Microbial–Organic Inputs with Glycine Supplementation Enhance Growth and Heat Stress Tolerance in Lettuce
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Microbial Inoculum
2.2. Plant Material and Growing Conditions
2.3. Growth and Physiological Parameters
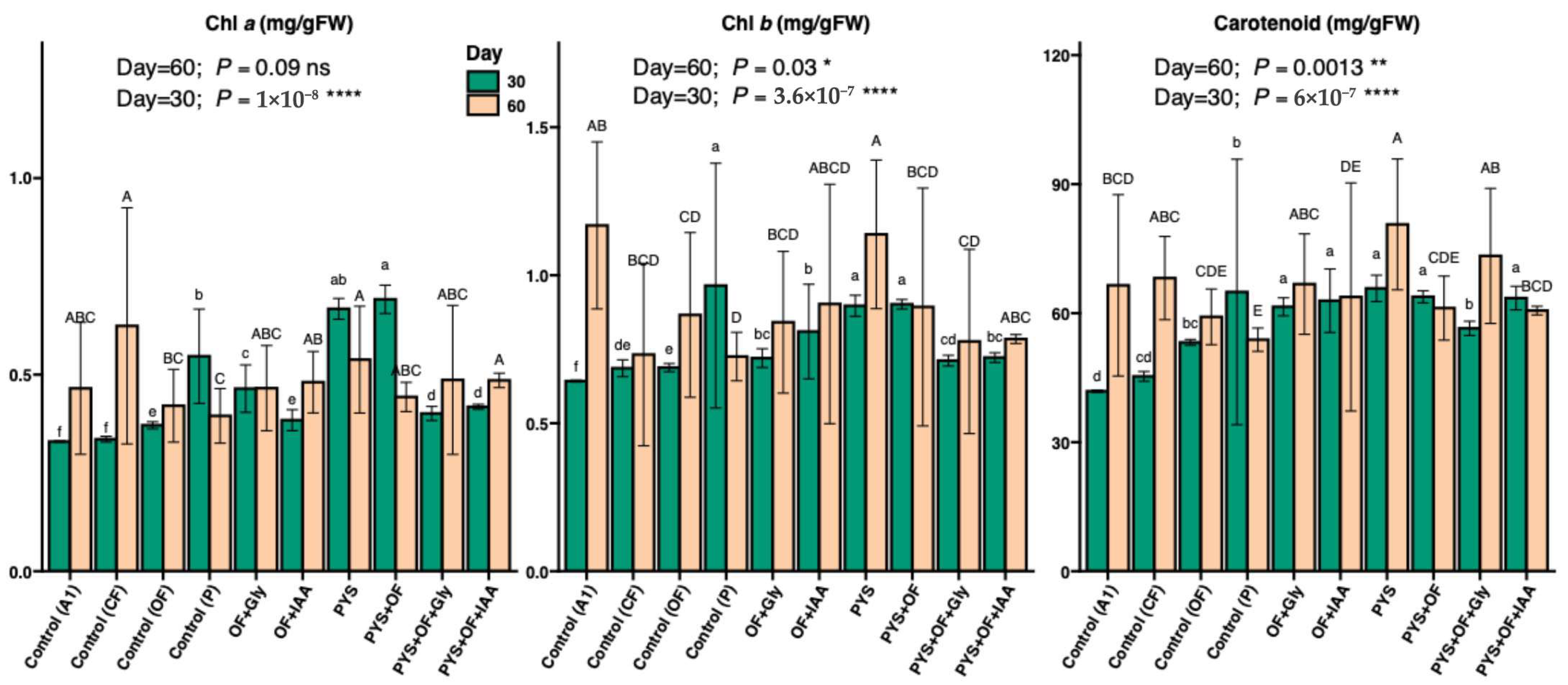
2.4. Chlorophyll and Carotenoid Content
2.5. Biochemical Parameters
2.5.1. Ascorbic Acid
2.5.2. Total Phenolic Content
2.5.3. Total Flavonoid Content
2.5.4. Antioxidant Enzyme Activities
2.5.5. DPPH Radical Scavenging Assay
2.5.6. Hydrogen Peroxide (H2O2) and Lipid Peroxidation
2.5.7. Glycine Betaine Analysis
2.6. Statistical Analysis
3. Results
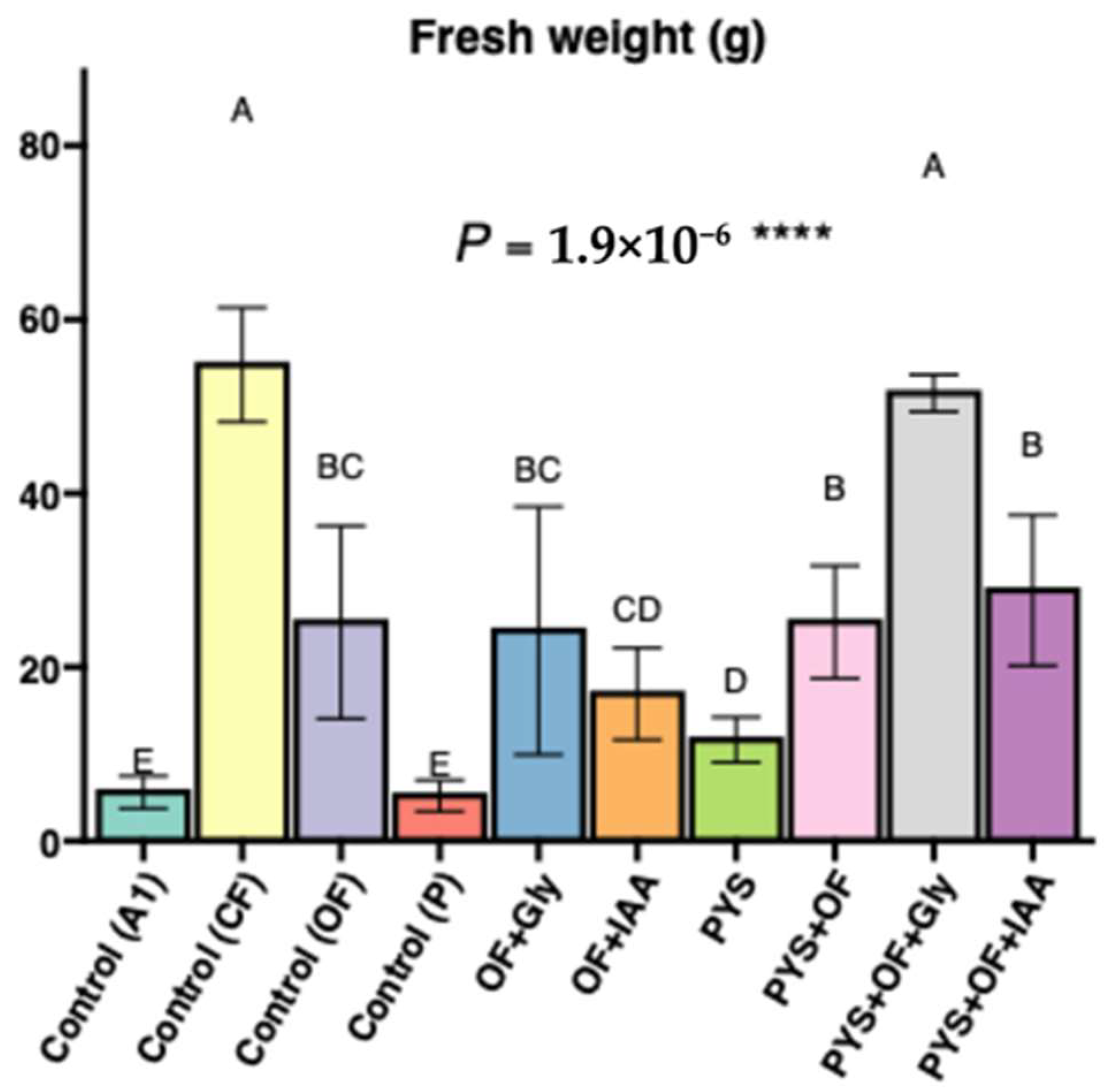
3.1. The Effect of Glycine and IAA Supplementation Combined with PYS and Organic Fertilizer on Lettuce Growth
3.2. Total Phenolic Compounds, Flavonoid Content, and Vitamin C in Lettuce Under Different Treatments
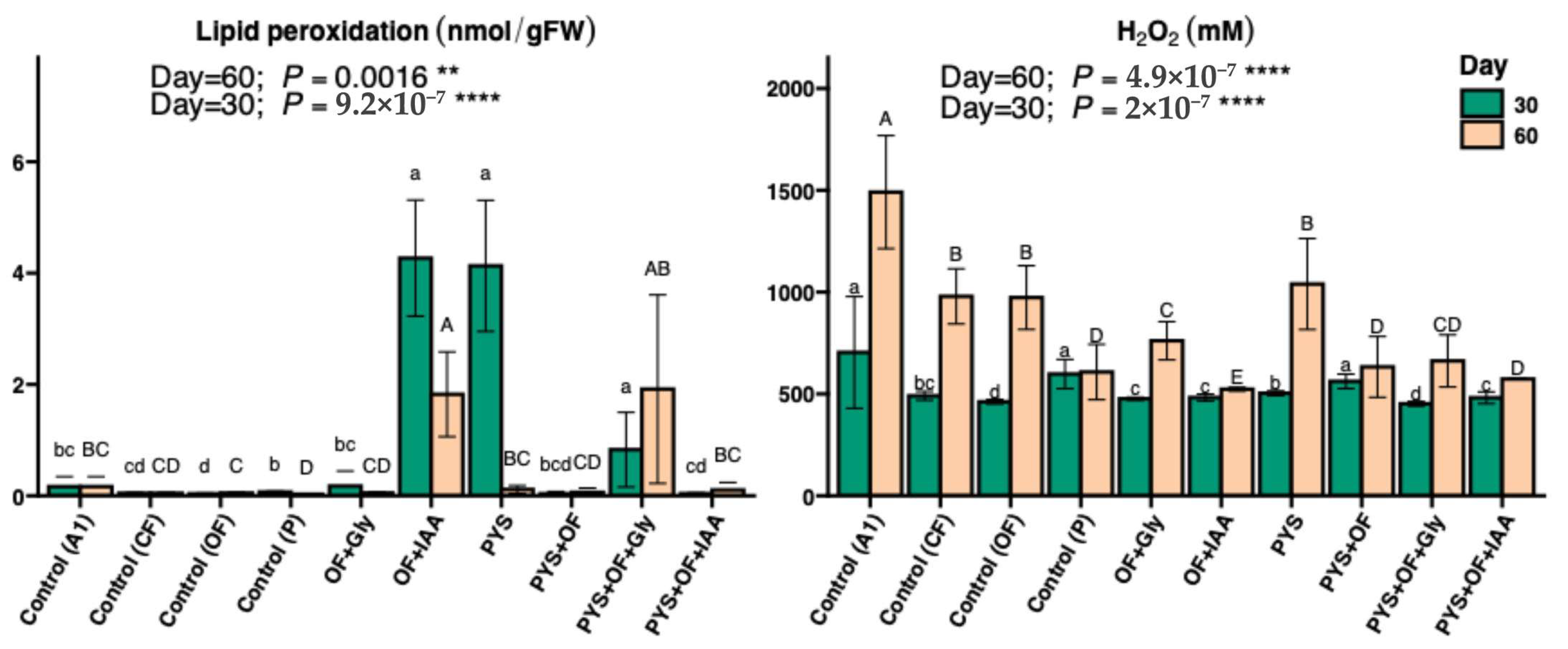
3.3. Lipid Peroxidation and Hydrogen Peroxide Content in Lettuce Leaves
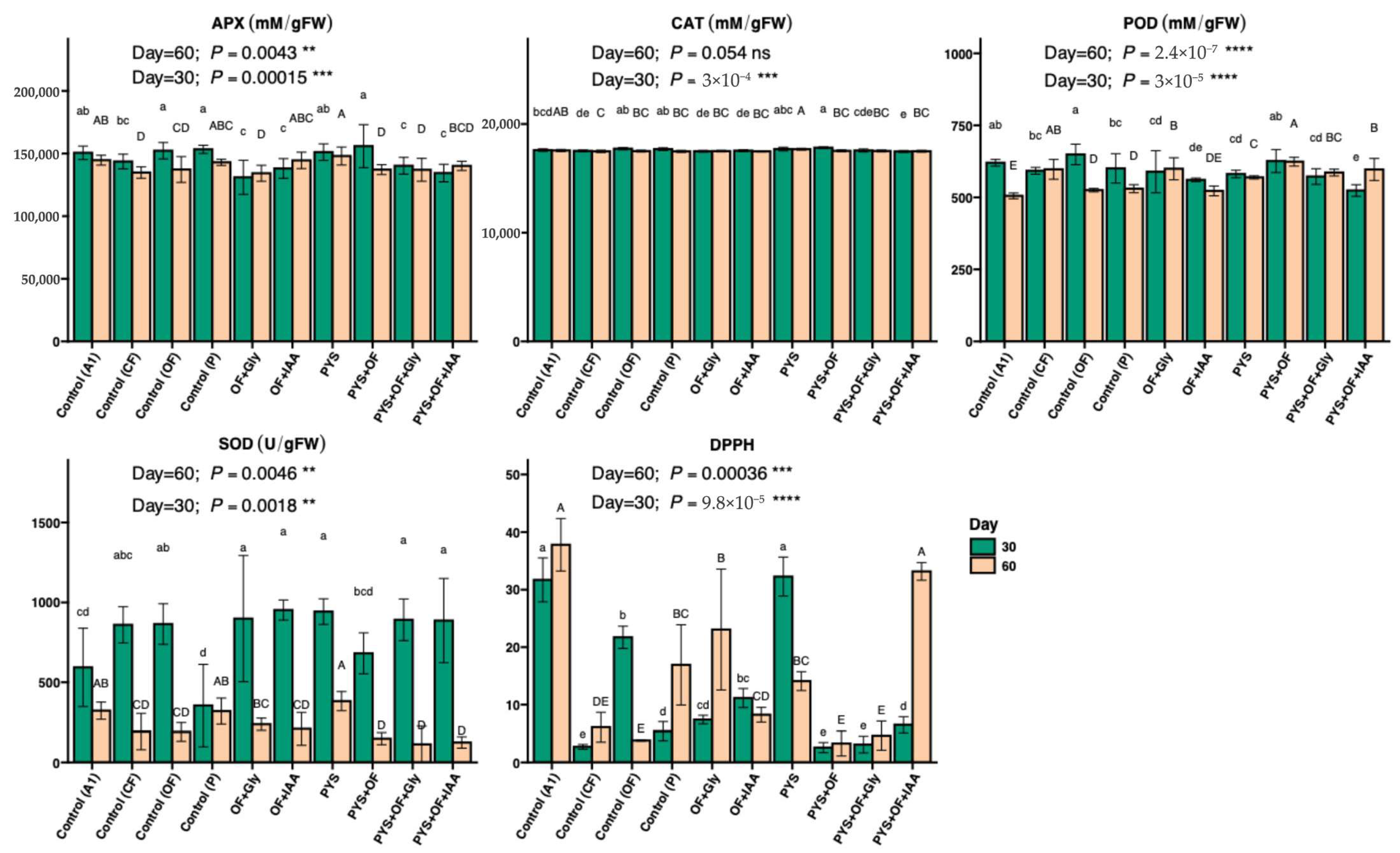
3.4. Antioxidant and Stress Responses in Lettuce Under Different Treatments
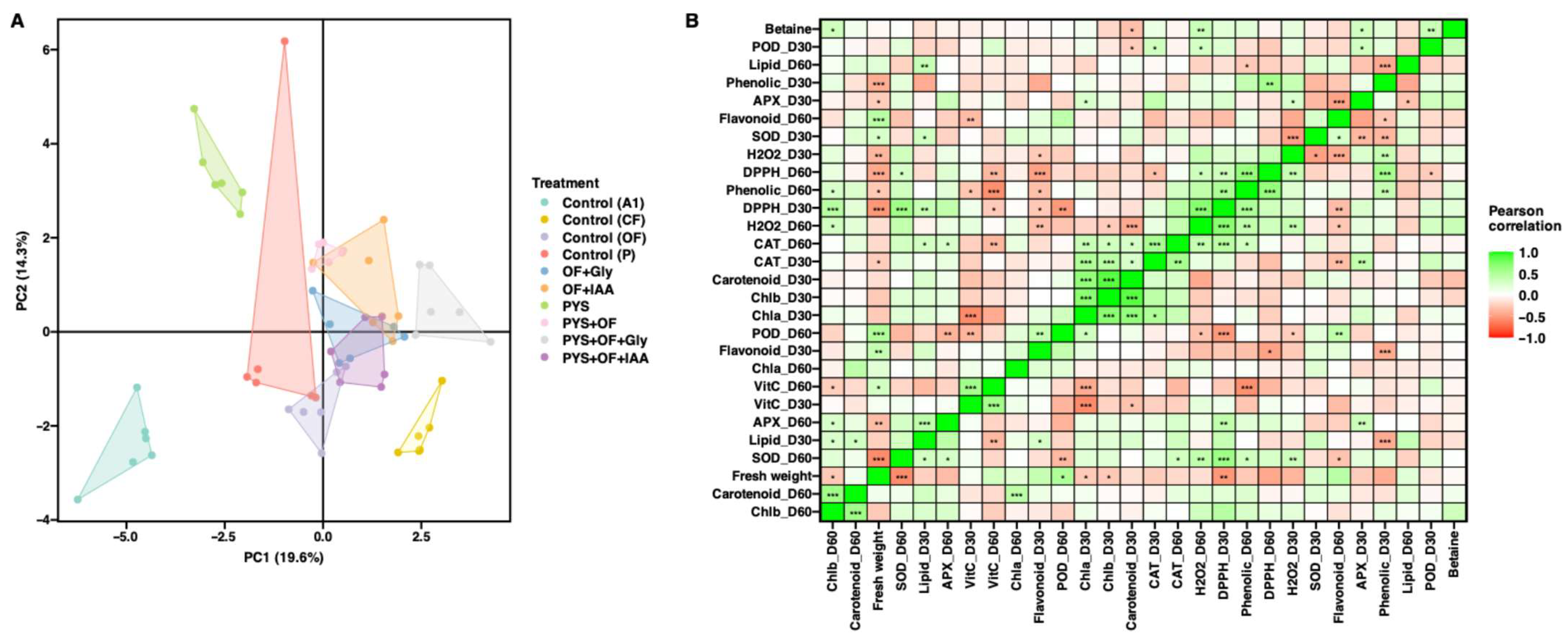
3.5. Principal Component and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| CF | Chemical fertilizer |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| Gly | Glycine (Gly) |
| H2O2 | Hydrogen peroxide |
| IAA | Indole-3-acetic acid |
| OF | Organic fertilizer |
| PGPB | Plant growth-promoting bacteria |
| POD | Peroxidase |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
References
- Khoiri, A.N.; Cheevadhanarak, S.; Jirakkakul, J.; Dulsawat, S.; Prommeenate, P.; Tachaleat, A.; Kusonmano, K.; Wattanachaisaereekul, S.; Sutheeworapong, S. Comparative metagenomics reveals microbial signatures of sugarcane phyllosphere in organic management. Front. Microbiol. 2021, 12, 623799. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Balasuriya, B.T.G.; Ghose, A.; Gheewala, S.H.; Prapaspongsa, T. Assessment of eutrophication potential from fertiliser application in agricultural systems in Thailand. Sci. Total Environ. 2022, 833, 154993. [Google Scholar] [CrossRef]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate change and future of agri-food production. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 49–79. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, N.; Verma, A.; Singh, H.; Siddique, K.H.M.; Singh, N.P. Novel approaches to mitigate heat stress impacts on crop growth and development. Plant Physiol. Rep. 2020, 25, 627–644. [Google Scholar] [CrossRef]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox signaling in plant heat stress response. Antioxidants 2023, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Li, Y.; Yang, Q.; Yang, T.; Zhou, Z.; Li, Y.; Zhang, N.; Lyu, Y.; Zhu, Y.; et al. Heat shock transcription factors regulate thermotolerance gene networks in tomato (Solanum lycopersicum) flower buds. Hortic. Plant J. 2025, 11, 199–210. [Google Scholar] [CrossRef]
- Wang, X.; Tan, N.W.K.; Chung, F.Y.; Yamaguchi, N.; Gan, E.-S.; Ito, T. Transcriptional regulators of plant adaptation to heat stress. Int. J. Mol. Sci. 2023, 24, 13297. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef]
- Tian, F.; Hu, X.-L.; Yao, T.; Yang, X.; Chen, J.-G.; Lu, M.-Z.; Zhang, J. Recent advances in the roles of HSFs and HSPs in heat stress response in woody plants. Front. Plant Sci. 2021, 12, 704905. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, C.; Zhuang, Y.; Zhang, M.; Chen, B. Glycine betaine induces tolerance to oxidative stress in cherry radishes under high-temperature conditions. Agronomy 2024, 14, 1294. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Jarin, A.; Ghosh, U.K.; Hossain, M.S.; Mahmud, A.; Khan, M.A.R. Glycine betaine in plant responses and tolerance to abiotic stresses. Discov. Agric. 2024, 2, 127. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- Quan, J.; Zheng, W.; Wu, M.; Shen, Z.; Tan, J.; Li, Z.; Zhu, B.; Hong, S.-B.; Zhao, Y.; Zhu, Z.; et al. Glycine betaine and β-Aminobutyric acid mitigate the detrimental effects of heat stress on Chinese cabbage (Brassica rapa L. ssp. pekinensis) seedlings with improved photosynthetic performance and antioxidant system. Plants 2022, 11, 1213. [Google Scholar] [CrossRef]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and its role in plant development: Structure, signalling, regulation and response mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed]
- Khawula, S.; Daniel, A.I.; Nyawo, N.; Ndlazi, K.; Sibiya, S.; Ntshalintshali, S.; Nzuza, G.; Gokul, A.; Keyster, M.; Klein, A.; et al. Optimizing plant resilience with growth-promoting Rhizobacteria under abiotic and biotic stress conditions. Plant Stress 2025, 17, 100949. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of plant growth promotion properties and induction of antioxidative defense mechanism by tea rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Wang, L.; Yue, Y.; Wang, L.; Yang, X. The effects of plant growth-promoting rhizobacteria on plants under temperature stress: A meta-analysis. Rhizosphere 2023, 28, 100788. [Google Scholar] [CrossRef]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.-M.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.-H.; et al. A review on the role of endophytes and plant growth Promoting rhizobacteria in mitigating heat stress in plants. Microorganisms 2022, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Khoiri, A.N.; Duangfoo, T.; Kusonmano, K.; Kittichotirat, W.; Laomettachit, T.; Cheevadhanarak, S.; Prommeenate, P.; Jirakkakul, J. Context-dependent effects of various synthetic communities on the ecological dynamics of sugarcane rhizosphere. Appl. Soil Ecol. 2025, 209, 106009. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Kavvadias, V.; Ioannou, Z.; Vavoulidou, E.; Paschalidis, C. Short term effects of chemical fertilizer, compost and zeolite on yield of lettuce, nutrient composition and soil properties. Agriculture 2023, 13, 1022. [Google Scholar] [CrossRef]
- Jirakkakul, J.; Khoiri, A.N.; Duangfoo, T.; Dulsawat, S.; Sutheeworapong, S.; Petsong, K.; Wattanachaisaereekul, S.; Paenkaew, P.; Tachaleat, A.; Cheevadhanarak, S.; et al. Insights into the genome of Methylobacterium sp. NMS14P, a novel bacterium for growth promotion of maize, chili, and sugarcane. PLoS ONE 2023, 18, e0281505. [Google Scholar] [CrossRef]
- Petropoulou, A.S.; van Marrewijk, B.; de Zwart, F.; Elings, A.; Bijlaard, M.; van Daalen, T.; Jansen, G.; Hemming, S. Lettuce production in intelligent greenhouses—3D imaging and computer vision for plant spacing decisions. Sensors 2023, 23, 2929. [Google Scholar] [CrossRef]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant factories are heating up: Hunting for the best combination of light intensity, air temperature and root-zone temperature in lettuce production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef]
- Jenni, S.; Yan, W. Genotype by environment interactions of heat stress disorder resistance in crisphead lettuce. Plant Breed. 2009, 128, 374–380. [Google Scholar] [CrossRef]
- Soengas, P.; Rodríguez, V.M.; Velasco, P.; Cartea, M.E. Effect of temperature stress on antioxidant defenses in Brassica oleracea. ACS Omega 2018, 3, 5237–5243. [Google Scholar] [CrossRef]
- Meena, N.L.; Kumari, A.; Maheshwari, C.; Bhardwaj, R.; Dhaka, A.S.; Hasan, M. Antioxidant defense mechanism and high-temperature stress tolerance in plants. In Molecular Dynamics of Plant Stress and Its Management; Shahid, M., Gaur, R., Eds.; Springer Nature: Singapore, 2024; pp. 193–210. [Google Scholar] [CrossRef]
- Dahdouh, A.; Bachir-Bey, M.; Kati, D.E. Optimization of peroxidase activity of turnip (Brassica rapa) using response surface methodology. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 186–194. [Google Scholar] [CrossRef]
- Tyagi, S.; Shumayla; Verma, P.C.; Singh, K.; Upadhyay, S.K. Molecular characterization of ascorbate peroxidase (APX) and APX-related (APX-R) genes in Triticum aestivum L. Genomics 2020, 112, 4208–4223. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org (accessed on 11 March 2025).
- Mendiburu, F.d.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.5.0. 2020. Available online: https://myaseen208.github.io/agricolae/ (accessed on 11 March 2025).
- Shaji, H.; Chandran, V.; Mathew, L. Organic fertilizers as a route to controlled release of nutrients. In Controlled Release Fertilizers for Sustainable Agriculture; Lewu, F.B., Volova, T., Thomas, S., Rakhimol, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 231–245. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Li, C. Organic fertilizer has a greater effect on soil microbial community structure and carbon and nitrogen mineralization than planting pattern in rainfed farmland of the Loess Plateau. Front. Environ. Sci. 2023, 11, 1232527. [Google Scholar] [CrossRef]
- Mondal, S.; Pramanik, K.; Ghosh, S.K.; Pal, P.; Mondal, T.; Soren, T.; Maiti, T.K. Unraveling the role of plant growth-promoting rhizobacteria in the alleviation of arsenic phytotoxicity: A review. Microbiol. Res. 2021, 250, 126809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jian, Q.; Yao, X.; Guan, L.; Li, L.; Liu, F.; Zhang, C.; Li, D.; Tang, H.; Lu, L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and quality of several crops. Heliyon 2024, 10, e31553. [Google Scholar] [CrossRef]
- Aly, A.A.; Eliwa, N.E.; Safwat, G. Role of gamma-irradiated sodium alginate on growth, physiological and active components of iceberg lettuce (Lactuca sativa) plant. BMC Plant Biol. 2024, 24, 185. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research progress in the interconversion, turnover and degradation of chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Khan, I.; Zada, A.; Jia, T.; Hu, X. Effect of the enhanced production of chlorophyll b on the light acclimation of tomato. Int. J. Mol. Sci. 2023, 24, 3377. [Google Scholar] [CrossRef]
- Zoufan, P.; Zare Bavani, M.R.; Tousi, S.; Rahnama, A. Effect of exogenous melatonin on improvement of chlorophyll content and photochemical efficiency of PSII in mallow plants (Malva parviflora L.) treated with cadmium. Physiol. Mol. Biol. Plants 2023, 29, 145–157. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Azeem, M.; Sultana, R.; Ahmed, N.; Abbasi, M.W.; Hasan, K.A.; Dong, R.; Alamri, S.; Alfagham, A.T. Salinity stress resilience in Sorghum bicolor through Pseudomonas-mediated modulation of growth, antioxidant system, and eco-physiological adaptations. ACS Omega 2025, 10, 940–954. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.; Esmaeel, Q.; Sanchez, L.; Courteaux, B.; Guise, J.-F.; Gibon, Y.; Ballias, P.; Clément, C.; Jacquard, C.; Vaillant-Gaveau, N.; et al. Impacts of Paraburkholderia phytofirmans strain PsJN on tomato (Lycopersicon esculentum L.) under high temperature. Front. Plant Sci. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Gómez-Sagasti, M.T.; López-Pozo, M.; Artetxe, U.; Becerril, J.M.; Hernández, A.; García-Plazaola, J.I.; Esteban, R. Carotenoids and their derivatives: A “Swiss Army knife-like” multifunctional tool for fine-tuning plant-environment interactions. Environ. Exp. Bot. 2023, 207, 105229. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- Savchenko, T.; Tikhonov, K. Oxidative stress-induced alteration of plant central metabolism. Life 2021, 11, 304. [Google Scholar] [CrossRef]
- Patil, J.R.; Mhatre, K.J.; Yadav, K.; Yadav, L.S.; Srivastava, S.; Nikalje, G.C. Flavonoids in plant-environment interactions and stress responses. Discov. Plants 2024, 1, 68. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef]
- Yang, X.; Cui, X.; Zhao, L.; Guo, D.; Feng, L.; Wei, S.; Zhao, C.; Huang, D. Exogenous glycine nitrogen enhances accumulation of glycosylated flavonoids and antioxidant activity in lettuce (Lactuca sativa L.). Front. Plant Sci. 2017, 8, 2098. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Aluko, O.O.; Yan, J.; Zeng, H.; Liu, G.; Zhao, J.; Li, H.; Chen, S.; Dakora, F.D. Phenylpropanoids metabolism: Recent insight into stress tolerance and plant development cues. Front. Plant Sci. 2025, 16, 1571825. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in Plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Mu, K.; Cai, W.; Zhao, Y.; Shen, H.; Wang, X.; Ma, H. Phenylalanine ammonia lyase GmPAL1.1 promotes seed vigor under high-temperature and -humidity stress and enhances seed germination under salt and drought stress in transgenic Arabidopsis. Plants 2022, 11, 3239. [Google Scholar] [CrossRef]
- Nanda, A.K.; Andrio, E.; Marino, D.; Pauly, N.; Dunand, C. Reactive oxygen species during plant-microorganism early interactions. J. Integr. Plant Biol. 2010, 52, 195–204. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Asaf, S.; Imran, M.; Al-Harrasi, A.; Lee, I.-J. Efficacy of endophytic SB10 and glycine betaine duo in alleviating phytotoxic impact of combined heat and salinity in Glycine max L. via regulation of redox homeostasis and physiological and molecular responses. Environ. Pollut. 2023, 316, 120658. [Google Scholar] [CrossRef]
- Gechev, T.S.; Hille, J. Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell Biol. 2005, 168, 17–20. [Google Scholar] [CrossRef]
- Liu, H.; Mu, Y.; Xuan, Y.; Wu, X.; Wang, W.; Zhang, H. Hydrogen peroxide signaling in the maintenance of plant root apical meristem activity. Antioxidants 2024, 13, 554. [Google Scholar] [CrossRef]
- Neshat, M.; Abbasi, A.; Hosseinzadeh, A.; Sarikhani, M.R.; Dadashi Chavan, D.; Rasoulnia, A. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol. Mol. Biol. Plants 2022, 28, 347–361. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.K.; Patel, J.S. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci. Rep. 2020, 10, 4818. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, S.; Thangappan, S.; Uthandi, S. Plant growth-promoting Bacillus sp. cahoots moisture stress alleviation in rice genotypes by triggering antioxidant defense system. Microbiol. Res. 2020, 239, 126518. [Google Scholar] [CrossRef] [PubMed]
- Al Methyeb, M.; Ruppel, S.; Eichler-Löbermann, B.; Vassilev, N. The combined applications of microbial inoculants and organic fertilizer Improve plant growth under unfavorable soil conditions. Microorganisms 2023, 11, 1721. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, H.; Lian, J.; Zhang, W.; Li, G.; Zhang, J. Combined application of organic fertilizer with microbial inoculum improved aggregate formation and salt leaching in a secondary salinized soil. Plants 2023, 12, 2945. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, M.; Zhao, Q.; Wang, F.; Gao, J.; Sheng, H.; An, L. Complete genome sequence of Sphingomonas sp. Cra20, a drought resistant and plant growth promoting rhizobacteria. Genomics 2020, 112, 3648–3657. [Google Scholar] [CrossRef]
- Mujumdar, S.; Bhoyar, J.; Akkar, A.; Hundekar, S.; Agnihotri, N.; Jaybhay, P.; Bhuyan, S. Acinetobacter: A versatile plant growth-promoting rhizobacteria (PGPR). In Plant-Microbe Interaction—Recent Advances in Molecular and Biochemical Approaches; Swapnil, P., Meena, M., Harish, Marwal, A., Vijayalakshmi, S., Zehra, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 327–362. [Google Scholar] [CrossRef]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants. Plant Signal. Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. How do plant growth-promoting bacteria use plant hormones to regulate stress reactions? Plants 2024, 13, 2371. [Google Scholar] [CrossRef]


| Activity | Methylobacterium sp. NMS14P | Sphingomonas sp. NMS25Y | Acinetobacter sp. SCRE97 |
|---|---|---|---|
| Nitrogen fixation | – | – | ✓ |
| Phosphorus solubilization | ✓ | ✓ | ✓ |
| Potassium solubilization | – | – | ✓ |
| Alkaline phosphatase activity | ✓ | – | – |
| Urease activity | ✓ | ✓ | ✓ |
| ACC deaminase activity | ✓ | – | – |
| Indole-3-acetic acid (IAA) production | ✓ | ✓ | ✓ |
| Positive results in plant–bacterium association assays | Chili, maize, sugarcane | Chili, sugarcane | Chili, sugarcane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudpeng, K.; Khoiri, A.N.; Duangfoo, T.; Cheevadhanarak, S.; Jirakkakul, J. Microbial–Organic Inputs with Glycine Supplementation Enhance Growth and Heat Stress Tolerance in Lettuce. Horticulturae 2025, 11, 935. https://doi.org/10.3390/horticulturae11080935
Kudpeng K, Khoiri AN, Duangfoo T, Cheevadhanarak S, Jirakkakul J. Microbial–Organic Inputs with Glycine Supplementation Enhance Growth and Heat Stress Tolerance in Lettuce. Horticulturae. 2025; 11(8):935. https://doi.org/10.3390/horticulturae11080935
Chicago/Turabian StyleKudpeng, Kanjana, Ahmad Nuruddin Khoiri, Thanawat Duangfoo, Supapon Cheevadhanarak, and Jiraporn Jirakkakul. 2025. "Microbial–Organic Inputs with Glycine Supplementation Enhance Growth and Heat Stress Tolerance in Lettuce" Horticulturae 11, no. 8: 935. https://doi.org/10.3390/horticulturae11080935
APA StyleKudpeng, K., Khoiri, A. N., Duangfoo, T., Cheevadhanarak, S., & Jirakkakul, J. (2025). Microbial–Organic Inputs with Glycine Supplementation Enhance Growth and Heat Stress Tolerance in Lettuce. Horticulturae, 11(8), 935. https://doi.org/10.3390/horticulturae11080935






