Integrated Assays and Microscopy to Study the Botrytis cinerea–Strawberry Interaction Reveal Tissue-Specific Stomatal Penetration
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Fungal Transformation
2.3. B. cinerea Transformant Evaluation
2.4. Confocal Microscopy
2.5. Scanning Electron Microscopy
2.6. Strawberry Plant Material
2.7. Axenic Strawberry Plant Material
2.8. Pathogenicity Assays—General
2.9. Pathogenicity Assays—Leaves
2.10. Pathogenicity Assays—Detached Petals
2.11. Pathogenicity Assays—Compound Fruit
2.12. Image Analysis of Infected Plant Material
2.13. Chlorophyll Image Analysis
2.14. DNA Extractions
2.15. Fungal Biomass Quantification in Petals by qPCR
2.16. Statistical Analysis
3. Results
3.1. B. cinerea GFP Transformant Has Typical Morphology and Pathogenicity
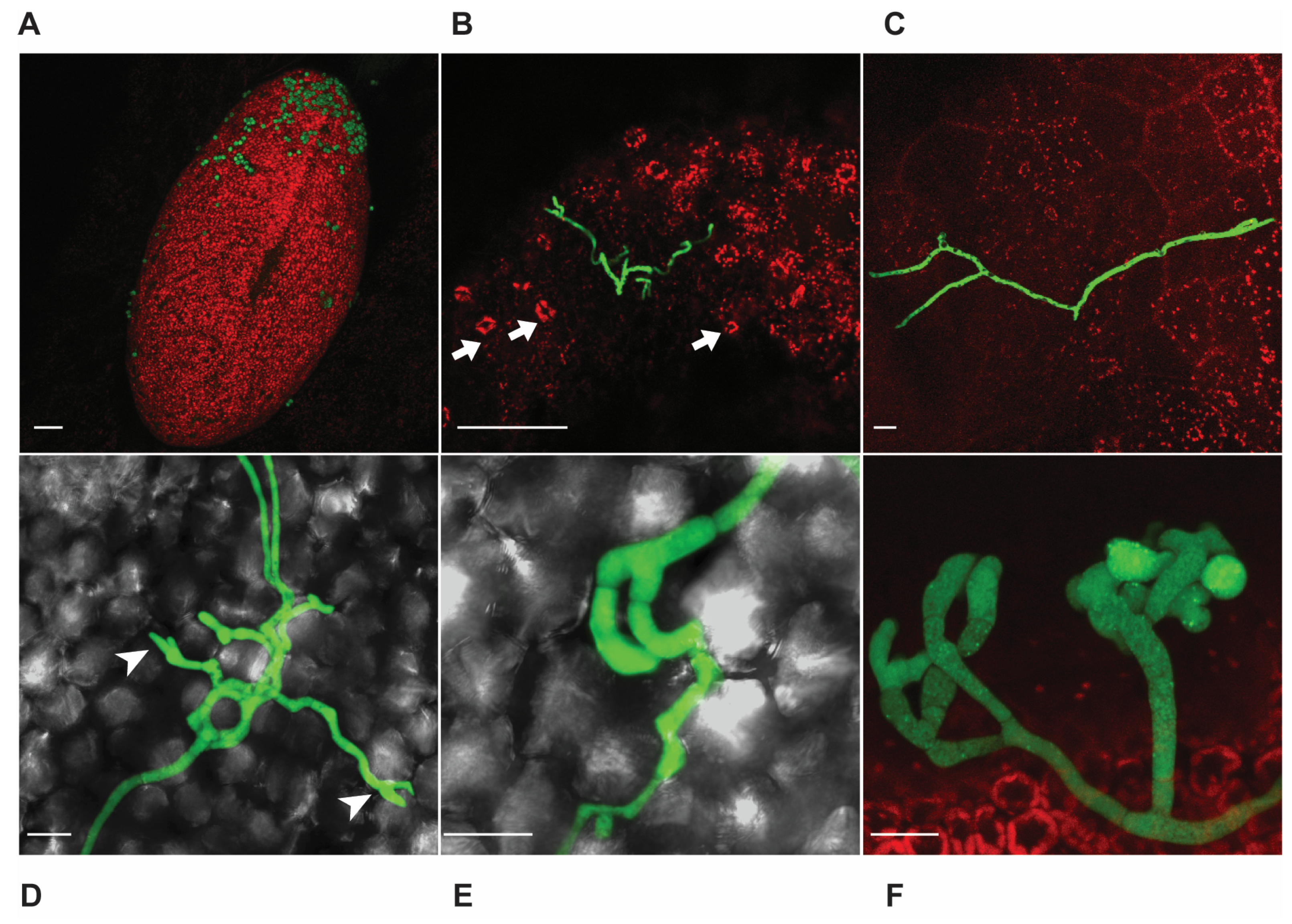
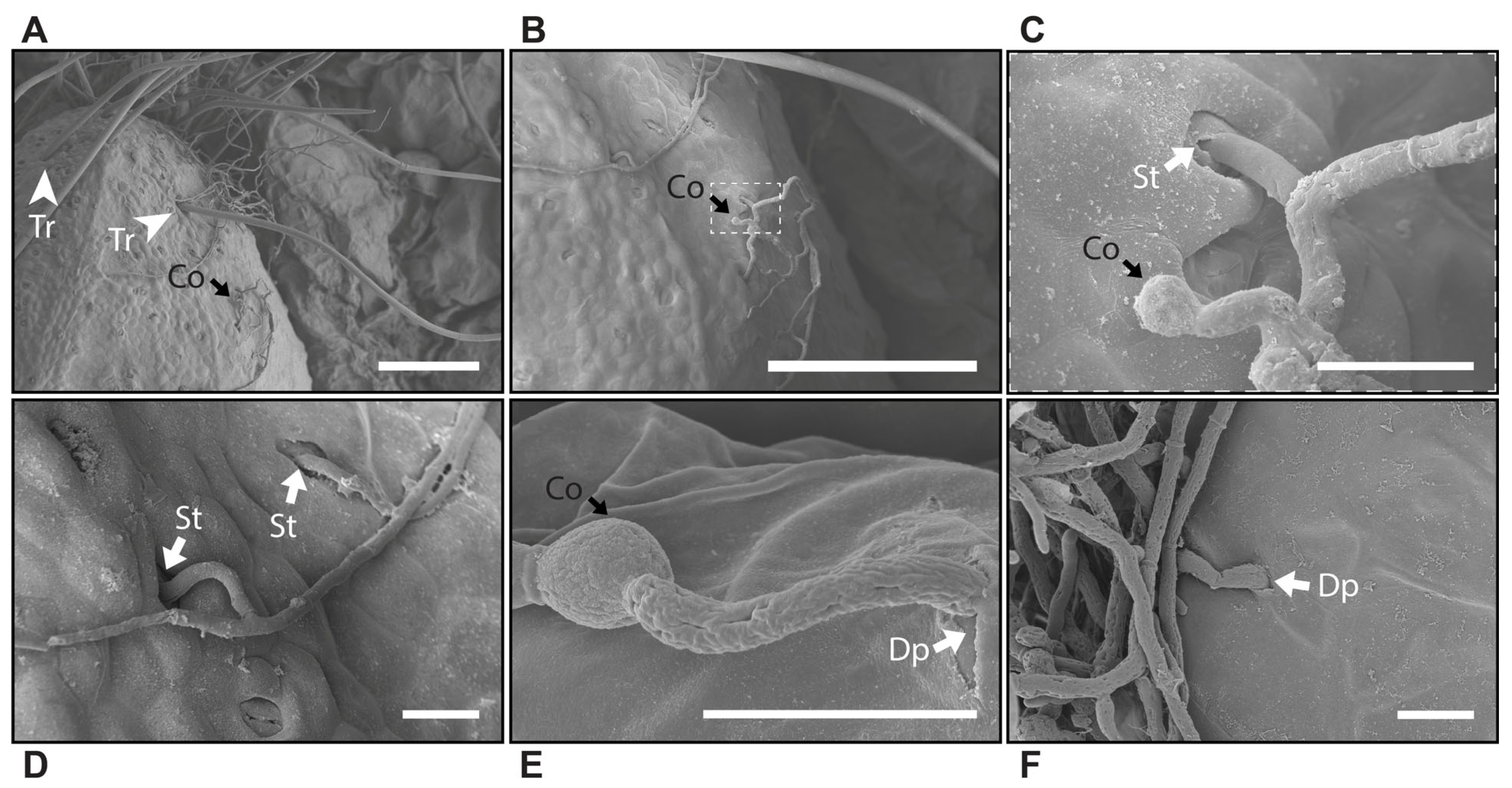
3.2. Microscopy Reveals Different Modes of B. cinerea Penetration in Strawberry Tissues
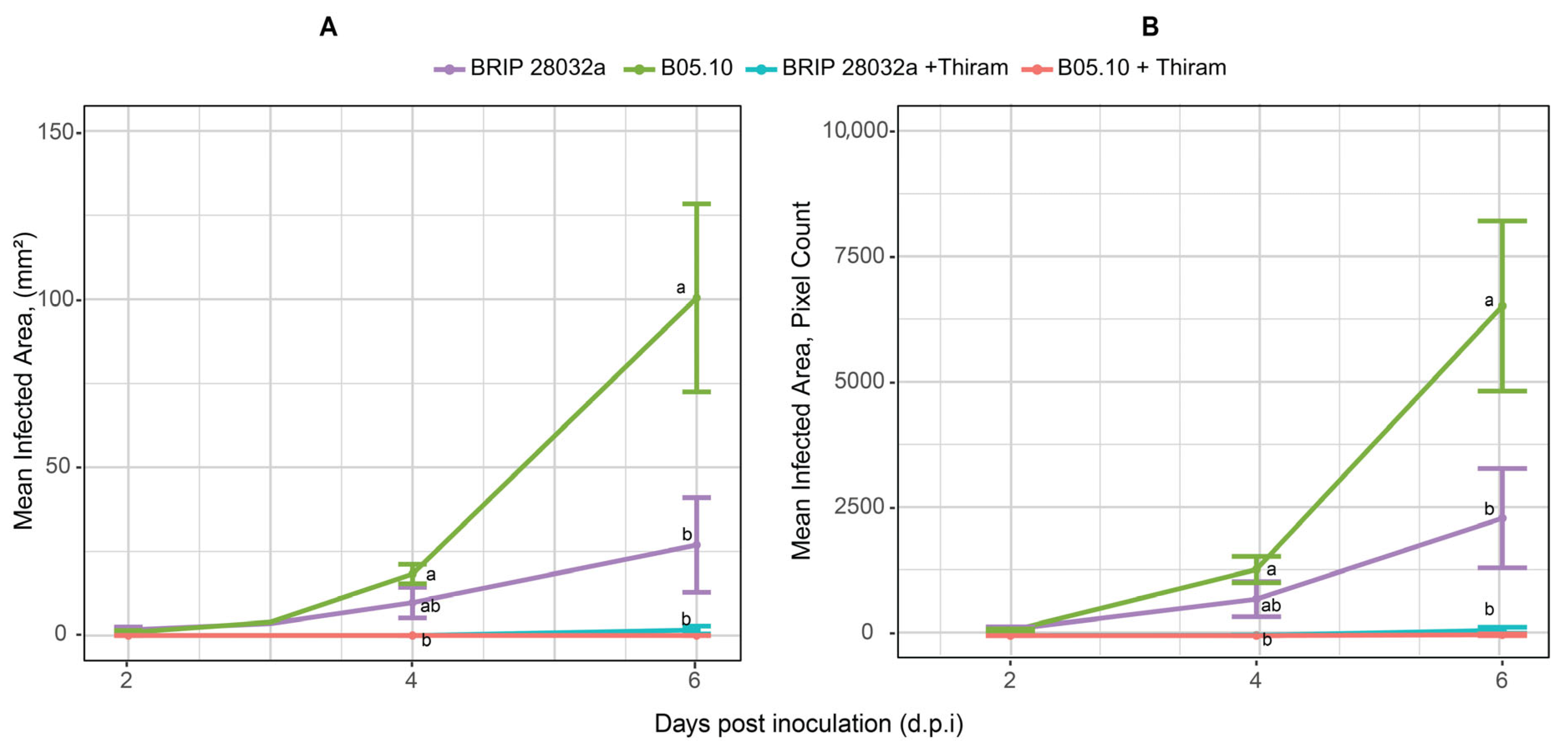
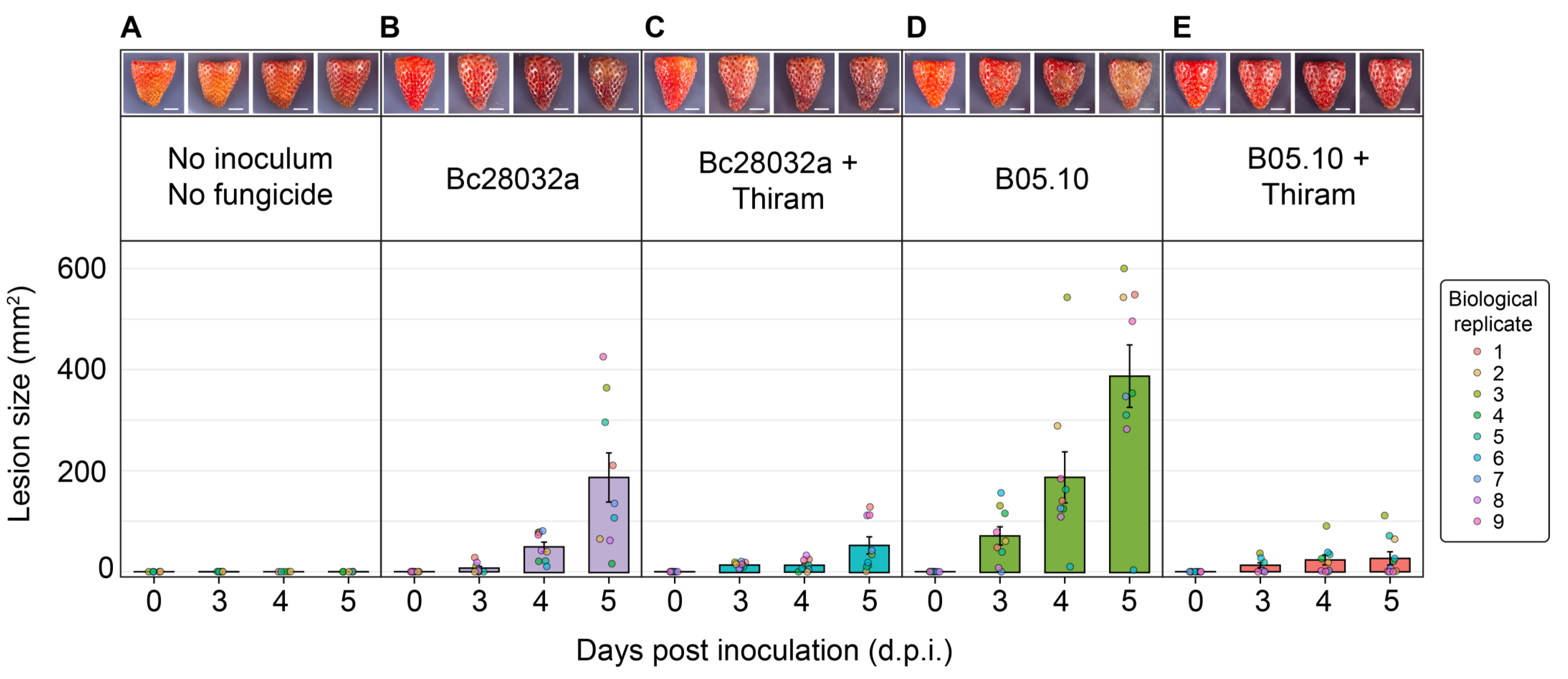
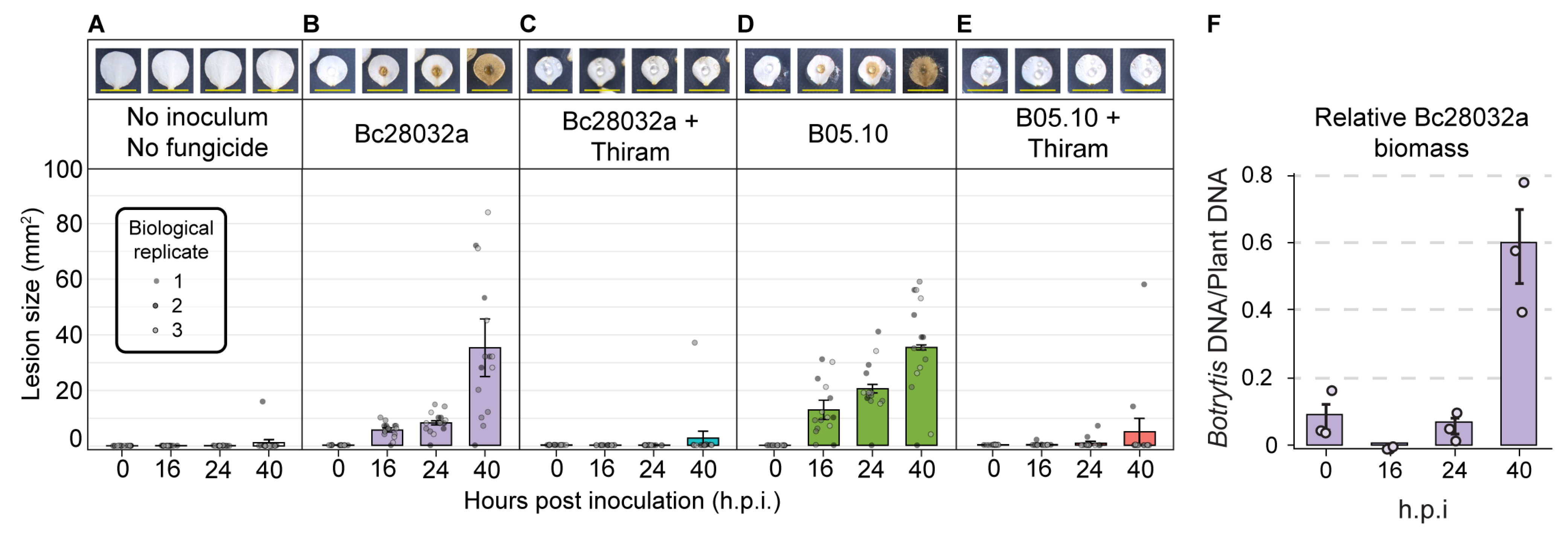
3.3. In Vitro Assays Allow for a Sensitive Measurement of B. cinerea Disease Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Samykanno, K.; Pang, E.; Marriott, P.J. Chemical characterisation of two Australian-grown strawberry varieties by using comprehensive two-dimensional gas chromatography-mass spectrometry. Food Chem. 2013, 141, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- FAO. Crops and Livestock Products. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 August 2024).
- Hort Innovation. Berry Strategic Investment Plan 2022–2026; Hort Innovation: North Sydney, NSW, Australia, 2021. [Google Scholar]
- Samtani, J.B.; Rom, C.R.; Friedrich, H.; Fennimore, S.A.; Finn, C.E.; Petran, A.; Wallace, R.W.; Pritts, M.P.; Fernandez, G.; Chase, C.A.; et al. The Status and Future of the Strawberry Industry in the United States. HortTechnology 2019, 29, 11–24. [Google Scholar] [CrossRef]
- Jarvis, W.R. Botryotinia and Botrytis Species: Taxonomy, Physiology, and Pathogenicity: A Guide to the Literature; Research Branch, Canada Department of Agriculture: Ottawa, ON, Canada, 1977. [Google Scholar]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis spp. and Diseases They Cause in Agricultural Systems—An Introduction. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–8. [Google Scholar]
- Singh, R.; Caseys, C.; Kliebenstein, D.J. Genetic and molecular landscapes of the generalist phytopathogen Botrytis cinerea. Mol. Plant Pathol. 2024, 25, e13404. [Google Scholar] [CrossRef]
- Qin, S.; Veloso, J.; Baak, M.; Boogmans, B.; Bosman, T.; Puccetti, G.; Shi-Kunne, X.; Smit, S.; Grant-Downton, R.; Leisen, T.; et al. Molecular characterization reveals no functional evidence for naturally occurring cross-kingdom RNA interference in the early stages of Botrytis cinerea-tomato interaction. Mol. Plant Pathol. 2023, 24, 3–15. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Ries, M.S. RPD No. 704—Gray Mold of Strawberry; Reports on Plant Diseases; University of Illinois Urbana-Champaign: Champaign, IL, USA, 1995. [Google Scholar]
- Mertely, J.; Oliveira, M.S.; Peres, N.A. Botrytis Fruit Rot or Gray Mold of Strawberry: PP230 PP152, Rev. 2 2018. EDIS 2018, 2018, 230. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Management of Botrytis blossom blight in wild blueberries by biological control agents under field conditions. Crop Prot. 2020, 131, 105078. [Google Scholar] [CrossRef]
- Bristow, P.R.; McNicol, R.J.; Williamson, B. Infection of strawberry flowers by Botrytis cinerea and its relevance to grey mould development. Ann. Appl. Biol. 1986, 109, 545–554. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; Van Kan, J.A.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef]
- Garrido, C.; Carbú, M.; Fernández-Acero, F.J.; González-Rodríguez, V.E.; Cantoral, J.M. New Insights in the Study of Strawberry Fungal Pathogens. Genes Genomes Genom. 2011, 5, 24–39. [Google Scholar]
- Washington, W.S.; Engleitner, S.; Boontjes, G.; Shanmuganathan, N. Effect of fungicides, seaweed extracts, tea tree oil, and fungal agents on fruit rot and yield in strawberry. Aust. J. Exp. Agric. 1999, 39, 487–494. [Google Scholar] [CrossRef]
- Washington, W.S.; Shanmuganathan, N.; Forbes, C. Fungicide control of strawberry fruit rots, and the field occurrence of resistance of Botrytis cinerea to iprodione, benomyl and dichlofluanid. Crop Prot. 1992, 11, 355–360. [Google Scholar] [CrossRef]
- Berries Australia. Next Generation of Strawberry Disease Control. Aust. Berry J. Winter 2023, 15, 80–83. [Google Scholar]
- Menzel, C.; Gomez, A.; Smith, L. Control of grey mould and stem-end rot in strawberry plants growing in a subtropical environment. Australas. Plant Pathol. 2016, 45, 489–498. [Google Scholar] [CrossRef]
- Leroch, M.; Plesken, C.; Weber, R.W.S.; Kauff, F.; Scalliet, G.; Hahn, M. Gray mold populations in german strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl. Environ. Microbiol. 2013, 79, 159–167. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Grabke, A.; Bryson, P.K.; Amiri, A.; Peres, N.A.; Schnabel, G. Fungicide Resistance Profiles in Botrytis cinerea from Strawberry Fields of Seven Southern U.S. States. Plant Dis. 2014, 98, 825–833. [Google Scholar] [CrossRef]
- Sowley, E.N.K.; Dewey, F.M.; Shaw, M.W. Persistent, symptomless, systemic, and seed-borne infection of lettuce by Botrytis cinerea. Eur. J. Plant Pathol. 2010, 126, 61–71. [Google Scholar] [CrossRef]
- Dewey, F.; Grant-Downton, R. Botrytis-Biology, Detection and Quantification. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Germany, 2016; pp. 17–34. [Google Scholar]
- Braun, P.G.; Sutton, J.C. Infection cycles and population dynamics of Botrytis cinerea in strawberry leaves. Can. J. Plant Pathol. 1988, 10, 133–141. [Google Scholar] [CrossRef]
- Backhouse, D.; Willetts, H.J. Development and structure of infection cushions of Botrytis cinerea. Trans. Br. Mycol. Soc. 1987, 89, 89–95. [Google Scholar] [CrossRef]
- Choquer, M.; Rascle, C.; Gonçalves, I.R.; de Vallée, A.; Ribot, C.; Loisel, E.; Smilevski, P.; Ferria, J.; Savadogo, M.; Souibgui, E.; et al. The infection cushion of Botrytis cinerea: A fungal ‘weapon’ of plant-biomass destruction. Environ. Microbiol. 2021, 23, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Mattner, S.W.; Porter, I.; Gounder, R.; Shanks, A.; Wren, D.; Allen, D. Factors that impact on the ability of biofumigants to suppress fungal pathogens and weeds of strawberry. Crop Prot. 2008, 27, 1165–1173. [Google Scholar] [CrossRef]
- Pavicic, M.; Overmyer, K.; Rehman, A.U.; Jones, P.; Jacobson, D.; Himanen, K. Image-Based Methods to Score Fungal Pathogen Symptom Progression and Severity in Excised Arabidopsis Leaves. Plants 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Audenaert, K.; Van Labeke, M.-C.; Höfte, M. Detection of Botrytis cinerea on strawberry leaves upon mycelial infection through imaging technique. Sci. Hortic. 2024, 330, 113071. [Google Scholar] [CrossRef]
- Reboledo, G.; Agorio, A.; Vignale, L.; Batista-García, R.A.; De León, I.P. Botrytis cinerea Transcriptome during the Infection Process of the Bryophyte Physcomitrium patens and Angiosperms. J. Fungi 2020, 7, 11. [Google Scholar] [CrossRef]
- You, Y.; Suraj, H.M.; Matz, L.; Valderrama, A.L.H.; Ruigrok, P.; Shi-Kunne, X.; Pieterse, F.P.J.; Oostlander, A.; Beenen, H.G.; Chavarro-Carrero, E.A.; et al. Botrytis cinerea combines four molecular strategies to tolerate membrane-permeating plant compounds and to increase virulence. Nat. Commun. 2024, 15, 6448. [Google Scholar] [CrossRef]
- Asadollahi, M.; Fekete, E.; Karaffa, L.; Flipphi, M.; Árnyasi, M.; Esmaeili, M.; Váczy, K.Z.; Sándor, E. Comparison of Botrytis cinerea populations isolated from two open-field cultivated host plants. Microbiol. Res. 2013, 168, 379–388. [Google Scholar] [CrossRef]
- Koch, F.; Voigt, K.; Quidde, T.; Risch, S.; Blaich, R.; Tudzynski, P.; Büttner, P.; Brückner, B. Variations in ploidy among isolates of Botrytis cinerea: Implications for genetic and molecular analyses. Curr. Genet. 1994, 25, 445–450. [Google Scholar] [CrossRef]
- Sabburg, R.; Gregson, A.; Urquhart, A.S.; Aitken, E.A.; Smith, L.; Thatcher, L.F.; Gardiner, D.M. A method for high-throughput image-based antifungal screening. J. Microbiol. Methods 2021, 190, 106342. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Howlett, B.J. Negative selection using thymidine kinase increases the efficiency of recovery of transformants with targeted genes in the filamentous fungus Leptosphaeria maculans. Curr. Genet. 2004, 45, 249–255. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Galvão, A.; Resende, L.V.; Guimaraes, R.M.; Ferraz, A.K.L.; Morales, R.G.F.; Marodin, J.C.; Catão, H.C.R.M. Overcoming strawberry achene dormancy for improved seedling production in breeding programs. Idesia 2014, 32, 57–62. [Google Scholar] [CrossRef]
- Baroncelli, R.; Sarrocco, S.; Zapparata, A.; Tavarini, S.; Angelini, L.G.; Vannacci, G. Characterization and epidemiology of Colletotrichum acutatum sensu lato (C. chrysanthemi) causing arthamus tinctorius anthracnose. Plant Pathol. 2015, 64, 375–384. [Google Scholar] [CrossRef]
- Green, M.J.; Thompson, D.A.; MacKenzie, D.J. Easy and Efficient DNA Extraction from Woody Plants for the Detection of Phytoplasmas by Polymerase Chain Reaction. Plant Dis. 1999, 83, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.B.; Walsh, K.; Boonham, N.; O’Neill, T.; Pearson, S.; Barker, I. Development of real-time PCR (TaqMan) assays for the detection and quantification of Botrytis cinerea in planta. Plant Physiol. Biochem. 2005, 43, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, J.; Hao, J.; Liu, P.; Liu, X. Understanding Efflux-Mediated Multidrug Resistance in Botrytis cinerea for Improved Management of Fungicide Resistance. Microb. Biotechnol. 2025, 18, e70074. [Google Scholar] [CrossRef] [PubMed]
- Straube, J.; Hurtado, G.; Zeisler-Diehl, V.; Schreiber, L.; Knoche, M. Cuticle deposition ceases during strawberry fruit development. BMC Plant Biol. 2024, 24, 623. [Google Scholar] [CrossRef]
- Moya-León, M.A.; Mattus-Araya, E.; Herrera, R. Molecular Events Occurring During Softening of Strawberry Fruit. Front. Plant Sci. 2019, 10, 615. [Google Scholar] [CrossRef]
- Jiang, Y.; Peng, Y.; Hou, G.; Yang, M.; He, C.; She, M.; Li, X.; Li, M.; Chen, Q.; Zhang, Y.; et al. A high epicuticular wax strawberry mutant reveals enhanced resistance to Tetranychus urticae Koch and Botrytis cinerea. Sci. Hortic. 2024, 324, 112636. [Google Scholar] [CrossRef]
- Yu, H.; Sutton, J.C. Morphological development and interactions of Gliocladium roseum and Botrytis cinerea in raspberry. Can. J. Plant Pathol. 1997, 19, 237–246. [Google Scholar] [CrossRef]
- Dinh, S.Q.; Joyce, D.C.; Irving, D.E.; Wearing, A.H. Histology of waxflower (Chamelaucium spp.) flower infection by Botrytis cinerea. Plant Pathol. 2011, 60, 278–287. [Google Scholar] [CrossRef]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef]
- Nehra, N.S.; Kartha, K.K.; Stushnoff, C.; Giles, K.L. Effect of in vitro propagation methods on field performance of two strawberry cultivars. Euphytica 1994, 76, 107–115. [Google Scholar] [CrossRef]
- Ullah, I.; Demirsoy, H.; Soysal, D.; Lizalo, A.; Doğan, D.E.; Demirsoy, L. Evaluation of Strawberry Cultivars Based on Growth-Related Attributes. Appl. Fruit Sci. 2024, 66, 431–439. [Google Scholar] [CrossRef]
- Blanke, M. Photosynthesis of strawberry fruit. Acta Hortic. 2002, 567, 373–376. [Google Scholar] [CrossRef]
- Wang, Z.; Narciso, J.; Biotteau, A.; Plotto, A.; Baldwin, E.; Bai, J. Improving storability of fresh strawberries with controlled release chlorine dioxide in perforated clamshell packaging. Food Bioprocess Technol. 2014, 7, 3516–3524. [Google Scholar] [CrossRef]
- Mohammadi, L.; Ramezanian, A.; Tanaka, F. Impact of Aloe vera gel coating enriched with basil (Ocimum basilicum L.) essential oil on postharvest quality of strawberry fruit. J. Food Meas. Charact. 2021, 15, 353–362. [Google Scholar] [CrossRef]
- Wang, L.; Hasenstein, K.H. Seed coat stomata of several Iris species. Flora 2016, 224, 24–29. [Google Scholar] [CrossRef]
- Paiva, É.A.S.; Lemos-Filho, J.P.; Oliveira, D.M.T. Imbibition of Swietenia macrophylla (Meliaceae) Seeds: The Role of Stomata. Ann. Bot. 2006, 98, 213–217. [Google Scholar] [CrossRef]
- Boesewinkel, F.D.; Bouman, F. The Seed: Structure. In Embryology of Angiosperms; Johri, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 567–610. [Google Scholar]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Coertze, S.; Holz, G. Surface Colonization, Penetration, and Lesion Formation on Grapes Inoculated Fresh or After Cold Storage with Single Airborne Conidia of Botrytis cinerea. Plant Dis. 1999, 83, 917–924. [Google Scholar] [CrossRef]
- Viret, O.; Keller, M.; Jaudzems, V.G.; Cole, F.M. Botrytis cinerea Infection of Grape Flowers: Light and Electron Microscopical Studies of Infection Sites. Phytopathology 2004, 94, 850–857. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. In Plant Cell Walls; Carpita, N.C., Campbell, M., Tierney, M., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 311–340. [Google Scholar]
- Blanco-Ulate, B.; Labavitch, J.M.; Vincenti, E.; Powell, A.L.; Cantu, D. Hitting the Wall: Plant Cell Walls During Botrytis cinerea Infections. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Germany, 2016; pp. 361–386. [Google Scholar]
- Cheung, N.; Tian, L.; Liu, X.; Li, X. The Destructive Fungal Pathogen Botrytis cinerea-Insights from Genes Studied with Mutant Analysis. Pathogens 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holz, G.; Coertze, S.; Williamson, B. The Ecology of Botrytis on Plant Surfaces. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 9–27. [Google Scholar]
- van Kan, J.A. Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Steentjes, M.B.F.; Tonn, S.; Coolman, H.; Langebeeke, S.; Scholten, O.E.; van Kan, J.A.L. Visualization of Three Sclerotiniaceae Species Pathogenic on Onion Reveals Distinct Biology and Infection Strategies. Int. J. Mol. Sci. 2021, 22, 1865. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.J.; Gutiérrez-Sánchez, G.; Brito, N.; Shah, P.; Orlando, R.; González, C. The Botrytis cinerea early secretome. Proteomics 2010, 10, 3020–3034. [Google Scholar] [CrossRef]
- Srivastava, D.A.; Yakubov, M.; Feldbaum, R.; Tish, N.; Shoyhet, H.; Manasherova, E.; Pandaranayaka, E.P.J.; Rav-David, D.; Elad, Y.; Harel, A. Multiparametric analysis of diversity in Botrytis cinerea isolates from Israel. Phytoparasitica 2018, 46, 569–581. [Google Scholar] [CrossRef]
- Kumari, S.; Tayal, P.; Sharma, E.; Kapoor, R. Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiol. Res. 2014, 169, 862–872. [Google Scholar] [CrossRef]
- Brauna-Morževska, E.; Stoddard, F.L.; Bankina, B.; Kaņeps, J.; Bimšteine, G.; Petrova, I.; Neusa-Luca, I.; Roga, A.; Fridmanis, D. Evaluation of pathogenicity of Botrytis species isolated from different legumes. Front. Plant Sci. 2023, 14, 1069126. [Google Scholar] [CrossRef]
- Ahn, I.I.P.; Lee, Y.-H. A Viral Double-Stranded RNA Up Regulates the Fungal Virulence of Nectria radicicola. Mol. Plant-Microbe Interact. 2001, 14, 496–507. [Google Scholar] [CrossRef]
- Hao, F.; Ding, T.; Wu, M.; Zhang, J.; Yang, L.; Chen, W.; Li, G. Two Novel Hypovirulence-Associated Mycoviruses in the Phytopathogenic Fungus Botrytis cinerea: Molecular Characterization and Suppression of Infection Cushion Formation. Viruses 2018, 10, 254. [Google Scholar] [CrossRef]
- Kuckenberg, J.; Tartachnyk, I.; Noga, G. Temporal and spatial changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves. Pres. Agric. 2009, 10, 34–44. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Zumaquero, A.; Ariza, M.T.; Barceló, A.; Pliego, C. Nondestructive Detection of White Root Rot Disease in Avocado Rootstocks by Leaf Chlorophyll Fluorescence. Plant Dis. 2016, 100, 49–58. [Google Scholar] [CrossRef]
- Meng, L.; Mestdagh, H.; Ameye, M.; Audenaert, K.; Höfte, M.; Van Labeke, M.-C. Phenotypic Variation of Botrytis cinerea Isolates Is Influenced by Spectral Light Quality. Front. Plant Sci. 2020, 11, 1233. [Google Scholar] [CrossRef]
- Martinez, F.; Dubos, B.; Fermaud, M. The Role of Saprotrophy and Virulence in the Population Dynamics of Botrytis cinerea in Vineyards. Phytopathology 2005, 95, 692–700. [Google Scholar] [CrossRef]
- Mehli, L.; Kjellsen, T.D.; Dewey, F.M.; Hietala, A.M. A case study from the interaction of strawberry and Botrytis cinerea highlights the benefits of comonitoring both partners at genomic and mRNA level. New Phytol. 2005, 168, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, L.; Ruiz-Padilla, A.; Rodríguez-Romero, J.; Ayllón, M.A. Construction and Characterization of a Botrytis Virus F Infectious Clone. J. Fungi 2022, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yan, H.; Liu, X.; Li, D.; Sui, M.; Wu, J.; Yu, H.; Zhang, Z. A detached petal disc assay and virus-induced gene silencing facilitate the study of Botrytis cinerea resistance in rose flowers. Hortic. Res. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lei, X.; Wu, Z.; Cao, X.; Zhang, D.; Teng, N. An efficient and novel method to screen Botrytis cinerea resistance genes based on TRV-induced gene silencing with lily petal discs. Physiol. Mol. Plant Pathol. 2022, 122, 101923. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClayTM prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez Coy, L.; Garcia-Ceron, D.; Mattner, S.W.; Gardiner, D.M.; Gendall, A.R. Integrated Assays and Microscopy to Study the Botrytis cinerea–Strawberry Interaction Reveal Tissue-Specific Stomatal Penetration. Horticulturae 2025, 11, 954. https://doi.org/10.3390/horticulturae11080954
Rodriguez Coy L, Garcia-Ceron D, Mattner SW, Gardiner DM, Gendall AR. Integrated Assays and Microscopy to Study the Botrytis cinerea–Strawberry Interaction Reveal Tissue-Specific Stomatal Penetration. Horticulturae. 2025; 11(8):954. https://doi.org/10.3390/horticulturae11080954
Chicago/Turabian StyleRodriguez Coy, Lorena, Donovan Garcia-Ceron, Scott W. Mattner, Donald M. Gardiner, and Anthony R. Gendall. 2025. "Integrated Assays and Microscopy to Study the Botrytis cinerea–Strawberry Interaction Reveal Tissue-Specific Stomatal Penetration" Horticulturae 11, no. 8: 954. https://doi.org/10.3390/horticulturae11080954
APA StyleRodriguez Coy, L., Garcia-Ceron, D., Mattner, S. W., Gardiner, D. M., & Gendall, A. R. (2025). Integrated Assays and Microscopy to Study the Botrytis cinerea–Strawberry Interaction Reveal Tissue-Specific Stomatal Penetration. Horticulturae, 11(8), 954. https://doi.org/10.3390/horticulturae11080954








