Abstract
We examined the modulation of ‘Sweet Gold’ and ‘Goldone’ kiwifruit (Actinidia spp.) ripening using modified atmosphere and humidity (MAH), 1-methylcyclopropene (1-MCP), and edible coating treatments up to 35 days after storage (DAS) at room temperature. The 1-MCP and coating treatments decreased [CO2] in both cultivars, whereas MAH treatment rapidly increased or decreased [CO2]. Use of 1-MCP highly preserved firmness in both cultivars, followed by coating. MAH sharply reduced approximately 17% of ‘Goldone’ fruit firmness at 7 DAS compared to other treatments. MAH, 1-MCP, and coating reduced weight loss in ‘Sweet Gold’ kiwifruits from 14 to 35 DAS. Coating prevented approximately 14% of weight loss in ‘Goldone’ fruits during storage by strong adherence to the fruit surface. The flesh of control and MAH-treated fruits of both cultivars exhibited reduced acidity during storage, increasing the soluble solids content to acidity ratio. The use of 1-MCP delayed a reduction in L* values of the peel color of ‘Sweet Gold’ kiwifruits, while reduced L* values of flesh color were mostly observed with control and MAH treatment in both fruit cultivars. The use of 1-MCP, coating, and MAH maintained high total phenolics, ABTS, and vitamin C levels in both cultivars at 14 and 28 DAS. Fruit ripening was delayed by coating and promoted by MAH treatment, while maintaining the quality and functional substances of the fruit.
1. Introduction
Kiwifruit (Actinidia spp.) is classified as a climacteric fruit at the onset of ripening [1,2,3]. In 2002, China recorded the highest kiwifruit production (2.38 million metric tons), followed by New Zealand, Italy, Greece, and Iran, with South Korea ranking 15th [4]. Green-fleshed ‘Hayward’ kiwifruit (Actinidia deliciosa) is the most widely distributed worldwide, including in South Korea, owing to its ease of cultivation and high storability [5,6]. Yellow-fleshed ‘Hort16A’ kiwifruit (Actinidia chinensis), introduced in New Zealand in 2000, is widely appreciated for its high nutritional value and antioxidant content, notably carotenoids and total polyphenols [5,6]. The yellow-fleshed cultivars ‘Haegeum’, ‘Jecy Gold’, ‘Sweet Gold’, and ‘Goldone’ were recently developed through South Korea’s national kiwifruit breeding program to meet growing consumer demand for health-promoting polyphenols [5,6]. However, most kiwifruit cultivars, particularly ‘Sweet Gold’ and ‘Goldone’, are often difficult to ripen due to the accumulation of calcium oxalate, which delays the ripening process and results in a bright green peel, high acidity, and low sugar content [2,5].
On the one hand, kiwifruit exhibit undetectable production of endogenous ethylene during storage at temperatures below 5.0 °C and display non-climacteric ripening behavior [1,2,3,7,8]. On the other hand, kiwifruit are sensitive to exposure to low ethylene concentrations (1.0 μL·L−1) and produce autocatalytic ethylene to accelerate ripening in a climacteric pattern [1,2,3,7,8], leading to cell wall breakdown and increased susceptibility to softening even under low temperatures. In addition, increased ethylene production in kiwifruit is accelerated by flesh dehydration and shrinkage at room temperature and/or when fruits are stored in low relative humidity during winter [3]. ‘Sweet Gold’ and ‘Goldone’ kiwifruit are conventionally air-stored and display variable softening rates depending on exposure to exogenous ethylene, orchard and storage conditions, and harvest and postharvest handling. Prestorage treatments are needed to modulate softening and delay senescence in kiwifruit [7,8,9].
The application of 1-methylcyclopropene (1-MCP) in postharvest kiwifruit effectively regulates ethylene biosynthesis, respiration, enzyme activity, maturity, and softening [10,11,12]. Postharvest coatings containing sucrose, lipids, and proteins mixed with other natural biopolymers form a thin, semipermeable layer on the fruit surface, extending shelf life by regulating CO2 diffusion and creating a modified atmosphere across various kiwifruit cultivars [8,10,13,14,15,16,17]. However, limited data are available for the postharvest behavior of coated ‘Sweet Gold’ and ‘Goldone’ kiwifruit. Modified atmosphere and humidity (MAH) is a novel postharvest approach to inducing ripening of firm fruits by applying moisture stress while maintaining storability by controlling O2 and CO2 within small eco-friendly simple containers, with low costs and non-requirement of harmful chemicals [8,18].
This study aimed to investigate the modulation of ripening through MAH, 1-MCP, and coating treatments with the goal of improving fruit quality and antioxidant levels in two newly bred kiwifruit cultivars during 35 DAS at room temperature to avoid the limitations under cold storage.
2. Materials and Methods
2.1. Sample Preparation
The ‘Sweet Gold’ and ‘Goldone’ kiwifruit samples used in the study were obtained two days after picking from a packing house in a commercial orchard located in Seogwipo-si, South Korea, on 30 October 2023. A total of 320 healthy fruit samples with uniform size and quality for each cultivar were placed on trays in a university fruit laboratory in Anseong-si, South Korea, and stored at room temperature [20.0 ± 0.5 °C/50 ± 5% relative humidity (RH)].
2.2. Treatment
Eighty fruits from each cultivar were collected every 7 days over 35 days of room temperature storage (35 DAS) from each treatment: control (no treatment), MAH, and 1-MCP treatment. Next, for the MAH treatment, 1000 mL of water was added to a small plastic container (50 cm × 50 cm). Thereafter, all fruit samples from each cultivar were treated by exposure to a humid environment without immersion under a sealed plastic container for 24 h, such as in the 1-MCP treatment conditions presented below, which were passive MA storage with simple high humidity that automatically reduces the respiratory volume in fruits. For the MCP-1 treatment, fruits were exposed to 1.0 ppm of 1-MCP gas through vaporization in a sealed plastic container for 24 h at room temperature. The fruit was treated with a 2% solution of sucrose-based fatty acid monoester coating agent. The ingredients of the coating agent are as follows: 15.0% (w/v) ethanol, 5.0% α-d-glucopyranoside, β-d-fructofuranans, combined palmitates and stearates, 2.0% water, and 78% of unknown ingredients (Naturcover Extra Preservation®; Decco Co., Valencia, Spain). Fruit samples were treated by dipping them into a 10 L solution containing 5.0% coating material prepared with tap water for 5 min [19].
2.3. Fruit Quality Parameters
Experimental analyses were conducted on two main groups for each fruit cultivar, with measurements taken every 7 days over 35 days. One group (10 fruits per treatment for each cultivar per DAS) was subjected to non-destructive analysis to monitor CO2 production, % weight loss, and peel color development. The other group (seven fruits per treatment for each cultivar per DAS) was subjected to destructive analysis to determine flesh color development, total soluble solids (TSS), total acidity (TA), TSS/TA, and antioxidant activity.
Scanning electron microscopy (SEM; SU-3500, Hitachi Co., Ltd., Tokyo, Japan) images were presented, with 100× zoom, of 2 mm thick samples of peel from control and coated fruits at 14 DAS.
For non-destructive analysis, each fruit was placed in a 1000 mL plastic bag containing a CO2 sensor and monitored for 1 h using a portable CO2 detector (Gastiger-6000; Shenzhen Wandi Science and Technology Co., Ltd., Hangzhou, China) to assess CO2 concentrations within fruit tissue [19]. The fresh weight of each kiwifruit was measured using an electronic balance (EB-430HU; Shimadzu Corporation, Kyoto, Japan). Weight loss percentage was calculated as the difference between the initial weight and the weight recorded after DAS, divided by the initial weight and multiplied by 100. Fruit peel color changes were monitored using a Minolta CR-400 colorimeter equipped with an 8.0 mm aperture and a 2.0° observer (Konica Minolta, Inc., Tokyo, Japan) at three points on the equatorial region of each fruit surface. The L* value indicates lightness on a scale from 0.0 (black) to 100.0 (white). The a* value indicates the shift from greenness (negative values) to redness (positive values) of the fruit. Positive b* values reflect yellowness, with negative values representing blueness.
For destructive analysis, fruits were cut in half along the equatorial axis and color changes were monitored using a colorimeter (CR-400; Konica Minolta), with color expressed quantitatively as L*, a*, and b* as measured for the peel samples above. Fruit flesh firmness was recorded at three central points by inserting a plunger using a penetrometer with an 8.0 mm diameter cylindrical tip (KM-1; Fujiwara Factory Co., Ltd., Tokyo, Japan) and expressed in Newtons (N). Subsequently, the fruits were freshly squeezed with cheesecloth. The extracted kiwifruit juice was used to determine TSS and diluted 100-fold to measure fruit acidity using a Brix-acidity meter (GMK-706R; G-WON Hitech Co., Ltd., Seoul, Republic of Korea). The TSS/TA ratio was calculated by dividing TSS by acidity (%), and the juice was immediately stored at −80 °C for subsequent antioxidant analysis.
2.4. Antioxidant Analysis
The juice extracted from the fruits was centrifuged at 3000× g for 20 min and subjected to colorimetry to determine total phenolic content using the Folin–Ciocalteu method with some modifications [20]. After extraction with the Folin–Ciocalteu reagent, the juice sample was analyzed colorimetrically at 725 nm using a UV-visible spectrophotometer (UV-1800 spectrophotometer; Shimadzu Corporation). The absorbance of the sample solution was compared with the calibration curve of gallic acid equivalents to determine the total phenolic content.
An ABTS [2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)] assay was performed on the juice extract following the methods of Arnao et al. [21] with some modifications [20]. After mixing the juice sample with ABTS stock solution, comprising 7.4 mM ABTS and 2.6 mM potassium persulfate solutions, the absorbance was measured colorimetrically at 734 nm using a spectrophotometer (UV-1800 spectrophotometer; Shimadzu Corporation).
Vitamin C was extracted from 1.0 mL of fruit juice extract using a solution containing 5.0% (w/v) meta-phosphoric (100.0 mL) acid and 10.0% acetic acid. The mixture was centrifuged at 3000 rpm for 10 min, diluted with 100.0 mL distilled water, and analyzed using high-performance liquid chromatography (Waters Alliance 2695; Waters Corporation, Milford, MA, USA) to determine vitamin C content, according to previous studies [19,22].
2.5. Statistical Analysis
Data from the treatment results for each fruit cultivar were analyzed using Minitab software v. 15.1 (Minitab LLC, State College, PA, USA). One-way analysis of variance was used to assess statistical differences in each variable by DAS among the experimental groups. Duncan’s new multiple range test was performed to test the significance of differences at p < 0.05. Also, a two-way ANOVA was used to determine the effects of two independent variables which produced the main effects, treatment and storage, on each dependent variable (respiration rate, firmness, and weight loss), as well as the interaction effects of treatment × storage.
3. Results and Discussion
3.1. Changes in Fruit Respiration Rates
MAH treatment considerably increased external CO2 concentration by 7.35 mL·kg−1·h−1 in ‘Sweet Gold’ kiwifruit at room temperature at 7 DAS, with CO2 concentrations declining from 14 to 35 DAS (Figure 1A). CO2 concentrations increased more rapidly in control fruits than those in MAH-, 1-MCP-, and coating-treated ‘Sweet Gold’ kiwifruit between 28 and 35 DAS. CO2 concentrations increased in MAH-treated ‘Goldone’ kiwifruit at 0, 21, and 35 DAS (Figure 1B), presumably due to oxidative stress induced by excessive moisture and anaerobic conditions within the confined plastic modified atmosphere (MA) storage during the 24 h of treatment. High respiration rates in coated fruits at 0 DAS were likely induced by water-soaking damage under anaerobic treatment conditions and may have been exacerbated by gas exchange through improperly coated stomata and lenticels, as observed in other coated fruits [19,23]. Ethylene accumulation, and elevated CO2 and low O2 levels in pullulan-coated kiwifruit may be due to the restriction of ethylene translocation from the internal to external fruit atmosphere during treatment [14,15]. However, low CO2 concentrations were primarily obtained in fruits treated with 1-MCP and coating. 1-MCP competitively inhibited ethylene action by binding the ethylene receptor and could have reduced the ethylene-induced respiration process of kiwifruit [8,9,10,11,12,17]. The CO2 concentrations were mainly affected by effects such as treatment and storage time as well as the interaction effects between them (p < 0.001; Figure 1). The SEM images showed coating evenly spread onto the peel surface in fine fragments (Figure 2B,D) compared to uncoated fruits (Figure 2A,C). The coating served as a barrier to CO2 and O2 exchange between the fruit and the air, a phenomenon widely reported in other climacteric fruit species such as bananas (Musa spp.), mangos (Mangifera indica), apples (Malus domestica), peaches (Prunus persica), and plums (Prunus domestica) [19,23,24,25,26,27,28].
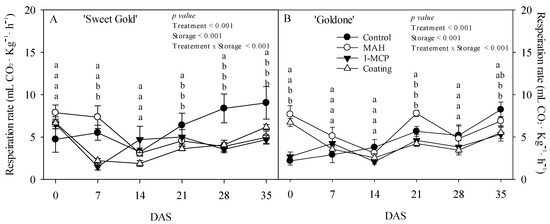
Figure 1.
Changes in respiration rate in ‘Sweet Gold’ (A) and ‘Goldone’ (B) kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS). Each observation is the mean ± SD. Different lower-case letters adjacent to each datum point at each DAS indicate significant differences between the treatments at p < 0.05.
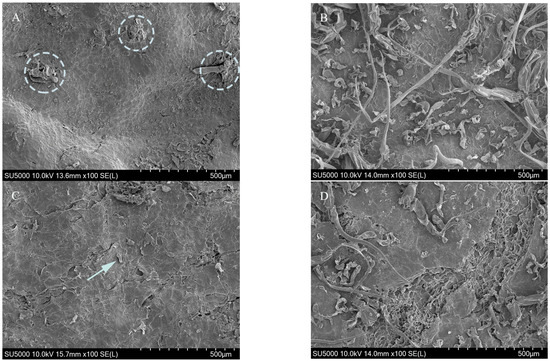
Figure 2.
Scanning electron microscopy images showing the cuticles of ‘Sweet Gold’ (top) and ‘Goldone’ (bottom) kiwifruit following control (A,C) and coating (B,D) treatments. Arrows and circles indicate pores and epicuticular wax, respectively. Magnification = 100×.
3.2. Changes in Fruit Quality Parameters
Coated ‘Sweet Gold’ kiwifruit exhibited higher flesh firmness at 7 DAS than that of other treated fruits, a key maturity indicator particularly relevant for assessing the end of storage life in kiwifruits [8,29] (Figure 3A). Fruit firmness was strongly retained at 14 and 28 DAS by both 1-MCP and coating treatments, consistent with other studies reporting increased fruit firmness across kiwifruit cultivars [8,11,12,13,14,15,16,17]. At 14 DAS, ‘Goldone’ kiwifruit treated with 1-MCP exhibited greater firmness than did ‘Sweet Gold’ kiwifruit, contributing to preserving storability and shelf life during the storage period (Figure 3B). ‘Sweet Gold’ kiwifruit are known for their high initial firmness [5], and the variation in softening rates across kiwifruit cultivars results from differential enzyme activities, cell wall structures, and the accessibility of specific cell wall polysaccharides [30,31]. The flesh firmness of MAH-treated ‘Goldone’ kiwifruit decreased sharply to 4.3 N at 7 DAS, reaching the eating-ripe stage preferred by consumers [1,11]. MAH, 1-MCP, and coating treatments resulted in a clear reduction in the weight loss rate of ‘Sweet Gold’ kiwifruit at 14–35 DAS (Figure 3C). The small plastic MA storage used during treatment created a high-humidity environment, minimizing fruit water loss [8,18]. Coated ‘Goldone’ kiwifruit exhibited the maximum reduction in weight loss from 7 to 35 DAS (Figure 3D) through coating on the fruit surface (Figure 2), slowing down moisture loss during storage and reducing rapid fruit shrivel, consistent with the findings of other kiwifruit studies [8,13,14,16]. Coated fruits with low weight loss may have softened, resulting in firmness levels similar to those of control or MAH fruits during the storage period, as severe shrivel has been observed to elevate hardness and toughness in apple fruits [32]. At 35 DAS, fruit weight loss was significantly higher in ‘Sweet Gold’ kiwifruit (12.4–13.9%) compared with that in ‘Goldone’ kiwifruit (8.9–10.5%). The fruit surface of ‘Goldone’ kiwifruit exhibited higher epicuticular wax density and larger lenticels due to enhanced biosynthesis (Figure 2), reducing transpirational water loss and providing protection against fruit desiccation compared with ‘Sweet Gold’ kiwifruit [33]. This is an area where deeper analysis is needed, such as regarding differences in cell wall structure or metabolic activity between the cultivars. Fruit firmness and weight loss were not affected by interaction effects but were affected by the main effects, treatment and storage time (Figure 3).
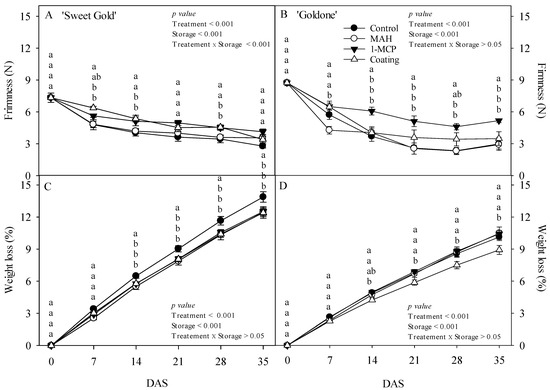
Figure 3.
Changes in fruit firmness (A,B) and weight loss (C,D) in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS). Each observation is the mean ± SD. Each observation is the mean ± SD. Different lower-case letters adjacent to each datum point at each DAS indicate significant differences between the treatments at p < 0.05.
Flesh TSS contents in both kiwifruit cultivars did not significantly differ across all treatments, except for a higher TSS content in MAH-treated ‘Sweet Gold’ at 7 DAS and ‘Goldone’ at 21 DAS (Figure 4A,B). TSS did not show a relative increase in either ‘Sweet Gold’ or ‘Goldone’ kiwifruit from 0 to 35 DAS, in contrast to previous findings where starch degradation contributed to oligosaccharide accumulation during post-ripening in yellow- and red-fleshed kiwifruit [1,11,12,17]. During storage, total acidity in the flesh predominantly decreased in control ‘Sweet Gold’ and MAH-treated ‘Goldone’ kiwifruit (Figure 4C,D), likely due to enhanced respiratory metabolism, which led to an increase in the SSC-to-acidity ratio [1,11] in Figure 5A,B. Notably, MAH-treated and control ‘Sweet Gold’ and ‘Goldone’ kiwifruit exhibited dramatically increased TSS/acidity at 7 and 14 DAS. Yellow-fleshed kiwifruit harvested early at the end of November retained high flesh firmness after removal from storage, and MAH treatment helped avoid the hard-core stage within the short eating window of a few days [34].
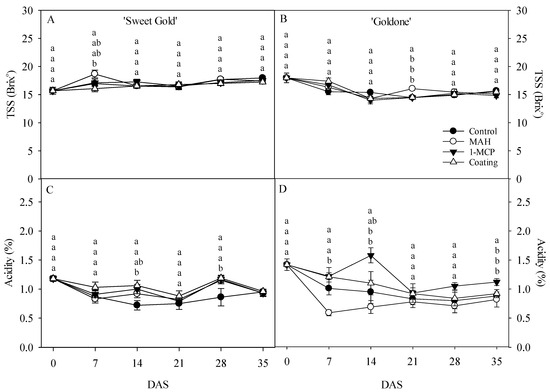
Figure 4.
Changes in total soluble solids TSS; (A,B) and acidity (C,D) in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS). Each observation is the mean ± SD. Each observation is the mean ± SD. Different lower-case letters adjacent to each datum point at each DAS indicate significant differences between the treatments at p < 0.05.
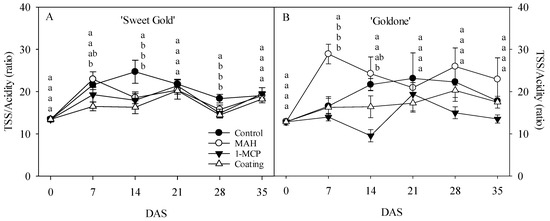
Figure 5.
Changes in TSS/acidity ratio (A,B) in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS). Each observation is the mean ± SD. Different lower-case letters adjacent to each datum point at each DAS indicate significant differences between the treatments at p < 0.05.
1-MCP treatment delayed a reduction in L* values of the background peel color in ‘Sweet Gold’ kiwifruit during storage, which was closely associated with high market prices, whereas control fruits exhibited lower L* values [1,11] in Table 1. The L* values of peel color in ‘Goldone’ kiwifruit did not significantly differ between the treatments post-storage, presumably due to cultivar-specific pigment stability. The flesh L* values for ‘Sweet Gold’ kiwifruit decreased in the control and MAH treatment at 7 and 14 DAS but were similar to those of all treated fruits during storage. The flesh L* values for control and MAH-treated ‘Goldone’ kiwifruit declined during storage. Low L* values were associated with color degradation and the onset of dark pigment accumulation in the pulp due to oxidative browning reactions [17,35]. Also, oxidative enzymes, PPO and POD, catalyzed the oxidation of phenols to quinones and then to melanins, linking this directly to the reduction in L*. The flesh L* values decreased considerably by 26.6% in all ‘Goldone’ kiwifruits compared with the 14% reduction in ‘Sweet Gold’ kiwifruit. ‘Sweet Gold’ was reported to have a later maturity period than ‘Goldone’ during fruit growth, which may affect the slower color change in the flesh of ‘Sweet Gold’ than that of ‘Gold One’ [36].

Table 1.
Color L* values of peel and flesh tissues in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS).
Fruit peel a* values in ‘Sweet Gold’ kiwifruit remained similar across all treatments during storage, with the highest values were observed in MAH-treated ‘Goldone’ kiwifruit at 28 and 35 DAS (Table 2). Control fruit showed a* values closest to 0 for ‘Sweet Gold’ kiwifruit flesh at 14 and 28 DAS and for ‘Goldone’ at 28 DAS, indicating red-flesh color development. The a* values significantly increased in both the peel and flesh tissues of all ‘Sweet Gold’ kiwifruit compared with those of ‘Goldone’ kiwifruit throughout storage, partially reflecting chlorophyll and carotenoid breakdown and anthocyanin accumulation [17,35,37].

Table 2.
Color a* values of peel and flesh tissues in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS).
Increased peel b* values were primarily observed in ‘Sweet Gold’ kiwifruit treated with 1-MCP and coating, as well as in coated ‘Goldone’ during storage (Table 3). Fruit flesh b* values were well retained at 28 DAS in coated ‘Sweet Gold’ kiwifruit, with the highest b* values observed in 1-MCP-treated ‘Goldone’ kiwifruit, which retained yellow-colored flesh at ripening [5,17]. Flesh b* values considerably decreased in all ‘Sweet Gold’ kiwifruit, which was associated with rapid softening and weight loss, compared with those in ‘Goldone’ kiwifruit.

Table 3.
Color b* values of peel and flesh tissues in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS).
3.3. Changes in Antioxidants
An increase in total phenolics in the flesh tissue was observed in 1-MCP-treated and coated ‘Sweet Gold’ kiwifruit at 14 DAS, with levels at 28 DAS following the order of 1-MCP > MAH > coating > control (Figure 6A). The total phenolic content significantly increased in MAH- and coating-treated ‘Goldone’ kiwifruit at 14 DAS (Figure 6B), presumably due to an increase in the respiration rate and ethylene production at 0 DAS during treatment and increased stress and physiological disorders [19,23]. Phenolic compounds are secondary metabolites involved in fruit chemical defense against oxidative stress associated with pathogen invasion, ripening, heat and drought stress, and ethylene production [12,16,17]. Total phenolics in MAH-treated ‘Goldone’ kiwifruit significantly decreased from 14 to 28 DAS due to the oxidation of phenolics during fruit ripening. This could result in enzymatic fruit browning and reduced L* values observed in MAH-treated fruits [36,38,39] in Table 1.
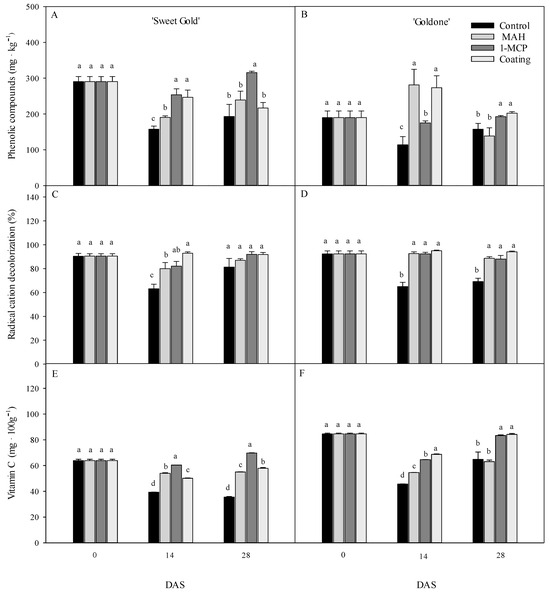
Figure 6.
Total phenolic content (A,B), radical cation decolorization (C,D), and vitamin C content (E,F) in ‘Sweet Gold’ and ‘Goldone’ kiwifruit following control (untreated), MAH, 1-MCP, and coating treatments at 0, 7, 14, 21, 28, and 35 days after room temperature storage (DAS). Error bars represent the error of the means when larger than the dimension of the symbol. Each observation is the mean ± SD. Different lower-case letters adjacent to each datum point at each DAS indicate significant differences between the treatments at p < 0.05.
Coating treatment enhanced resistance-related ABTS radical scavenging activity in ‘Sweet Gold’ kiwifruit at 14 DAS, with some degree of increased ABTS in 1-MCP- and MAH-treated fruits (Figure 6C). No significant difference in ABTS was observed between the treatment fruits at 28 DAS. ABTS activity in ‘Goldone’ kiwifruit remained relatively high in 1-MCP-, MAH-, and coating-treated fruits at 14 DAS, continuing to increase through 28 DAS (Figure 6D). Vitamin C accumulation during ripening (14–28 DAS) was enhanced by 1-MCP, coating, or MAH treatments in both kiwifruit cultivars (Figure 6E,F), which is in agreement with values reported for ripe yellow- and red-fleshed kiwifruit species under low oxygen permeability conditions [13,14,16,17]. Polyphenol and vitamin C levels are strongly correlated with antioxidant activities—ABTS (r2 = 0.929), FRAP (r2 = 0.952), and CUPRAC (r2 = 0.909)—in proportion to the suppression of ethylene action in kiwifruit during long-term storage [12,16,17,39,40]. 1-MCP treatment increased expression levels of genes in ascorbic acid in kiwifruit, enhancing the ascorbate metabolism for scavenging ROS [41].
4. Conclusions
The application of 1-MCP and coating treatments slowed wilting, ripening, and oxidative browning in early-harvested, newly developed kiwifruit cultivars, ‘Sweet Gold’ and ‘Goldone’, during room temperature storage, which will contribute to improving the storage properties and commercialization of the two cultivars. There was another very impactful finding with practical implications that passive MA storage in simple high-humidity and somewhat anaerobic conditions reduced weight loss in ‘Sweet Gold’ kiwifruit by inhibiting dehydration during storage. It also increased TSS/acidity in both cultivars, preserved high antioxidant levels, and enhanced consumer acceptance during the early stages of fruit storage. We recommend the application of 1-MCP and coating to slow the ripening of the two newly bred kiwifruit cultivars and of MAH to accelerate the ripening of ‘Goldone’ when applied within 7 DAS during 35-day storage at room temperature in actual agricultural product distribution. The limitation of this experiment was that treatment time and concentrations of 1-MCP, coating, and MAH should be adjusted for different harvest maturities of ‘Sweet Gold’ and ‘Goldone’. Future research should replicate this study across multiple harvest seasons to validate the treatment effects on different batches of ‘Sweet Gold’ and ‘Goldone’.
Author Contributions
Conceptualization, S.-K.J. and H.-S.C.; Methodology, S.-K.J.; Formal analysis, S.-K.J., H.-W.B., and H.-J.H.; Investigation, S.-K.J., H.-W.B., and H.-J.H.; Resources, S.-K.J.; Data curation, S.-K.J.; Writing—original draft, H.-S.C.; Writing—review and editing, S.-K.J. and H.-S.C.; Visualization, S.-K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research grant from Hankyong National University in the year of 2023.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 1-MCP | 1-Methylcyclopropene |
| ABTS | 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) |
| DAS | Days after storage |
| MA | Modified atmosphere |
| MAH | Modified atmosphere and humidity |
| TSS | Total soluble solids |
References
- Chen, Y.; Hu, X.; Shi, Q.; Lu, Y.; Yan, J.; Wu, D.T.; Qin, W. Changes in the fruit quality, phenolic compounds, and antioxidant potential of red-fleshed kiwifruit during postharvest ripening. Foods 2023, 12, 1509. [Google Scholar] [CrossRef]
- Ferguson, A.R. Kiwifruit cultivars: Breeding and selection. Acta Hortic. 1999, 498, 43–52. [Google Scholar] [CrossRef]
- Huang, W.; Billing, D.; Cooney, J.; Wang, R.; Burdon, J. The role of ethylene and abscisic acid in kiwifruit ripening during postharvest dehydration. Postharvest Biol. Technol. 2021, 178, 111559. [Google Scholar] [CrossRef]
- Statista. Statista Research Department Report, Production Volume of Kiwis Worldwide in 2022, by Leading Country. 2024. Available online: https://www.statista.com/statistics/812434/production-volume-of-leading-kiwi-producing-countries/ (accessed on 1 August 2025).
- Kim, S.C.; Kim, C.H.; Lim, C.K.; Song, E.Y. ‘Sweet Gold’, A kiwifruit variety with high firmness. Korean J. Breed. Sci. 2018, 50, 245–248. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.G.; Kim, H.L.; Lee, M.H.; Chae, W.B.; Kang, S.K.; Kwack, Y.B. Fruit characteristics of kiwifruit as affected by weather conditions during the period of fruit growth. Korean J. Int. Agric. 2021, 33, 75–81. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Gunaseelan, K.; Wang, M.Y.; Luo, L.; Wang, T.; Norling, C.L.; Johnston, S.L.; Maddumage, R.; Schröder, R.; Schaffer, R.J. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J. Exp. Bot. 2011, 62, 3821–3835. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wu, D.T.; Ali, M.; Liu, Y.; Zhuang, Q.G.; Wadood, S.A.; Liao, Q.H.; Liu, H.Y.; Gan, R.Y. Innovative postharvest strategies for maintaining the quality of kiwifruit during storage: An updated review. Food Front. 2024, 5, 1933–1950. [Google Scholar] [CrossRef]
- Goldberg, T.; Agra, H.; Ben-Arie, R. Quality of “Hayward” kiwifruit in prolonged cold storage as affected by the stage of maturity at harvest. Horticulturae 2021, 7, 358. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Wang, Y.; Liu, Y.; Yong, K.; Liu, Z. 1-MCP extends the shelf life of ready-to-eat “Hayward” and “Qihong” kiwifruit stored at room temperature. Sci. Hortic. 2021, 289, 110437. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Gorinstein, S. Shelf life extension and antioxidant activity of “Hayward” kiwi fruit as a result of prestorage conditioning and 1-methylcyclopropene treatment. J. Food Sci. Technol. 2015, 52, 2711–2720. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of “Hayward” kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Pujolà, M.; Sepulcre, F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: A case of study with kiwifruit slices. LWT Food Sci. Technol. 2015, 61, 184–193. [Google Scholar] [CrossRef]
- Diab, T.; Biliaderis, C.G.; Gerasopoulos, D.; Sfakiotakis, E. Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. J. Sci. Food Agric. 2001, 81, 988–1000. [Google Scholar] [CrossRef]
- Kumarihami, H.M.P.C.; Kim, Y.H.; Kwack, Y.B.; Kim, J.; Kim, J.G. Application of chitosan as edible coating to enhance storability and fruit quality of kiwifruit: A review. Sci. Hortic. 2022, 292, 110647. [Google Scholar] [CrossRef]
- Xia, Y.; Zhuo, R.; Li, B.; Tian, S. Effects of 1-methylcyclopropene on disease resistance of red-fleshed kiwifruit during long-term cold storage and the possible mechanisms. N. Z. J. Crop Hortic. Sci. 2021, 49, 182–195. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Mahajan, P.V.; Opara, U.L. Design of active modified atmosphere and humidity packaging (MAHP) for “Wonderful” pomegranate arils. Food Bioprocess Technol. 2018, 11, 1478–1494. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, J.K.; Choi, H.S. Antioxidants and shelf-life of “Changbang” peaches as affected by coating after cooling. Sustainability 2023, 15, 14242. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Odriozolaserrano, I.; Hernandezjover, T.; Martinbelloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovation in development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Ali, S.; Ishtiaq, S.; Nawaz, A.; Naz, S.; Ejaz, S.; Haider, M.W.; Shah, A.A.; Ali, M.M.; Javad, S. Layer by layer application of chitosan and carboxymethyl cellulose coatings delays ripening of mango fruit by suppressing cell wall polysaccharides disassembly. Int. J. Biol. Macromol. 2024, 256, 128429. [Google Scholar] [CrossRef]
- Bansal, H.; Singh, H.P.; Singh, S.; Sharma, A.; Singh, J.; Kaur, K.; Mehta, S.K. Preserving plum perfection: Buckwheat starch edible coating with xanthan gum and lemongrass essential oil. Int. J. Biol. Macromol. 2024, 274, 133239. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl chitosan-pullulan edible films enriched with galangal essential oil: Characterization and application in mango preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Manolopoulou, H.; Papadopoulou, P. A study of respiratory and physico-chemical changes of four kiwi fruit cultivars during cool-storage. Food Chem. 1998, 63, 529–534. [Google Scholar] [CrossRef]
- Fullerton, C.G.; Prakash, R.; Ninan, A.S.; Atkinson, R.G.; Schaffer, R.J.; Hallett, I.C.; Schröder, R. Fruit from two kiwifruit genotypes with contrasting softening rates show differences in the xyloglucan and pectin domains of the cell wall. Front. Plant Sci. 2020, 11, 964. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, R.; Suo, J.; Ding, Y.; Tan, J.; Zhu, Q.; Ma, Y. Understanding quality differences between kiwifruit varieties during softening. Food Chem. 2024, 430, 136983. [Google Scholar] [CrossRef]
- Hatfield, S.G.S.; Knee, M. Effects of water loss on apples in storage. Int. J. Food Sci. Technol. 1988, 23, 575–583. [Google Scholar] [CrossRef]
- Celano, G.; Minnocci, A.; Sebastiani, L.; D’Auria, M.; Xiloyannis, C. Changes in the structure of the skin of kiwifruit in relation to water loss. J. Hortic. Sci. Biotechnol. 2009, 84, 41–46. [Google Scholar] [CrossRef]
- Choi, H.R.; Tilanhun, S.; Park, D.S.; Lee, Y.M.; Choi, J.H.; Back, M.W.; Jeong, C.S. Harvest time affects quality and storability of kiwifruit (Actinidia spp.): Cultivars during long-term cool storage. Sci. Hortic. 2019, 256, 108523. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Liguori, G.; Vonella, V.; Zappia, R.; Inglese, P. Evaluation of fruit quality and antioxidant activity of kiwifruit during ripening and after storage. J. Berry Res. 2016, 6, 25–35. [Google Scholar] [CrossRef]
- Kang, H.H.; Oh, E.U.; Lee, K.U.; Kwack, Y.B.; Lee, M.H.; Song, K.J. Comparison of fruit development characteristics and sucrose metabolizing enzyme activity in different kiwifruit cultivars. Hortic. Sci. Technol. 2021, 39, 213–223. [Google Scholar] [CrossRef]
- Xiong, Y.; He, J.; Li, M.; Du, K.; Lang, H.; Gao, P.; Xie, Y. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in yellow-fleshed kiwifruit. Int. J. Mol. Sci. 2023, 24, 1573. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, Y.; Chen, X.; He, H.; Liu, Z.; Zhang, Z.; Ren, Y.; Ren, X. Phenolic compounds and antioxidant activity in red- and in green-fleshed kiwifruits. Food Res. Int. 2019, 116, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Park, Y. Antioxidant activity, total phenolics and vitamin C contents of the unripe and ripe fruit of hardy kiwi (Actinidia arguta) “Saehan” as honey plant. J. Apic. Sci. 2017, 32, 133–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Xiao, X.; Cao, S.; Chen, W.; Yang, Z.; Shi, L. Effect of 1-MCP on the regulation processes involved in ascorbate metabolism in kiwifruit. Postharvest Biol. Technol. 2021, 179, 111563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).