 2022, 8, 893. [Google Scholar] [CrossRef]
- Barbieri, P.; Pellerin, S.; Nesme, T. Comparing crop rotations between organic and conventional farming. Sci. Rep. 2017, 7, 13761. [Google Scholar] [CrossRef] [PubMed]
- Litskas, V.; Chrysargyris, A.; Stavrinides, M.; Tzortzakis, N. Water-energy-food nexus: A case study on medicinal and aromatic plants. J. Clean. Prod. 2019, 233, 1334–1343. [Google Scholar] [CrossRef]
- Carrascosa, Á.; Pascual, J.A.; Ros, M.; Petropoulos, S.; del Mar Alguacil, M. The effect of fertilization regime on growth parameters of Sonchus oleraceus and two genotypes of Portulaca oleracea. Biol. Life Sci. Forum 2022, 16, 7. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Koutsoumpeli, E.; Xylia, P.; Fytrou, A.; Konstantopoulou, M.; Tzortzakis, N. Organic cultivation and deficit irrigation practices to improve chemical and biological activity of Mentha spicata plants. Agronomy 2021, 11, 599. [Google Scholar] [CrossRef]
- Molina, M.; Tardío, J.; Aceituno-Mata, L.; Morales, R.; Reyes-García, V.; Pardo-De-Santayana, M. Weeds and food diversity: Natural yield assessment and future alternatives for traditionally consumed wild vegetables. J. Ethnobiol. 2014, 34, 44–67. [Google Scholar] [CrossRef]
- Paschoalinotto, B.H.; Polyzos, N.; Compocholi, M.; Rouphael, Y.; Alexopoulos, A.; Dias, M.I.; Barros, L.; Petropoulos, S.A. Domestication of wild edible species: The response of Scolymus hispanicus plants to different fertigation regimes. Horticulturae 2023, 9, 103. [Google Scholar] [CrossRef]
- Herencia, J.F.; García-Galavís, P.A.; Dorado, J.A.R.; Maqueda, C. Comparison of nutritional quality of the crops grown in an organic and conventional fertilized soil. Sci. Hortic. 2011, 129, 882–888. [Google Scholar] [CrossRef]
- Hallmann, E.; Sabała, P. Organic and conventional herbs quality reflected by their antioxidant compounds concentration. Appl. Sci. 2020, 10, 3468. [Google Scholar] [CrossRef]
- Raei, Y.; Alami-milani, M. Organic cultivation of medicinal plants: A review. J. Biodivers. Environ. Sci. 2014, 4, 6–18. [Google Scholar]
- Malik, A.A.; Suryapani, S.; Ahmad, J. Chemical vs organic cultivation of medicinal and aromatic plants: The choice is clear. Int. J. Med. Aromat. Plants 2011, 1, 5–13. [Google Scholar]
- Lv, J.; Huang, H.; Yu, L.; Whent, M.; Niu, Y.; Shi, H.; Wang, T.T.Y.; Luthria, D.; Charles, D.; Yu, L.L. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012, 132, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.R.; Mohan Jain, S. (Eds.) Genetic Diversity and Erosion in Plants: Indicators and Prevention; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, ISBN 9783319256351. [Google Scholar]
- Nazari, M.; Zarinkamar, F.; Mohammad Soltani, B.; Niknam, V. Manganese-induced changes in glandular trichomes density and essential oils production of Mentha aquatica L. at different growth stages. J. Trace Elem. Med. Biol. 2018, 50, 57–66. [Google Scholar] [CrossRef]
- Elhindi, K.; Al-Suhaibani, N.A.; Sharaf El-Din, A.F.; Yakout, S.M.; Al-Amri, S.M. Effect of foliar-applied iron and zinc on growth rate and essential oil in sweet basil (Ocimum basilicum L.) under saline conditions. Prog. Nutr. 2016, 18, 288–298. [Google Scholar]
- Jahani, F.; Tohidi-Moghadam, H.R.; Larijani, H.R.; Ghooshchi, F.; Oveysi, M. Influence of zinc and salicylic acid foliar application on total chlorophyll, phenolic components, yield and essential oil composition of peppermint (Mentha piperita L.) under drought stress condition. Arab. J. Geosci. 2021, 14, 691. [Google Scholar] [CrossRef]
- Shoormij, F.; Mirlohi, A.; Saeidi, G.; Shirvani, M. Combined foliar application of Zn and Fe increases grain micronutrient concentrations and alleviates water stress across diverse wheat species and ploidal levels. Sci. Rep. 2022, 12, 20328. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Neofytou, G.; Chrysargyris, A. Nitrogen fertilization coupled with zinc foliar applications modulate the production, quality, and stress response of Sideritis cypria plants grown hydroponically under excess copper concentrations. Plants 2025, 14, 691. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Hussein, M.S.; Abd El-Kader, A.A. Effect of nitrogen fertilizer and/or some foliar application on growth, herb yield, essential oil and chemical composition of dragonhead. J. Med. Food Plants 2010, 2, 12–28. [Google Scholar]
- Chrysargyris, A.; Tzortzakis, N. Iron and zinc foliar spraying affected Sideritis cypria Post. growth, mineral content and antioxidant properties. Plants 2025, 14, 840. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Mehrabani, L.V.; Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr. 2019, 20, 335–345. [Google Scholar] [CrossRef]
- Nasiri, Y.; Zehtab-Salmasi, S.; Nasrullahzadeh, S.; Najafi, N.; Ghassemi-Golezani, K. Effects of foliar application of micronutrients (Fe and Zn) on flower yield and essential oil of chamomile (Matricaria chamomilla L.). J. Med. Plants Res. 2010, 4, 1733–1737. [Google Scholar] [CrossRef]
- Ruiz-Torres, N.; Flores-Naveda, A.; Barriga-Castro, E.D.; Camposeco-Montejo, N.; Ramírez-Barrón, S.; Borrego-Escalante, F.; Niño-Medina, G.; Hernández-Juárez, A.; Garza-Alonso, C.; Rodríguez-Salinas, P.; et al. Zinc oxide nanoparticles and zinc sulfate impact physiological parameters and boosts lipid peroxidation in soil grown coriander plants (Coriandrum sativum). Molecules 2021, 26, 1998. [Google Scholar] [CrossRef]
- Senanayake, S.P.J.N. Rosemary extract as a natural source of bioactive compounds. J. Food Bioact. 2018, 2, 51–57. [Google Scholar] [CrossRef]
- Priss, O.; Yevlash, V. Technology of fresh herbs storage using hydrogel and antioxidant composition. Food Environ. Saf. 2017, 16, 256–261. [Google Scholar]
- Lytra, K.; Tomou, E.; Chrysargyris, A.; Drouza, C.C.; Skaltsa, H.; Tzortzakis, N. Traditionally used Sideritis cypria Post: Phytochemistry, nutritional content, bioactive compounds of cultivated populations. Front. Pharmacol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Ekinci, M.; Ercisli, S.; Ozturk, H.I.; Aydin, M.; Ilhan, E.; Vicas, S.I.; Iancu, C.V.; Gitea, D.; et al. Composition of anthocyanins, specific sugars, and organic acids in wild edible aromatic and medicinal vegetables. Horticulturae 2025, 11, 145. [Google Scholar] [CrossRef]
- Hanoğlu, D.Y.; Hanoğlu, A.; Güvenir, M.; Süer, K.; Demirci, B.; Başer, K.H.C.; Yavuz, D.Ö. Chemical composition and antimicrobial activity of the essential oil of Sideritis cypria Post endemic in Northern Cyprus. J. Essent. Oil Res. 2017, 29, 228–232. [Google Scholar] [CrossRef]
- Karioti, A.; Vrahimi-Hadjilouca, T.; Droushiotis, D.; Rancic, A.; Hadjipavlou-Litina, D.; Skaltsa, H. Analysis of the essential oil of Origanum dubium growing wild in Cyprus. Investigation of its antioxidant capacity and antimicrobial activity. Planta Med. 2006, 72, 1330–1334. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Miltiadous, P.; Tzortzakis, N. Origanum dubium (cypriot oregano) as a promising sanitizing agent against Salmonella enterica and Listeria monocytogenes on tomato and cucumber fruits. Biology 2022, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Karousou, R.; Deirmentzoglou, S. The herbal market of Cyprus: Traditional links and cultural exchanges. J. Ethnopharmacol. 2011, 133, 191–203. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Optimizing nitrogen, phosphorus, and potassium requirements to improve Origanum dubium Boiss. growth, nutrient and water use efficiency, essential oil yield and composition. Ind. Crop. Prod. 2025, 224, 120291. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Nitrogen, phosphorus, and potassium requirements to improve Sideritis cypria growth, nutrient and water use efficiency in hydroponic cultivation. Heliyon 2025, 11, e40755. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Petrovic, J.D.; Tomou, E.; Kyriakou, K.; Xylia, P.; Kotsoni, A.; Gkretsi, V.; Miltiadous, P.; Skaltsa, H.; Sokovi, M.D.; et al. Phytochemical profiles and biological activities of plant extracts from aromatic plants cultivated in Cyprus. Biology 2024, 13, 45. [Google Scholar] [CrossRef]
- Bergstrand, K.-J. Organic fertilizers in greenhouse production systems–a review. Sci. Hortic. 2022, 295, 110855. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A. Olive-mill and grape-mill residue impact the growth, physiology and nutrient status of grapevines young cuttings. Sustain. Chem. Pharm. 2024, 37, 101362. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Číž, M.; Čížová, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Hajisolomou, E.; Neofytou, G.; Petropoulos, S.A.; Tzortzakis, N. The application of conventional and organic fertilizers during wild edible species cultivation: A case study of purslane and common sowthistle. Horticulturae 2024, 10, 1222. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Carrascosa, A.; Pascual, J.A.A.; Ros, M.; Petropoulos, S.A.; Alguacil, M.d.M. Agronomical practices and management for commercial cultivation of Portulaca oleracea as a crop: A review. Plants 2023, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, R.; Hallmann, E.; Rembiałkowska, E. Effects of organic and conventional production systems on the content of bioactive substances in four species of medicinal plants. Biol. Agric. Hortic. 2015, 31, 118–127. [Google Scholar] [CrossRef]
- Peng, L.-C.; Ng, L.-T. Impacts of nitrogen and phosphorus fertilization on biomass, polyphenol contents, and essential oil yield and composition of Vitex negundo Linn. Agriculture 2022, 12, 859. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. Improvement in drought tolerance of lemon balm, Melissa officinalis L. under the pre-treatment of LED lighting. Plant Physiol. Biochem. 2019, 139, 548–557. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Wolf, W.M. The influence of copper and zinc on photosynthesis and phenolic levels in basil (Ocimum basilicum L.), borage (Borago officinalis L.), common nettle (Urtica dioica L.) and peppermint (Mentha piperita L.). Int. J. Mol. Sci. 2024, 25, 3612. [Google Scholar] [CrossRef]
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum (Sorghum bicolour (L.) Moench). African J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar] [CrossRef]
- Aslam, W.; Arfan, M.; Shahid, S.A.; Anwar, F.; Mahmood, Z.; Rashid, U. Effects of exogenously applied Zn on the growth, yield, chlorophyll contents and nutrient accumulation in wheat line L-5066. Int. J. Pharm. Chem. Biol. Sci 2014, 5, 11–15. [Google Scholar]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y.; Guo, L.; Shao, X. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.A.M.; Rady, M.M.; Semida, W.M.; Belal, E.E.; Omran, W.M.; Al-Yasi, H.M.; Ali, E.F. Foliar nourishment with different zinc-containing forms effectively sustains carrot performance in zinc-deficient soil. Agronomy 2021, 11, 1853. [Google Scholar] [CrossRef]
- Xue, Y.; Yan, W.; Gao, Y.; Zhang, H.; Jiang, L.; Qian, X.; Cui, Z.; Zhang, C.; Liu, S.; Wang, H.; et al. Interaction effects of nitrogen rates and forms combined with and without zinc supply on plant growth and nutrient uptake in maize seedlings. Front. Plant Sci. 2021, 12, 722752. [Google Scholar] [CrossRef]
- Roosta, H.R.; Mohsenian, Y. Effects of foliar spray of different Fe sources on pepper (Capsicum annum L.) plants in aquaponic system. Sci. Hortic. 2012, 146, 182–191. [Google Scholar] [CrossRef]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.A.M.; Haque, A.N.A. Impact of foliar application of zinc and zinc oxide nanoparticles on growth, yield, nutrient uptake and quality of tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Alinejad Elahshah, A.; Moradi, H.; Sadeghi, H. Boron and zinc foliar application enhanced the morphophysiological responses and mineral absorption in the hydroponically grown ‘Aromas’ strawberry. J. Plant Nutr. 2023, 46, 3487–3498. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by soil fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef]
- Neofytou, G.; Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Foliar iron and zinc modulate the qualitative and nutritional status of Sideritis cypria with diverse rates of phosphorus in hydroponic cultivation. Agronomy 2025, 15, 1178. [Google Scholar] [CrossRef]
- Bagheri, H.; Ladan Moghadam, A.; Danaee, E.; Abdossi, V. Morphophysiological and phytochemical changes of Mentha piperita using calcium, potassium, iron and manganese nano-fertilizers. Eur. J. Hortic. Sci. 2021, 86, 419–430. [Google Scholar] [CrossRef]
- Rouphael, Y.; Raimondi, G.; Paduano, A.; Sacchi, R.; Barbieri, G.; De Pascale, S. Influence of organic and conventional farming on seed yield, fatty acid composition and tocopherols of Perilla. Aust. J. Crop Sci. 2015, 9, 303–308. [Google Scholar]
- Farnoosh, S.; Masoudian, N.; Safipour Afshar, A.; Nematpour, F.S.; Roudi, B. Foliar-applied iron and zinc nanoparticles improved plant growth, phenolic compounds, essential oil yield, and rosmarinic acid production of lemon balm (Melissa officinalis L.). Environ. Sci. Pollut. Res. 2024, 31, 36882–36893. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var. Sheva). Sci. Hortic. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Pagare, S.S.; Bhatia, M.; Tripathi, N.; Pagare, S.S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Liu, Y.; Lan, X.; Hou, H.; Ji, J.; Liu, X.; Lv, Z. Multifaceted ability of organic fertilizers to improve crop productivity and abiotic stress tolerance: Review and perspectives. Agronomy 2024, 14, 1141. [Google Scholar] [CrossRef]
- Mogazy, A.M.; Hanafy, R.S. Foliar spray of biosynthesized zinc oxide nanoparticles alleviate salinity stress effect on Vicia faba plants. J. Soil Sci. Plant Nutr. 2022, 22, 2647–2662. [Google Scholar] [CrossRef]
- Klofac, D.; Antosovsky, J.; Skarpa, P. Effect of zinc foliar fertilization alone and combined with trehalose on maize (Zea mays L.) growth under the drought. Plants 2023, 12, 2539. [Google Scholar] [CrossRef]
- Liu, Z.; Su, J.; Luo, X.; Meng, J.; Zhang, H.; Li, P.; Sun, Y.; Song, J.; Peng, X.; Yu, C. Nitrogen limits zinc-mediated stimulation of tillering in rice by modifying phytohormone balance under low-temperature stress. Food Energy Secur. 2022, 11, e359. [Google Scholar] [CrossRef]
- Sida-Arreola, J.P.; Sánchez-Chávez, E.; Ávila-Quezada, G.D.; Zamudio-Flores, P.B.; Acosta Muñíz, C.H. Iron biofortification and its impact on antioxidant system, yield and biomass in common bean. Plant Soil Environ. 2015, 61, 573–576. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, X.; Duan, X.; Song, L.; Fan, M.; Wang, C.; Zhao, L. Effects of tillage and substitution of chemical fertilizers with organic fertilizers on leaf physiological characteristics and yield of maize. Int. J. Plant Prod. 2025, 19, 449–467. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; López-Delacalle, M.; Ródenas, R.; Martínez, V.; Rubio, F.; Rivero, R.M. Critical responses to nutrient deprivation: A comprehensive review on the role of ROS and RNS. Environ. Exp. Bot. 2019, 161, 74–85. [Google Scholar] [CrossRef]
- Lay-Pruitt, K.S.; Wang, W.; Prom-u-thai, C.; Pandey, A.; Zheng, L.; Rouached, H. A tale of two players: The role of phosphate in iron and zinc homeostatic interactions. Planta 2022, 256, 23. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Khalilzadeh, R.; Khan, S.; Zaib-un-Nisa; Bashir, R.; Pirzad, A.; Malik, A. Mitigation of drought stress and yield improvement in wheat by zinc foliar spray relates to enhanced water use efficiency and zinc contents. Int. J. Plant Prod. 2021, 15, 377–389. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-talks between macro- and micronutrient uptake and signaling in plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Walder, F.; van der Heijden, M.G.A. Organic management and soil health promote nutrient use efficiency. J. Sustain. Agric. Environ. 2023, 2, 215–224. [Google Scholar] [CrossRef]




| Sideritis cypria | Origanum dubium | |||||
|---|---|---|---|---|---|---|
| Fertilization | Foliar | Fertilization × Foliar | Fertilization | Foliar | Fertilization × Foliar | |
| Plant FW | *** | ns | * | *** | ns | * |
| Plant DW | ns | ns | ns | ns | ns | ns |
| Plant DM | *** | * | ns | *** | * | ns |
| SPAD | ns | ns | ns | ns | ns | ns |
| Chlorophyll a | ns | ns | ns | ** | ** | *** |
| Chlorophyll b | ns | ns | ns | ns | * | *** |
| Total chlorophylls | ns | ns | ns | ns | ** | *** |
| Carotenoids | ns | ns | ns | *** | ns | *** |
| Chl a/Chl b | * | ns | ns | ** | ns | *** |
| Car/Total Chls | ns | ns | ns | ** | ns | *** |
| N | *** | *** | *** | *** | *** | *** |
| P | *** | *** | *** | *** | *** | *** |
| K | *** | *** | *** | *** | *** | *** |
| Na | *** | *** | *** | *** | *** | *** |
| Phenols | *** | *** | *** | * | *** | *** |
| DPPH | *** | *** | *** | * | *** | *** |
| FRAP | *** | ** | *** | ** | *** | ** |
| ABTS | *** | * | *** | *** | *** | *** |
| Flavonoids | *** | * | *** | *** | *** | *** |
| MDA | *** | *** | *** | *** | *** | *** |
| H2O2 | *** | *** | *** | *** | *** | *** |
| Fertilization | Foliar | Plant FW | Plant DW | Plant DM | |
|---|---|---|---|---|---|
| Sideritis cypria | NoFert | H2O | 193.37 ± 13.84 cd | 83.54 ± 7.64 d | 43.60 ± 3.89 cd |
| Fe | 260.43 ± 36.26 abc | 111.08 ± 12.58 bcd | 43.59 ± 1.83 cd | ||
| Zn | 305.58 ± 21.65 ab | 150.09 ± 8.30 a | 49.78 ± 2.66 bc | ||
| OrFert | H2O | 243.12 ± 23.29 bc | 121.30 ± 11.56 abc | 50.06 ± 1.26 bc | |
| Fe | 234.87 ± 22.56 bcd | 126.81 ± 10.40 ab | 54.48 ± 1.57 ab | ||
| Zn | 335.65 ± 37.20 a | 140.88 ± 12.56 ab | 42.58 ± 1.54 d | ||
| CoFert | H2O | 254.80 ± 26.39 abc | 111.34 ± 7.00 bcd | 44.83 ± 2.68 cd | |
| Fe | 224.48 ± 32.52 bcd | 109.50 ± 15.18 bcd | 48.73 ± 2.02 bcd | ||
| Zn | 155.45 ± 15.36 d | 88.63 ± 8.40c | 57.12 ± 1.25 a | ||
| Fertilization | Foliar | Plant FW | Plant DW | Plant DM | |
| Origanum dubium | NoFert | H2O | 164.90 ± 31.92 c | 66.03 ± 13.42 | 40.49 ± 2.48 ab |
| Fe | 209.62 ± 29.20 bc | 84.20 ± 10.19 | 40.60 ± 0.92 ab | ||
| Zn | 172.43 ± 16.10 c | 69.42 ± 6.21 | 40.32 ± 0.83 ab | ||
| OrFert | H2O | 140.42 ± 15.20 c | 61.98 ± 6.81 | 42.73 ± 1.34 a | |
| Fe | 141.53 ± 19.16 c | 58.82 ± 10.11 | 40.73 ± 1.54 ab | ||
| Zn | 234.68 ± 40.20 bc | 74.09 ± 11.39 | 33.74 ± 3.69 b | ||
| CoFert | H2O | 350.77 ± 34.28 a | 93.14 ± 14.24 | 25.73 ± 1.73 c | |
| Fe | 283.75 ± 24.20 ab | 70.95 ± 10.38 | 24.09 ± 2.34 c | ||
| Zn | 287.35 ± 40.72 ab | 70.06 ± 16.53 | 22.18 ± 3.18 c |
| Fertilization | Foliar | SPAD | Chl a | Chl b | Total Chls | Car | Chl a/Chl b | Car/Total Chl | |
|---|---|---|---|---|---|---|---|---|---|
| Sideritis cypria | NoFert | H2O | 56.10 ± 1.65 ab | 0.76 ± 0.04 a | 0.38 ± 0.03 ab | 1.13 ± 0.07 a | 0.118 ± 0.003 | 2.05 ± 0.07 ab | 0.105 ± 0.005 |
| Fe | 67.45 ± 5.21 a | 0.70 ± 0.03 ab | 0.35 ± 0.02 ab | 1.04 ± 0.05 ab | 0.115 ± 0.005 | 2.04 ± 0.05 ab | 0.108 ± 0.003 | ||
| Zn | 49.35 ± 3.69 b | 0.81 ± 0.03 a | 0.42 ± 0.03 a | 1.23 ± 0.05 a | 0.120 ± 0.007 | 1.93 ± 0.09 b | 0.098 ± 0.006 | ||
| OrFert | H2O | 57.98 ± 5.56 ab | 0.71 ± 0.04 ab | 0.35 ± 0.03 ab | 1.06 ± 0.07 ab | 0.110 ± 0.009 | 2.02 ± 0.04 ab | 0.105 ± 0.005 | |
| Fe | 56.75 ± 2.33 ab | 0.72 ± 0.05 ab | 0.37 ± 0.05 ab | 1.10 ± 0.10 ab | 0.110 ± 0.004 | 1.98 ± 0.12 b | 0.103 ± 0.009 | ||
| Zn | 47.30 ± 3.79 b | 0.74 ± 0.02 ab | 0.38 ± 0.01 ab | 1.12 ± 0.03 a | 0.110 ± 0.007 | 1.96 ± 0.03 b | 0.103 ± 0.006 | ||
| CoFert | H2O | 53.68 ± 3.35 ab | 0.72 ± 0.02 ab | 0.34 ± 0.01 ab | 1.06 ± 0.03 ab | 0.115 ± 0.003 | 2.11 ± 0.02 ab | 0.110 ± 0.004 | |
| Fe | 57.45 ± 6.83 ab | 0.62 ± 0.04 b | 0.28 ± 0.02 b | 0.89 ± 0.06 b | 0.103 ± 0.005 | 2.22 ± 0.03 a | 0.115 ± 0.003 | ||
| Zn | 66.73 ± 3.76 a | 0.71 ± 0.06 ab | 0.35 ± 0.05 ab | 1.07 ± 0.10 ab | 0.115 ± 0.003 | 2.06 ± 0.12 ab | 0.113 ± 0.013 | ||
| Fertilization | Foliar | SPAD | Chl a | Chl b | Total chls | Car | Chl a/Chl b | Car/Total chl | |
| Origanum dubium | NoFert | H2O | 36.93 ± 2.96 | 0.76 ± 0.02 de | 0.46 ± 0.01 cd | 1.22 ± 0.03 de | 0.10 ± 0.00 abc | 1.63 ± 0.03 bc | 0.08 ± 0.00 bcd |
| Fe | 40.55 ± 2.49 | 0.84 ± 0.02 ab | 0.61 ± 0.03 a | 1.45 ± 0.04 a | 0.06 ± 0.01 d | 1.39 ± 0.07 d | 0.04 ± 0.01 e | ||
| Zn | 40.23 ± 3.96 | 0.69 ± 0.01 f | 0.37 ± 0.01 e | 1.06 ± 0.02 f | 0.09 ± 0.00 bc | 1.84 ± 0.03 a | 0.08 ± 0.00 abc | ||
| OrFert | H2O | 36.95 ± 3.60 | 0.78 ± 0.03 cde | 0.49 ± 0.04 bcd | 1.26 ± 0.06 cd | 0.09 ± 0.01 c | 1.63 ± 0.08 bc | 0.07 ± 0.01 cd | |
| Fe | 43.95 ± 3.21 | 0.80 ± 0.01 bcd | 0.42 ± 0.01 de | 1.21 ± 0.02 de | 0.10 ± 0.00 abc | 1.91 ± 0.02 a | 0.08 ± 0.00 abc | ||
| Zn | 41.28 ± 2.24 | 0.81 ± 0.01 bc | 0.53 ± 0.03 bc | 1.34 ± 0.04 bc | 0.09 ± 0.01 c | 1.54 ± 0.06 cd | 0.06 ± 0.01 d | ||
| CoFert | H2O | 37.78 ± 3.10 | 0.75 ± 0.01 e | 0.38 ± 0.01 e | 1.13 ± 0.02 ef | 0.11 ± 0.00 ab | 1.98 ± 0.04 a | 0.10 ± 0.00 a | |
| Fe | 38.93 ± 3.22 | 0.80 ± 0.01 bcde | 0.45 ± 0.02 de | 1.24 ± 0.03 cd | 0.11 ± 0.00 a | 1.80 ± 0.09 ab | 0.09 ± 0.00 ab | ||
| Zn | 44.45 ± 2.45 | 0.86 ± 0.00 a | 0.54 ± 0.03 b | 1.40 ± 0.02 ab | 0.09 ± 0.01 abc | 1.62 ± 0.09 bc | 0.07 ± 0.01 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzortzakis, N.; Hajisolomou, E.; Zaravelli, N.; Chrysargyris, A. Foliar Application of Iron and Zinc Affected Aromatic Plants Grown Under Conventional and Organic Agriculture Differently. Horticulturae 2025, 11, 967. https://doi.org/10.3390/horticulturae11080967
Tzortzakis N, Hajisolomou E, Zaravelli N, Chrysargyris A. Foliar Application of Iron and Zinc Affected Aromatic Plants Grown Under Conventional and Organic Agriculture Differently. Horticulturae. 2025; 11(8):967. https://doi.org/10.3390/horticulturae11080967
Chicago/Turabian StyleTzortzakis, Nikolaos, Efraimia Hajisolomou, Nikoletta Zaravelli, and Antonios Chrysargyris. 2025. "Foliar Application of Iron and Zinc Affected Aromatic Plants Grown Under Conventional and Organic Agriculture Differently" Horticulturae 11, no. 8: 967. https://doi.org/10.3390/horticulturae11080967
APA StyleTzortzakis, N., Hajisolomou, E., Zaravelli, N., & Chrysargyris, A. (2025). Foliar Application of Iron and Zinc Affected Aromatic Plants Grown Under Conventional and Organic Agriculture Differently. Horticulturae, 11(8), 967. https://doi.org/10.3390/horticulturae11080967







