How Many Acerola (Malpighia emarginata DC.) Fruit Are Required for Reliable Postharvest Quality Assessment?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Plant Material
2.2. Assessment of Fruit Quality Attributes
2.2.1. Weight, Diameter, and Length
2.2.2. Color
2.2.3. Firmness
2.2.4. Vitamin C
2.2.5. Soluble Solids Content (SSC) and Titratable Acidity (TA)
2.3. Statistical Analysis
3. Results
3.1. Descriptive Statistics and Comparison of Fruit Quality Attributes Among Cultivars
3.2. Determination of OSS Based on the MCP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSS | Optimal sample size |
| SFV | São Francisco Valley, Brazil |
| SD | Standard deviation |
| MCP | Maximum curvature point |
| SSC | Soluble solids content |
| TA | Titratable acidity |
| CI95% | 95% confidence interval |
| GCF | General curvature function |
| PD | Perpendicular distances |
| LRP | Linear response plateau |
References
- Carneiro Ferreira, I.; Silva, V.P.d.; Vilvert, J.C.; Souza, F.d.F.; de Freitas, S.T.d.; Lima, M.d.S. Brazilian Varieties of Acerola (Malpighia emarginata DC.) Produced Under Tropical Semi—Arid Conditions: Bioactive Phenolic Compounds, Sugars, Organic Acids, and Antioxidant Capacity. J. Food Biochem. 2021, 45, e13829. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.P.d.; Costa, R.C.; Morais, C.d.L.M.d.; Medeiros, F.G.M.d.; Fernandes, T.R.N.; Hoskin, R.T.; Lima, K.M.G.d. Estimation of Ascorbic Acid in Intact Acerola (Malpighia emarginata DC) Fruit by NIRS and Chemometric Analysis. Horticulturae 2019, 5, 12. [Google Scholar] [CrossRef]
- Poletto, P.; Álvarez-Rivera, G.; López, G.D.; Borges, O.M.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Recovery of Ascorbic Acid, Phenolic Compounds and Carotenoids from Acerola By-Products: An Opportunity for Their Valorization. LWT 2021, 146, 111654. [Google Scholar] [CrossRef]
- Vilvert, J.C.; de Freitas, S.T.d.; Santos, L.F.d.; Ribeiro, T.d.S.; Veloso, C.M. Phenolic Compounds in Acerola Fruit and By-Products: An Overview on Identification, Quantification, Influencing Factors, and Biological Properties. J. Food Meas. Charact. 2024, 18, 216–239. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, Antioxidant Efficacies, and Health Effects—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef] [PubMed]
- Müller Carneiro, J.; Dias, A.F.; Barros, V.d.S.; Giongo, V.; Folegatti Matsuura, M.I.d.S.; Brito de Figueirêdo, M.C. Carbon and Water Footprints of Brazilian Mango Produced in the Semiarid Region. Int. J. Life Cycle Assess. 2019, 24, 735–752. [Google Scholar] [CrossRef]
- Leão, P.C.d.S.; Carvalho, J.N.d. Tropical Viticulture in Brazil: São Francisco Valley as an Important Supplier of Table Grapes to the World Market. In Latin American Viticulture Adaptation to Climate Change: Perspectives and Challenges of Viticulture Facing up Global Warming; Gamboa, G.G., Fourment, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 47–59. [Google Scholar]
- Vilvert, J.C.; de Freitas, S.T.d.; Ferreira, M.A.R.; Costa, E.B.S.d.; Aroucha, E.M.M. Sample Size for Postharvest Quality Traits of ‘Palmer’ Mangoes. Rev. Bras. Frutic. 2021, 43, e014. [Google Scholar] [CrossRef]
- Toebe, M.; Both, V.; Thewes, F.R.; Cargnelutti Filho, A.; Brackmann, A. Sample Size for Estimate the Average of Characters in Apples. Ciência Rural 2014, 44, 759–767. [Google Scholar] [CrossRef][Green Version]
- Schmildt, E.R.; Alexandre, R.S.; Siqueira, A.L.; Mayrinck, L.G.; Schmildt, O. Dimensionamento Amostral para Analisar Caracteres Físicos e Químicos de Frutos de Maracujá-Fedorento [Sample Dimension for Evaluating Physical and Chemical Characters of Wild Passion Fruit]. Rev. Ceres 2017, 64, 115–121. [Google Scholar] [CrossRef][Green Version]
- Schmildt, E.R.; Schmildt, O.; Salinas, I.; Hueso, J.J.; Pinillos, V.; Cuevas, J. Sample Size for the Evaluation of ‘BH-65’ Papaya Fruits Under Protected Cultivation. Rev. Bras. Frutic. 2019, 41, e-107. [Google Scholar] [CrossRef]
- Toebe, M.; Both, V.; Brackmann, A.; Cargnelutti Filho, A.; Thewes, F.R. Tamanho de Amostra para a Estimação da Média de Caracteres de Pêssego na Colheita e Após o Armazenamento Refrigerado [Sample Size to Estimate the Average Peach Characters at Harvest and After Cold Storage]. Ciência Rural 2012, 42, 209–212. [Google Scholar] [CrossRef]
- Silva, W.; Bianco, A.C.; Oliari, L.S.; Giles, J.A.D.; Schmildt, O.; Schmildt, E.R. Dimensionamento Amostral para Caracterização Física e Química em Frutos de Ciriguela [Sample Size in the Physical and Chemical Characterisation of the Red Mombin]. Rev. Agro@Mbiente-Line 2016, 10, 178–182. [Google Scholar] [CrossRef][Green Version]
- Rossetti, A.G.; Moura, C.F.H.; Silva, E.d.O.; Vidal Neto, F.d.C.; Ribeiro, L.B.; Almeida, A.C.P.d. Sample Size for the Physical and Physico-Chemical Characteristics of the Cashew. Rev. Bras. Frutic. 2022, 44, e-614. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Mallawaarachchi, I.; Alvarado, L.A. Analysis of Small Sample Size Studies Using Nonparametric Bootstrap Test with Pooled Resampling Method. Stat. Med. 2017, 36, 2187–2205. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T. Bootstrap. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 497–526. [Google Scholar] [CrossRef]
- Chernick, M.R.; LaBudde, R.A. An Introduction to Bootstrap Methods with Applications to R; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–24. [Google Scholar]
- Silva, A.R.d.; Lima, R.P.d. Determination of Maximum Curvature Point with the R Package soilphysics. Int. J. Curr. Res. 2017, 9, 45241–45245. [Google Scholar]
- Souza, R.R.d.; Toebe, M.; Mello, A.C.; Bittencourt, K.C. Sample Size and Shapiro-Wilk Test: An Analysis for Soybean Grain Yield. Eur. J. Agron. 2023, 142, 126666. [Google Scholar] [CrossRef]
- Neu, I.M.M.; Cargnelutti Filho, A.; Toebe, M.; Carini, F.; Pezzini, R.V.; Silveira, D.L. Sample Size to Evaluate the Degree of Multicollinearity in Rye Morphological Traits. Rev. Caatinga 2023, 36, 215–225. [Google Scholar] [CrossRef]
- Bittencourt, K.C.; Souza, R.R.d.; Pazetto, S.B.; Toebe, M.; Toebe, I.C.D. What Is the Best Way to Define Sample Size for Cauliflower Seedlings? Ciência Rural 2022, 52, e20210747. [Google Scholar] [CrossRef]
- Ritzinger, R.; Kobayashi, A.K.; Oliveira, J.R.P. A Cultura da Aceroleira [The Acerola Crop]; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2003; pp. 72–93. [Google Scholar]
- Ferreira, M.A.R.; Vilvert, J.C.; Silva, B.O.S.d.; Ferreira, I.C.; Souza, F.d.F.; de Freitas, S.T.d. Multivariate Selection Index of Acerola Genotypes for Fresh Consumption Based on Fruit Physicochemical Attributes. Euphytica 2022, 218, 25. [Google Scholar] [CrossRef]
- Calgaro, M.; Braga, M.B. A Cultura da Acerola [The Acerola Crop]; Embrapa Semiárido: Petrolina, Brazil, 2012; pp. 23–30. [Google Scholar]
- AOAC International. Method 967.21: Ascorbic Acid in Vitamin Preparations and Juices—2,6-Dichloroindophenol Titrimetric Method. In Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International: Gaithersburg, MD, USA, 2023. [Google Scholar]
- Efron, B. Bootstrap Methods: Another Look at the Jacknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Guide for its Bootstrap Procedures in Multiple Comparisons. Ciênc. Agrotec. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Vilvert, J.C.; de Freitas, S.T.d.; Veloso, C.M.; Amaral, C.L.F. Genetic Diversity on Acerola Quality: A Systematic Review. Braz. Arch. Biol. Technol. 2024, 67, e24220490. [Google Scholar] [CrossRef]
- Silva, R.d.S.; Souza, F.d.F.; Vilvert, J.C.; Ribeiro, T.d.S.; Costa, C.d.S.R.; de Freitas, S.T.d. Acerola Genetic Diversity and Multitrait Selection for Fresh Consumption and Processing Industry. Sci. Agr. 2025; in press. [Google Scholar]
- Magalhães, D.S.; Rufini, J.C.M.; Alburquerque, A.S.; Viol, R.E.; Fagundes, M.C.P.; Menezes, T.P.d. Genetic Diversity Among Accessions of Acerola Based on the Quality of Fruits. Comum. Sci. 2018, 9, 133–141. [Google Scholar] [CrossRef]
- Carpentieri-Pípolo, V.; Destro, D.; Prete, C.E.C.; Gonzales, M.G.N.; Popper, I.; Zanatta, S.; Silva, A.M.d. Seleção de Genótipos Parentais de Acerola com Base na Divergência Genética Multivariada [West Indian Cherry Parental Genotype Selection Based on Multivariate Genetic Divergence]. Pesqui. Agropecu. Bras. 2000, 35, 1613–1619. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Chen, H.; Liu, R.; Chen, H.; Fang, X.; Xiao, J.; Wu, W.; Gao, H. The Crucial Evaluation Indexes and Relative Measurement Methods of Edible Value for Fresh Fruits and Vegetables: A Review. Futur. Postharvest Food 2024, 1, 222–236. [Google Scholar] [CrossRef]
- Souza, K.O.d.; Moura, C.F.H.; Lopes, M.M.d.A.; Rabelo, M.C.; Miranda, M.R.A.d. Quality of Acerola (Malpighia emarginata) Treated with Gibberelic Acid and Stored Under Refrigeration. Rev. Bras. Frutic. 2017, 39, e-574. [Google Scholar] [CrossRef]
- Mamede, M.E.D.O.; Miranda, M.D.P.S.; Ritzinger, R.; Godoy, R.C.B.D.; Velozo, E.D.S. Physico—Chemical and Sensorial Evaluation of New Varieties of Acerola. Br. Food J. 2009, 111, 387–395. [Google Scholar] [CrossRef]
- Sousa, E.d.S.; Lobo, J.T.; Carreiro, D.d.A.; Dias, D.d.N.; Sanches, L.G.; Cavalcante, Í.H.L. Paclobutrazol in the Flowering Management Affects the Quality of Malpighia emarginata Fruits. Pesq. Agropec. Trop. 2020, 50, e62805. [Google Scholar] [CrossRef]
- Coelho, B.E.S.; Araújo, A.A.d.; Santos, J.C.d.; Braga, A.C.D.; Azevedo, M.C.d.; Figueiredo Neto, A.; Sousa, K.d.S.M.d. Production and Characterization of Powdered Acerola Juice Obtained by Atomization. Acta Sci. Technol. 2025, 47, e70517. [Google Scholar] [CrossRef]
- Dias, D.d.N.; Sousa, K.d.S.M.d.; Lima, A.M.N.; Cavalcante, Í.H.L.; Santos, L.P.A.d.; Cunha, J.C. Nutritional Status, Production and Fruit Quality of West Indian Cherry Fertigated with Nitrogen and Humic Substance. Rev. Bras. Frutic. 2020, 42, e-254. [Google Scholar] [CrossRef]
- Nogueira, R.J.M.C.; Moraes, J.A.P.V.d.; Burity, H.A.; Silva Junior, J.F.d. Efeito do Estádio de Maturação dos Frutos nas Características Físico-Químicas de Acerola [Physicochemical Characteristics of Barbados Cherry Influenced by Fruit Maturation Stage]. Pesq. Agropec. Bras. 2002, 37, 463–470. [Google Scholar] [CrossRef][Green Version]
- Matsuura, F.C.A.U.; Cardoso, R.L.; Folegatti, M.I.d.S.; Oliveira, J.R.P.; Oliveira, J.A.B.d.; Santos, D.B.d. Avaliações Físico-Químicas em Frutos de Diferentes Genótipos de Acerola (Malpighia punicifolia L.) [Physicochemical Evaluation In Fruits from Different Genotypes of Barbados Cherry (Malpighia punicifolia L.)]. Rev. Bras. Frutic. 2001, 23, 602–606. [Google Scholar] [CrossRef][Green Version]
- Lima, P.C.C.; Souza, B.S.; Souza, P.S.; Borges, S.d.S.; Assis, M.D.O.d. Caracterização e Avaliação de Frutos de Aceroleira [Characterization and Evaluation of Fruits of West Indian Cherry]. Rev. Bras. Frutic. 2014, 36, 550–555. [Google Scholar] [CrossRef][Green Version]
- Farinelli, D.; Portarena, S.; Silva, D.F.d.; Traini, C.; Silva, G.M.d.; Silva, E.C.d.; Veiga, J.F.d.; Pollegioni, P.; Villa, F. Variability of Fruit Quality among 103 Acerola (Malpighia emarginata DC) Phenotypes from the Subtropical Region of Brazil. Agriculture 2021, 11, 1078. [Google Scholar] [CrossRef]
- Bittencourt, K.C.; Toebe, M.; Souza, R.R.d.; Pazetto, S.B.; Toebe, I.C.D. Sample Size Affects the Precision of the Analysis of Variance in Experiments with Cauliflower Seedlings. Ciência Rural 2022, 53, e20220180. [Google Scholar] [CrossRef]
- Bittencourt, K.C.; Souza, R.R.d.; Pazetto, S.B.; Toebe, M.; Toebe, I.C.D.; Cargnelutti Filho, A. How Many Cauliflower Seedlings are Necessary to Estimate Experimental Precision Statistics Reliably? Sci. Hortic. 2023, 310, 111788. [Google Scholar] [CrossRef]
- Rajput, D.; Wang, W.J.; Chen, C.C. Evaluation of a Decided Sample Size in Machine Learning Applications. BMC Bioinform. 2023, 24, 48. [Google Scholar] [CrossRef] [PubMed]

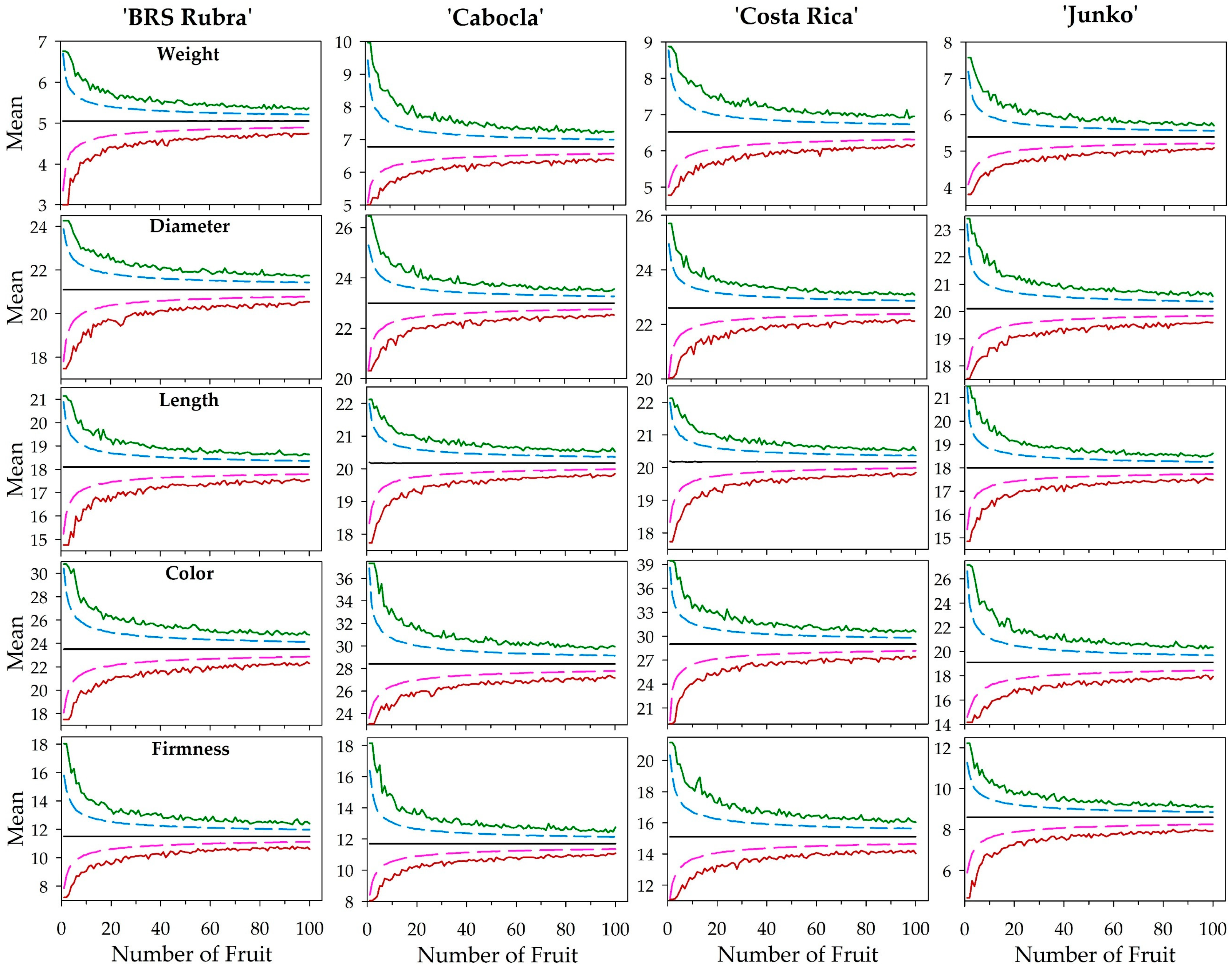
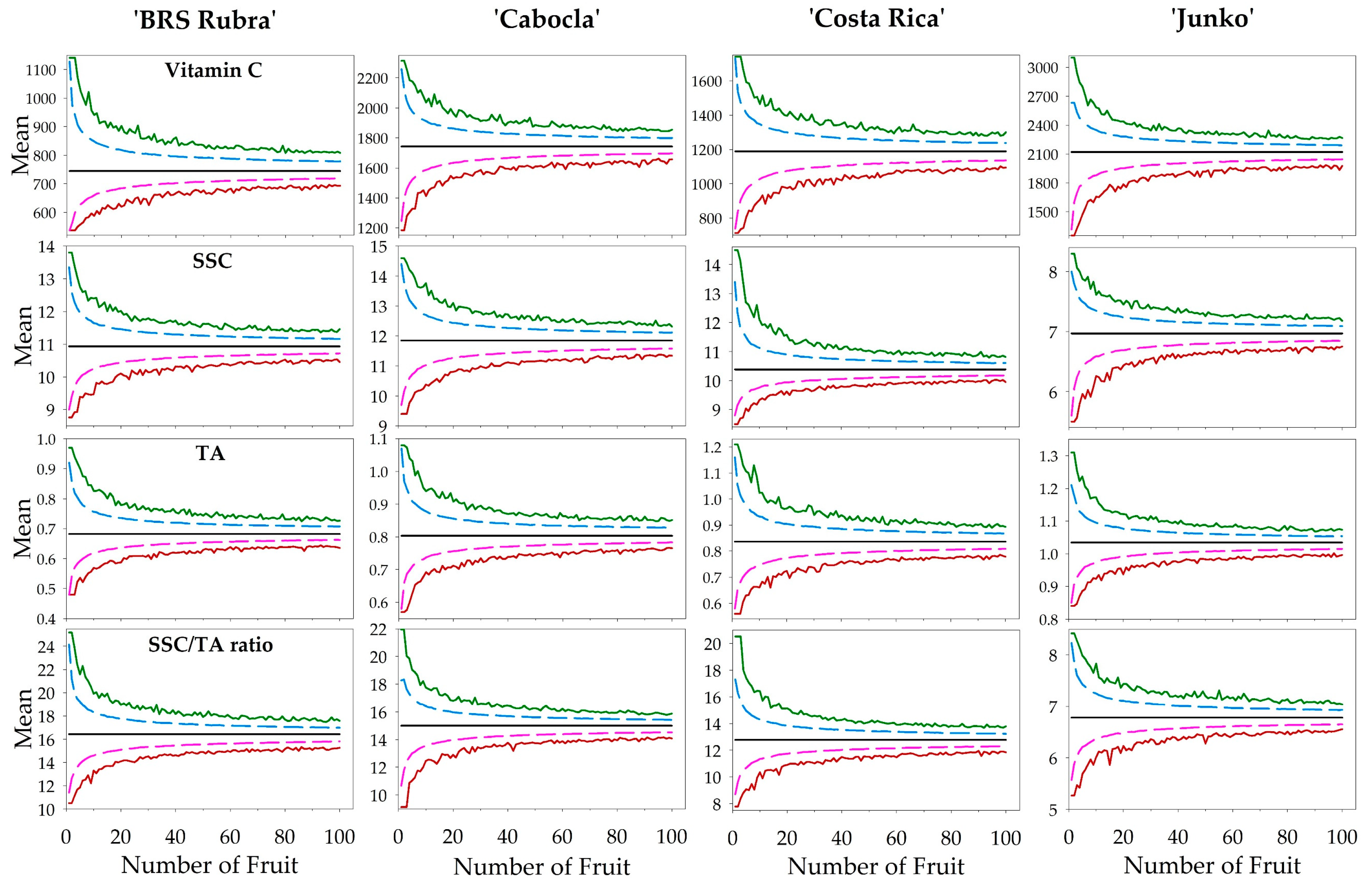
| Quality Attribute | Cultivar | Minimum | Mean 1 | Maximum | Median | SD 2 | CV 3 |
|---|---|---|---|---|---|---|---|
| Weight | BRS Rubra | 3.00 | 5.05 b | 6.76 | 5.14 | 0.81 | 16.12 |
| Cabocla | 5.02 | 6.78 a | 9.96 | 6.50 | 1.11 | 16.36 | |
| Costa Rica | 4.78 | 6.52 a | 8.87 | 6.33 | 1.08 | 16.50 | |
| Junko | 3.81 | 5.39 b | 7.57 | 5.30 | 0.89 | 16.51 | |
| Diameter | BRS Rubra | 17.5 | 21.1 b | 24.3 | 21.3 | 1.7 | 7.91 |
| Cabocla | 20.3 | 23.0 a | 26.5 | 23.2 | 1.3 | 5.75 | |
| Costa Rica | 20.0 | 22.6 a | 25.7 | 22.7 | 1.3 | 5.51 | |
| Junko | 17.5 | 20.1 c | 23.4 | 20.4 | 1.4 | 6.71 | |
| Length | BRS Rubra | 14.8 | 18.1 b | 21.1 | 18.2 | 1.5 | 8.05 |
| Cabocla | 17.7 | 20.2 a | 22.1 | 20.1 | 1.0 | 4.85 | |
| Costa Rica | 17.6 | 19.7 a | 22.2 | 19.7 | 1.1 | 5.45 | |
| Junko | 14.9 | 18.0 b | 21.5 | 17.9 | 1.3 | 7.43 | |
| Color | BRS Rubra | 17.5 | 23.5 b | 30.8 | 23.1 | 3.3 | 14.01 |
| Cabocla | 23.1 | 28.4 c | 37.3 | 27.5 | 3.5 | 12.38 | |
| Costa Rica | 19.1 | 29.0 c | 39.5 | 28.7 | 4.2 | 14.49 | |
| Junko | 14.2 | 19.1 a | 27.1 | 18.8 | 3.2 | 16.88 | |
| Firmness | BRS Rubra | 7.2 | 11.5 b | 18.0 | 11.4 | 2.2 | 19.18 |
| Cabocla | 8.1 | 11.7 b | 18.2 | 11.8 | 2.0 | 17.15 | |
| Costa Rica | 11.1 | 15.1 a | 21.2 | 14.7 | 2.5 | 16.69 | |
| Junko | 4.7 | 8.6 c | 12.2 | 8.7 | 1.6 | 18.08 | |
| Vitamin C | BRS Rubra | 537 | 749 d | 1141 | 752 | 153 | 20.45 |
| Cabocla | 1184 | 1748 b | 2315 | 1736 | 262 | 15.02 | |
| Costa Rica | 712 | 1187 c | 1741 | 1201 | 260 | 21.92 | |
| Junko | 1253 | 2119 a | 3102 | 2162 | 377 | 17.80 | |
| SSC | BRS Rubra | 8.8 | 10.9 b | 13.8 | 11.0 | 1.2 | 10.58 |
| Cabocla | 9.4 | 11.8 a | 14.6 | 11.6 | 1.4 | 11.46 | |
| Costa Rica | 8.5 | 10.4 b | 14.5 | 10.3 | 1.1 | 10.64 | |
| Junko | 5.5 | 7.0 c | 8.3 | 6.9 | 0.6 | 9.03 | |
| TA | BRS Rubra | 0.48 | 0.68 c | 0.97 | 0.66 | 0.11 | 16.60 |
| Cabocla | 0.57 | 0.80 b | 1.08 | 0.80 | 0.12 | 14.41 | |
| Costa Rica | 0.56 | 0.84 b | 1.21 | 0.83 | 0.15 | 18.00 | |
| Junko | 0.84 | 1.03 a | 1.31 | 1.04 | 0.10 | 9.51 | |
| SSC/TA ratio | BRS Rubra | 10.5 | 16.4 a | 25.2 | 16.0 | 3.1 | 18.64 |
| Cabocla | 9.1 | 15.0 b | 21.9 | 15.1 | 2.3 | 15.50 | |
| Costa Rica | 7.8 | 12.8 c | 20.5 | 12.5 | 2.4 | 19.01 | |
| Junko | 5.3 | 6.8 d | 8.4 | 6.7 | 0.7 | 10.54 |
| Quality Attribute | Cultivar | Power Model | R2 | RMSE | d Index |
|---|---|---|---|---|---|
| Weight | BRS Rubra | CI95% = 3.2632 × n–0.5106 | 0.9988 | 0.0149 | 0.9997 |
| Cabocla | CI95% = 4.2581 × n–0.4984 | 0.9990 | 0.0176 | 0.9998 | |
| Costa Rica | CI95% = 3.9015 × n–0.4800 | 0.9984 | 0.0207 | 0.9996 | |
| Junko | CI95% = 3.2514 × n–0.4816 | 0.9975 | 0.0217 | 0.9994 | |
| Diameter | BRS Rubra | CI95% = 6.1982 × n–0.4871 | 0.9992 | 0.0227 | 0.9998 |
| Cabocla | CI95% = 5.0317 × n–0.4937 | 0.9995 | 0.0153 | 0.9999 | |
| Costa Rica | CI95% = 4.8549 × n–0.5022 | 0.9998 | 0.0099 | 0.9999 | |
| Junko | CI95% = 5.3321 × n–0.5052 | 0.9995 | 0.0150 | 0.9999 | |
| Length | BRS Rubra | CI95% = 5.6467 × n–0.5005 | 0.9998 | 0.0105 | 0.9999 |
| Cabocla | CI95% = 3.7081 × n–0.4925 | 0.9995 | 0.0108 | 0.9999 | |
| Costa Rica | CI95% = 3.8000 × n–0.4722 | 0.9949 | 0.0361 | 0.9987 | |
| Junko | CI95% = 5.7841 × n–0.5348 | 0.9949 | 0.0534 | 0.9987 | |
| Color | BRS Rubra | CI95% = 12.529 × n–0.4944 | 0.9995 | 0.0361 | 0.9999 |
| Cabocla | CI95% = 13.1691 × n–0.4905 | 0.9996 | 0.0355 | 0.9999 | |
| Costa Rica | CI95% = 18.0854 × n–0.5342 | 0.9946 | 0.1718 | 0.9987 | |
| Junko | CI95% = 12.1620 × n–0.4925 | 0.9995 | 0.0353 | 0.9999 | |
| Firmness | BRS Rubra | CI95% = 8.2023 × n–0.4852 | 0.9983 | 0.0445 | 0.9996 |
| Cabocla | CI95% = 8.0114 × n–0.5066 | 0.9997 | 0.0191 | 0.9999 | |
| Costa Rica | CI95% = 9.3704 × n–0.4873 | 0.9992 | 0.0344 | 0.9998 | |
| Junko | CI95% = 5.6593 × n–0.4818 | 0.9968 | 0.0428 | 0.9992 | |
| Vitamin C | BRS Rubra | CI95% = 586.4827 × n–0.4972 | 0.9997 | 1.2452 | 0.9999 |
| Cabocla | CI95% = 1012.465 × n–0.4984 | 0.9998 | 1.5869 | 0.9999 | |
| Costa Rica | CI95% = 990.848 × n–0.4952 | 0.9998 | 1.9818 | 0.9999 | |
| Junko | CI95% = 1378.287 × n–0.4813 | 0.9972 | 9.7784 | 0.9992 | |
| SSC | BRS Rubra | CI95% = 4.4027 × n–0.4938 | 0.9996 | 0.0110 | 0.9999 |
| Cabocla | CI95% = 4.9162 × n–0.4788 | 0.9974 | 0.0335 | 0.9993 | |
| Costa Rica | CI95% = 4.5390 × n–0.5181 | 0.9992 | 0.0165 | 0.9998 | |
| Junko | CI95% = 2.4248 × n–0.4979 | 0.9996 | 0.0067 | 0.9999 | |
| TA | BRS Rubra | CI95% = 0.4387 × n–0.4980 | 0.9998 | 0.0009 | 0.9999 |
| Cabocla | CI95% = 0.4706 × n–0.5166 | 0.9974 | 0.0032 | 0.9993 | |
| Costa Rica | CI95% = 0.5801 × n–0.4967 | 0.9997 | 0.0013 | 0.9999 | |
| Junko | CI95% = 0.3715 × n–0.4904 | 0.9985 | 0.0019 | 0.9996 | |
| SSC/TA ratio | BRS Rubra | CI95% = 12.3633 × n–0.5141 | 0.9985 | 0.0617 | 0.9996 |
| Cabocla | CI95% = 8.3038 × n–0.4742 | 0.9910 | 0.1059 | 0.9977 | |
| Costa Rica | CI95% = 8.9859 × n–0.4843 | 0.9973 | 0.0616 | 0.9993 | |
| Junko | CI95% = 2.7115 × n–0.4931 | 0.9993 | 0.0096 | 0.9998 |
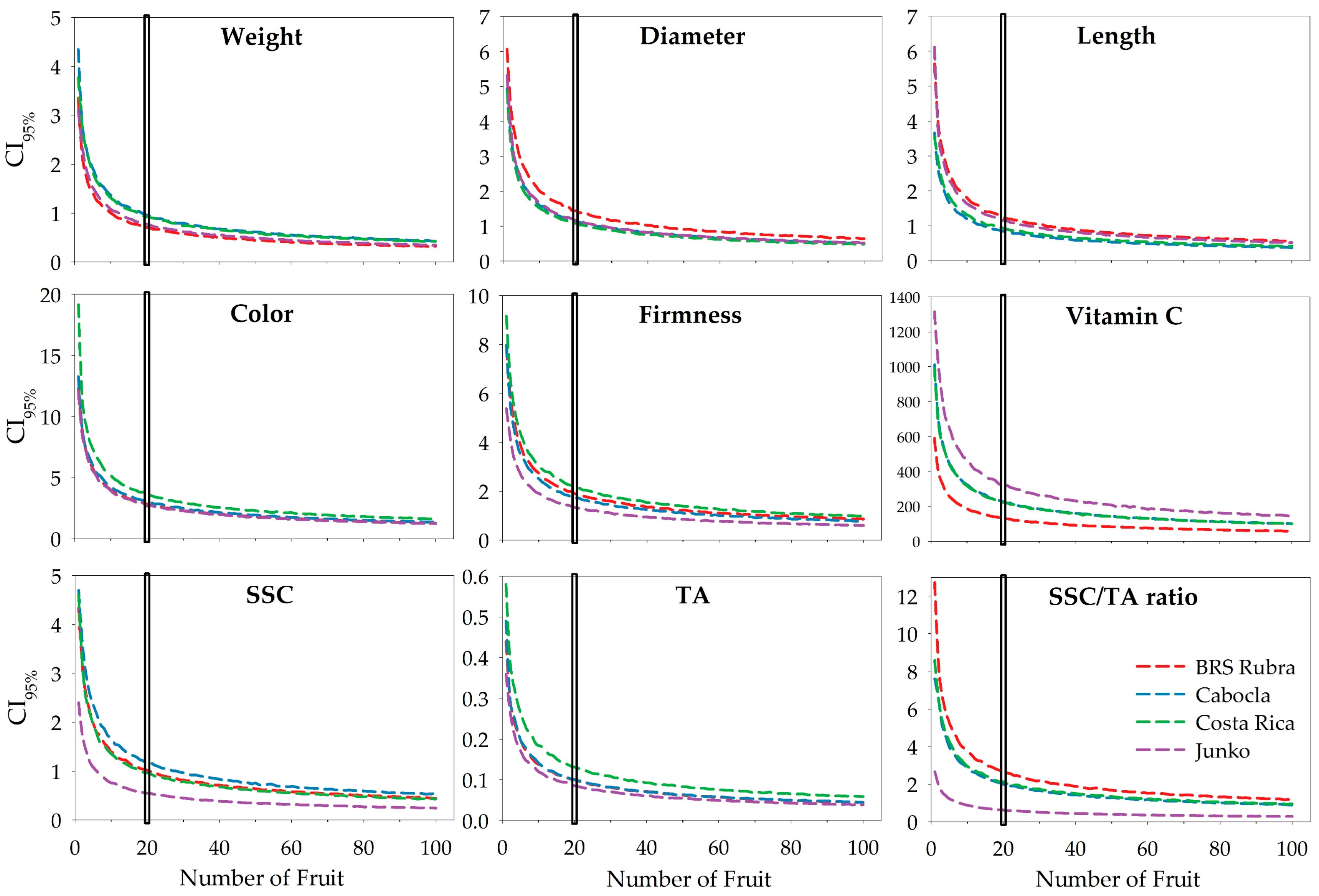
| Quality Attribute | Cultivar | General Curvature Function Method | Perpendicular Distances Method | Linear Response Plateau Method | |||
|---|---|---|---|---|---|---|---|
| Maximum CI95% | Sample Size | Maximum CI95% | Sample Size | Maximum CI95% | Sample Size | ||
| Weight | BRS Rubra | 2.2500 | 2 | 0.9000 | 12 | 0.7718 | 17 |
| Cabocla | 2.9101 | 2 | 1.1677 | 13 | 1.0289 | 18 | |
| Costa Rica | 2.8650 | 2 | 1.1022 | 14 | 0.8460 | 25 | |
| Junko | 2.4300 | 2 | 0.8259 | 17 | 0.8195 | 18 | |
| Diameter | BRS Rubra | 4.4800 | 2 | 1.5306 | 17 | 1.4430 | 20 |
| Cabocla | 3.6200 | 2 | 1.3140 | 15 | 1.2042 | 19 | |
| Costa Rica | 3.4400 | 2 | 1.3247 | 13 | 1.0855 | 20 | |
| Junko | 3.8601 | 2 | 1.3460 | 15 | 1.2483 | 18 | |
| Length | BRS Rubra | 3.9750 | 2 | 1.4093 | 15 | 1.4056 | 16 |
| Cabocla | 2.6300 | 2 | 1.0385 | 13 | 0.7838 | 24 | |
| Costa Rica | 2.3533 | 3 | 1.0427 | 15 | 1.0253 | 17 | |
| Junko | 3.7800 | 2 | 1.4362 | 13 | 1.1601 | 20 | |
| Color | BRS Rubra | 9.1000 | 2 | 3.0335 | 17 | 2.9585 | 19 |
| Cabocla | 9.1900 | 2 | 3.4947 | 15 | 2.8609 | 23 | |
| Costa Rica | 11.7700 | 2 | 4.4723 | 13 | 3.8190 | 19 | |
| Junko | 8.6701 | 2 | 3.3101 | 14 | 2.7182 | 21 | |
| Firmness | BRS Rubra | 6.0200 | 2 | 1.9956 | 18 | 1.9837 | 19 |
| Cabocla | 5.7302 | 2 | 1.9960 | 15 | 1.7230 | 21 | |
| Costa Rica | 6.8500 | 2 | 2.3560 | 17 | 2.7732 | 13 | |
| Junko | 3.4267 | 3 | 1.5387 | 15 | 1.2068 | 25 | |
| Vitamin C content | BRS Rubra | 409.5100 | 2 | 158.5673 | 14 | 120.8430 | 24 |
| Cabocla | 716.1900 | 2 | 252.1048 | 16 | 219.8540 | 22 | |
| Costa Rica | 694.9400 | 2 | 257.1668 | 15 | 211.3904 | 23 | |
| Junko | 1033.8350 | 2 | 371.7634 | 15 | 357.5112 | 17 | |
| SSC | BRS Rubra | 3.1704 | 2 | 1.1131 | 16 | 1.2250 | 14 |
| Cabocla | 3.6500 | 2 | 1.3468 | 15 | 1.0000 | 28 | |
| Costa Rica | 3.2000 | 2 | 1.2250 | 12 | 0.9650 | 20 | |
| Junko | 1.7500 | 2 | 0.6267 | 15 | 0.5062 | 24 | |
| TA | BRS Rubra | 0.3100 | 2 | 0.1127 | 15 | 0.1005 | 20 |
| Cabocla | 0.3100 | 2 | 0.1267 | 12 | 0.1207 | 14 | |
| Costa Rica | 0.4050 | 2 | 0.1450 | 16 | 0.1146 | 26 | |
| Junko | 0.2750 | 2 | 0.1046 | 13 | 0.0796 | 24 | |
| SSC/TA ratio | BRS Rubra | 8.5250 | 2 | 2.9953 | 15 | 2.9557 | 16 |
| Cabocla | 5.1802 | 3 | 2.2407 | 16 | 1.8931 | 23 | |
| Costa Rica | 6.6561 | 2 | 2.1861 | 18 | 2.1285 | 20 | |
| Junko | 1.9850 | 2 | 0.7080 | 15 | 0.5918 | 22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilvert, J.C.; Veloso, C.M.; Souza, F.d.F.; de Freitas, S.T. How Many Acerola (Malpighia emarginata DC.) Fruit Are Required for Reliable Postharvest Quality Assessment? Horticulturae 2025, 11, 941. https://doi.org/10.3390/horticulturae11080941
Vilvert JC, Veloso CM, Souza FdF, de Freitas ST. How Many Acerola (Malpighia emarginata DC.) Fruit Are Required for Reliable Postharvest Quality Assessment? Horticulturae. 2025; 11(8):941. https://doi.org/10.3390/horticulturae11080941
Chicago/Turabian StyleVilvert, João Claudio, Cristiane Martins Veloso, Flávio de França Souza, and Sérgio Tonetto de Freitas. 2025. "How Many Acerola (Malpighia emarginata DC.) Fruit Are Required for Reliable Postharvest Quality Assessment?" Horticulturae 11, no. 8: 941. https://doi.org/10.3390/horticulturae11080941
APA StyleVilvert, J. C., Veloso, C. M., Souza, F. d. F., & de Freitas, S. T. (2025). How Many Acerola (Malpighia emarginata DC.) Fruit Are Required for Reliable Postharvest Quality Assessment? Horticulturae, 11(8), 941. https://doi.org/10.3390/horticulturae11080941









