Enhancing Soilless Production of Portulaca oleracea, Mesembryanthemum crystallinum and Valerianella locusta Through Nitrogen Form Ratio Optimization and Biostimulant Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Experimental Design
2.3. Sampling and Laboratory Analysis
2.4. Partial Budget Analysis
2.5. Statistical Analyses
3. Results
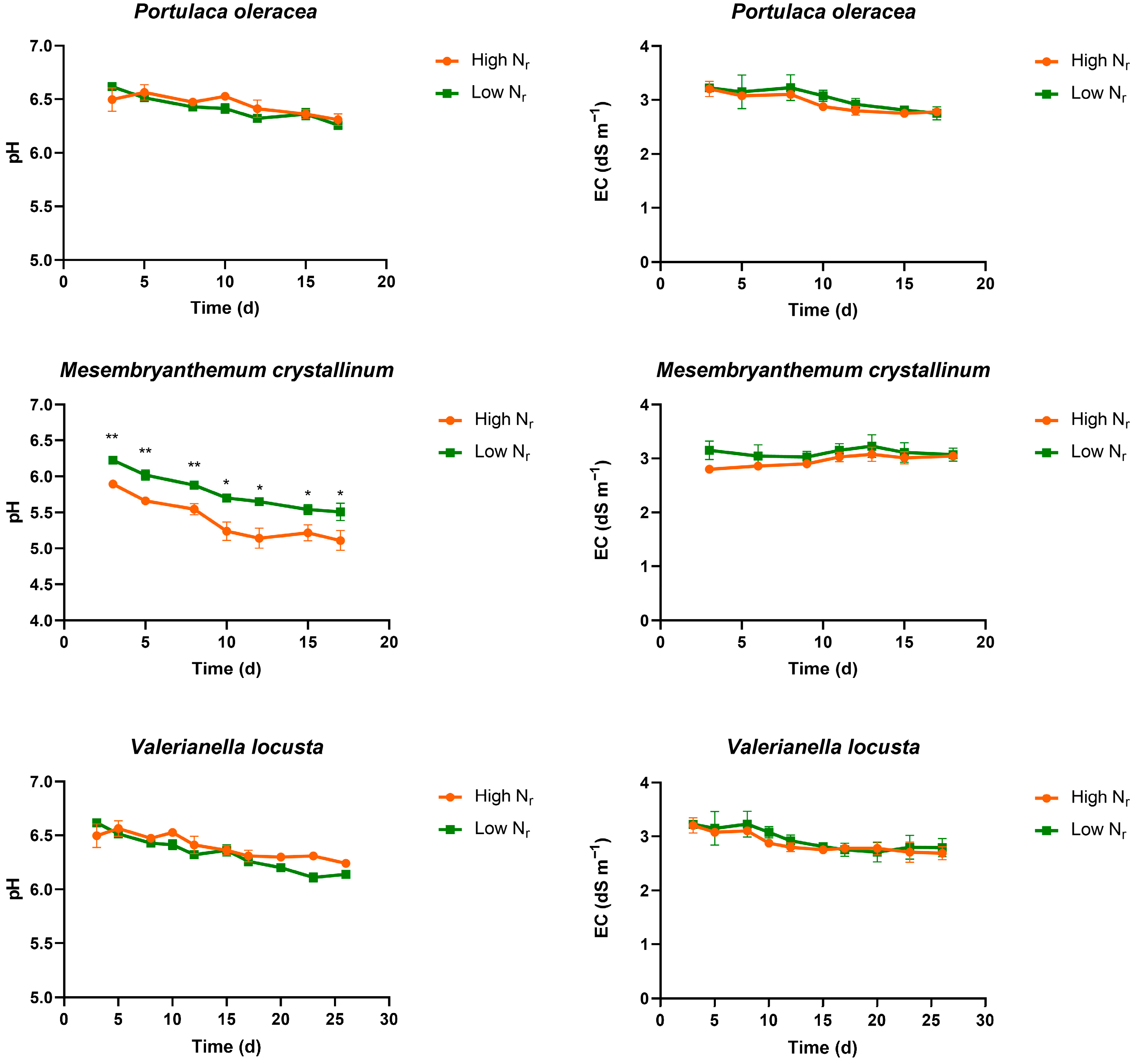
3.1. Evolution of EC and pH in the Drainage Solution
3.2. Yield and Dry Matter Content
3.3. Nitrogen and Leaf Nitrate Content
3.4. Nutrient Concentrations in Plant Tissues
3.5. Partial Budget Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Commission. EU Agricultural Outlook for Markets and Income, 2019–2030; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Pinto, E.; Ferreira, H.; Santos, C.S.; da Silva, M.N.; Styles, D.; Migliorini, P.; Ntatsi, G.; Karkanis, A.; Brémaud, M.F.; de Mey, Y.; et al. Healthier and Sustainable Food Systems: Integrating Underutilised Crops in a ‘Theory of Change Approach.’ In Biodiversity, Functional Ecosystems and Sustainable Food Production; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 275–323. ISBN 9783031074349. [Google Scholar]
- Lowe, N.M. The Global Challenge of Hidden Hunger: Perspectives from the Field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef]
- Peduruhewa, P.S.; Jayathunge, K.G.L.R.; Liyanage, R. Potential of Underutilized Wild Edible Plants as the Food for the Future—A Review. J. Food Secur. 2021, 9, 136–147. [Google Scholar] [CrossRef]
- Platis, D.P.; Papoui, E.; Bantis, F.; Katsiotis, A.; Koukounaras, A.; Mamolos, A.P.; Mattas, K. Underutilized Vegetable Crops in the Mediterranean Region: A Literature Review of Their Requirements and the Ecosystem Services Provided. Sustainability 2023, 15, 4921. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Savvas, D.; Papadopoulou, E.A.; Aliferis, K.A.; Ntatsi, G. Combined Effect of Salt Stress and Nitrogen Level on the Primary Metabolism of Two Contrasting Hydroponically Grown Cichorium spinosum L. Ecotypes. Biomolecules 2023, 13, 607. [Google Scholar] [CrossRef] [PubMed]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of Nitrogen Source and Supply Level on Growth, Yield and Nutritional Value of Two Contrasting Ecotypes of Cichorium spinosum L. Grown Hydroponically. J. Sci. Food Agric. 2018, 98, 1615–1624. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Savvas, D.; Liakopoulos, G.; Karavidas, I.; Ntanasi, T.; Sabatino, L.; Marcelis, L.F.M.; Ntatsi, G. Optimizing Vertical Farm Cultivation of Cichorium spinosum L.: White Light’s Influence and Nutrition Management. Heliyon 2024, 10, e37146. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Karavidas, I.; Savvas, D.; Ntanasi, T.; Kaimpalis, V.; Consentino, B.B.; Aliferis, K.A.; Karkanis, A.; Sabatino, L.; Ntatsi, G. Impact of Nitrogen Limitation, Irrigation Levels, and Nitrogen-Rich Biostimulant Application on Agronomical and Chemical Traits of Hydroponically Grown Cichorium spinosum L. Horticulturae 2024, 10, 1063. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Karavidas, I.; Petropoulos, D.; Zioviris, G.; Fortis, D.; Ntanasi, T.; Ropokis, A.; Karkanis, A.; Sabatino, L.; Savvas, D.; et al. Effects of NaCl and CaCl2 as Eustress Factors on Growth, Yield, and Mineral Composition of Hydroponically Grown Valerianella locusta. Plants 2023, 12, 1454. [Google Scholar] [CrossRef] [PubMed]
- Uddin, K.; Quan, L.; Hasan, M.; Selamat Madom, M. Purslane: A Perspective Plant Source of Nutrition and Antioxidant. Plant Arch. 2020, 20, 1624–1630. [Google Scholar]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A Review on Bioactive Phytochemicals and Ethnopharmacological Potential of Purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.U.; Ali, M.E.; Rahman, M.M. Purslane Weed (Portulaca oleracea): A Prospective Plant Source of Nutrition, Omega-3 Fatty Acid, and Antioxidant Attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.H.; Ghalavand, A.; Mashhadi-Akbar-Boojar, M.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Increased Medicinal Contents of Purslane by Nitrogen and Arbuscular Mycorrhiza under Drought Stress. Commun. Soil Sci. Plant Anal. 2019, 51, 118–135. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; Volke-Haller, V.H.; Trinidad-Santos, A.; Villanueva-Verduzco, C. Change in the Contents of Fatty Acids and Antioxidant Capacity of Purslane in Relation to Fertilization. Sci. Hort. 2018, 234, 152–159. [Google Scholar] [CrossRef]
- Popescu, C.; Popescu, C.; Manea, S.; Vladut, V.; Caba, I.; Covaliu, I.C.; Abbas, H.; Dune, A.; Lupuleasa, D. Study on Portulaca oleracea Native Species as Vegetal Source of Omega-3 and Omega-6 Fatty Acids. Rev. Chim. 2018, 69, 2973–2980. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and Metabolic Changes of Purslane (Portulaca oleracea L.) in Response to Drought, Heat, and Combined Stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef]
- Xing, J.C.; Dong, J.; Wang, M.W.; Liu, C.; Zhao, B.Q.; Wen, Z.G.; Zhu, X.M.; Ding, H.R.; Zhao, X.H.; Hong, L.Z. Effects of NaCl Stress on Growth of Portulaca oleracea and Underlying Mechanisms. Braz. J. Bot. 2019, 42, 217–226. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Zengin, G.; Tzortzakis, N. Purslane (Portulaca oleracea L.) Growth, Nutritional, and Antioxidant Status under Different Nitrogen Levels in Hydroponics. Horticulturae 2024, 10, 1007. [Google Scholar] [CrossRef]
- Loconsole, D.; Murillo-Amador, B.; Cristiano, G.; De Lucia, B. Halophyte Common Ice Plants: A Future Solution to Arable Land Salinization. Sustainability 2019, 11, 6076. [Google Scholar] [CrossRef]
- Barkla, B.J.; Vera-Estrella, R. Single Cell-Type Comparative Metabolomics of Epidermal Bladder Cells from the Halophyte Mesembryanthemum crystallinum. Front. Plant Sci. 2015, 6, 435. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ksouri, R.; Medini, F.; Guyot, S.; Abdelly, C.; Magné, C. Antioxidant Activity and Phenolic Composition of the Medicinal and Edible Halophyte Mesembryanthemum edule L. Ind. Crops Prod. 2011, 34, 1066–1071. [Google Scholar] [CrossRef]
- Eoh, G.; Kim, C.; Bae, J.; Park, J. Evaluation of Sodium Chloride Concentrations on Growth and Phytochemical Production of Mesembryanthemum crystallinum L. in a Hydroponic System. Horticulturae 2024, 10, 1304. [Google Scholar] [CrossRef]
- Mndi, O.; Sogoni, A.; Jimoh, M.O.; Wilmot, C.M.; Rautenbach, F.; Laubscher, C.P. Interactive Effects of Salinity Stress and Irrigation Intervals on Plant Growth, Nutritional Value, and Phytochemical Content in Mesembryanthemum crystallinum L. Agriculture 2023, 13, 1026. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Sousa, J.; Bento-Silva, A.; Duarte, B.; Caçador, I.; Salazar, M.; Mecha, E.; Serra, A.T.; Bronze, M.R. Soilless Cultivated Halophyte Plants: Volatile, Nutritional, Phytochemical, and Biological Differences. Antioxidants 2023, 12, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, P.; Verbeke, W.; Devlieghere, F.; Debevere, J. Consumer Perception and Choice of Minimally Processed Vegetables and Packaged Fruits. Food Qual. Prefer. 2003, 15, 259–270. [Google Scholar] [CrossRef]
- Fontana, E.; Nicola, S. Traditional and Soilless Culture Systems to Produce Corn Salad (Valerianella olitoria L.) and Rocket (Eruca sativa Mill.) with Low Nitrate Content. J. Food Agric. Environ. 2009, 7, 405–410. [Google Scholar]
- Hernández, V.; Ángeles Botella, M.; Hellín, P.; Cava, J.; Fenoll, J.; Mestre, T.; Martínez, V.; Flores, P. Phenolic and Carotenoid Profile of Lamb’s Lettuce and Improvement of the Bioactive Content by Preharvest Conditions. Foods 2021, 10, 188. [Google Scholar] [CrossRef]
- Schmitzer, V.; Senica, M.; Slatnar, A.; Stampar, F.; Jakopic, J. Changes in Metabolite Patterns During Refrigerated Storage of Lamb’s Lettuce (Valerianella locusta L. Betcke). Front. Nutr. 2021, 8, 731869. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Rincón-Cervera, M.A.; González-Fernández, M.J.; Guil-Guerrero, J.L. Phytochemical Composition and Antitumor Activities of New Salad Greens: Rucola (Diplotaxis tenuifolia) and Corn Salad (Valerianella locusta). Plant Foods Hum. Nutr. 2016, 71, 197–203. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002; ISBN 0878938230. [Google Scholar]
- Voogt, W.; Bar-Yosef, B. Water and Nutrient Management and Crops Response to Nutrient Solution Recycling in Soilless Growing Systems in Greenhouses. In Soilless Culture: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–507. ISBN 9780444636966. [Google Scholar]
- Boschiero, B.N.; Mariano, E.; Azevedo, R.A.; Ocheuze Trivelin, P.C. Influence of Nitrate—Ammonium Ratio on the Growth, Nutrition, and Metabolism of Sugarcane. Plant Physiol. Biochem. 2019, 139, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Effect of Seaweed Extract (Ulva rigida) on the Water Deficit Tolerance of Salvia officinalis L. J. Appl. Phycol. 2016, 28, 1363–1370. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Ali, R.M.; Hemida, K.A.; Sayed, M.A. Role of Ulva Lactuca Extract in Alleviation of Salinity Stress on Wheat Seedlings. Sci. World J. 2014, 2014, 847290. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum Nodosum-Based Algal Extracts Act as Enhancers of Growth, Fruit Quality, and Adaptation to Stress in Salinized Tomato Plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Sánchez-Hernández, C.V.; Palmeros-Suárez, P.A.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Meza-Canales, I.D.; Becerril-Espinosa, A. Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy 2022, 12, 2495. [Google Scholar] [CrossRef]
- Sogoni, A.; Ngcobo, B.L.; Jimoh, M.O.; Kambizi, L.; Laubscher, C.P. Seaweed-Derived Bio-Stimulant (Kelpak®) Enhanced the Morphophysiological, Biochemical, and Nutritional Quality of Salt-Stressed Spinach (Spinacia oleracea L.). Horticulturae 2024, 10, 1340. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Zioviris, G.; Ziogas, I.; Karaolani, M.; Fortis, D.; Conesa, M.À.; Schubert, A.; Savvas, D.; Ntatsi, G. Assessment of Growth, Yield, and Nutrient Uptake of Mediterranean Tomato Landraces in Response to Salinity Stress. Plants 2023, 12, 3551. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Svecová, E.; Cardarelli, M.; Rouphael, Y.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a Plant-Derived Protein Hydrolysate to Improve Crop Performances under Different Growing Conditions. Acta Hortic. 2013, 1009, 175–179. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous Pig Blood-Derived Protein Hydrolysates as a Promising Method for Alleviation of Salt Stress in Tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Sorrentino, M.; Panzarová, K.; Spyroglou, I.; Spíchal, L.; Buffagni, V.; Ganugi, P.; Rouphael, Y.; Colla, G.; Lucini, L.; De Diego, N. Integration of Phenomics and Metabolomics Datasets Reveals Different Mode of Action of Biostimulants Based on Protein Hydrolysates in Lactuca sativa L. and Solanum lycopersicum L. Under Salinity. Front. Plant Sci. 2022, 12, 808711. [Google Scholar] [CrossRef]
- Campbell, C.R.; Plank, C.O. Preparation of Plant Tissue for Laboratory Analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press: Boca Raton, FL, USA, 1997; p. 320. ISBN 9780367802233. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Gurav, R.G.; Jadhav, J.P. A Novel Source of Biofertilizer from Feather Biomass for Banana Cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef]
- Spyrou, G.P.; Karavidas, I.; Ntanasi, T.; Marka, S.; Giannothanasis, E.; Gohari, G.; Allevato, E.; Sabatino, L.; Savvas, D.; Ntatsi, G. Chloride as a Partial Nitrate Substitute in Hydroponics: Effects on Purslane Yield and Quality. Plants 2025, 14, 2160. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; ISBN 9789048125326. [Google Scholar]
- Akl, I.A.; Savvas, D.; Papadantonakis, N.; Lydakis-Simantiris, N.; Kefalas, P. Influence of Ammonium to Total Nitrogen Supply Ratio on Growth, Yield and Fruit Quality of Tomato Grown in a Closed Hydroponic System. Eur. J. Hortic. Sci. 2003, 68, 204–211. [Google Scholar] [CrossRef]
- Wenceslau, D.d.S.L.; Daniele, F.d.O.; Hudson, d.O.R.; Guilherme, F.F.; Luiz, A.A.G.; Erica, C.A.L.; Gustavo, C. Nitrate Concentration and Nitrate/Ammonium Ratio on Lettuce Grown in Hydroponics in Southern Amazon. Afr. J. Agric. Res. 2021, 17, 862–868. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; ISBN 978-0-12-384905-2. [Google Scholar]
- Savvas, D.; Passam, H.C.; Olympios, C.; Nasi, E.; Moustaka, E.; Mantzos, N.; Barouchas, P. Effects of Ammonium Nitrogen on Lettuce Grown on Pumice in a Closed Hydroponic System. HortScience 2006, 41, 1667–1673. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Prinsi, B.; Espen, L.; Ferrante, A. Influence of Different Ammonium and Nitrate Ratios on Quality of Rocket. Acta Hortic. 2021, 1321, 103–108. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Optimising Fertigation of Hydroponically Grown Sowthistle (Sonchus oleraceus L.): The Impact of the Nitrogen Source and Supply Concentration. Agric. Water Manag. 2023, 289, 108528. [Google Scholar] [CrossRef]
- Papadimitriou, D.M.; Daliakopoulos, I.N.; Lydakis-Simantiris, N.; Cheiladaki, I.; Manios, T.; Savvas, D. Nitrogen Source and Supply Level Impact Water Uptake, Yield, and Nutrient Status of Golden Thistle in a Soilless Culture. Sci. Hortic. 2024, 336, 113384. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Ammonium to Total Nitrogen Ratio Affects the Purslane (Portulaca oleracea L.) Growth, Nutritional, and Antioxidant Status. Heliyon 2023, 9, e21644. [Google Scholar] [CrossRef] [PubMed]
- Mattson, N.; Lieth, J.H. Liquid Culture Hydroponic System Operation. In Soilless Culture: Theory and Practice Theory and Practice; Elsevier: Amsterdam, The Netherlands, 2019; pp. 567–585. ISBN 9780444636966. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of Crops with Seven Mineral Elements Often Lacking in Human Diets—Iron, Zinc, Copper, Calcium, Magnesium, Selenium and Iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Pagan Loeiro da Cunha-Chiamolera, T.; Chileh-Chelh, T.; Carmona-Fernández, M.; Urrestarazu, M.; Guil-Guerrero, J.L. Growth Parameters, Phytochemicals, and Antitumor Activity of Wild and Cultivated Ice Plants (Mesembryanthemum crystallinum L.). Food Sci. Nutr. 2024, 12, 6548–6562. [Google Scholar] [CrossRef]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H. Effects of Vegetal Protein Hydrolysate Application Method, Nitrogen Level, and Nitrate-to-ammonium Ratio on Growth and Composition of Hydroponic Lettuce. J. Sci. Food Agric. 2025, 105, 599–610. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Bryan, N.S.; Alexander, D.D.; Coughlin, J.R.; Milkowski, A.L.; Boffetta, P. Ingested Nitrate and Nitrite and Stomach Cancer Risk: An Updated Review. Food Chem. Toxicol. 2012, 50, 3646–3665. [Google Scholar] [CrossRef]
- Zhang, F.-X.; Miao, Y.; Ruan, J.-G.; Meng, S.-P.; Dong, J.-D.; Yin, H.; Huang, Y.; Chen, F.-R.; Wang, Z.-C.; Lai, Y.-F. Association Between Nitrite and Nitrate Intake and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2019, 25, 1788–1799. [Google Scholar] [CrossRef]
- Manzocco, L.; Foschia, M.; Tomasi, N.; Maifreni, M.; Dalla Costa, L.; Marino, M.; Cortella, G.; Cesco, S. Influence of Hydroponic and Soil Cultivation on Quality and Shelf Life of Ready-to-Eat Lamb’s Lettuce (Valerianella locusta L. Laterr). J. Sci. Food Agric. 2011, 91, 1373–1380. [Google Scholar] [CrossRef]
- Agusta, H.; Kartika, J.G.; Sari, K.R. Nitrate Concentration and Accumulation on Vegetables Related to Altitude and Sunlight Intensity. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012052. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Xu, H.; Liang, X.; Hu, Y.; Jin, C.; Lu, L.; Lin, X. Short-Term Nitrate Limitation Prior to Harvest Improves Phenolic Compound Accumulation in Hydroponic-Cultivated Lettuce (Lactuca sativa L.) without Reducing Shoot Fresh Weight. J. Agric. Food. Chem. 2018, 66, 10353–10361. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Zhang, Y.; Li, K.; Jin, B.; Lu, L.; Jin, C.; Lin, X. Reduced Nitrogen Supply Enhances the Cellular Antioxidant Potential of Phenolic Extracts through Alteration of the Phenolic Composition in Lettuce (Lactuca sativa L.). J. Sci. Food. Agric. 2019, 99, 4761–4771. [Google Scholar] [CrossRef]
- Voutsinos-Frantzis, O.; Savvas, D.; Antoniadou, N.; Karavidas, I.; Ntanasi, T.; Sabatino, L.; Ntatsi, G. Innovative Cultivation Practices for Reducing Nitrate Content in Baby Leaf Lettuce Grown in a Vertical Farm. Horticulturae 2024, 10, 375. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an Alfalfa Protein Hydrolysate on the Gene Expression and Activity of Enzymes of the Tricarboxylic Acid (TCA) Cycle and Nitrogen Metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa Plant-Derived Biostimulant Stimulate Short-Term Growth of Salt Stressed Zea mays L. Plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant Activity of Two Protein Hydrolyzates in the Growth and Nitrogen Metabolism of Maize Seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Ryan, C.A.; Pearce, G.; Scheer, J.; Moura, D.S. Polypeptide Hormones. Plant Cell 2002, 14, S251–S264. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino Acids and Nitrate as Signals for the Regulation of Nitrogen Acquisition. J. Exp. Bot. 2007, 59, 111–119. [Google Scholar] [CrossRef]
- Fan, X.; Gordon-Weeks, R.; Shen, Q.; Miller, A.J. Glutamine Transport and Feedback Regulation of Nitrate Reductase Activity in Barley Roots Leads to Changes in Cytosolic Nitrate Pools. J. Exp. Bot. 2006, 57, 1333–1340. [Google Scholar] [CrossRef]
- White, P.J. Ion Uptake Mechanisms of Individual Cells and Roots: Short-Distance Transport. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 7–48. [Google Scholar]
| Parameter | Unit | Water | Starter | High Nr | Low Nr |
|---|---|---|---|---|---|
| EC | dS m−1 | 0.30 | 3.00 | 2.40 | 2.30 |
| pH | 7.55 | 5.60 | 5.60 | 5.60 | |
| K+ | mM | 0.00 | 8.72 | 7.43 | 7.43 |
| Ca2+ | mM | 1.00 | 5.25 | 4.10 | 4.10 |
| Mg2+ | mM | 0.20 | 3.42 | 2.29 | 2.29 |
| NH4+ | mM | 0.00 | 0.89 | 1.79 | 0.64 |
| SO42− | mM | 0.20 | 5.02 | 3.88 | 2.73 |
| NO3− | mM | 0.00 | 15.05 | 12.63 | 13.78 |
| P | mM | 0.00 | 1.65 | 1.40 | 1.40 |
| Fe | μM | 0.00 | 40.00 | 25.00 | 25.00 |
| Mn | μM | 0.00 | 5.00 | 8.00 | 8.00 |
| Zn | μM | 0.20 | 7.00 | 5.00 | 5.00 |
| Cu | μM | 0.00 | 0.80 | 0.75 | 0.75 |
| B | μM | 0.00 | 40.00 | 30.00 | 30.00 |
| Cl− | mM | 0.30 | 0.40 | 0.40 | 0.40 |
| Na+ | mM | 0.60 | 0.60 | 0.60 | 0.60 |
| HCO3− | mM | 2.20 | 0.40 | 0.40 | 0.40 |
| Nr | mol:mol | 0.06 | 0.12 | 0.04 |
| Purslane | Iceplant | Corn Salad | |||||
|---|---|---|---|---|---|---|---|
| Nr | Biostimulants | FW | DMC (%) | FW | DMC (%) | FW | DMC (%) |
| High | 152.18 | 4.53 | 79.33 b | 3.37 a | 11.43 a | 9.49 b | |
| Low | 147.29 | 4.37 | 110.72 a | 2.76 b | 9.34 b | 9.98 a | |
| Control | 154.58 | 4.43 | 89.15 c | 3.16 | 10.23 b | 9.72 | |
| SW | 143.38 | 4.43 | 99.05 a | 3.05 | 10.11 b | 9.81 | |
| PH | 151.13 | 4.47 | 97.56 ab | 2.99 | 10.64 a | 9.65 | |
| Interactions | |||||||
| High | Control | 160.93 | 4.53 | 77.96 c | 3.54 | 11.48 a | 9.40 |
| SW | 145.44 | 4.49 | 80.49 c | 3.34 | 11.47 a | 9.54 | |
| PH | 150.84 | 4.57 | 79.45 c | 3.26 | 11.32 a | 9.50 | |
| Low | Control | 149.35 | 4.35 | 97.65 b | 2.85 | 8.8 c | 10.03 |
| SW | 141.57 | 4.38 | 117.61 a | 2.75 | 8.83 c | 10.08 | |
| PH | 151.43 | 4.36 | 120.17 a | 2.66 | 10.22 b | 9.79 | |
| Statistical significance | |||||||
| Nr | NS | NS | *** | *** | *** | *** | |
| Biostimulants | NS | NS | * | NS | * | NS | |
| Nr X Biostimulants | NS | NS | * | NS | ** | NS | |
| Main Effects | Purslane | |||||
| Nr | Biostimulants | Nred (%) | Nmin (%) | Total-N (%) | Nass (%) | NO3− (ppm) |
| High | 4.98 a | 0.37 b | 5.35 a | 93.16 a | 732 b | |
| Low | 4.32 b | 0.47 a | 4.79 b | 90.15 b | 907 a | |
| Control | 4.64 | 0.44 a | 5.07 | 91.35 b | 855 a | |
| SW | 4.60 | 0.45 a | 5.04 | 91.01 b | 873 a | |
| PH | 4.75 | 0.37 b | 5.12 | 92.56 a | 731 b | |
| Statistical significance | ||||||
| Nr | *** | *** | *** | *** | *** | |
| Biostimulants | NS | *** | NS | ** | *** | |
| Nr X Biostimulants | NS | NS | NS | NS | NS | |
| Nr | Biostimulants | Iceplant | ||||
| Nred (%) | Nmin (%) | Total-N (%) | Nass (%) | NO3− (ppm) | ||
| High | 4.85 b | 0.33 b | 5.18 b | 93.52 a | 496 | |
| Low | 5.06 a | 0.40 a | 5.46 a | 92.69 b | 481 | |
| Control | 4.90 b | 0.38 | 5.28 b | 92.78 b | 530 a | |
| SW | 4.72 b | 0.36 | 5.08 b | 92.91 b | 470 b | |
| PH | 5.24 a | 0.36 | 5.60 a | 93.64 a | 457 b | |
| Statistical significance | ||||||
| Nr | * | *** | ** | *** | NS | |
| Biostimulants | *** | NS | *** | ** | ** | |
| Nr X Biostimulants | NS | NS | NS | NS | NS | |
| Nr | Biostimulants | Corn salad | ||||
| Nred (%) | Nmin (%) | Total-N (%) | Nass (%) | NO3− (ppm) | ||
| High | 4.88 | 0.28 b | 5.16 | 94.50 a | 1191 b | |
| Low | 4.73 | 0.31 a | 5.04 | 93.89 b | 1358 a | |
| Control | 4.83 | 0.30 a | 5.13 | 94.15 ab | 1295 a | |
| SW | 4.77 | 0.31 a | 5.08 | 93.92 b | 1340 a | |
| PH | 4.81 | 0.28 b | 5.09 | 94.52 a | 1194 b | |
| Statistical significance | ||||||
| Nr | NS | *** | NS | *** | *** | |
| Biostimulants | NS | *** | NS | ** | *** | |
| Nr X Biostimulants | NS | NS | NS | NS | NS | |
| Main Effects | Purslane | ||||
| Nr | Biostimulants | K (mg g−1) | Ca (mg g−1) | Mg (mg g−1) | Na (mg g−1) |
| High | 63.69 | 3.77 b | 10.81 | 1.38 | |
| Low | 67.00 | 5.40 a | 10.74 | 1.32 | |
| Control | 64.89 | 4.91 | 10.94 | 1.29 | |
| SW | 64.29 | 4.64 | 10.91 | 1.47 | |
| PH | 66.44 | 4.13 | 10.50 | 1.32 | |
| Statistical significance | |||||
| Nr | NS | *** | NS | NS | |
| Biostimulants | NS | NS | NS | NS | |
| Nr X Biostimulants | NS | NS | NS | NS | |
| Nr | Biostimulants | Iceplant | |||
| K (mg g−1) | Ca (mg g−1) | Mg (mg g−1) | Na (mg g−1) | ||
| High | 20.69 | 0.72 | 4.74 | 10.23 | |
| Low | 20.15 | 0.74 | 4.65 | 8.42 | |
| Control | 20.00 | 0.68 | 4.52 | 9.17 | |
| SW | 19.75 | 0.70 | 4.77 | 9.50 | |
| PH | 21.44 | 0.80 | 4.81 | 9.33 | |
| Statistical significance | |||||
| Nr | NS | NS | NS | NS | |
| Biostimulants | NS | NS | NS | NS | |
| Nr X Biostimulants | NS | NS | NS | NS | |
| Nr | Biostimulants | Corn salad | |||
| K (mg g−1) | Ca (mg g−1) | Mg (mg g−1) | Na (mg g−1) | ||
| High | 57.80 | 0.58 | 4.07 | 0.44 | |
| Low | 58.17 | 0.61 | 4.35 | 0.47 | |
| Control | 57.33 | 0.57 | 4.08 | 0.43 | |
| SW | 58.25 | 0.61 | 4.27 | 0.43 | |
| PH | 58.25 | 0.60 | 4.28 | 0.50 | |
| Statistical significance | |||||
| Nr | NS | NS | NS | NS | |
| Biostimulants | NS | NS | NS | NS | |
| Nr X Biostimulants | NS | NS | NS | NS | |
| Main Effects | Purslane | |||
| Nr | Biostimulants | Fe (μg g−1) | Mn (μg g−1) | Zn (μg g−1) |
| High | 64.30 | 75.61 | 49.60 a | |
| Low | 62.49 | 77.28 | 37.89 b | |
| Control | 64.58 | 79.28 | 43.25 | |
| SW | 65.31 | 73.87 | 44.82 | |
| PH | 60.83 | 75.53 | 44.06 | |
| Statistical significance | ||||
| Nr | NS | NS | *** | |
| Biostimulants | NS | NS | NS | |
| Nr X Biostimulants | NS | NS | NS | |
| Nr | Biostimulants | Iceplant | ||
| Fe (μg g−1) | Mn (μg g−1) | Zn (μg g−1) | ||
| High | 170.63 a | 70.38 | 83.04 a | |
| Low | 81.70 b | 70.29 | 59.34 b | |
| Control | 125.12 | 68.80 | 69.82 | |
| SW | 133.74 | 68.04 | 73.16 | |
| PH | 120.48 | 73.92 | 70.81 | |
| Statistical significance | ||||
| Nr | *** | NS | *** | |
| Biostimulants | NS | NS | NS | |
| Nr X Biostimulants | NS | NS | NS | |
| Nr | Biostimulants | Corn salad | ||
| Fe (μg g−1) | Mn (μg g−1) | Zn (μg g−1) | ||
| High | 94.26 a | 86.09 a | 45.62 a | |
| Low | 87.54 b | 78.52 b | 38.59 b | |
| Control | 91.73 | 84.62 | 44.30 | |
| SW | 87.70 | 79.8 | 38.75 | |
| PH | 92.64 | 82.12 | 42.94 | |
| Statistical significance | ||||
| Nr | ** | * | * | |
| Biostimulants | NS | NS | NS | |
| Nr X Biostimulants | NS | NS | NS | |
| Species | Biostimulant | Yield Increase (kg ha−1) | Price (EUR kg−1) | Added Gross Return (EUR ha−1) | Added Variable Cost (EUR ha−1) | Added Net Return (EUR ha−1) | ||
|---|---|---|---|---|---|---|---|---|
| Biostimulant Treatment | Application | Total | ||||||
| Purslane | SW | −2800.0 | 8.5 | −23,800.0 | 81.0 | 200.0 | 281.0 | −24,081.0 |
| PH | −862.5 | 8.5 | −7331.3 | 51.0 | 200.0 | 251.0 | −7582.3 | |
| Iceplant | SW | 2475.0 | 41.0 | 101,475.0 | 81.0 | 200.0 | 281.0 | 101,194.0 |
| PH | 2102.5 | 41.0 | 86,202.5 | 51.0 | 200.0 | 251.0 | 85,951.5 | |
| Corn salad | SW | −30.0 | 17.6 | −528.0 | 81.0 | 200.0 | 281.0 | −809.0 |
| PH | 102.5 | 17.6 | 1804.0 | 51.0 | 200.0 | 251.0 | 1553.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntanasi, T.; Karavidas, I.; Giannothanasis, E.; Spyrou, G.P.; Karaviti, T.; Marka, S.; Napoli, S.; Neocleous, D.; Ntatsi, G. Enhancing Soilless Production of Portulaca oleracea, Mesembryanthemum crystallinum and Valerianella locusta Through Nitrogen Form Ratio Optimization and Biostimulant Application. Horticulturae 2025, 11, 1076. https://doi.org/10.3390/horticulturae11091076
Ntanasi T, Karavidas I, Giannothanasis E, Spyrou GP, Karaviti T, Marka S, Napoli S, Neocleous D, Ntatsi G. Enhancing Soilless Production of Portulaca oleracea, Mesembryanthemum crystallinum and Valerianella locusta Through Nitrogen Form Ratio Optimization and Biostimulant Application. Horticulturae. 2025; 11(9):1076. https://doi.org/10.3390/horticulturae11091076
Chicago/Turabian StyleNtanasi, Theodora, Ioannis Karavidas, Evangelos Giannothanasis, George P. Spyrou, Theoni Karaviti, Sofia Marka, Simona Napoli, Damianos Neocleous, and Georgia Ntatsi. 2025. "Enhancing Soilless Production of Portulaca oleracea, Mesembryanthemum crystallinum and Valerianella locusta Through Nitrogen Form Ratio Optimization and Biostimulant Application" Horticulturae 11, no. 9: 1076. https://doi.org/10.3390/horticulturae11091076
APA StyleNtanasi, T., Karavidas, I., Giannothanasis, E., Spyrou, G. P., Karaviti, T., Marka, S., Napoli, S., Neocleous, D., & Ntatsi, G. (2025). Enhancing Soilless Production of Portulaca oleracea, Mesembryanthemum crystallinum and Valerianella locusta Through Nitrogen Form Ratio Optimization and Biostimulant Application. Horticulturae, 11(9), 1076. https://doi.org/10.3390/horticulturae11091076









