Effect of the Combined Application of Aqueous Cabbage Seed Extract and Chitosan Solutions on the Shelf Life of Fresh-Cut Apple Cubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Aqueous Seed Extracts, Chitosan-Based Coatings, and pH Adjustment
2.4. Sample Preparation and Application of Treatments
2.5. Quality Assessment Parameters
2.5.1. Determination of Phenolics, Antioxidant Capacity, and Total Glucosinolates
2.5.2. Chromatometric Color Measurement and Weight Loss
2.5.3. Hardness Determination
2.5.4. FTIR FT-IR Spectroscopy
2.5.5. Visual Evaluation of Browning
2.5.6. Shelf Life Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolics, Antioxidant Capacity, and Total Glucosinolates of Cabbage Seeds Aqueous Extracts
3.2. Analysis of Variance
3.3. Pearson Linear Correlation and Principal Component Analysis
3.4. FTIR
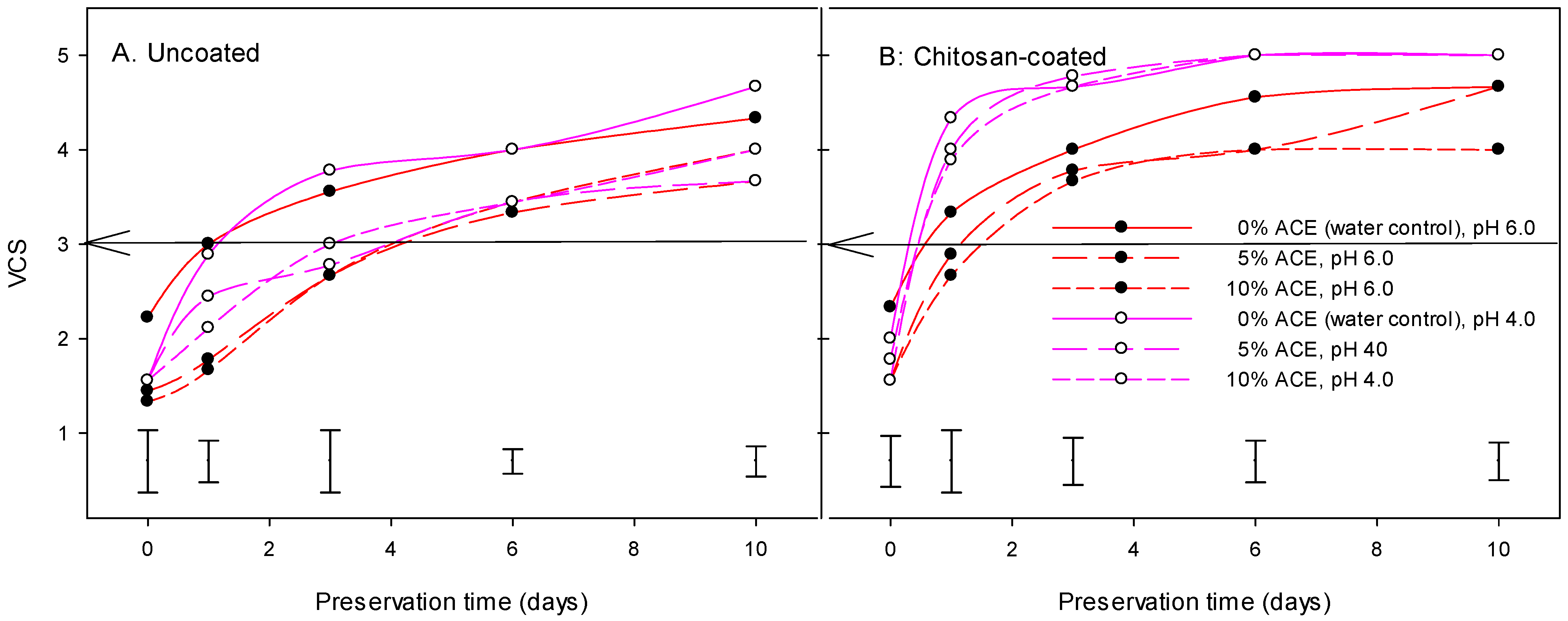
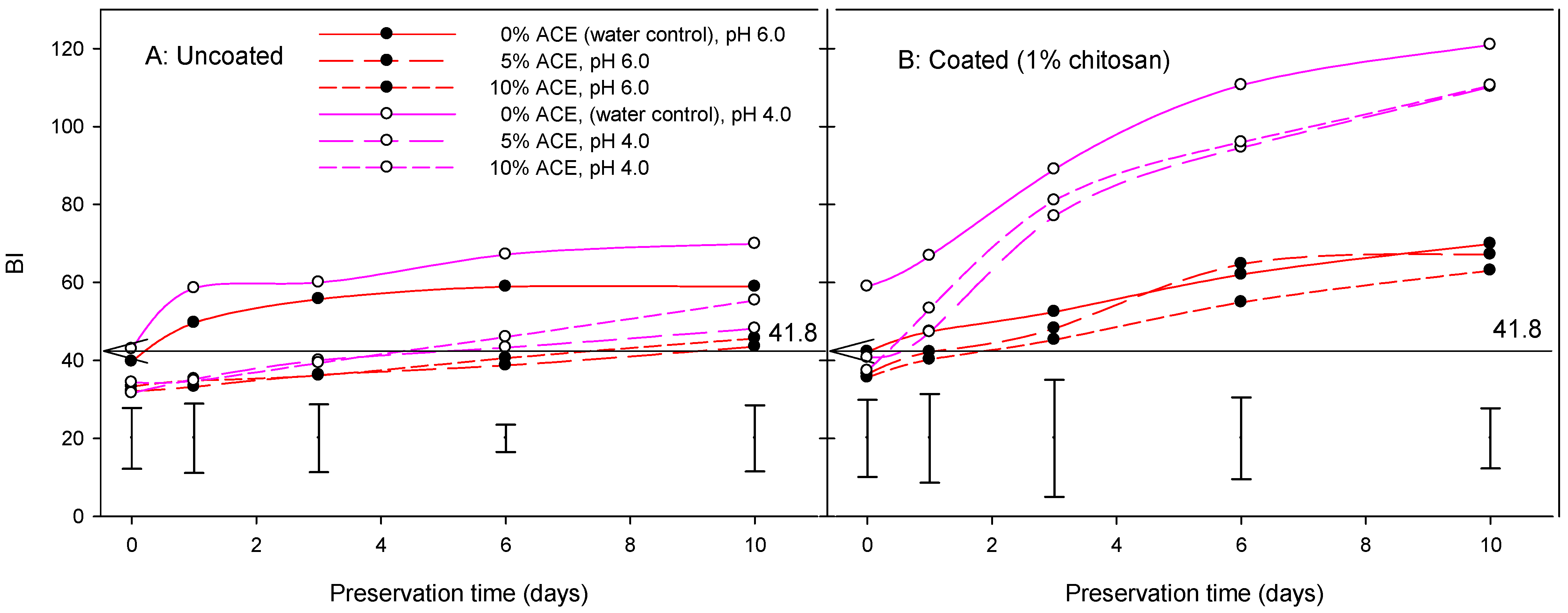
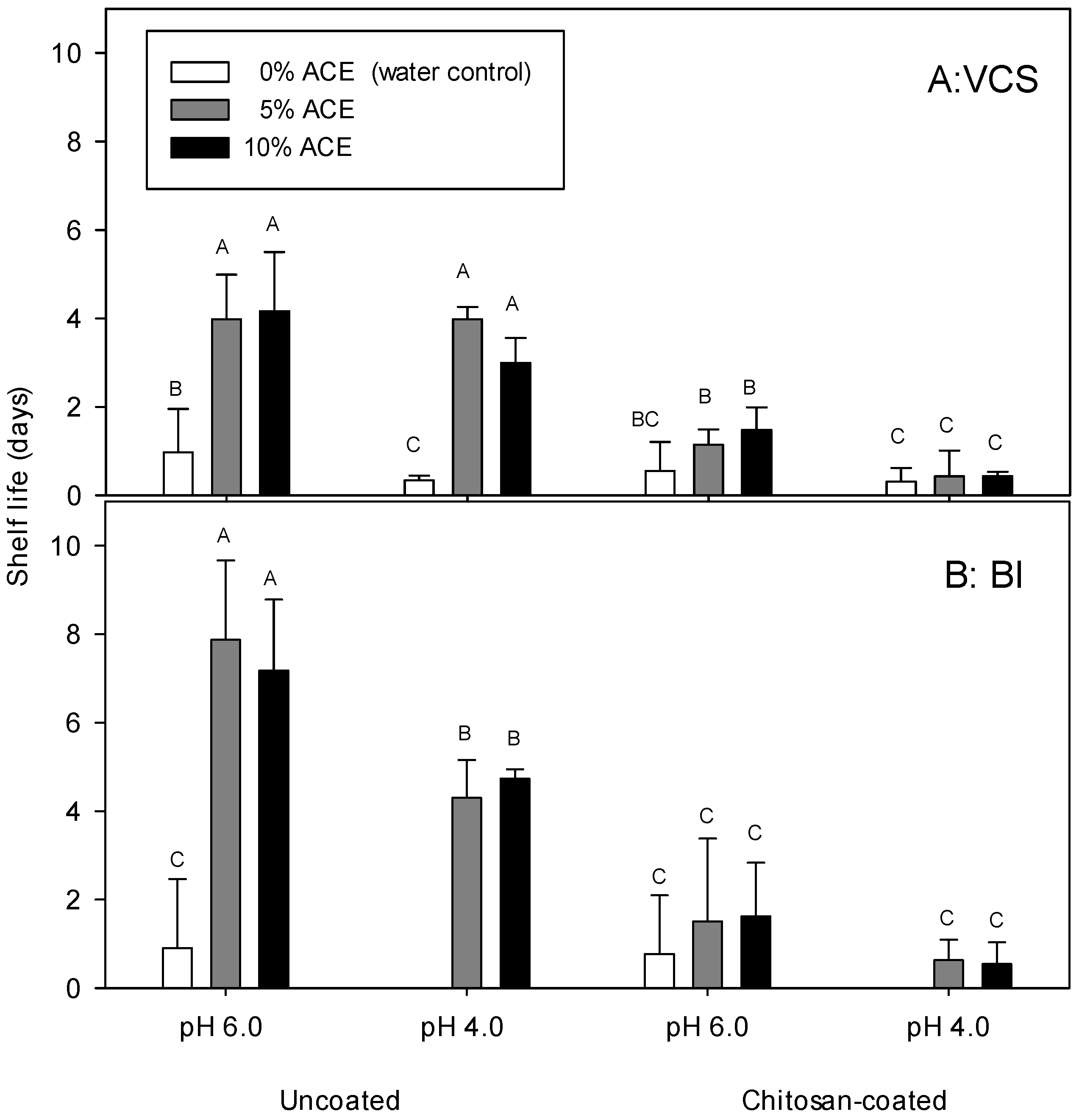
3.5. Visual Color Score, Browning Index, and Shelf Life
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xin, Y.; Yang, C.; Zhang, J.; Xiong, L. Application of Whey Protein-Based Emulsion Coating Treatment in Fresh-Cut Apple Preservation. Foods 2023, 12, 1140. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, D.; Wang, X.; Zhang, F.; Chang, D.; You, C.; Zhang, S.; Wang, X. Effects of Cellulose Nanofibrils Treatment on Antioxidant Properties and Aroma of Fresh-Cut Apples. Food Chem. 2023, 415, 135797. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical Properties and Function of Plant Polyphenol Oxidase: A Review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; De Siqueira Elias, H.H.; Fukushima, K.L.; Silva, E.K.; De Deus Souza Carneiro, J.; De Fátima Ferreira Soares, N.; Borges, S.V. Effect of Whey Protein Isolate Films Incorporated with Montmorillonite and Citric Acid on the Preservation of Fresh-Cut Apples. Food Res. Int. 2018, 107, 306–313. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, A.; Liu, C.; Wagstaff, C.; Zhao, Q.; Zhang, Y.; Hu, W. Hydrogen Sulfide Inhibits the Browning of Fresh-Cut Apple by Regulating the Antioxidant, Energy and Lipid Metabolism. Postharvest Biol. Technol. 2021, 175, 111487. [Google Scholar] [CrossRef]
- Shrestha, L.; Kulig, B.; Moscetti, R.; Massantini, R.; Pawelzik, E.; Hensel, O.; Sturm, B. Optimisation of Physical and Chemical Treatments to Control Browning Development and Enzymatic Activity on Fresh-Cut Apple Slices. Foods 2020, 9, 76. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Anti-Browning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Lyengar, R.; McEvily, A.J. Anti-browning Agents: Alternatives to the Use of Sulfites in Foods. Trends Food Sci. Tech. 1992, 3, 60–64. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Munuera, I.P.; Fiszman, S.; Martín-Belloso, O. Recent Approaches Using Chemical Treatments to Preserve Quality of Fresh-Cut Fruit: A Review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Mouzahim, M.E.; Eddarai, E.M.; Eladaoui, S.; Guenbour, A.; Bellaouchou, A.; Zarrouk, A.; Boussen, R. Effect of Kaolin Clay and Ficus carica Mediated Silver Nanoparticles on Chitosan Food Packaging Film for Fresh Apple Slice Preservation. Food Chem. 2023, 410, 135470. [Google Scholar] [CrossRef]
- Du, T.; Li, X.; Wang, S.; Su, Z.; Sun, H.; Wang, J.; Zhang, W. Phytochemicals-Based Edible Coating for Photodynamic Preservation of Fresh-Cut Apples. Food Res. Int. 2023, 163, 112293. [Google Scholar] [CrossRef]
- Hu, K.; Wang, Q.; Hu, L.; Gao, S.; Wu, J.; Li, Y.; Zheng, J.; Han, Y.; Liu, Y.; Zhang, H. Hydrogen Sulfide Prolongs Postharvest Storage of Fresh-Cut Pears (Pyrus pyrifolia) by Alleviation of Oxidative Damage and Inhibition of Fungal Growth. PLoS ONE 2014, 9, e85524. [Google Scholar] [CrossRef]
- Chaisakdanugull, C.; Theerakulkait, C.; Wrolstad, R.E. Pineapple Juice and Its Fractions in Enzymatic Browning Inhibition of Banana [Musa (AAA Group) Gros Michel]. J. Agric. Food Chem. 2007, 55, 4252–4257. [Google Scholar] [CrossRef] [PubMed]
- Martín-Diana, A.B.; Rico, D.; Barry-Ryan, C. Green Tea Extract As a Natural Antioxidant to Extend The Shelf-Life of Fresh-Cut Lettuce. Innov. Food Sci. Emerg. Technol. 2008, 9, 593–603. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Z.; Zeng, W. Investigation of antibrowning activity of pine needle (Cedrus deodara) extract with fresh-cut apple slice model and identification of the primary active components. Eur. Food Res. Technol. 2014, 239, 669–678. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, C.Y.; Park, I. Prevention of Enzymatic Browning of Pear by Onion Extract. Food Chem. 2008, 89, 181–184. [Google Scholar] [CrossRef]
- Lee, M.K. Inhibitory Effect of Banana Polyphenol Oxidase During Ripening of Banana by Onion Extract and Maillard Reaction Products. Food Chem. 2007, 102, 146–149. [Google Scholar] [CrossRef]
- Sukhontha, S.; Kunwadee, K.; Chockchai, T. Inhibitory Effect of Rice Bran Extracts and Its Phenolic Compounds on Polyphenol Oxidase Activity and Browning in Potato and Apple Puree. Food Chem. 2016, 190, 922–927. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of Purslane (Portulaca oleracea L.) Extract on Anti-Browning of Fresh-Cut Potato Slices during Storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef]
- Supapvanich, S.; Yimpong, A.; Srisuwanwichan, J. Browning Inhibition on Fresh-Cut Apple by the Immersion of Liquid Endosperm from Mature Coconuts. J. Food Sci. Technol. 2020, 57, 4424–4431. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, H.; Shao, J.; Lu, L.; Tian, J.; Liu, X. A Novel Mitigator of Enzymatic Browning—Hawthorn Leaf Extract and its Application in the Preservation of Fresh-Cut Potatoes. Food Qual. Saf. 2021, 5, 1399–2399. [Google Scholar] [CrossRef]
- Chen, X.; Ren, L.; Li, M.; Qian, J.; Fan, J.; Du, B. Effects of Clove Essential Oil and Eugenol on Quality and Browning Control of Fresh-Cut Lettuce. Food Chem. 2017, 214, 432–439. [Google Scholar] [CrossRef]
- Bin, Y.; Tian, M.; Xie, J.; Wang, M.; Chen, C.; Jiang, A. Bamboo Leaf Extract Treatment Alleviates the Surface Browning of Fresh-Cut Apple by Regulating Membrane Lipid Metabolism and Antioxidant Properties. J. Sci. Food Agric. 2023, 104, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Degl’Innocenti, E.; Guglielminetti, L.; Guidi, L. Role of Ascorbic Acid in the Inhibition of Polyphenol Oxidase and the Prevention of Browning in Different Browning-Sensitive Lactuca sativa var. capitata (L.) and Eruca sativa (Mill.) Stored as Fresh-Cut Produce. J. Sci. Food Agric. 2013, 93, 1814–1819. [Google Scholar] [CrossRef]
- Zocca, F.; Lomolino, G.; Lante, A. Antibrowning Potential of Brassicacaea Processing Water. Biores. Technol. 2010, 101, 3791–3795. [Google Scholar] [CrossRef]
- Bustos, M.C.; Mazzobre, M.F.; Buera, M.P. Stabilization of Refrigerated Avocado Pulp: Chemometrics-Assessed Antibrowning Allium and Brassica Extracts as Effective Lipid Oxidation Retardants. Food Bioprocess Technol. 2017, 10, 1142–1153. [Google Scholar] [CrossRef]
- Androudis, E.; Gerasopoulos, A.; Koukounaras, A.; Siomos, A.S.; Gerasopoulos, D. Water Extracts of Cruciferous Vegetable Seeds Inhibit Enzymic Browning of Fresh-Cut Mid Ribs of Romaine Lettuce. Horticulturae 2024, 10, 500. [Google Scholar] [CrossRef]
- Wessels, B.; Damm, S.; Kunz, B.; Schulze-Kaysers, N. Effect of Selected Plant Extracts on the Inhibition of Enzymatic Browning in Fresh-Cut Apple. J. Appl. Bot. Food Qual. 2014, 87, 16–23. [Google Scholar] [CrossRef]
- Gerasopoulos, A.; Mantzouridou, F.T.; Nenadis, N. Physicochemical Properties of Olive Leaf Powders and Incorporation in Chitosan-Based Edible Films for Improved Functionality. Food Hydrocoll. 2024, 160, 110748. [Google Scholar] [CrossRef]
- Diab, T.; Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical Properties and Application of Pullulan Edible Films and Coatings in Fruit Preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- Demircan, B.; Velioglu, Y.S. Control of Browning, Enzyme Activity, and Quality in Stored Fresh-cut Fruit Salads through Chitosan Coating Enriched with Bergamot Juice Powder. Foods 2024, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.M.; Saxena, A.; Kaur, C. Effect of Chitosan and Alginate Based Coatings Enriched with Pomegranate Peel Extract to Extend the Postharvest Quality of Guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Q.; Shi, J.; Chen, Y.; Zhang, X. Optimization and Release Evaluation for Tea Polyphenols and Chitosan Composite Films with Regulation of Glycerol and Tween. Food Sci. Technol. 2019, 40, 162–170. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 4th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1990. [Google Scholar]
- Scalbert, A.; Monties, B.; Janin, G. Tannins in Wood: Comparison of Different Estimation Methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nenadis, N.; Kyriakoudi, A.; Tsimidou, M.Z. Impact of Alkaline or Acid Digestion to Antioxidant Activity, Phenolic Content and Composition of Rice Hull Extracts. LWT-Food Sci. Technol. 2013, 54, 207–215. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate Metabolism, Functionality and Breeding for the Improvement of Brassicaceae Vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Lister, C.E.; Reay, P.F.; Triggs, C.M. Influence of Pigment Composition on Skin Color in a Wide Range of Fruit and Vegetables. J. Amer. Soc. Hortic. Sci. 1997, 122, 594–598. Available online: https://journals.ashs.org/downloadpdf/view/journals/jashs/122/4/article-p594.pdf (accessed on 7 March 2025). [CrossRef]
- Palou, E.; Lopez-Malo, A.; Barbosa-Canovas, G.V.; Welti-Chanes, J.; Swanson, G.B. Polyphenoloxidase Activity and Colour of Balanced and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Bolin, H.R.; Huxsoll, C.C. Control of Minimally Process Carrot (Daucus carota) Surface Discoloration Caused by Abrasion Peeling. J. Food Sci. 1991, 56, 416–418. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Effect of Chitosan-Ascorbic Acid Coatings on the Refrigerated Storage Stability of Fresh-Cut Apples. Coatings 2019, 9, 503. [Google Scholar] [CrossRef]
- Bustos, M.C.; Agudelo-Laverde, L.M.; Mazzobre, F.; Buera, M.P. The Relationship between Antibrowning, Anti-Radical and Reducing Capacity of Brassica and Allium Extracts. Int. J. Food Stud. 2014, 3, 82–92. [Google Scholar] [CrossRef]
- Bustos, M.C.; Pérez, G.; León, A.E. Structure and Quality of Pasta Enriched with Functional Ingredients. RSC Adv. 2015, 5, 30780–30792. [Google Scholar] [CrossRef]
- Sarıkamış, G.; Yıldırım, A.; Alkan, D. Glucosinolates in Seeds, Sprouts and Seedlings of Cabbage and Black Radish as Sources of Bioactive Compounds. Can. J. Plant Sci. 2015, 95, 681–687. [Google Scholar] [CrossRef]
- Kołodziejski, D.; Piekarska, A.; Hanschen, F.S.; Pilipczuk, T.; Tietz, F.; Kusznierewicz, B.; Bartoszek, A. Relationship between Conversion Rate of Glucosinolates to Isothiocyanates/Indoles and Genotoxicity of Individual Parts of Brassica Vegetables. Eur. Food Res. Technol. 2018, 245, 383–400. [Google Scholar] [CrossRef]
- Fan, X. Chemical Inhibition of Polyphenol Oxidase and Cut Surface Browning of Fresh-Cut Apples. Crit. Rev. Food Sci. Nutr. 2022, 63, 8737–8751. [Google Scholar] [CrossRef]
- Tappi, S.; Ragni, L.; Tylewicz, U.; Romani, S.; Ramazzina, I.; Rocculi, P. Browning Response of Fresh-Cut Apples of Different Cultivars to Cold Gas Plasma Treatment. Innov. Food Sci. Emerg. Technol. 2017, 53, 56–62. [Google Scholar] [CrossRef]
- Eissa, H.A.; Saad, S.M.; El-Aleem, I.M.A.; Ibrahim, W.A.M.; Elmoniem, G.M.A.; Helmy, A.M. Effect of Natural Cabbage and Taro Extracts on Oxidative Enzymes Activity of Frozen and Dried Apple Products. J. Am. Sci. 2010, 6, 454–464. [Google Scholar] [CrossRef]
- Incardona, A.; Amodio, M.A.; Derossi, A.; Colelli, G. Investigating the Impact of the Degree of Sharpness on the Microstructure of Fresh-Cut Apples. Foods 2025, 14, 636. [Google Scholar] [CrossRef]
- Luo, Z.; Li, G.; Du, Y.; Yi, J.; Hu, X.; Jiang, Y. Enhancing Fresh-Cut Apple Preservation: Impact of Slightly Acidic Electrolyzed Water and Chitosan–Apple Essence Microencapsulation Coating on Browning and Flavor. Foods 2024, 13, 1585. [Google Scholar] [CrossRef]
- Moreira, M.R.; Tomadoni, B.; Martín-Belloso, O.; Soliva-Fortuny, R. Preservation of Fresh-Cut Apple Quality Attributes by Pulsed Light in Combination with Gellan Gum-Based Prebiotic Edible Coatings. LWT-Food Sci. Technol. 2015, 64, 1130–1137. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of Thermal Treatment on Glucosinolates and Antioxidant-Related Parameters in Red Cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Hansen, M.; Wicklund, T.; Bengtsson, G.B. Processing (Blanching, Boiling, Steaming) Effects on the Content of Glucosinolates and Antioxidant-Related Parameters in Cauliflower (Brassica oleracea L. ssp. botrytis). LWT-Food Sci. Technol. 2009, 42, 63–73. [Google Scholar] [CrossRef]
- Zou, H.; Xiao, G.; Li, G.; Wei, X.; Tian, X.; Zhu, L.; Ma, F.; Li, M. Revisiting the Advancements in Plant Polyphenol Oxidases Research. Sci. Hortic. 2025, 341, 113960. [Google Scholar] [CrossRef]
- Karaibrahimoglu, Y.; Fan, Χ.; Sapers, G.M.; Sokorai, K. Effect of pH on the Survival of Listeria innocua in Calcium Ascorbate Solutions and on Quality of Fresh-Cut Apples. J. Food Prot. 2004, 67, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Hu, W.; Jiang, A.; Tian, M.; Li, Y. Extending Shelf-Life of Fresh-Cut ‘Fuji’ Apples with Chitosan-Coatings. Innov. Food Sci. Emerg. Technol. 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Shyu, Y.S.; Chen, G.W.; Chiang, S.C.; Sung, W.C. Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples. Molecules 2019, 24, 2008. [Google Scholar] [CrossRef]
- Karagöz, Ş.; Demirdöven, A. Effect of Chitosan Coatings with and without Stevia rebaudiana and Modified Atmosphere Packaging on Quality of Cold Stored Fresh-Cut Apples. LWT-Food Sci. Technol. 2019, 108, 332–337. [Google Scholar] [CrossRef]
- Eissa, H.A.A. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J. Food Qual. 2007, 30, 623–645. [Google Scholar] [CrossRef]
- Batkan, A.; Kundakçi, A.; Ergönül, B. Effect of Holding Period prior to Storage on the Chemical Attributes of Starking Delicious Apples during Refrigerated Storage. Food Sci. Technol. 2012, 32, 223–227. [Google Scholar] [CrossRef][Green Version]
- Ackah, S.; Xue, S.; Osei, R.; Kweku-Amagloh, F.; Zong, Y.; Prusky, D.; Bi, Y. Chitosan Treatment Promotes Wound Healing of Apple by Eliciting Phenylpropanoid Pathway and Enzymatic Browning of Wounds. Front. Microbiol. 2022, 13, 828914. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Luo, Y.; Luo, Y.; Wang, Q. Combined Effects of Sodium Chlorite Dip Treatment and Chitosan Coatings on the Quality of Fresh-Cut d’Anjou Pears. Postharvest Biol. Technol. 2011, 62, 319–326. [Google Scholar] [CrossRef]
- Zeb, S.; Sajid, M.; Shah, S.T.; Ali, M.; Ali, S.; Nawaz, Z.; Rauf, K.; Khan, A.; Shah, H.; Shah, S.A.A.; et al. Influence of Post-Harvest Application of Chitosan on Physico-Chemical Changes of Apple Fruit during Storage. Pure Appl. Biol. 2020, 9, 2554–2562. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Wang, D.; Zhong, Y.; Deng, Y. Combination of Chitosan Coating and Heat Shock Treatments to Maintain Postharvest Quality and Alleviate Cracking of Akebia trifoliate Fruit during Cold Storage. Food Chem. 2022, 394, 133330. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Q.; Cao, J.; Jiang, W. Effects of Chitosan Coating on Postharvest Quality of Mango (Mangifera indica L. cv. Tainong) Fruits. J. Food Process. Preserv. 2008, 32, 770–784. [Google Scholar] [CrossRef]
- Fernades, S.D.S.; Ribeiro, C.A.d.S.; Raposo, M.F.d.J.; de Morais, R.M.S.; de Morais, A.M.M.B. Polyphenol Oxidase Activity and Colour Changes of ‘Starking’ Apple Cubes Coated with Alginate and Dehydrated with Air. Food Nutr. Sci. 2011, 2, 451–457. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Free Phenolics and Polyphenol Oxidase (PPO): The Factors Affecting Post-Cut Browning in Eggplant (Solanum melongena). Food Chem. 2013, 139, 105–114. [Google Scholar] [CrossRef]
- Piagentini, A.M.; Pirovani, M.E. Total phenolics content, antioxidant capacity, physicochemical attributes, and browning susceptibility of different apple cultivars for minimal processing. Int. J. Fruit Sci. 2017, 17, 102–116. [Google Scholar] [CrossRef]
- Cvetković, B.; Bajić, A.; Belović, M.; Pezo, L.; Dragojlović, D.; Šimurina, O.; Djordjević, M.; Korntheuer, K.; Philipp, C.; Eder, R. Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach. Foods 2024, 13, 2291. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, W.; Chen, Y.; Peng, Y. Browning Inhibition of Plant Extracts on Fresh-Cut Fruits and Vegetables—A Review. J. Food Process. Preserv. 2022, 46. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Li, X.; Liu, H.; Li, F.; Zhang, X. Combined Effects of 1-methylcyclopropene and Tea Polyphenols Coating Treatment on the Postharvest Senescence and Reactive Oxygen Species Metabolism of Bracken (Pteridium aquilinum var. latiusculum). Postharvest Biol. Technol. 2022, 185, 111813. [Google Scholar] [CrossRef]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Pre-harvest Application of Chitosan and Postharvest Aloe vera Gel Coating Enhances Quality of Table Grape (Vitis vinifera L. cv.‘Yaghouti’) during Postharvest Period. Food Chem. 2021, 347, 129012. [Google Scholar] [CrossRef]
- Sabir, F.K.; Unal, S.; Aydın, S.; Sabir, A. Pre- and Postharvest Chitosan Coatings Extend the physicochemical and Bioactive Qualities of Minimally Processed ‘Crimson Seedless’ grapes during Cold Storage. J. Sci. Food Agric. 2024, 104, 7834–7842. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Li, D.; Guo, L.; Hong, X.; He, S.; Huo, J.; Sui, X.; Zhang, Y. Enhancing Postharvest Quality and Antioxidant Capacity of Blue Honeysuckle cv. ‘Lanjingling’ with Chitosan and Aloe vera Gel Edible Coatings during Storage. Foods 2024, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; Downey, G.; Rawson, A.; Warriner, K.; Gernigon, G. Application of Principal Component and Hierarchical Cluster Analysis to Classify Fruits and Vegetables Commonly Consumed in Ireland Based on in vitro Antioxidant Activity. J. Food Compos. Anal. 2010, 24, 250–256. [Google Scholar] [CrossRef]
- Minh, T.N.; Van Thinh, P.; Anh, H.L.T.; Anh, L.V.; Khanh, N.H.; Van Nhan, L.; Trung, N.Q.; Dat, N.T. Chemometric Classification of Vietnamese Green Tea (Camellia sinensis) Varieties and Origins Using Elemental Profiling and FTIR Spectroscopy. Int. J. Food Sci. Technol. 2024, 59, 9234–9244. [Google Scholar] [CrossRef]
- Hamzah, H.T.; Sridevi, V.; Surya, D.V.; Palla, S.; Yadav, A.; Rao, P.V. Conventional and Microwave-Assisted Acid Pretreatment of Tea Waste Powder: Analysis of Functional Groups Using FTIR. Environ. Sci. Pollut. Res. 2023, 31, 57523–57532. [Google Scholar] [CrossRef]
- Rajhard, S.; Hladnik, L.; Vicente, F.A.; Srčič, S.; Grilc, M.; Likozar, B. Solubility of Luteolin and Other Polyphenolic Compounds in Water, Nonpolar, Polar Aprotic and Protic Solvents by Applying FTIR/HPLC. Processes 2021, 9, 1952. [Google Scholar] [CrossRef]
- Irchad, A.; Hssaini, L.; Razouk, R.; Chabbi, M.; Charafi, J.; Bouassab, A. Exogenous Salicylic and ascorbic Acids Delay Peel Enzymatic Browning and Improve Quality of Dried Figs under Low-Temperature Storage. Biointerface Res. Appl. Chem. 2021, 12, 8367–8384. [Google Scholar] [CrossRef]
- Hssaini, L.; Ouaabou, R.; Charafi, J.; Razouk, R.; Houmanat, K.; Irchad, A. Effects of Pre-Storage Ascorbic and Salicylic Acids Treatments on the Enzymatic Browning and Nutritional Quality of Dried Fig: Combined Use of Biochemical and ATR-FTIR Analyses. Vib. Spectrosc. 2021, 115, 103269. [Google Scholar] [CrossRef]
- Ma, S.; Ma, J.; Wang, Q.; Wang, C.; Shi, H.; Shi, J. NaCl Treatment with Low Density Polyethylene Packaging Bags Can Enhance the Aroma of Fresh-Cut Apple, not only Inhibit its Browning. Food Packag. Shelf Life 2023, 39, 101148. [Google Scholar] [CrossRef]
- Karseno, N.; Erminawati, N.; Yanto, N.T.; Setyawati, R.; Haryanti, P. Effect of pH and Temperature on Browning Intensity of Coconut Sugar and its Antioxidant Activity. Food Res. 2017, 2, 32–38. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martin-Belloso, O. Effect of Natural Antibrowning Agents on Color and Related Enzymes in Fresh-Cut Fuji Apples as an Alternative to the Use of Ascorbic Acid. J. Food Sci. 2008, 73, S267–S272. [Google Scholar] [CrossRef]


| Degree of Browning Score | Acceptability/Browning Degree | Color Range |
|---|---|---|
| 1 | Absolutely acceptable No sign of browning |  |
| 2 | Accepted Evidence of browning beyond doubt |  |
| 3 | Borderline acceptable/unacceptable Noticeable browning |  |
| 4 | Unacceptable Obvious time wear—inappropriate |  |
| 5 | Unacceptable Decomposition image |  |
| ACE % Coating | TPC | DPPH | GSL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg GA/L) | (%Inh) | (mg Sin/g Seed FW *) | |||||||
| ACE 5% | 799.8 | ± | 15.6C ** | 51.55 | ± | 1.38C | 5.41 | ± | 0.27 |
| ACE 10% | 1497.0 | ± | 9.6A | 78.43 | ± | 2.03B | 7.24 | ± | 0.84 |
| Chitosan | n.d. *** | n.d. | 11.35 | ± | 1.97D | n.d. | n.d. | ||
| Chitosan/ACE 5% | 792.6 | ± | 3.6C | 54.75 | ± | 1.68C | n.d. | n.d. | |
| Chitosan/ACE 10% | 1443.0 | ± | 13.2B | 82.97 | ± | 2.36A | n.d. | n.d. | |
| MAIN EFFECTS | DF | L* | a* | b* | h° | C | BI | VCS | HNS | WL | TPC | DPPH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACE | Water/control | 68.70B | 2.06A | 31.40A | 90.70A | 31.71A | 63.75A | 3.77A | 681.5A | 4.47A | 344.5B | 33.85A |
| 5% | 72.11A | 0.35B | 28.27B | 90.72A | 28.56B | 49.96B | 3.20B | 703.8A | 4.08A | 521.6A | 46.46B | |
| 10% | 72.02A | 0.22B | 27.80B | 90.35A | 28.10B | 50.91B | 3.18B | 709.5A | 4.53A | 445.1A | 36.56B | |
| pH | pH 4.0 | 69.63Β | 2.57A | 30.70A | 86.94B | 31.13A | 63.21A | 3.60A | 697.8A | 4.18A | 434.3A | 35.33A |
| pH 6.0 | 72.25A | −0.81B | 27.61B | 94.29A | 27.78B | 46.53B | 3.16B | 698.7A | 4.54A | 439.8A | 42.58B | |
| Coating | Non-coated/control | 73.67A | −1.10B | 27.35B | 93.09A | 27.44B | 44.41B | 2.99B | 639.9B | 4.41A | 500.4A | 42.47A |
| Coated, 1% chitosan | 68.22B | 2.86A | 30.97A | 88.09B | 31.47A | 65.33A | 3.78A | 756.6A | 4.31A | 373.7B | 35.44B | |
| Preservation (days) | 0 | 74.56A | −1.90E | 25.52D | 94.78A | 25.65E | 38.76E | 1.75E | 788.6A | 0.00C | - | - |
| 1 | 74.09A | −0.89D | 27.82C | 92.81A | 27.93D | 45.27D | 3.01D | 735.9A | 2.36B | 459.7A | 40.98A | |
| 3 | 71.28B | 0.76C | 29.77B | 90.01A | 29.95C | 54.77C | 3.70C | 661.7B | 5.84A | - | - | |
| 6 | 67.95C | 2.47B | 31.10A | 86.67A | 31.46B | 64.85B | 4.10B | 651.1B | 6.60A | 414.3A | 36.93A | |
| 10 | 66.83D | 3.95A | 31.59A | 88.68A | 32.28A | 70.71A | 4.34A | 653.9B | 7.01A | - | - |
| L* | a* | b* | ho | C | BI | VCS | HNS | WL | TPC | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | -- | ||||||||||
| a* | −0.939 *** | -- | |||||||||
| b* | −0.776 *** | 0.846 *** | -- | ||||||||
| ho | 0.712 *** | −0.76 *** | −0.71 *** | -- | |||||||
| C | −0.823 *** | 0.888 *** | 0.996 *** | −0.733 *** | -- | ||||||
| BI | −0.953 *** | 0.977 *** | 0.902 *** | −0.758 *** | 0.937 *** | -- | |||||
| VCS | −0.74 *** | 0.782 *** | 0.82 *** | −0.663 *** | 0.824 *** | 0.792 *** | -- | ||||
| HNS | −0.038 | 0.043 | −0.008 | −0.056 | 0.001 | 0.021 | −0.065 | -- | |||
| WL | −0.345 *** | 0.371 *** | 0.375 *** | −0.302 ** | 0.375 *** | 0.365 *** | 0.601 *** | −0.34 | -- | ||
| TPC | 0.554 *** | −0.5 *** | −0.505 *** | 0.319 | −0.514 *** | −0.531 *** | −0.495 *** | −0.205 | −0.034 | -- | |
| DPPH | 0.458 *** | −0.469 *** | −0.4 *** | 0.345 ** | −0.419 *** | −0.468 *** | −0.401 *** | −0.174 | 0.006 | 0.797 *** | -- |
| UNCOATED (No Chitosan) | COATED (1% Chitosan) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preservation | ACE 0% | ACE 5% | ACE 10% | ACE 0% | ACE 5% | ACE 10% | ||||||
| (days) | pH 4.0 | pH 6.0 | pH 4.0 | pH 6.0 | pH 4.0 | pH 6.0 | pH 4.0 | pH 6.0 | pH 4.0 | pH 6.0 | pH 4.0 | pH 6.0 |
| 0 |  | 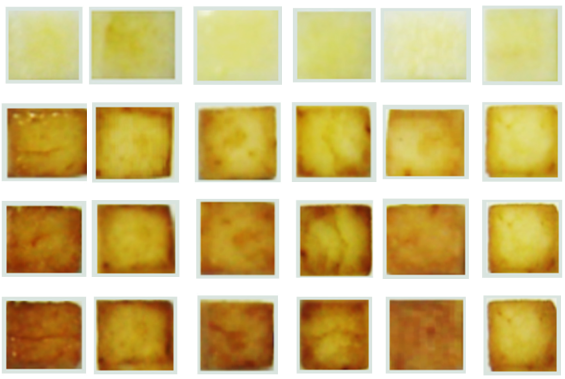 | ||||||||||
| 3 | ||||||||||||
| 6 | ||||||||||||
| 10 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexaki, D.; Gerasopoulos, A.; Gerasopoulos, D. Effect of the Combined Application of Aqueous Cabbage Seed Extract and Chitosan Solutions on the Shelf Life of Fresh-Cut Apple Cubes. Horticulturae 2025, 11, 953. https://doi.org/10.3390/horticulturae11080953
Alexaki D, Gerasopoulos A, Gerasopoulos D. Effect of the Combined Application of Aqueous Cabbage Seed Extract and Chitosan Solutions on the Shelf Life of Fresh-Cut Apple Cubes. Horticulturae. 2025; 11(8):953. https://doi.org/10.3390/horticulturae11080953
Chicago/Turabian StyleAlexaki, Despina, Athanasios Gerasopoulos, and Dimitrios Gerasopoulos. 2025. "Effect of the Combined Application of Aqueous Cabbage Seed Extract and Chitosan Solutions on the Shelf Life of Fresh-Cut Apple Cubes" Horticulturae 11, no. 8: 953. https://doi.org/10.3390/horticulturae11080953
APA StyleAlexaki, D., Gerasopoulos, A., & Gerasopoulos, D. (2025). Effect of the Combined Application of Aqueous Cabbage Seed Extract and Chitosan Solutions on the Shelf Life of Fresh-Cut Apple Cubes. Horticulturae, 11(8), 953. https://doi.org/10.3390/horticulturae11080953








