Nanoextract of Zataria multiflora Boiss. Enhances Salt Stress Tolerance in Hydroponically Grown Ocimum basilicum L. var. Genovese
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Experimental Design, and Cultivation System
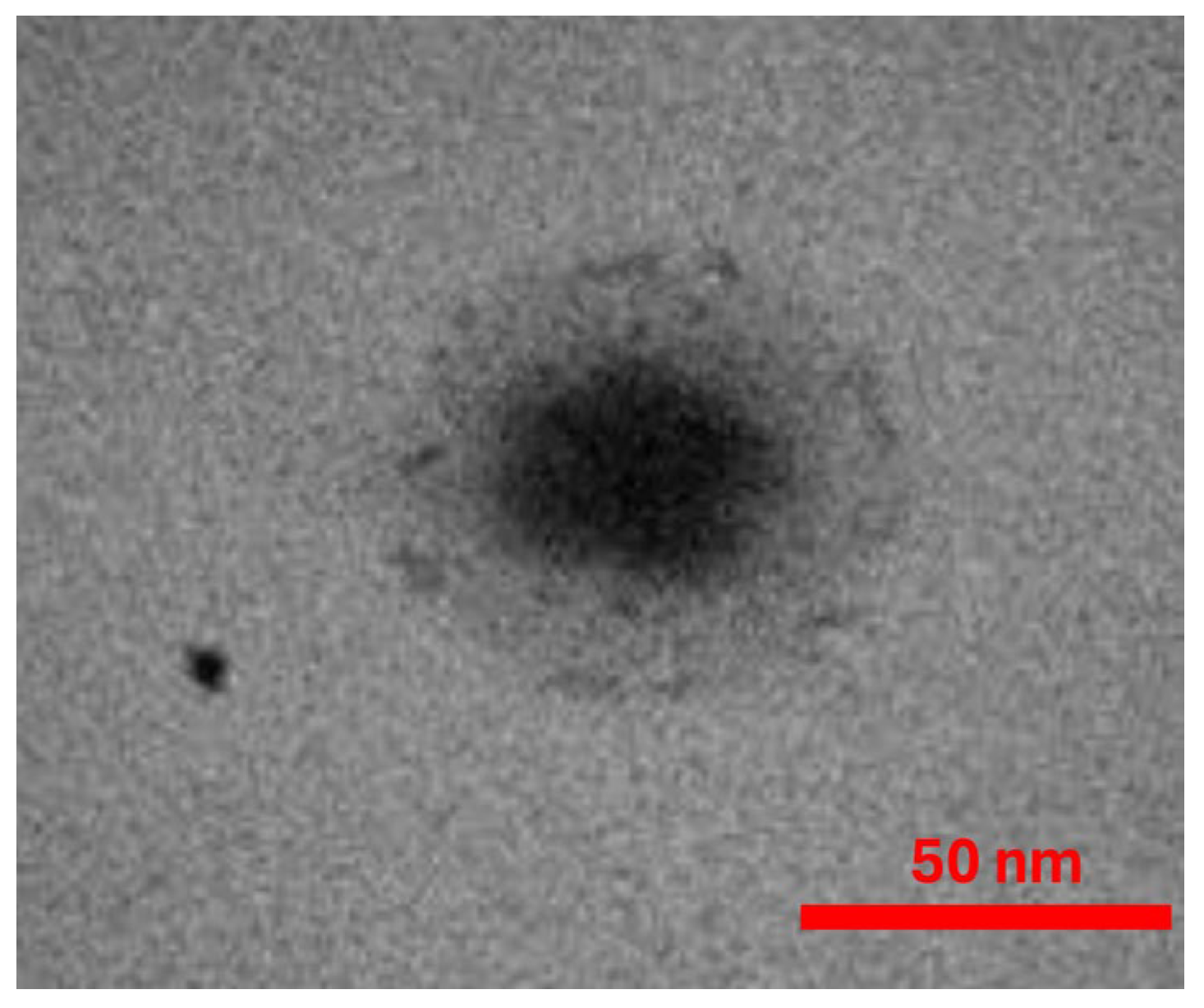
2.2. Preparation of Extracts and Nanoemulsions
2.3. Sampling Methods and Determination Indicators
2.3.1. Total Chlorophyll Content
2.3.2. Total Phenol and Flavonoid Content
2.3.3. Proline Content
2.3.4. Catalase (CAT) and Phenylalanine Ammonia-Lyase (PAL) Enzyme Activity
2.3.5. Antioxidant Activity by DPPH and FRAP Methods
2.3.6. Sodium and Potassium Content
2.4. Essential Oil Extraction and GC-MS Analysis
2.5. Statistical Analysis
3. Results
3.1. Vegetative Traits
3.2. Total Chlorophyll
3.3. Sodium (Na) and Potassium (K) Content
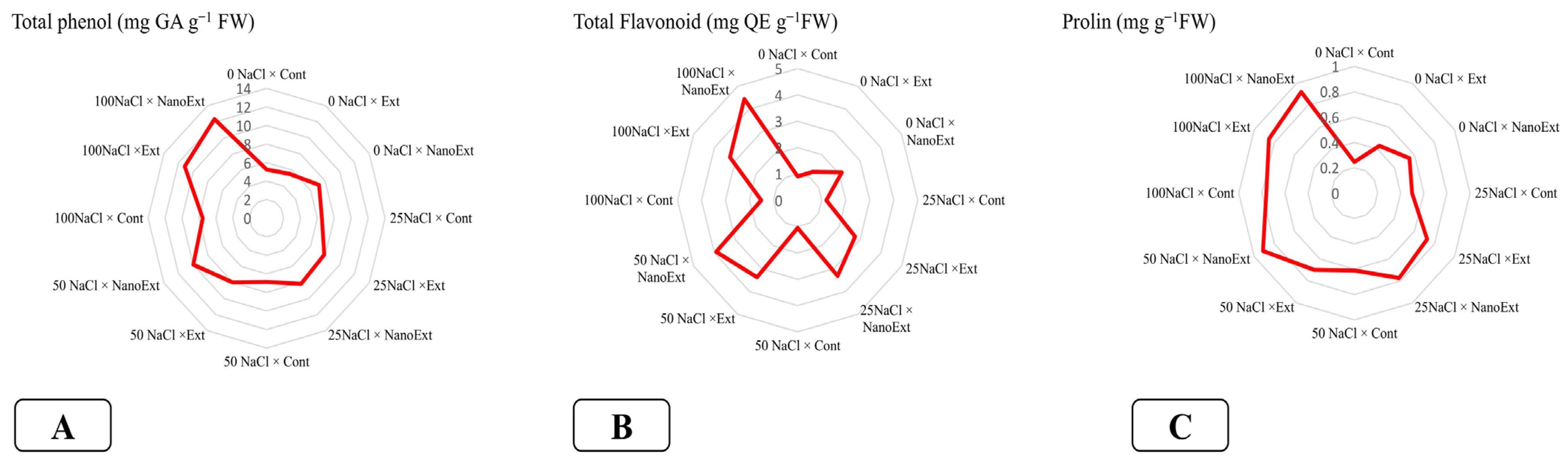
3.4. Total Phenols and Flavonoid Content
3.5. Proline
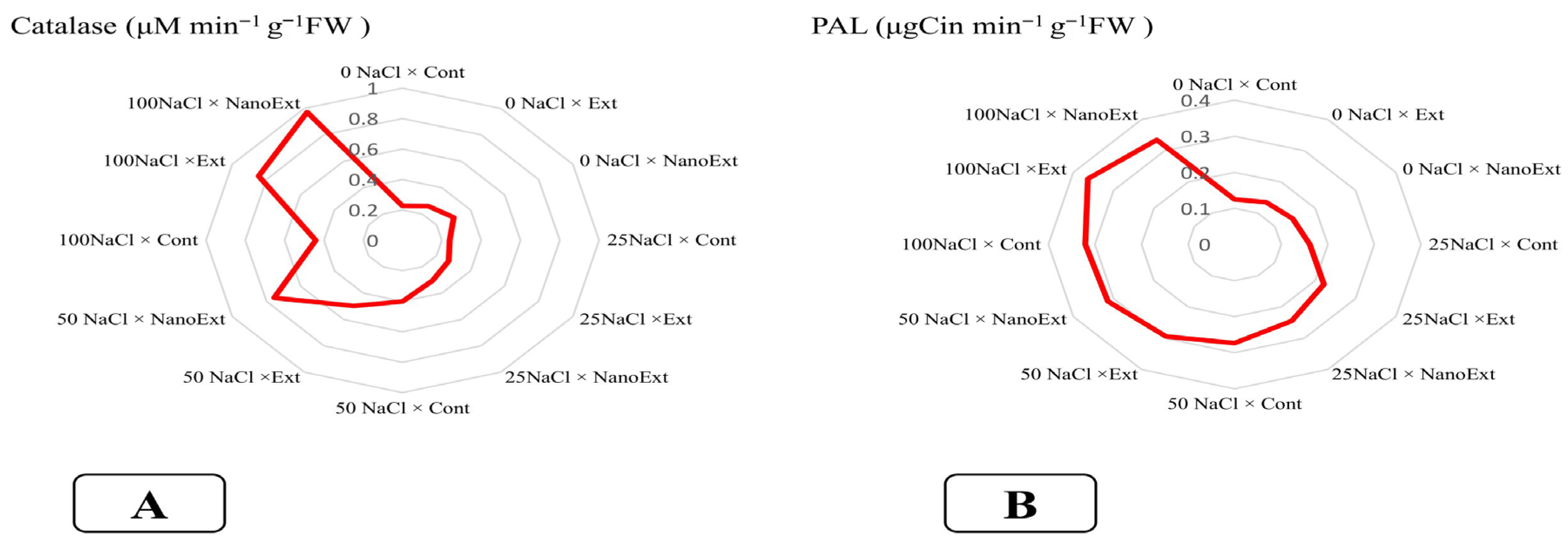
3.6. Catalase Enzyme and Phenolalanine Ammonialyase (PAL) Enzyme
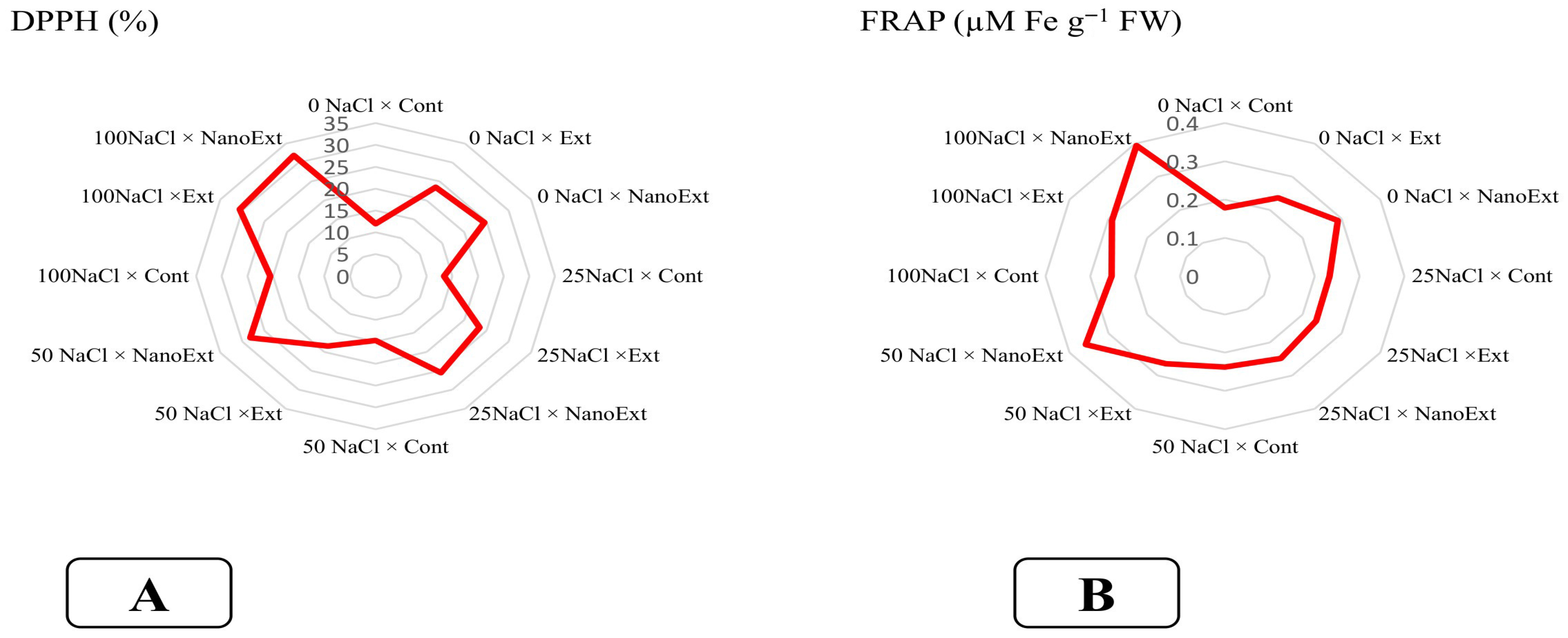
3.7. Antioxidant Activity (DPPH and FARP)
3.8. Identification of Essential Oil Compounds with GC-MS
3.9. Correlation and Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAL | phenol alanine ammonia lyase |
| ROS | reactive oxygen species |
| CAT | catalase enzyme |
| MDA | malondialdehyde |
References
- Saia, S.; Corrado, G.; Vitaglione, P.; Colla, G.; Bonini, P.; Giordano, M.; Fiorillo, A.; Rouphael, Y. An Endophytic Fungi-Based Biostimulant Modulates Volatile and Non-Volatile Secondary Metabolites and Yield of Greenhouse Basil (Ocimum basilicum L.) through Variable Mechanisms Dependent on Salinity Stress Level. Pathogens 2021, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Kanber, R.; Elhindi, K.M.; Alotaibi, M.A. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol. Environ. Saf. 2020, 206, 111396. [Google Scholar] [CrossRef] [PubMed]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in growth parameter and essential oil composition of sage (Salvia officinalis L.) leaves in response to various salt stresses. Ind. Crops Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Jakovljević, D.; Momčilović, J.; Bojović, B.; Stanković, M. The Short-Term Metabolic Modulation of Basil (Ocimum basilicum L. cv. ‘Genovese’) after Exposure to Cold or Heat. Plants 2021, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.S.; Fernandes, C.S.; Sousa-Neto, O.N.; Silva, C.R.; Ferreira, J.F.S.; Sa, F.V.S.; Cosme, C.R.; Souza, A.C.M.S.; Oliveira, A.M.; Batista, C.N.O. Potential agricultural use of reject brine from desalination plants in family farming areas. Saline and Alkaline Soils in Latin America. In Natural Resources, Management and Productive Alternatives; Springer: New York, NY, USA, 2021; pp. 101–118. [Google Scholar] [CrossRef]
- Ghaemi, A.A.; Salimi, M.H.; Tabarza, A. The interaction of fishery effluent and plant residues on the yield and water consumption efficiency of cherry tomatoes under drip irrigation system in the greenhouse. J. Plant Interact. 2016, 8, 41–49. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Kyriacou, M.C.; Rouphael, Y. Successive harvests modulate the productive and physiological behavior of three genovese pesto basil cultivars. Agronomy 2021, 11, 560. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Shabani, E.; Bolandnazar, S.; Tabatabaei, S.J. Magnetized nutrient solution and arbuscular mycorrhizal affect essential oil and physiological aspects of sweet basil (Ocimum basilicum L.) grown in various P concentrations. J. Plant Nutr. 2022, 45, 883–895. [Google Scholar] [CrossRef]
- Maggio, A.; Roscigno, G.; Bruno, M.; De Falco, E.; Senatore, F. Essential-Oil Variability in a Collection of Ocimum basilicum L. Cultivars. Chem. Biodivers. 2016, 13, 1357–1368. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of genovese basil for pesto sauce production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; López-Serrano, M.; Egea-Gilabert, C. An agroindustrial compost as alternative to peat for production of baby leaf red lettuce in a floating system. Sci. Hortic. 2019, 246, 907–915. [Google Scholar] [CrossRef]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of nutrient solution pH on the growth, yield, and quality of Taraxacum officinale and Reichardia picroides in a floating hydroponic system. Agronomy 2021, 11, 1118. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of basil (Ocimum basilicum L.) under different soilless cultures. Adv. Environ. Sci. Technol. 2021, 11, 12754. [Google Scholar] [CrossRef] [PubMed]
- Moncada, A.; Miceli, A.; Vetrano, F. Use of plant growth-promoting rhizobacteria (PGPR) and organic fertilization for soilless cultivation of basil. Sci. Hortic. 2021, 275, 109733. [Google Scholar] [CrossRef]
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic production of tilapia and basil: Comparing a batch and staggered cropping system. SPSCC 2003, 648, 63–69. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Stoia, M.; Oancea, S. Low-molecular-weight synthetic antioxidants: Classification, pharmacological profile, effectiveness and trends. Antioxidants 2022, 11, 638. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Sobarzo-Sánchez, E. Polyphenols: Natural preservatives with promising applications in food, cosmetics and pharma industries; problems and toxicity associated with synthetic preservatives; impact of misleading advertisements; recent trends in preservation and legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef]
- Rathee-Gomes, E.A.; Mejia-da-Silva, L.d.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; de Carvalho, J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Shahid, I.; Mehnaz, S. Microbial secondary metabolites: Effectual armors to improve stress survivability in crop plants. In Microbial Services in Restoration Ecology; Springer: New York, NY, USA, 2020; pp. 47–70. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, S. Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud. Univ. Sci. 2020, 32, 643–647. [Google Scholar] [CrossRef]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Kumaran, A. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Ahari, H.; Yousefi, S. Green synthesis of three-component Ag/AgCl/TiO2 nanocomposite using Zataria Multiflora plant. J. Food Sci. Technol. 2023, 20, 94–112. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. JAFC 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Bonesi, S.; Pimoradloo, E.; Bonesi, M.; Vessal, M. Evaluation of antioxidant potentials and α-amylase inhibition of different fractions of labiatae plants extracts: As a model of antidiabetic compounds properties. BioMed Res. Int. 2017, 2017, 7319507. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Mosavat, N.; Jalali, S.A.H. Essential oils, chemical constituents, antioxidant, antibacterial and in vitro cytotoxic activity of different Thymus species and Zataria multiflora collected from Iran. South Afr. J. Bot. 2020, 130, 250–258. [Google Scholar] [CrossRef]
- Diab, F.; Khalil, M.; Lupidi, G.; Zbeeb, H.; Salis, A.; Damonte, G.; Bramucci, M.; Portincasa, P.; Vergani, L. Influence of simulated in vitro gastrointestinal digestion on the phenolic profile, antioxidant, and biological activity of Thymbra spicata L. extracts. Antioxidants 2022, 11, 1778. [Google Scholar] [CrossRef] [PubMed]
- Solgi, M.; Bagnazari, M.; Mohammadi, M.; Azizi, A. Thymbra spicata extract and arbuscular mycorrhizae improved the morphophysiological traits, biochemical properties, and essential oil content and composition of Rosemary (Rosmarinus officinalis L.) under salinity stress. BMC Plant Biol. 2025, 25, 220. [Google Scholar] [CrossRef]
- Pandey, G. Agri-Nanotechnology for sustainable agriculture. In Ecological and Practical Applications for Sustainable Agriculture; Springer: New York, NY, USA, 2020; pp. 229–249. [Google Scholar] [CrossRef]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Wilkinson, K.J. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Elsadek, M.S.A.; Kordrostami, M.; Tran, L.S.P. Titanium dioxide nanoparticles improve growth and enhance the tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Soleymanzadeh, R.; Iranbakhsh, A.; Habibi, G.; Ardebili, Z.O. Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta Biol. Cracov. Bot. 2020, 62, 33–42. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Caser, M.; Lovisolo, C.; Scariot, V. The influence of water stress on growth, ecophysiology and ornamental quality of potted Primula vulgaris ‘Heidy’ plants. New insights to increase water use efficiency in plant production. J. Plant Biochem. Physiol. 2024, 196, 111–122. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mittal, V.; Wahab, S.; Alsayari, A.; Bin Muhsinah, A.; Almaghaslah, D. Intravenous nanocarrier for improved efficacy of quercetin and curcumin against breast cancer cells: Development and comparison of single and dual drug–loaded formulations using hemolysis, cytotoxicity and cellular uptake studies. Membranes 2022, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- García, D.J.; Fernández-Culma, M.; Upegui, Y.A.; Ríos-Vásquez, L.A.; Quiñones, W.; Ocampo-Cardona, R.; Robledo, S.M. Nanoemulsions for increased penetrability and sustained release of leishmanicidal compounds. Archiv Der Pharmazie. 2023, 356, 2300108. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, V.; Hekmatimoghaddam, S.; Jebali, A.; Khalili Sadrabad, E.; Akrami Mohajeri, F. Chemical composition and antifungal activity of essential oil of Zataria Multiflora. J. Nutr. Food Secur. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Aryaeian, N.; Agh, F.; Nouri, A.; Ghoreishy, S.M.; Ahmadi, A.R.; Dehghanseresht, N.; Morvaridi, M. The effect of Zataria multiflora on respiratory symptoms, pulmonary functions, and oxidative stress parameters: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2025, 25, 1–14. [Google Scholar] [CrossRef]
- Ghazy, O.A.; Fouad, M.T.; Saleh, H.H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and its bioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef] [PubMed]
- Arnon, A.N. Method of extraction of chlorophyll in the plants. Agron. J. 1967, 23, 112–121. [Google Scholar]
- Pandjaitan, N.; Howard, L.R.; Morelock, T.; Gil, M.I. Antioxidant capacity and phenolic content of spinach as affected by genetics and maturation. J. Agric. Food Chem. 2005, 53, 8618–8623. [Google Scholar] [CrossRef]
- Krizek, D.T.; Britz, S.J.; Mirecki, R.M. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cv. New Red Fire lettuce. Physiol. Plant. 1998, 103, 1–7. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. J. Plant Nutr. Soil. Sci. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Method Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Wu, J.; Tan, R. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and Taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide 2006, 15, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tandon, H.L.S. Methods of Analysis of Soils, Plants, Water and Fertilizers; FDCO: New Delhi, India, 1995. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Rezende, R.A.L.S.; Rodrigues, F.A.; Soares, J.D.R.; Silveira, H.R.O.D.; Pasqual, M.; Dias, G.M.G. Nitrogen removal during the cold season by constructed floating wetlands planted with Oenanthe javanica. Mar. Freshw. Res. 2018, 69, 635–647. [Google Scholar] [CrossRef]
- Abdel-Latif, A.; El-Demerdash, F.M. The ameliorative effects of silicon on salt-stressed sorghum seedlings and its influence on the activities of sucrose synthase and PEP carboxylase. Physiol. Plant Pathol. 2017, 5, 2–8. [Google Scholar] [CrossRef]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Marashi, A.M.; Falah, N.A. Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J. Chem. Health Risks 2014, 4, 49–55. [Google Scholar]
- Chehregani Rad, A.; Khorzaman, N.; LariYazdi, H.; Shirkhani, Z. Changes in growth characteristics and physiological indices in Zn-Stressed Phaseolus vulgaris plants on hydroponic medium. J. Dev. Biol. 2016, 8, 31–39. [Google Scholar]
- Mahlooji, M. Effects of salinity stress and Zinc application and some physiological traits on grain filling of three barley cultivars. J. Plant Process. Funct. 2022, 11, 211–227. [Google Scholar]
- Rahmani, Z.; Karimi, M.; Saffari, I.; Mirzaei, H.; Nejati, M.; Sharafati Chaleshtori, R. Nanoemulsion and nanoencapsulation of a hydroethanolic extract of nettle (Urtica dioica) and wormwood (Artemisia absinthium): Comparison of antibacterial and anticancer activity. Front. Chem. 2024, 12, 1266573. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 2021, 160, 257–268. [Google Scholar] [CrossRef]
- Hniličková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil. Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Sofy, M.R.; Elhawat, N.; Alshaal, T. Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol. Environ. Saf. 2020, 200, 110732. [Google Scholar] [CrossRef]
- Summart, J.; Thanonkeo, P.; Panichajakul, S.; Prathepha, P.; McManus, M.T. Effect of salt stress on growth, inorganic ion and proline accumulation in Thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr. J. Biotechnol. 2010, 9, 145–152. [Google Scholar]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. J. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized calcium phosphates as novel macronutrient nano-fertilizers. J. Nanomater. 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Muzolf-Panek, M.; Golinski, P. Phenolic content changes in plants under salt stress. In Ecophysiology and Responses of Plants Under Salt Stress; Springer: New York, NY, USA, 2013; pp. 283–314. [Google Scholar] [CrossRef]
- Firoozeh, R.; Khavarinejad, R.; Najafi, F.; Saadatmand, S. Effects of gibberellin on contents of photosynthetic pigments, proline, phenol and flavonoid in savory plants (Satureja hortensis L.) under salt stress. Iran. J. Plant Physiol. 2019, 31, 894–908. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. J. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Jurkow, R.; Sękara, A.; Pokluda, R.; Smoleń, S.; Kalisz, A. Biochemical response of oakleaf lettuce seedlings to different concentrations of some metal (oid) oxide nanoparticles. Agronomy 2020, 10, 997. [Google Scholar] [CrossRef]
- Amist, N.; Singh, N.B. Responses of enzymes involved in proline biosynthesis and degradation in wheat seedlings under stress. J. Biol. Res. 2017, 42, 195–206. [Google Scholar] [CrossRef]
- Hmidi, D.; Abdelly, C.; Athar, H.U.R.; Ashraf, M.; Messedi, D. Effect of salinity on osmotic adjustment, proline accumulation and possible role of ornithine-δ-ami notransferase in proline biosynthesis in Cakile maritima. Physiol. Mol. Biol. Plants 2018, 24, 1017–1033. [Google Scholar] [CrossRef]
- Sarkar, M.M.; Pradhan, N.; Subba, R.; Saha, P.; Roy, S. Sugar-terminated carbon-nanodots stimulate osmolyte accumulation and ROS detoxification for the alleviation of salinity stress in Vigna radiata. Sci. Rep. 2022, 12, 17567. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Hong-mei, M. Higher plant antioxidants and redox signaling under environ-mental stresses. Comptes Rendus Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Wen, P.F.; Chen, J.Y.; Wan, S.B.; Kong, W.F.; Zhang, P.; Wang, W.; Huang, W.D. Salicylic acid activates phenylalanine ammo-nia-lyase in grape berry in response to high temperature stress. J. Plant Growth Regul. 2008, 55, 1–10. [Google Scholar] [CrossRef]
- Rezaie, R.; Abdollahi Mandoulakani, B.; Fattahi, M. Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci. Rep. 2020, 10, 5290. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V.; Niazi, A.; Moghadam, A. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: From gene to metabolite. J. Hortic. Sci. Biotechnol. 2019, 94, 389–399. [Google Scholar] [CrossRef]
- El Amerany, F. The role of terpenoids in plant development and stress tolerance. In Molecular and Physiological Insights into Plant Stress Tolerance and Applications in Agriculture-Part 2; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 71–98. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Rafeie, M.; Shabani, L.; Sabzalian, M.R.; Gharibi, S. Pretreatment with LEDs regulates antioxidant capacity and polyphenolic profile in two genotypes of basil under salinity stress. Protoplasma 2022, 259, 1567–1583. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Anbazhagan, V.; Dhankher, O.P.; Prasad, P.V. Uptake, translocation, toxicity, and impact of nanoparticles on plant physiological processes. Plants 2024, 13, 3137. [Google Scholar] [CrossRef]
- Lo Presti, M.; Ragusa, S.; Trozzi, A.; Dugo, P.; Visinoni, F.; Fazio, A.; Dugo, G.; Mondello, L. A comparison between different techniques for the isolation of rosemary essential oil. J. Sep. Sci. 2005, 28, 273–280. [Google Scholar] [CrossRef]
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Karray-Bouraoui, N.; Rabhi, M.; Neffati, M.; Baldan, B.; Ranieri, A.; Marzouk, B.; Lachaal, M.; Smaoui, A. Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. Ind. Crop Prod. 2009, 30, 338–343. [Google Scholar] [CrossRef]
- Bernstein, N.; Kravchik, M.; Dudai, N. Salinity-induced changes in essential oil, pigments and salts accumulation in sweet basil (Ocimum basilicum) in relation to alterations of morphological development. Ann. Appl. Biol. 2010, 156, 167–177. [Google Scholar] [CrossRef]

| Treatments | Fresh Weight of Shoot (g) | Dry Weight of Shoot (g) | Fresh Weight of Root (g) | Dry Weight of Root (g) | Total Chlorophyll Content (mg g−1 FW) | Na Content (mg g−1 DW) | K Content (mg g−1 DW) |
|---|---|---|---|---|---|---|---|
| Salinity × Extract spray | |||||||
| 0 NaCl × Cont | 53.35 c | 7.45 c | 76.84 c | 7.42 c | 5.68 d | 0.24 fg | 4.97 c |
| 0 NaCl × Ext | 57.81 b | 8.16 b | 89.18 b | 8.64 b | 7.42 b | 0.23 g | 5.42 b |
| 0 NaCl × NanoExt | 71.64 a | 10.01a | 103.13a | 9.99 a | 8.25 a | 0.20 h | 6.11 a |
| 25 NaCl × Cont | 37.79 f | 5.28 f | 58.46 f | 5.78 f | 4.74 ef | 0.27 e | 4.73 cd |
| 25 NaCl × Ext | 42.01 e | 5.87 e | 64.14 e | 6.27 e | 5.18 de | 0.27 e | 4.82 cd |
| 25 NaCl × NanoExt | 46.83 d | 6.70 d | 70.23 d | 6.86 d | 5.80 cd | 0.25 f | 4.74 cd |
| 50 NaCl × Cont | 25.79 h | 3.60 h | 46.42 g | 4.60 gh | 3.87 g | 0.32 c | 4.55 d |
| 50 NaCl × Ext | 33.03 g | 4.61 g | 48.54 g | 4.70 g | 5.65 d | 0.29 d | 4.81 cd |
| 50 NaCl × NanoExt | 35.56 fg | 4.96 fg | 54.46 f | 5.43 f | 6.49 c | 0.27 e | 4.71 cd |
| 100 NaCl × Cont | 12.10 k | 1.46 k | 26.86 j | 2.80 j | 3.15 h | 0.46 a | 3.59 e |
| 100 NaCl × Ext | 18.06 j | 2.52 j | 33.48 i | 3.30 i | 4.20 fg | 0.37 b | 3.64 e |
| 100 NaCl × NanoExt | 22.35 i | 3.12 i | 41.54 h | 4.20 h | 4.70 ef | 0.36 b | 3.61 e |
| Significance | ** | ** | ** | ** | * | ** | ** |
| Treatment | df | Phenol | Flavonoid | Proline | Catalase | Phenylalanine Ammonia Lyase | DPPH | FRAP |
|---|---|---|---|---|---|---|---|---|
| Block | 2 | 2.05 ** | 0.03 NS | 0.00 NS | 0.006 ** | 0.00 NS | 18.16 * | 0.002 * |
| Salinity (S) | 3 | 29.27 ** | 4.65 ** | 0.31 ** | 0.50 ** | 0.07 ** | 119.25 ** | 0.01 ** |
| Error a | 4 | 0.46 | 0.07 | 0.00 | 0.001 | 0.00 | 3.95 | 0.00 |
| Extract spray (E) | 2 | 11.26 ** | 10.07 ** | 0.19 ** | 0.06 ** | 0.005 ** | 378.77 ** | 0.01 ** |
| S×E | 4 | 1.23 ** | 0.86 ** | 0.007 ** | 0.02 ** | 0.00 * | 19.81 * | 0.002 * |
| Error b | 18 | 0.31 | 0.08 | 0.0002 | 0.00 | 0.00 | 4.68 | 0.00 |
| CV (%) | - | 6.93 | 11.90 | 2.38 | 0.02 | 0.01 | 9.74 | 10.01 |
| 0 NaCl × Cont | 0 NaCl × Ext | 0 NaCl × NanoExt | 25 NaCl × Cont | 25 NaCl × Ext | 25 NaCl × NanoExt | 50 NaCl × Cont | 50 NaCl × Ext | 50 NaCl × NanoExt | 100 NaCl × Cont | 100 NaCl × Ext | 100 NaCl × NanoExt | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | KI | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% | Area% |
| a-Pinene | 939 | 0.14 | 0.32 | 0.45 | 0.61 | 0.85 | 0.54 | 1.25 | 1.12 | 1.36 | 0.63 | 0.85 | 0.93 |
| Sabinene | 975 | 0.97 | 1.12 | 1.36 | 1.49 | 1.64 | 1.96 | 2.36 | 1.39 | 0.48 | 0.63 | 0.78 | 0.96 |
| Myrcene | 989 | 0.41 | 0.36 | 0.89 | 0.92 | 1.26 | 0.15 | 0.54 | 0.64 | 0.78 | 0.37 | 0.54 | 0.24 |
| 1,8-Cineol | 1030 | 1.52 | 1.45 | 2.58 | 3.26 | 2.31 | 2.13 | 3.79 | 2.15 | 1.65 | 2.98 | 2.45 | 2.47 |
| Linalool | 1088 | 12.3 | 13.25 | 15.3 | 22.64 | 18.26 | 21.26 | 22.25 | 23.13 | 19.94 | 21.47 | 22.68 | 23.91 |
| Eugenol | 1355 | 2.4 | 3.4 | 3.85 | 3.95 | 5.84 | 6.84 | 7.26 | 7.96 | 5.95 | 4.78 | 4.55 | 4.15 |
| Camphor | 1145 | 0.74 | 0.89 | 0.65 | 0.74 | 0.61 | 0.64 | 0.97 | 1.14 | 8.56 | 5.14 | 6.37 | 6.29 |
| Terpinen-4-ol | 1177 | 0.41 | 0.61 | 0.74 | 0.85 | 0.45 | 0.79 | 0.85 | 1.29 | 2.14 | 1.34 | 2.78 | 2.18 |
| a-Terpineol | 1188 | 1.54 | 1.2 | 2.3 | 3.2 | 3.28 | 2.15 | 1.02 | 2.46 | 3.26 | 2.96 | 3.79 | 2.65 |
| Cis-Carveol | 1229 | 0.65 | 0.64 | 0.78 | 0.64 | 0.79 | 0.75 | 0.63 | 0.98 | 1.24 | 2.78 | 2.36 | 2.06 |
| Geraniol | 1265 | 0.41 | 0.18 | 0.52 | 0.56 | 0.14 | 0.62 | 2.36 | 0 | 0 | 0 | 0 | 0 |
| Cubenol | 1511 | 2.7 | 1.36 | 1.25 | 1.39 | 2.31 | 1.12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Epi-a-Muurolol | 1642 | 1.47 | 1.78 | 1.97 | 3.24 | 5.6 | 2.03 | 0 | 0 | 0 | 0 | 0 | 0 |
| E-B-Ocimene | 1045 | 2.1 | 1.2 | 2.5 | 2.6 | 3.1 | 1.46 | 1.25 | 2.15 | 1.02 | 3.98 | 3.94 | 4.18 |
| Carvone | 1243 | 15.9 | 16.6 | 16.4 | 18.23 | 9.24 | 12.4 | 14.26 | 13.6 | 7.6 | 7.63 | 7.37 | 7.7 |
| 6-Methyl-5-hepten-2-one | 989 | 0.35 | 0.21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
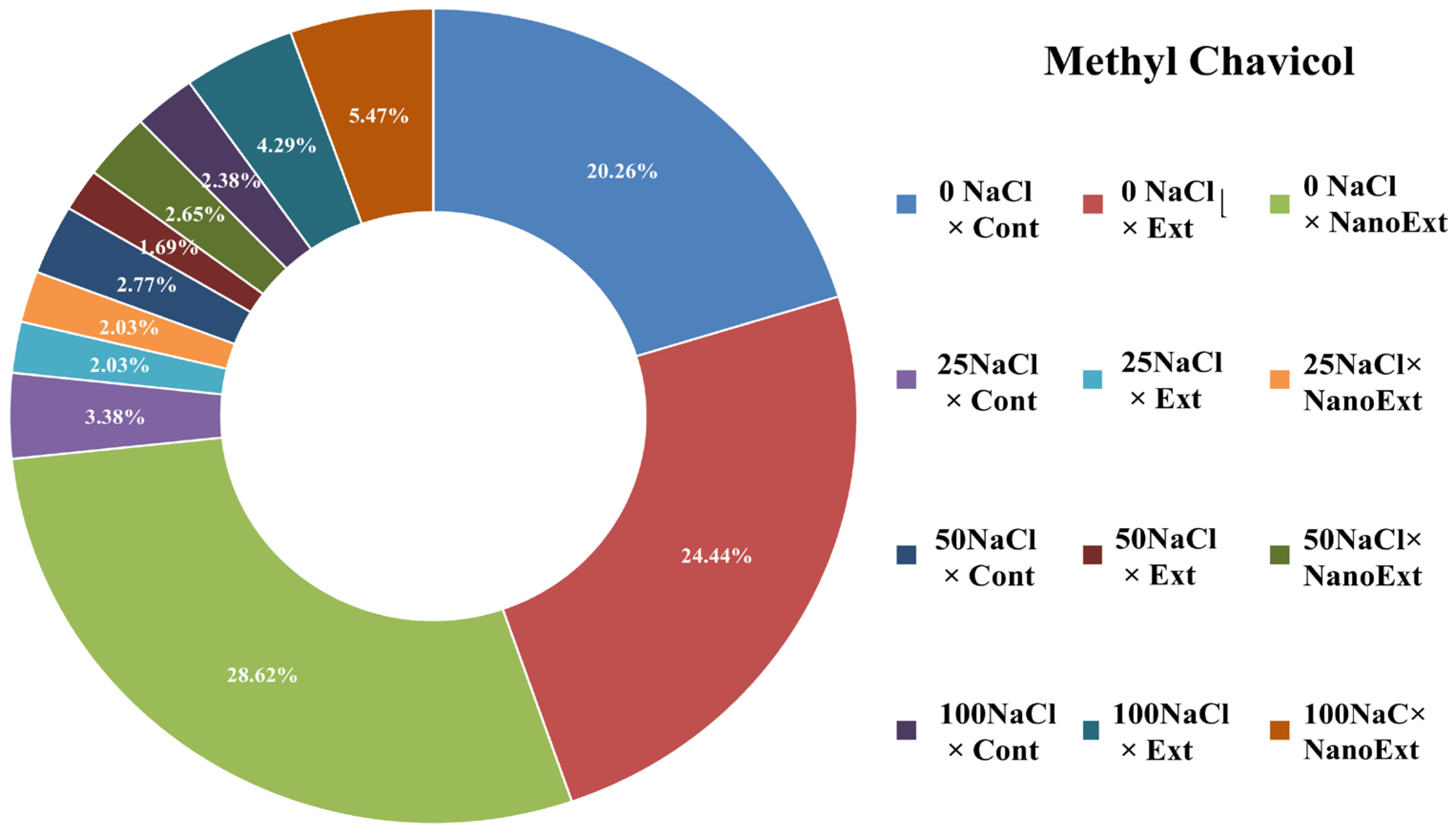
| Methyl Chavicol | 1196 | 12.6 | 15.2 | 17.8 | 2.1 | 1.26 | 1.26 | 1.72 | 1.05 | 1.65 | 1.48 | 2.67 | 3.4 |
| Nero | 1230 | 0.62 | 0.85 | 1.25 | 1.24 | 2.12 | 1.02 | 2.36 | 3.65 | 2.15 | 1.96 | 1.64 | 1.85 |
| Neral | 1242 | 0.52 | 0.62 | 0.65 | 0.89 | 3.16 | 0.96 | 0.89 | 0.85 | 0.89 | 0.52 | 0.53 | 0.63 |
| A-Humulene | 1456 | 10.2 | 8.25 | 5.12 | 4.29 | 2.12 | 2.31 | 1.56 | 1.36 | 2.16 | 3.46 | 2.98 | 3.26 |
| Actanol acetate | 1213 | 0.5 | 0.52 | 0.62 | 0.89 | 1.21 | 0 | 0 | 0 | 0.39 | 0 | 0 | 0 |
| 1-Octen-3-ol | 980 | 0.35 | 0.41 | 0.23 | 0.27 | 0.36 | 0 | 0 | 0 | 0.46 | 0 | 0 | 0 |
| Geranial | 1272 | 6.2 | 0.74 | 0.84 | 0.82 | 0.8 | 1.96 | 1.36 | 1.96 | 0.52 | 0.45 | 0.63 | 0.59 |
| E-Caryophyllene | 1418 | 0.5 | 0.65 | 0.79 | 0.8 | 0.75 | 0.61 | 0.98 | 1.97 | 0.17 | 0.52 | 0.78 | 0.64 |
| Isopulego | 1150 | 0.41 | 0.12 | 0.15 | 0 | 0 | 0.45 | 0.45 | 0.89 | 0.61 | 0.61 | 0.96 | 0.79 |
| Italicence ether | 1537 | 0.65 | 0.87 | 1.25 | 1.24 | 1.25 | 2.89 | 1.26 | 1.52 | 0.36 | 0.89 | 1.02 | 1.36 |
| B-Pinene | 979 | 1.4 | 2.14 | 1.36 | 4.58 | 3.46 | 2.16 | 0.84 | 0.94 | 0.34 | 0.98 | 2.16 | 1.03 |
| Menthol | 1171 | 4.2 | 5.6 | 6.2 | 6.85 | 9.03 | 9.25 | 10.39 | 11.26 | 10.15 | 11.57 | 12.48 | 13.62 |
| DeltGuaie | 1025 | 0.95 | 0.89 | 0.65 | 0.45 | 0 | 0.45 | 0.65 | 0.64 | 0.12 | 1.36 | 1.65 | 2.49 |
| Cabbenol | 1136 | 1.14 | 2.15 | 0.46 | 0.35 | 0 | 0 | 0.45 | 0.35 | 0 | 0 | 0 | 0 |
| y-Eudesmol | 1145 | 0 | 0 | 0 | 0.12 | 0 | 0 | 0 | 0.6 | 0 | 0.78 | 1.79 | 0 |
| tau.-Cadinol | 1026 | 0 | 0 | 0 | 0 | 0.23 | 0 | 0 | 0 | 0 | 0.25 | 1.25 | 0.37 |
| y-Cadinene | 856 | 0 | 0 | 0 | 0 | 0.56 | 0 | 0 | 0 | 0 | 0 | 0 | 0.28 |
| Isothymol methyl ether | 1234 | 0 | 0 | 0 | 0 | 1.07 | 0 | 0 | 0 | 0 | 0 | 0 | 0.96 |
| (+)-2-Carene | 1126 | 0 | 0 | 0 | 0 | 0.21 | 1.63 | 0 | 0 | 0 | 0.16 | 0 | 0.19 |
| 6-Cadinene, (+)- | 975 | 0 | 0 | 0 | 0 | 0 | 1.3 | 1.25 | 0.48 | 2.03 | 1.89 | 0.48 | 0 |
| endo-Borneol | 1596 | 0 | 0 | 0 | 0 | 0 | 0 | 3.64 | 0.41 | 2.67 | 1.96 | 0.52 | 0.97 |
| Caryophyllene oxide | 1138 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.18 | 2.65 | 1.12 | 0.32 | 0.89 |
| 84.25 | 83.58 | 88.91 | 89.21 | 83.27 | 81.09 | 86.59 | 86.12 | 81.3 | 84.65 | 90.32 | 91.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabani, E.; Ghanbari, F.; Azizi, A.; Helalipour, E.; Caser, M. Nanoextract of Zataria multiflora Boiss. Enhances Salt Stress Tolerance in Hydroponically Grown Ocimum basilicum L. var. Genovese. Horticulturae 2025, 11, 970. https://doi.org/10.3390/horticulturae11080970
Shabani E, Ghanbari F, Azizi A, Helalipour E, Caser M. Nanoextract of Zataria multiflora Boiss. Enhances Salt Stress Tolerance in Hydroponically Grown Ocimum basilicum L. var. Genovese. Horticulturae. 2025; 11(8):970. https://doi.org/10.3390/horticulturae11080970
Chicago/Turabian StyleShabani, Edris, Fardin Ghanbari, Afsaneh Azizi, Elham Helalipour, and Matteo Caser. 2025. "Nanoextract of Zataria multiflora Boiss. Enhances Salt Stress Tolerance in Hydroponically Grown Ocimum basilicum L. var. Genovese" Horticulturae 11, no. 8: 970. https://doi.org/10.3390/horticulturae11080970
APA StyleShabani, E., Ghanbari, F., Azizi, A., Helalipour, E., & Caser, M. (2025). Nanoextract of Zataria multiflora Boiss. Enhances Salt Stress Tolerance in Hydroponically Grown Ocimum basilicum L. var. Genovese. Horticulturae, 11(8), 970. https://doi.org/10.3390/horticulturae11080970








