Emerging Ornamental Plant Diseases and Their Management Trends in Northern Italy
Abstract
1. Introduction
2. Factors That Affect the Emergence of New Diseases
3. New or Re-Emerging Diseases, with Special Focus on the Mediterranean Area
4. Italy as a Hot Spot for the Appearance of New Diseases
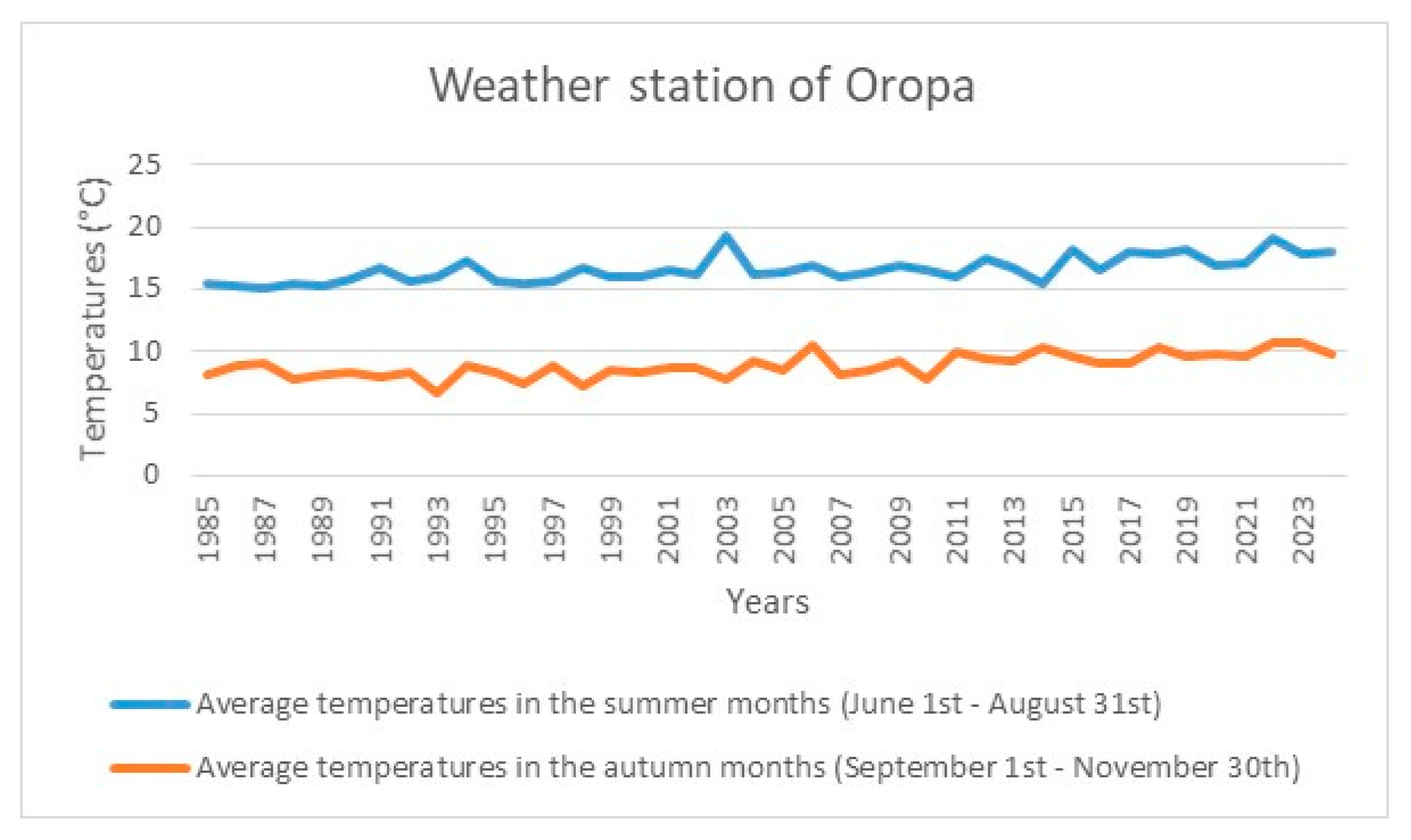
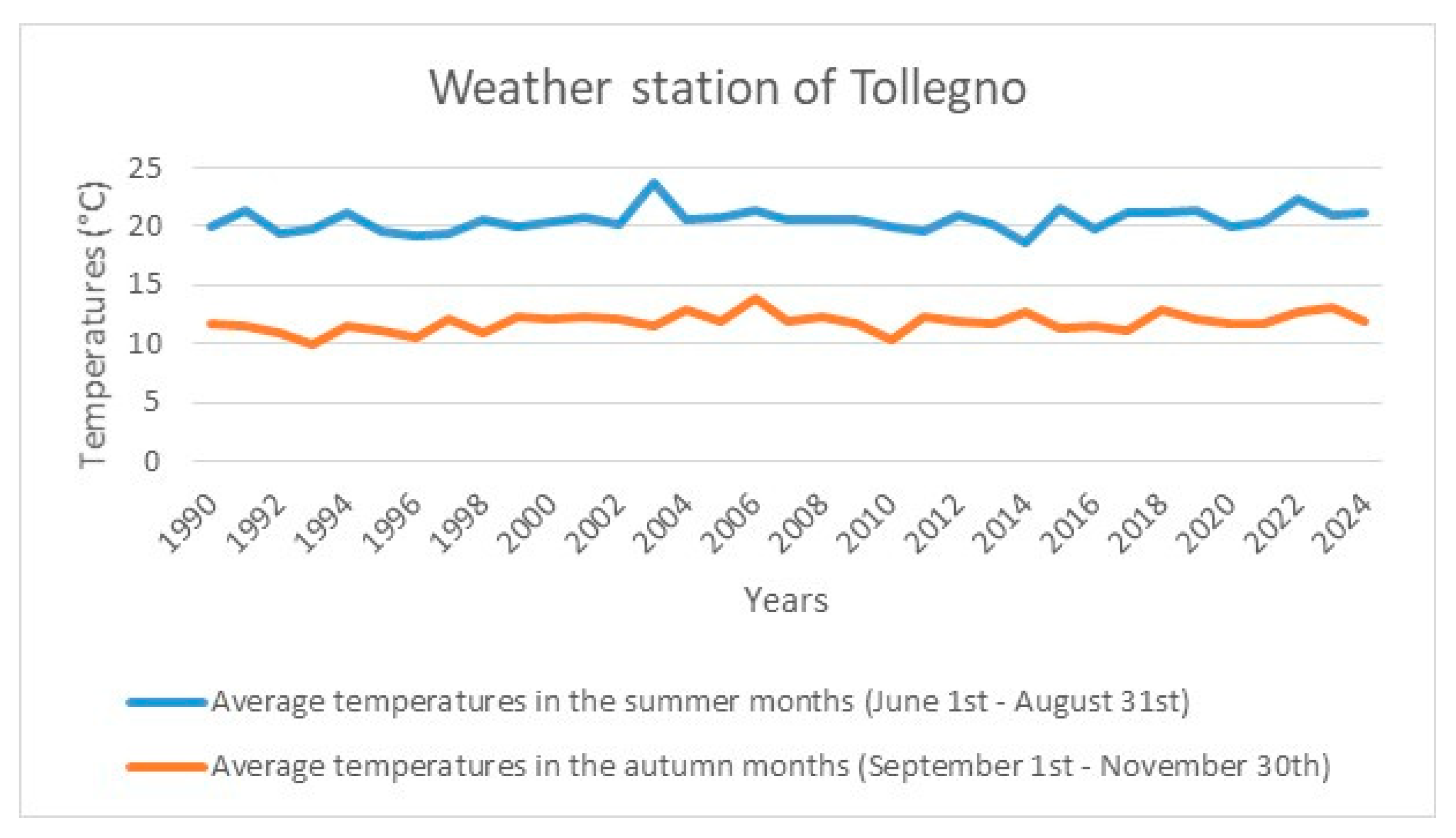
5. The Bariola Garden
- −
- investigate the impact of climate change on the prevalence and severity of plant diseases;
- −
- compare the disease resistance of different varieties/cultivars under natural infection pressure conditions, considering such factors as the weather and soil conditions;
- −
- evaluate the efficacy of new disease control methods (a novel fungicide, biocontrol agent or cultural practice) in reducing disease incidence and severity in a real-world agricultural setting.
6. New Trends in Disease Prevention and Management
Author Contributions
Funding
Conflicts of Interest
References
- Available online: https://www.agricultura.it/2024/01/09/litalia-seconda-potenza-europea-per-florovivaismo-nel-2022 (accessed on 4 August 2025).
- Available online: https://horticultureconnected.ie/news/floriculture-as-a-sector-of-italian-excellence-at-the-top-of-european-and-world-exports-urban-and-landscape-regeneration-as-future-focus/ (accessed on 4 August 2025).
- Garibaldi, A.; Bertetti, D.; Rapetti, S.; Gullino, M.L. Malattie delle Piante Ornamentali; Second updated reprint of the first edition; Edagricole: Bologna, Italy, 2024; pp. 45–148. [Google Scholar]
- Gullino, M.L.; Daughtrey, M.L.; Garibaldi, A.; Elmer, W.H. Fusarium wilts of ornamental crops and their management. Crop Prot. 2015, 73, 50–59. [Google Scholar] [CrossRef]
- Bahadur, A. Current Status of Fusarium and Their Management Strategies. In Fusarium—An Overview of the Genus; Mahyar Mirmajlessi, S., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Power, A.G. Release of invasive plants from fungal and viral pathogens. Nature 2003, 421, 625–627. [Google Scholar] [CrossRef]
- Available online: https://gd.eppo.int/taxon/BUDDA (accessed on 4 August 2025).
- Available online: https://www.invasiveplantatlas.org/subject.cfm?sub=3076 (accessed on 4 August 2025).
- Stadelmann, G.; Bugmann, H.; Wermelinger, B.; Bigler, C. Spatial interactions between storm damage and subsequent infestations by the European spruce bark beetle. For. Ecol. Manag. 2014, 318, 167–174. [Google Scholar] [CrossRef]
- Saravitz, C.H.; Joseph, C. NCSU Phytotron Procedural Manual—For Controlled-Environment Research at the Southeastern Plant Environment Laboratory; Technical Bulletin; North Carolina State University: Raleigh, NC, USA, 2019; p. 244. Available online: https://phytotron.ncsu.edu/procedural-manual/ (accessed on 4 August 2025).
- Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. Simulated elevated atmospheric CO2 and temperature affect the severity of bean and pelargonium rust. Phytoparasitica 2016, 44, 325–332. [Google Scholar] [CrossRef]
- Gullino, M.L.; Pugliese, M.; Gilardi, G.; Garibaldi, A. Effect of increased CO2 and temperature on plant diseases: A critical appraisal of results obtained in studies carried out under controlled environment facilities. J. Plant Pathol. 2018, 100, 371–389. [Google Scholar] [CrossRef]
- Pugliese, M.; Gilardi, G.; Garibaldi, A.; Gullino, M.L. The Impact of Climate Change on Vegetable Crop Diseases and Their Management: The Value of Phytotron Studies for the Agricultural Industry and Associated Stakeholders. Phytopathology 2024, 114, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Bindi, M.; Olesen, J.E. The responses of agriculture in Europe to climate change. Reg. Environ. Chang. 2011, 11, S151–S158. [Google Scholar] [CrossRef]
- Colombo, T.; Pelino, V.; Vergari, S.; Cristofanelli, P.; Bonasoni, P. Study of temperature and precipitation variations in Italy based on surface instrumental observations. Glob. Planet. Chang. 2007, 57, 308–318. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.L. Alternaria Leaf Spot Caused by Alternaria Species: An Emerging Problem on Ornamental Plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef]
- Raum, S.; Collins, C.M.; Urquhart, J.; Potter, C.; Pauleit, S.; Egerer, M. Tree insect pests and pathogens: A global systematic review of their impacts in urban areas. Urban Ecosyst. 2023, 26, 587–604. [Google Scholar] [CrossRef]
- Pest Risk Analysis for Thousand Cankers Disease (Geosmithia morbida and Pityophthorus juglandis); EPPO: Paris, France, 2015. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm (accessed on 4 August 2025).
- Saracchi, M.; Sardi, P.; Kunova, A.; Cortesi, P. Potential host range of Anthostoma decipiens and Endothiella sp., agents of hornbeam blight. J. Plant Pathol. 2015, 97, 93–97. [Google Scholar]
- Kowalski, T. Tubakia dryina, symptoms and pathogenicity to Quercus robur. Acta Mycol. 2006, 41, 299–304. [Google Scholar] [CrossRef][Green Version]
- Braun, U.; Nakashima, C.; Crous, P.W.; Groenewald, J.Z.; Moreno-Rico, O.; Rooney-Latham, S.; Blomquist, C.L.; Haas, J.; Marmolejo, J. Phylogeny and taxonomy of the genus Tubakia s. lat. Fungal Syst. Evol. 2018, 1, 41–99. [Google Scholar] [CrossRef]
- Mansfield, S.; McNeill, M.R.; Aalders, L.T.; Bell, N.L.; Kean, J.M.; Barratt, B.I.P.; Boyd-Wilson, K.; Teulon, D.A.J. The value of sentinel plants for risk assessment and surveillance to support biosecurity. NeoBiota 2019, 48, 1–24. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Li, H.-M.; Eschen, R.; Morales-Rodriguez, C.; Vannini, A. The sentinel tree nursery as an early warning system for pathway risk assessment: Fungal pathogens associated with Chinese woody plants commonly shipped to Europe. PLoS ONE 2017, 12, e0188800. [Google Scholar] [CrossRef]
- Chang, L.; Li, Y.; Gao, Z.; Bonello, P.; Cleary, M.; Munck, I.A.; Santini, A.; Sun, H. Novel Pathogen–Plant Host Interaction: Colletotrichum jiangxiense and Fraxinus americana L. (White Ash) in a Sentinel Garden in China. Plants 2023, 12, 4001. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Li, Y.-L.; Gao, Z.-W.; Bonello, P.; Cleary, M.; Munck, I.A.; Santini, A.; Sun, H. First report of Epicoccum latusicollum causing leaf spot disease on red maple (Acer rubrum L.) in China: Insights from a sentinel planting garden. Crop Prot. 2024, 175, 106439. [Google Scholar] [CrossRef]
- Marfleet, K.; Sharrock, S. The international plant sentinel Network: An update on phase 2. Sibbaldia 2020, 2, 105–116. [Google Scholar] [CrossRef]
- Daughtrey, M.L.; Benson, D.M. Principles of Plant Health Management for Ornamental Plants. Annu. Rev. Phytopathol. 2005, 43, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Daughtrey, M.; Buitenhuis, R. Integrated Pest and disease Management in Greenhouse ornamentals. In Integrated Pest and Disease Management in Greenhouse Crops, 2nd ed.; Gullino, M.L., Albajes, R., Nicot, P.C., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- van Lanteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Pugliese, M.; Gullino, M.L.; Garibaldi, A. Compost suppressiveness against Phytophthora spp. on Skimmia japonica and azalea. Commun. Agric. Appl. Biol. Sci. 2012, 77, 237–240. [Google Scholar] [PubMed]
- Bardin, M.; Pugliese, M. Biocontrol agents against diseases. In Integrated Pest and Disease Management in Greenhouse Crops; Gullino, M.L., Albajes, R., Nicot, P., Van Lenteren, J.C., Eds.; Springer: Dordrecht, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Havlin, J.L.; Schlegel, A.J. Review of Phosphite as a Plant Nutrient and Fungicide. Soil Syst. 2021, 5, 52. [Google Scholar] [CrossRef]
- Daughtrey, M.; Harlan, B.; Linderman, S.; Hausbeck, M.K. Coleus Cultivars and Downy Mildew. Special Research Report #136: Disease Management. 2014. Available online: https://endowment.org/wp-content/uploads/imported-files/136-ColeusDM-Cv-2014.pdf (accessed on 4 August 2025).
- Suffert, F.; Suffert, M. “Phytopathological strolls” in the dual context of COVID-19 lockdown and IYPH2020: Transforming constraints into an opportunity for public education about plant pathogens. Plant Pathol. 2021, 71, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, G.; Prasannath, K. Recent Advances in Molecular Diagnostics of Fungal Plant Pathogens: A Mini Review. Front. Cell. Infect. Microbiol. 2021, 10, 600234. [Google Scholar] [CrossRef]
- Webb, C.R.; Avramidis, E.; Castle, M.D.; Stutt, R.O.H.; Gilligan, C.A. Modelling the spread of tree pests and pathogens in urban forests. Urban For. Urban Green. 2023, 86, 128036. [Google Scholar] [CrossRef]
| Host | Forma Specialis | Region | Year |
|---|---|---|---|
| Gerbera jamesonii | Chrysanthemi | Liguria | 2004 |
| Osteospermum spp. | Chrysanthemi | Liguria | 2004 |
| Lewisia cotyledon | Unidentified | Piedmont | 2005 |
| Cereus peruvianus monstruosus | Opuntiarum | Liguria | 2011 |
| Crassula ovata | Crassulae * | Liguria | 2011 |
| Papaver nudicaule | Papaveris * | Liguria | 2012 |
| Echeveria agavoides | Echeveriae * | Liguria | 2013 |
| Cereus marginatus var. cristata | Opuntiarum | Liguria | 2014 |
| Echeveria tolimanensis | Echeveriae * | Liguria | 2015 |
| Euphorbia mammillaris var. variegata | Opuntiarum | Liguria | 2015 |
| Astrophytum myriostigma | Opuntiarum | Liguria | 2016 |
| Cereus peruvianus florida | Opuntiarum | Liguria | 2016 |
| Lavandula × allardii | Lavandulae * | Liguria | 2016 |
| Mammillaria zeilmanniana | Opuntiarum | Liguria | 2016 |
| Rudbeckia fulgida | Chrysanthemi | Piedmont | 2017 |
| Sulcorebutia heliosa | Opuntiarum | Liguria | 2019 |
| Mammillaria painteri | Opuntiarum | Liguria | 2020 |
| Sulcorebutia rauschii | Opuntiarum | Liguria | 2020 |
| Host | Pathogen | Region | Detected in the Bariola Garden | Year |
|---|---|---|---|---|
| Akebia quinata | Oidium sp. | Piedmont | Yes | 2004 |
| Aquilegia flabellata | Erysiphe aquilegiae | Piedmont | Yes | 2004 |
| Lonicera caprifolium | Oidium subgenus Pseudoidium | Piedmont | Yes | 2004 |
| Photinia × fraserii | Podosphaera leucotricha | Piedmont | 2005 | |
| Potentilla fruticosa | Podosphaera aphanis var. aphanis | Piedmont | Yes | 2005 |
| Veronica spicata | Golovinomyces orontii | Piedmont | Yes | 2006 |
| Coreopsis lanceolata | Podosphaera fusca | Piedmont | Yes | 2007 |
| Lamium galeobdolon | Golovinomyces orontii | Piedmont | Yes | 2007 |
| Petunia × hybrida | Golovinomyces orontii | Piedmont | Yes | 2007 |
| Argyranthemum frutescens | Golovinomyces cichoracearum | Liguria | 2008 | |
| Calendula officinalis | Podosphaera xanthii | Piedmont | Yes | 2008 |
| Hedera helix | Erysiphe heraclei | Liguria | 2008 | |
| Rudbeckia fulgida | Golovinomyces cichoracearum | Piedmont | 2008 | |
| Cleome hassleriana | Erysiphe cruciferarum | Piedmont | 2009 | |
| Cornus florida | Erysiphe pulchra | Piedmont | Yes | 2009 |
| Gerbera jamesonii | Golovinomyces cichoracearum | Piedmont | 2010 | |
| Phlox drummondii | Podosphaera sp. | Piedmont | Yes | 2011 |
| Verbascum blattaria | Golovinomyces cichoracearum | Piedmont | 2011 | |
| Aster novi-belgii | Golovinomyces cichoracearum | Piedmont | Yes | 2012 |
| Campanula rapunculoides | Golovinomyces orontii | Piedmont | Yes | 2012 |
| Euphorbia aggregata | Podosphaera sp. | Liguria | 2012 | |
| Euphorbia inermis | Podosphaera sp. | Liguria | 2012 | |
| Euphorbia perdorfiana | Podosphaera sp. | Liguria | 2012 | |
| Euphorbia susannae | Podosphaera sp. | Liguria | 2012 | |
| Oenothera biennis | Erysiphe sp. | Piedmont | Yes | 2012 |
| Phlox paniculata | Golovinomycesmagnicellulatus | Piedmont | Yes | 2016 |
| Thymus × citriodorus “Aureus” | Golovinomyces biocellatus | Liguria | 2016 | |
| Verbascum nigrum “Album” | Golovinomyces cichoracearum | Piedmont | Yes | 2016 |
| Campanula glomerata | Golovinomyces orontii | Piedmont | 2018 | |
| Echinacea purpurea | Golovinomyces cichoracearum | Piedmont | Yes | 2018 |
| Abelmoschus manihot | Golovinomyces orontii | Piedmont | 2019 | |
| Lavandula stoechas “Blueberry Ruffles” | Golovinomyces neosalviae | Liguria | 2020 | |
| Salvia nemorosa | Golovinomyces biocellatus | Piedmont | Yes | 2021 |
| Dianthus caryophyllus | Erysiphe buhrii | Liguria | 2022 | |
| Phlox maculata | Golovinomyces magnicellulatus | Piedmont | Yes | 2022 |
| Host | Pathogen | Year |
|---|---|---|
| Hydrangea macrophylla | Phoma exigua | 2006 |
| Clematis × jackmanii | Phoma sp. | 2007 |
| Hydrangea macrophylla | Alternaria alternata | 2007 |
| Hydrangea anomala | Alternaria compacta | 2008 |
| Cornus florida | Botrytis cinerea | 2009 |
| Platycodon grandiflorum | Botrytis cinerea | 2009 |
| Fuchsia × hybrida | Phoma multirostrata | 2010 |
| Rudbeckia fulgida | Phoma sp. | 2010 |
| Aquilegia flabellata | Phoma aquilegiicola | 2011 |
| Lupinus polyphyllus | Pleiochaeta setosa | 2012 |
| Saponaria officinalis | Alternaria nobilis | 2013 |
| Verbascum nigrum | Phoma novae-verbascicola | 2013 |
| Verbascum blattaria | Phoma novae-verbascicola | 2014 |
| Campanula glomerata | Alternaria sp. | 2015 |
| Campanula medium | Stagonosporopsis trachelii | 2015 |
| Rudbeckia fulgida | Alternaria sp. | 2015 |
| Salvia oxyphora | Botrytis cinerea | 2015 |
| Anemone japonica | Botrytis cinerea | 2016 |
| Campanula medium | Alternaria alternata | 2016 |
| Liquidambar styraciflua | Colletotrichum kahawae | 2016 |
| Salvia greggii | Boeremia exigua var. linicola | 2016 |
| Salvia leucantha | Colletotrichum fioriniae | 2016 |
| Campanula trachelium | Stagonosporopsis trachelii | 2017 |
| Ceratostigma willmottianum | Alternaria alternata | 2019 |
| Abelmoschus manihot | Alternaria alternata | 2020 |
| Coreopsis lanceolata | Colletotrichum fuscum | 2020 |
| Mahonia aquifolium | Colletotrichum fioriniae | 2020 |
| Phlox maculata | Alternaria alternata | 2020 |
| Aquilegia flabellata | Plectosphaerella cucumerina | 2021 |
| Campanula rapunculoides | Stagonosporopsis trachelii | 2022 |
| Hydrangea paniculata | Alternaria alternata | 2023 |
| Rhododendron arboreum | Botrytis cinerea | 2024 |
| Host | Pathogen | Year |
|---|---|---|
| Heuchera sanguinea | Rhizoctonia solani | 2007 |
| Lupinus polyphyllus | Verticillium dahliae | 2007 |
| Aquilegia flabellata | Rhizoctonia solani | 2009 |
| Digitalis purpurea | Rhizoctonia solani | 2009 |
| Rudbeckia hirta | Verticillium dahliae | 2012 |
| Phlox paniculata | Verticillium dahliae | 2014 |
| Campanula trachelium | Rhizoctonia solani | 2015 |
| Lychnis coronaria | Rhizoctonia solani | 2015 |
| Campanula carpatica | Rhizoctonia solani | 2018 |
| Echinacea purpurea | Rhizoctonia solani | 2019 |
| Coreopsis lanceolata | Phytopythium oedochilum | 2022 |
| Anemone japonica | Globisporangium sylvaticum | 2023 |
| Host | Pathogen | Year |
|---|---|---|
| Fuchsia × hybrida | Pucciniastrum circaeae | 2012 |
| Digitalis purpurea | Peronospora digitalidis | 2013 |
| Impatiens walleriana | Plasmopara obducens | 2013 |
| Oenothera biennis | Peronospora arthurii | 2018 |
| Host | Pathogen | Year |
|---|---|---|
| Phlox paniculata | Pseudomonas cichorii | 2005 |
| Viburnum sargentii | Pseudomonas syringae pv. viburnii | 2005 |
| Coreopsis lanceolata | Pseudomonas cichorii | 2009 |
| Verbena hybrida | Erwinia sp. | 2011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gullino, M.L.; Bertetti, D.; Pugliese, M.; Garibaldi, A. Emerging Ornamental Plant Diseases and Their Management Trends in Northern Italy. Horticulturae 2025, 11, 955. https://doi.org/10.3390/horticulturae11080955
Gullino ML, Bertetti D, Pugliese M, Garibaldi A. Emerging Ornamental Plant Diseases and Their Management Trends in Northern Italy. Horticulturae. 2025; 11(8):955. https://doi.org/10.3390/horticulturae11080955
Chicago/Turabian StyleGullino, Maria Lodovica, Domenico Bertetti, Massimo Pugliese, and Angelo Garibaldi. 2025. "Emerging Ornamental Plant Diseases and Their Management Trends in Northern Italy" Horticulturae 11, no. 8: 955. https://doi.org/10.3390/horticulturae11080955
APA StyleGullino, M. L., Bertetti, D., Pugliese, M., & Garibaldi, A. (2025). Emerging Ornamental Plant Diseases and Their Management Trends in Northern Italy. Horticulturae, 11(8), 955. https://doi.org/10.3390/horticulturae11080955








