Abstract
This study comprehensively characterized the pomological and biochemical properties of 255 wild pear (Pyrus elaeagnifolia Pall.) genotypes collected from 17 different locations in Denizli province, Türkiye, a region known for its significant genetic resources. A total of 19 parameters were investigated, including fruit dimensions, seed characteristics, firmness, soluble solids content (SSC), pH, titratable acidity, vitamin C, total phenolic and flavonoid contents, and antioxidant activity. Variance analysis revealed significant differences among locations for most of the evaluated traits (p ≤ 0.05). Correlation analyses elucidated the relationships between pomological and biochemical characteristics, while principal component analysis and cluster analysis reflected the genetic and geographical structure of the genotypes. Notably, genotypes from Çivril, Çal, Pamukkale, and Tavas locations exhibited superior characteristics. The high phenolic and flavonoid content, coupled with the strong antioxidant capacity of Pyrus elaeagnifolia, supports the species’ potential as a functional food. The findings provide valuable resources for conservation efforts, sustainable utilization, and breeding programs aimed at adapting to climate change. To the best of our knowledge, this study represents the first systematic and multi-trait assessment of wild pear genetic diversity in the Denizli province, thus providing a crucial scientific baseline for the development of effective conservation and breeding strategies.
1. Introduction
Wild pear (Pyrus elaeagnifolia Pall.), an ancient fruit species of Anatolia, belongs to the order Rosales, family Rosaceae, subfamily Maloideae, and genus Pyrus, with a cultivation history spanning 3000 years. Its distribution extends across Western Asia (Türkiye), the Caucasus (Armenia, Azerbaijan, and Georgia), Eastern Europe (Moldova and Ukraine/Crimea), and Southeast Europe (Albania, Bulgaria, Greece, Romania, and Serbia) [1,2,3]. Pyrus elaeagnifolia is one of the naturally growing Pyrus species in Türkiye [4].
Wild pear has significant applications in fruit cultivation and environmental management. Its robust root system makes it effective in erosion control [5,6]. It is particularly favored as a rootstock for cultivated pears due to its drought tolerance from a deep root system, adaptability to various soil types, and disease resistance [7]. One of the most notable characteristics of the species is its potential as a chlorosis-resistant pear rootstock, its tolerance to winter cold (−30 °C) and high soil pH, and its adaptation to calcareous and arid conditions [8]. Its high resistance to fire blight (Erwinia amylovora) further enhances its importance in modern fruit cultivation [9]. Indeed, Özlük [8] reported no incidence of fire blight in wild pear genotypes in their study.
In recent years, wild pear has also begun to be evaluated in the functional food industry. Its fruit is traditionally used to make jams, pekmez (grape molasses), marmalades, cookies, and pickles, and is also consumed dried [10,11,12,13]. In some regions, its fruits are fermented to produce vinegar [14]. From a health perspective, wild pear possesses important bioactive components. Studies have identified high amounts of phenolic compounds, flavonoids, arbutin, and vitamin C in fruit [15]. It stands out for its antioxidant and antimicrobial properties [15,16,17] and is used in traditional medicine as an antidiarrheal, laxative, and antipyretic. Its leaves and fruits have also been used in folk medicine for treating coughs, colds, and rheumatism [18,19,20,21].
Morphological characterization forms the foundation of breeding programs [22]. Since leaf characteristics alone are insufficient for classifying the genus Pyrus, a combined evaluation of fruit characteristics and tree traits is recommended [23,24]. Key parameters determining fruit quality include fruit flesh texture, soluble solids content, titratable acidity, and fruit flesh color [25,26]. The relationships between chemical and physical properties of genotypes can be complex. A high-yielding genotype might exhibit low sugar content, which allows genotypes unsuitable for fresh consumption to be utilized in the processing industry [27]. In plant breeding, genetic gain necessitates the optimization of multiple traits. Instead of focusing on a single trait, the goal is to combine desired characteristics in a balanced manner [28].
However, the accelerating impacts of global climate change pose significant threats to agricultural production and food security [29]. Rising temperatures, erratic precipitation regimes, and drought events challenge the adaptive capacities of traditionally cultivated crops [30]. Given the narrow genetic base and limited stress tolerance of existing commercial pear cultivars, the conservation and evaluation of genetic diversity that provides resilience against these changing environmental conditions is of great urgency [31]. Local and adapted genetic resources, such as wild pear, harbor unique genes with the potential for natural resistance mechanisms against drought, diseases, and various environmental stresses [32]. A comprehensive investigation of these genetic resources is critically important for developing resilient varieties for future generations and for conserving agro-biodiversity [33].
Indeed, a growing body of research has begun to map the rich diversity of wild pear across Türkiye, with valuable pomological and biochemical characterizations conducted in specific provinces spanning Central Anatolia [34,35,36], the Mediterranean [11], Eastern Anatolia [16,37,38,39], and the Aegean [17]. Furthermore, a comprehensive genetic survey using SSR markers has confirmed the vast national gene pool, sampling from multiple provinces, including a limited set of two accessions from Denizli [40]. However, while these studies confirm that significant diversity exists and that Denizli is part of this genetic landscape, a systematic and in-depth investigation focusing specifically on the pomological and biochemical potential within the Denizli population has not yet been undertaken.
Therefore, this study aims to fill this critical gap. To our knowledge, this is the first study to systematically evaluate the multi-trait diversity of wild pear genotypes across Denizli province. In this context, the objectives were to (1) determine the variation among genotypes based on 19 pomological and chemical properties, (2) identify relationships between these traits, (3) detect genotypes with superior characteristics, and (4) group the genotypes using multivariate statistical analyses. This research contributes to the comprehensive pomological and chemical characterization of wild pear genotypes in an under-explored region, aiding in the preservation and effective utilization of this important genetic resource.
2. Materials and Methods
2.1. Experimental Location and Plant Material
This study focused on the pomological and biochemical characterization of wild pear fruits. The field component, involving the collection of fruit samples, was carried out during the 2022–2023 period in 17 locations across Denizli province (Türkiye), while all subsequent analyses were performed under laboratory conditions. Denizli was selected as the study area because it is a significant region of genetic diversity within the natural distribution range of Pyrus elaeagnifolia and represents a transitional zone between the Aegean and Central Anatolian regions. The 17 sampling locations were not chosen randomly but were determined using a purposeful sampling strategy to represent the maximum environmental diversity across the province. This strategy specifically aimed to cover a wide altitudinal gradient, different ecological zones, and varied soil types. The selected locations and their respective altitudes, which demonstrate the captured range from 165 m (Sarayköy) to 1309 m (Çameli), are the following: Acıpayam (937 m), Babadağ (758 m), Baklan (964 m), Beyağaç (699 m), Bozkurt (860 m), Buldan (619 m), Çal (838 m), Çameli (1309 m), Çardak (857 m), Çivril (831 m), Güney (822 m), Honaz (502 m), Kale (1050 m), Pamukkale (392 m), Sarayköy (165 m), Serinhisar (972 m), and Tavas (933 m) (Figure 1).
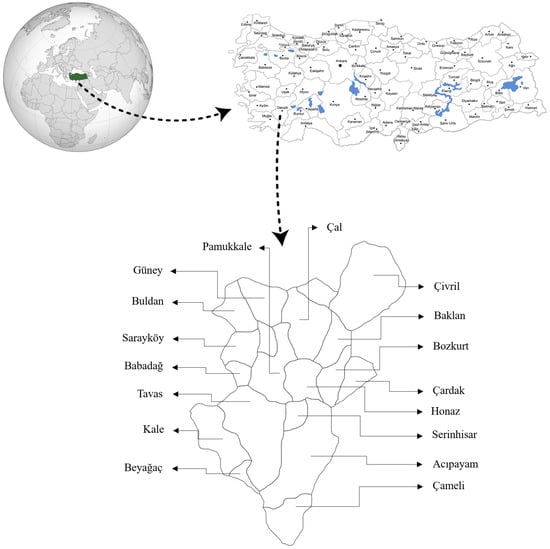
Figure 1.
Districts where wild pear genotype were collected.
Within these strategically chosen locations, the study examined fruit samples from 255 wild pear genotypes, collecting 15 samples from each district. In this study, the term “genotype” is used to refer to individual trees that are phenotypically distinguishable and collected from different locations, following the common practice in similar characterization studies. It should be noted that genetic distinctiveness was not confirmed through molecular analysis in this study. However, since Pyrus elaeagnifolia is an outcrossing species, each seedling arising from natural pollination can be considered a unique genotype. Therefore, the term “genotype” is employed here as a practical identifier for individual trees rather than a confirmed genetic differentiation. Future molecular studies are needed to definitively distinguish genotypic diversity from phenotypic variation.
This sample size was established in accordance with similar genetic resource characterization studies for wild fruit species [41,42,43] and is considered robust for capturing the principal patterns of morpho-biochemical variation within each location.
Furthermore, this approach provided a representative dataset for our primary objectives while maintaining logistical feasibility across the 17 distinct geographical sites. Genotype selection favored specimens free from Viscum album infestation. The research included the analysis of 7650 fruits, with 30 fruits sampled from each genotype. After harvest, fruits were placed in plastic bags, transported to the laboratory, and stored at −20 °C until analysis.
2.2. Pomological Analyses
Immediately after harvest, fruit weight (g) and seed weight (g) were measured using a digital scale sensitive to 0.0001 g (Shimadzu, ATX-224, Kyoto, Japan). Soluble solids content (%) was measured with a digital refractometer and expressed as Brix. pH values were measured by immersing a WTW inolab pH 7110 (Xylem Analytics, Weilheim, Germany) digital pH meter probe into prepared fruit juice, then titrating with 0.1 N NaOH until reaching pH 8.1. Titratable acidity was calculated as malic acid based on the base consumption [44]. Fruit flesh firmness was measured from both sides of the fruit using a digital penetrometer (PCE-PTR 200—PCE Instruments, Istanbul, Türkiye) and expressed as kg/cm2. Seed count was determined by counting filled seeds per fruit. Fruit length (mm), fruit width (mm), and stem length (mm) were measured using a digital caliper. Fruit shape was determined by dividing fruit length by fruit width.
2.3. Spectrophotometric Analyses
Total phenolic content was determined using the Folin–Ciocalteu method. A 0.5 mL appropriately diluted extract was combined with 2.5 mL Folin–Ciocalteu solution solution (Sigma-Aldrich, Darmstadt, Germany) (0.2 N) and 2 mL sodium carbonate solution solution (Sigma-Aldrich, Darmstadt, Germany) (75 g/L), stored in darkness for two hr, then measured at 765 nm wavelength. The total phenolic content was calculated using a gallic acid standard calibration curve and expressed as mg gallic acid equivalent (GAE)/100 g [45].
For total flavonoid content, 800 µL fresh fruit extract was mixed with 3.5 mL methanol, followed by the addition of 100 µL each of 10% ammonium acetate and 1 M ammonium nitrate. After 40 min incubation, absorbance values were measured at 517 nm using spectrophotometry. The results were expressed as mg catechin equivalent (CE)/100 g [46].
FRAP and DPPH antioxidant analyses were performed using a MECASYS OPTIZEN POP BIO spectrophotometer UV-VIS (Mecasys, Daejeon, Republic of Korea). The methods were performed according to previously described protocols [47,48]. All the analyses were conducted in triplicate. The results were expressed as mmol trolox equivalent/kg. While both the DPPH and FRAP results are expressed as mmol TE/kg using Trolox calibration curves for consistency, it is noteworthy that DPPH reflects radical scavenging activity (primarily SET mechanism), whereas FRAP indicates ferric reducing antioxidant power (SET mechanism), which can alternatively be expressed as μmol Fe2+ equivalents.
Total sugar content was determined using the Lane-Eynon method. The fruit samples were treated with acid hydrolysis and acid neutralization, then clarified with Carrez solution solution (Sigma-Aldrich, Darmstadt, Germany). The resulting clear solution was titrated with Fehling solution solution (Sigma-Aldrich, Darmstadt, Germany). Glucose solution solution (Sigma-Aldrich, Darmstadt, Germany) served as the standard solution. The total sugar content was expressed as g glucose equivalent (GE)/100 g [49].
2.4. HPLC Analyses
For vitamin C analysis, 5 g of the fruit sample was mixed with 5 mL of 2.5 M phosphoric acid solution and centrifuged at 6500× g for 10 min at +4 °C. A 0.5 mL aliquot of the clear supernatant was diluted to 10 mL with 2.5 M phosphoric acid, filtered through a 0.45 μm Teflon filter, and injected into the HPLC system. Analysis was performed on a C18 column (Phenomenex Luna C18) at 25 °C, using ultra-pure water adjusted to pH 2.2 as mobile phase at a 1 mL/min flow rate. Readings were taken at 254 nm using a DAD detector. Vitamin C content was determined using vitamin C standards prepared at different concentrations, and the results were expressed as mg/100 g [50].
2.5. Data Analysis
Statistical analyses of the study data were performed using R Studio 2024.12.0+467 [51] and JMP Pro 17.0.0 (SAS Institute Inc., Cary, NC, USA) software. Analysis of variance (ANOVA) and Tukey HSD multiple comparison test (p < 0.05) were applied to fruit characteristics data of wild pear genotypes using the ‘stats’ package [51], while descriptive statistics for examined traits were calculated using the ‘dplyr’ package [52]. Principal component analysis (PCA) was performed using ‘FactoMineR’ and ‘factoextra’ packages to determine relationships between examined characteristics and collection locations [53,54]. Cluster analysis was conducted using JMP Pro 17.0.0 statistical software to reveal similarities and differences between locations. Pearson correlation analysis was performed and visualized using the ‘corrplot’ package to determine relationships between examined characteristics [55]. The sample size included at least three technical replicates per wild pear genotype to ensure data reliability. Prior to statistical analyses, assumptions of normality and homogeneity of variances were verified. All the statistical tests were conducted at a significance level of p < 0.05.
3. Results
3.1. Variance Analysis and Descriptive Statistics
Variance analysis and descriptive statistics for the pomological characteristics of wild pear genotypes collected from different locations are presented in Table 1. Significant variations were observed among individual wild pear genotype samples within different locations regarding their physical and chemical properties (p ≤ 0.05).

Table 1.
Pomological characteristics of wild pear genotypes from different locations (mean of genotypes).
Fruit dimensions varied considerably. Fruit length ranged from 21.73 to 45.39 mm, with a coefficient of variation (CV) of 18.82%. The longest fruits were observed in the genotypes sampled from the “Güney” location, while the shortest were found in genotypes from the “Tavas” location. Fruit width ranged from 19.41 to 46.5 mm (17.84% CV), with “Bozkurt” exhibiting the widest fruits. The fruit shape index varied from 0.61 to 1.44, with “Pamukkale” and “Sarayköy” having the highest values. In terms of fruit weight, the “Honaz” (26.94 g) genotypes had the heaviest fruits, while the “Sarayköy” (10.73 g) genotypes had the lightest. The 23.08% coefficient of variation for fruit weight indicates a significant level of genetic diversity within the population, reflecting substantial variability in fruit weight among genotypes.
When examining seed characteristics (length, width, weight, and number), no significant differences were observed among genotypes for seed length, weight, and number. However, seed width showed significant differences (p ≤ 0.05), with the heaviest seeds (6.24 g) found in the “Beyağaç” region. Among the seed characteristics investigated, seed length (5.47%), width (16.93%), weight (4.88%), and number (2.33%) generally exhibited low coefficients of variation.
Significant differences were observed in fruit pedicel length and fruit flesh firmness parameters (p ≤ 0.05). The longest fruit pedicels were found in the “Beyağaç” genotypes (36.44 mm), whereas the shortest were in the “Pamukkale” genotypes (12.52 mm). For fruit flesh firmness, “Bozkurt” (14.41 kg/cm2) exhibited the highest values, while “Çardak” (8.61 kg/cm2) had the lowest. Fruit pedicel length showed a significant level of genetic diversity among genotypes, with a coefficient of variation of 25.43%.
Variance analysis and descriptive statistics for chemical properties are summarized in Table 2. Significant differences (p < 0.05) were observed among locations regarding the soluble solids content (SSC) of wild pear fruits. SSC ranged from 9.24% to 17.30%, with an average of 13.45%. A high coefficient of variation (CV) of 20.63% for SSC indicates substantial genetic diversity among genotypes for this trait.

Table 2.
Chemical properties of wild pear genotypes from different locations (mean of genotypes).
Fruit juice pH values ranged from 3.47 (“Acıpayam”) to 5.08 (“Honaz”), with an average of 4.39. Titratable acidity was highest in the “Acıpayam” genotypes (1.15%) and lowest in the “Beyağaç” (0.70%) and “Honaz” (0.71%) genotypes. Significant differences were detected among locations based on the mean genotype vitamin C content. The highest Vitamin C was found in the “Tavas” (29.90 mg/100 g) genotypes, while the lowest was in the “Kale” (11.36 mg/100 g) genotypes.
Significant differences were also observed in the total phenolic content among the genotypes from different regions. The highest total phenolic content was in “Çivril” (357.61 mg GAE/100 g), whereas the lowest values were in the “Acıpayam” (168.33 mg GAE/100 g) and “Babadağ” (182.66 mg GAE/100 g) genotypes. The total flavonoid content showed high variation among locations, with the “Çal” genotypes exhibiting the highest (46.50 mg CE/100 g) and the “Baklan” (21.11 mg CE/100 g), “Çivril” (22.03 mg CE/100 g), and “Çameli” (22.25 mg CE/100 g) genotypes showing the lowest.
Regarding antioxidant capacity, both DPPH (2,2-Diphenyl-1-picrylhydrazyl) and FRAP (ferric reducing antioxidant power) analyses revealed significant differences among locations. In the DPPH assay, the “Pamukkale” genotypes exhibited the highest activity (10.63 mmol TE/kg), while the “Serinhisar” and “Çardak” genotypes showed the lowest values (4.59 and 4.68 mmol TE/kg, respectively). For the FRAP assay, the “Çivril” and “Kale” genotypes (21.17 and 20.75 mmol TE/kg, respectively) had the highest antioxidant capacity, whereas the “Acıpayam” genotypes showed the lowest (9.5 mmol TE/kg).
When examining the coefficients of variation (CV%), the highest variability among the investigated traits was observed in the total flavonoid content (26.66%), followed by total sugar (25.91%), total phenolic compounds (22.84%), and DPPH antioxidant activity (22.71%). FRAP antioxidant activity (21.50%), vitamin C content (21.66%), and soluble solids content (SSC) (20.63%) showed moderate variation. The lowest coefficient of variation was found in pH (8.85%), while titratable acidity (TA) (12.00%) exhibited relatively low variability. Our findings suggest that bioactive components (flavonoids, phenolics, and antioxidant activity) are more influenced by genetic and environmental factors, while fundamental chemical properties like pH remain more stable.
3.2. Correlation Analysis
The Pearson correlation analysis conducted on all 255 individual wild pear genotypes revealed several significant relationships among morphological, physicochemical, and biochemical parameters (Figure 2). Varying degrees of positive and negative correlations were observed among these parameters, with statistically significant relationships identified. The strongest positive correlation was observed between fruit size and fruit shape (r = 0.57, p < 0.001), indicating that larger fruits tend to have a more elongated shape. Fruit size also showed significant positive correlations with seed weight (r = 0.24, p < 0.01) and SSC (r = 0.39, p < 0.001).
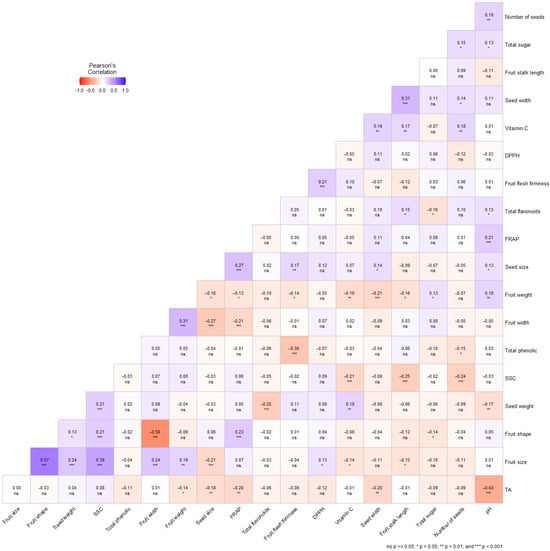
Figure 2.
Correlation matrix of studied traits. ns, not statistically significant; p ≥ 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001. Correlations are calculated based on individual genotype data rather than location means, providing a more statistically robust representation of trait relationships.
A notable negative correlation was found between fruit shape and fruit width (r = −0.58, p < 0.001), suggesting that more elongated fruits tend to be narrower. Fruit width showed a positive correlation with fruit size (r = 0.31, p < 0.001) but negative correlations with fruit shape (r = −0.27, p < 0.01) and seed size (r = −0.21, p < 0.01).
Regarding biochemical properties, the total phenolic content exhibited a significant negative correlation with fruit flesh firmness (r = −0.30, p < 0.01), indicating that the genotypes with higher phenolic content tend to have softer fruit flesh. Seed size showed positive correlations with seed weight (r = 0.27, p < 0.001) and vitamin C (r = 0.17, p < 0.01).
The analysis also revealed that pH and titratable acidity (TA) were negatively correlated (r = −0.43, p < 0.001), which is consistent with the fundamental inverse relationship between these parameters. Fruit weight showed negative correlations with several parameters, including seed size (r = −0.18, p < 0.01), total flavonoids (r = −0.21, p < 0.01), and fruit stalk length (r = −0.16, p < 0.05).
Interestingly, FRAP (antioxidant capacity) showed a positive correlation with total flavonoids (r = 0.21, p < 0.01) and seed size (r = 0.13, p < 0.05), suggesting a relationship between these bioactive compounds and antioxidant properties. The vitamin C content was positively correlated with seed width (r = 0.31, p < 0.001) and total flavonoids (r = 0.15, p < 0.05).
The number of seeds showed positive correlations with total sugar (r = 0.13, p < 0.05) and vitamin C (r = 0.18, p < 0.01), while SSC showed negative correlations with fruit flesh firmness (r = −0.21, p < 0.01), seed weight (r = −0.25, p < 0.01), and total sugar (r = −0.24, p < 0.01).
3.3. Principal Component Analysis (PCA)
We evaluated the physicochemical and bioactive properties of wild pear genotypes using principal component analysis, a multivariate statistical method. The findings are presented in Figure 3 and Figure 4. According to the principal component analysis results, the first two components explained 37.91% of the total variance (PC1: 20.93%, PC2: 16.98%) (Figure 4). The fact that the explained variance is not concentrated in the first two components reflects the complex structure of relationships among the studied traits and the multidimensional nature of the diversity among the genotypes. The cumulative variance explained by the first seven components was determined to be 83.91% (Figure 3). PC1 (20.93%) had the highest positive loadings from seed width (0.352) and seed number (0.320). Conversely, it showed negative correlations with soluble solids content (SSC) (−0.329) and fruit length (−0.310). PC2 (16.98%) correlated positively with fruit shape (0.470) and FRAP (0.334), while negatively correlating with fruit width (−0.344). PC3 (13.00%) displayed a positive relationship with pH (0.495) and fruit weight (0.473), and a negative relationship with titratable acidity (−0.457). PC4 (9.97%) showed a positive correlation with the total phenolic content (0.562) and a negative correlation with fruit flesh firmness (−0.536). PC5 (9.12%) had a positive relationship with seed weight (0.551) and a negative relationship with total flavonoid (−0.450). PC6 (7.60%) and PC7 (6.31%) contributed less to the explanation of the total variance.
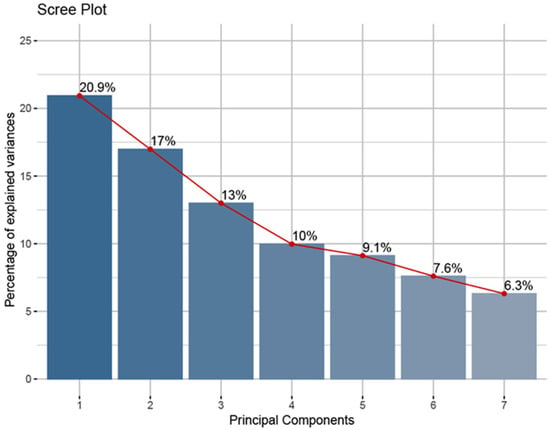
Figure 3.
Percentage of variance explained by each component in principal component analysis. PCA was performed on mean trait values aggregated at the location level.
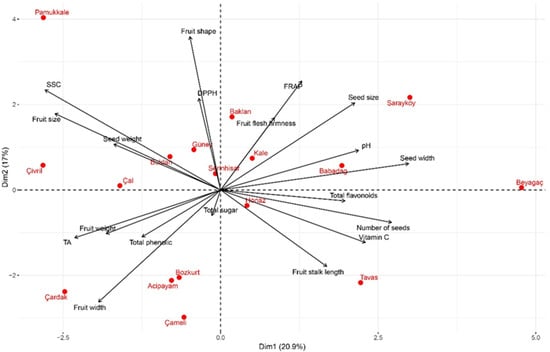
Figure 4.
Principal component analysis biplot of physicochemical and bioactive properties of wild pear genotypes. PCA biplot is based on location-level mean data.
3.4. Cluster Analysis
The results of the hierarchical cluster analysis, based on the physicochemical and bioactive properties of wild pear genotypes collected from different districts of Denizli province, are presented in Figure 5. The analysis clustered the locations into two main groups: Group A and Group B. Group A is divided into two subgroups, A1 and A2, while Group B is also divided into two subgroups, B1 and B2. Group A included genotypes from “Acıpayam”, “Bozkurt”, “Çameli”, “Çardak”, and “Çivril”. These genotypes were characterized by high phenolic content and antioxidant activity values. Group B encompassed the remaining locations. Genotypes in this group stood out primarily for their fruit size and sugar content. The highest genetic similarity was observed between the “Çal” and “Serinhisar” locations, while the lowest similarity was found between “Acıpayam” and “Babadağ”. The cluster analysis revealed that geographical proximity did not always reflect genetic similarity. Genotypes from distant locations were observed to exhibit similar characteristics, which suggests a complex nature of adaptation and dispersion processes within wild pear populations.
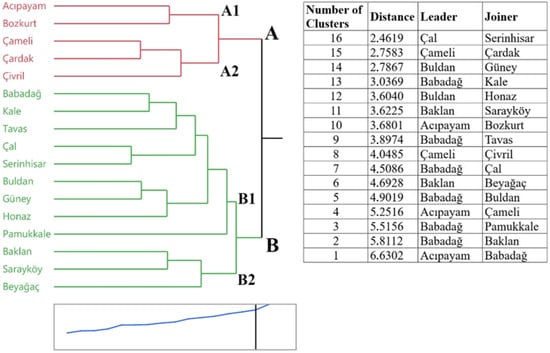
Figure 5.
Cluster analysis of locations based on examined characteristics. Cluster analysis was conducted using mean trait values aggregated at the location level.
4. Discussion
4.1. Analysis of Variance
Our investigation into the fruit characteristics of individual wild pear genotypes collected from various districts of Denizli province revealed significant variations in fruit length (21.73–45.39 mm), fruit width (19.41–46.50 mm), fruit shape index (0.61–1.44), fruit weight (10.73–26.94 g), and fruit pedicel length (12.52–36.44 mm). These findings align with previous studies; for instance, Yilmaz et al. [35] reported fruit lengths between 17.26 and 36.14 mm and fruit weights from 4.71 to 27.09 g for 30 wild pear genotypes collected in 2015 from the Kayseri region, using similar pomological measurement methods. Bozhüyük [38] found fruit lengths ranging from 25.5 to 42.3 mm and fruit weights from 8.36 to 16.11 g in a study conducted in Eastern Anatolia on 25 genotypes sampled in 2017, with fruit measurements performed under controlled laboratory conditions. Similarly, Karatas and Ercisli [56] reported fruit weights of 6.19–21.04 g and fruit pedicel lengths of 7.11–18.56 mm in wild pear samples from the Erzurum region. While our obtained values are largely consistent with published literature, some genotypes in our study exhibited higher values. These discrepancies are likely influenced by both genotypic diversity and environmental factors such as altitude, climate, soil composition, cultural practices, and harvest time. Previous studies have demonstrated that environmental gradients significantly affect phenotypic traits in fruit species [57,58], while genotypic variation remains a key driver of intraspecific diversity [59,60]. Therefore, the observed variation in our study aligns with established findings on the combined effects of genotype and environment on fruit characteristics. Considering Türkiye’s importance in terms of agro-biodiversity, this observed diversity represents a valuable resource for conservation and breeding programs.
Understanding the source of the diversity observed in pomological and biochemical traits in our study is crucial for the conservation and utilization of these genetic resources in breeding programs. Phenotypic plasticity is defined as the ability of the same genotypes to exhibit different phenotypic traits in response to different environmental conditions, while genotypic diversity refers to variation resulting from differences in genetic structure [57]. The distinct differences observed among the samples collected from different locations likely result from a combination of genotypic diversity and varying environmental conditions. Since each genotype represents a unique individual naturally established in its local environment, the observed variation reflects both inherent genetic differences and the influence of local ecological factors. However, the lack of molecular genetic data and controlled environmental experiments in this study highlights the need for future research to disentangle genetic and environmental contributions more precisely. Controlled cultivation and molecular marker analyses will provide more definitive insights into the sources of observed variation [40,57]. Molecular studies in Pyrus species support the presence of significant genotypic diversity contributing to trait variation [40]. Significant variation among samples within the same location further supports the presence of underlying genetic diversity, which environmental factors alone cannot fully explain [40,59,60]. Given that seed traits showed low coefficients of variation, these parameters may serve as more stable indicators for genetic selection in breeding programs [56]. Uzun et al. [40] detected significant genetic diversity among Pyrus elaeagnifolia populations collected from different regions of Turkey using SSR markers. These findings support that the variation observed among individual genotypes largely stems from genotypic diversity. However, to make a definitive distinction, advanced genetic studies such as controlled experiments where the same genotypes are grown under different environmental conditions or molecular marker analyses are necessary in future studies. Such studies will provide more reliable results for the selection of superior genotypes to be used in breeding programs.
When examining the seed characteristics of wild pear genotypes in our study, variations were identified in seed length (4.94–5.88 mm), seed width (3.41–6.24 mm), seed weight (0.81–0.98 g), and seed number (5.76–6.24 seeds/fruit). These results are comparable to those reported by Karatas and Ercisli [56], who found seed numbers ranging from 4.18 to 9.11 seeds/fruit in 20 wild pear genotypes collected in 2018 from the Erzurum region, applying standardized seed counting protocols similar to ours. Gercekcioglu et al. [61] reported seed numbers between 4 and 8 seeds/fruit, and Kececi [62] observed seed numbers ranging from 6.0 to 9.0 seeds/fruit in wild pear samples from the Hakkari region. The variations in seed characteristics observed in our study, besides genotypic diversity, might stem from ecological conditions and pollination efficiency. Notably, the low coefficients of variation for seed characteristics (seed length: 5.47%, seed weight: 4.88%, and seed number: 2.33%) suggest that these traits are less influenced by environmental factors. This indicates that seed characteristics could serve as more stable and reliable parameters for genetic selection in breeding programs.
Yılmaz et al. [35] emphasized the threatened status of Pyrus elaeagnifolia, highlighting this species’ extraordinary adaptation ability against adverse conditions such as extreme winter frosts (−30 °C), chlorosis, water stress, and high alkalinity. The researchers also noted that despite Turkey being the homeland of this species, studies on P. elaeagnifolia were insufficient. There has been a modest but significant increase in research interest in P. elaeagnifolia. Notable advances include molecular characterization studies using SSR markers [40], which provided information about the genetic structure of wild populations throughout Turkey. Additionally, significant progress has been made in understanding the species’ tolerance to salt stress, as demonstrated by Javadisaber et al. [62]. These researchers found that certain P. elaeagnifolia genotypes (especially AH-3) exhibited high survival rates (97.16%) even under severe salt stress conditions (300 mM NaCl). Their study revealed that P. elaeagnifolia’s salt tolerance is associated with enhanced antioxidant enzyme activity, proline accumulation, and early expression of dehydrin genes, confirming the species’ potential as a valuable rootstock for saline conditions. Despite these advances, comprehensive breeding programs specifically targeting P. elaeagnifolia have remained limited. The species’ resilience to environmental stresses positions it as a promising candidate for sustainable agriculture under climate change, emphasizing the urgency of coordinated conservation and breeding efforts [36,63]. The species is underutilized in commercial horticulture, with no registered cultivars developed from this genetic resource. This represents a significant missed opportunity, especially considering the increasing challenges of climate change and the species’ documented resilience to environmental stressors. Our current study contributes to filling this knowledge gap by providing detailed characterization of P. elaeagnifolia genotypes from Denizli province, but coordinated conservation efforts and dedicated breeding programs are urgently needed to harness the potential of this valuable genetic resource before its natural populations suffer further erosion.
Significant variations were also observed in the chemical and bioactive properties of wild pear genotypes collected from different districts of Denizli province. Fruit flesh firmness ranged from 8.61 to 14.41 kg/cm2, values similar to the 7.25–13.82 kg/cm2 range reported by Ercisli et al. [64] for wild pear samples from the Erzurum region. Soluble solids content (SSC) varied from 9.24 to 17.30%, which is consistent with the 10.00–20.00% range found by Yilmaz et al. [35] in 30 genotypes from Kayseri (2015) and the 11.90–20.35% range determined by Karatas and Ercisli [56] in 20 genotypes from Erzurum (2018), both using refractometry methods comparable to those employed in our study. pH values ranged from 3.47 to 5.08, and titratable acidity from 0.70 to 1.15%. The vitamin C content varied from 11.36 to 29.90 mg/100 g, comparable to the 15.25–32.45 mg/100 g range reported by Baltas [11] for wild pear samples from Antalya. The total phenolic content ranged from 168.33 to 357.61 mg GAE/100 g, while the total flavonoid content varied from 21.11 to 46.50 mg CE/100 g. These values were found to be higher when compared to the total phenolic (112–230 mg GAE/100 g) and total flavonoid (32–38 mg CE/100 g) values reported by Ercisli et al. [64] for wild pear samples from Erzurum. Antioxidant activity values were determined to be 4.59–10.63 mmol TE/kg by the DPPH method and 9.41–21.17 mmol TE/kg by the FRAP method. In the DPPH assay, the highest activity was observed in the Pamukkale genotype (10.63 mmol TE/kg), while the lowest values were found in Serinhisar (4.59 mmol TE/kg) and Çardak (4.68 mmol TE/kg) genotypes. In the FRAP assay, the highest values were in Çivril (21.17 mmol TE/kg) and Kale (20.75 mmol TE/kg) genotypes, with the lowest in the Acıpayam genotype (9.41 mmol TE/kg). The total sugar content ranged from 7.43 to 23.15 g/100 g, consistent with the 8.36–19.31 g/100 g range found by Yilmaz et al. [35] in the Kayseri region. The observed variation in chemical and bioactive components among wild pear genotypes is likely influenced by both genotypic diversity and environmental factors such as altitude, climate, soil composition, maturity stage, and harvest time. Previous studies have demonstrated that these environmental gradients significantly affect phytochemical profiles in fruit species [65,66]. Furthermore, genotypic variation is a critical determinant for the accumulation of bioactive compounds, as noted by Sevindik et al. [59], Çoban et al. [60], and Uzun et al. [40], who emphasized the role that genotype plays in the synthesis of these compounds irrespective of environmental influences [67]. Research on this topic has provided valuable insights into these interactions. These studies reveal that inherent genetic differences among plant varieties contribute significantly to biochemical and yield responses, but environmental factors can also result in variations in performance, supporting the notion that the combined effects of genotype and environment are important in understanding biochemical diversity [68,69,70]. Ultimately, understanding these complex interactions is crucial for optimizing the cultivation and utilization of wild pear genotypes, enabling the selection of varieties with superior chemical and bioactive profiles suited to specific environmental conditions.
The production of bioactive compounds is directly related to the complex interaction between genetic and environmental factors. To unravel these interactions and identify genotypes with superior biochemical traits, further studies involving controlled environmental conditions and molecular analyses are necessary. Özay and Pehlivan [71] emphasized the effects of genetic and environmental factors on the accumulation of these compounds by addressing the factors influencing the biosynthesis of secondary metabolites [71]. Additionally, a study by Dibek et al. [72] examined the potential uses and mechanisms of action of boron-containing bioactive compounds, evaluating their analysis at the molecular level and the role of environmental conditions [72]. Therefore, advanced studies aimed at determining the effects of genetic and environmental factors will enable a comprehensive evaluation of bioactive compounds and contribute to the development of beneficial strategies for breeding programs.
Expanding this comparison to a broader international context further highlights the significance of the Denizli population. A recent study on European wild pear (Pyrus pyraster) in Croatia, for instance, reported a notably narrower range for fruit weight, with population averages spanning from 7.9 g to 12.9 g [73], who examined 15 populations sampled between 2019 and 2022, applying fruit weight measurements under standardized conditions, allowing meaningful comparison with our Denizli genotypes. The fact that the upper range of our Pyrus elaeagnifolia genotypes (up to 26.94 g) substantially exceeds these values underscores the potential of Anatolian genetic resources for breeding programs targeting larger fruit size. Furthermore, a comprehensive 2023 comparative evaluation of 15 different Pyrus species places our findings within the vast diversity of the genus. That study reported an extreme range in fruit weight, from a mere 2.70 g (Prunus korshinskyi) to a remarkable 91.00 g for Pyrus caucasica [74]. This indicates that while our Denizli genotypes are superior to some European wild populations like Pyrus pyraster, they represent an intermediate fruit size within the broader Pyrus gene pool. In terms of biochemical potential, our results align with studies on other Mediterranean pear resources, which confirm that less-domesticated germplasm is a rich source of bioactive compounds [74]. Notably, Piluzza et al. [75] demonstrated that phenolics are most concentrated in the peel and core. This suggests that the whole-fruit values in our study may underestimate the potential of the peel fraction, and that our superior genotypes could be even more valuable than reported if analyzed by fruit part. Collectively, these comparisons position the genetic material from Denizli as a valuable resource, superior in key traits to some regional wild populations and holding significant, potentially concentrated, biochemical potential that warrants further exploration. This highlights the strategic importance of preserving and utilizing Anatolian wild pear germplasm in broader breeding programs aimed at enhancing fruit size and bioactive compound content [73,75].
The high phenolic and flavonoid content observed in wild pear genotypes, along with their strong antioxidant activities, align with findings in other fruit species where these compounds contribute to health-promoting properties such as anti-inflammatory and cardioprotective effects [75,76,77,78]. Although specific bioactivities were not directly tested in this study, extensive literature supports the potential of phenolic compounds abundant in pears—such as chlorogenic acid, catechin, and quercetin—to confer significant health benefits [79,80,81,82]. These findings suggest that selected wild pear genotypes could be prioritized for development into nutraceuticals or functional foods, supporting health management strategies for inflammatory and metabolic disorders [79,80,83]. For example, numerous in vitro studies have demonstrated that these compounds exhibit potent anti-inflammatory effects by suppressing the production of pro-inflammatory cytokines [83,84].
4.2. Correlation Analysis
Previous research on Pyrus elaeagnifolia characterization by Sagbas et al. [39] reported a moderately positive correlation between total phenolics and total flavonoids, and positive correlations between DPPH and FRAP, indicating a moderate positive relationship among these antioxidant activities. Similarly, Abaci et al. [85] found a positive correlation between total phenolics and DPPH in their study on bioactive compounds in pear genotypes. Azzini et al. [86] also reported a strong positive correlation between total phenolics and FRAP when examining the antioxidant and phytochemical content of pear fruit.
In contrast, our comprehensive analysis revealed different patterns of relationships among these parameters. Our findings did not show significant correlations between total phenolics and flavonoids or between total phenolics and antioxidant activities (DPPH and FRAP), diverging from previous studies. This difference is thought to be due to the larger and more diverse sample size in our study, which provides a more accurate representation of the natural variation in wild pear populations. Parvin et al. [87] reported positive correlations between SSC and TA, and between SSC and antioxidant activity with fruit weight in their work with Pyrus glabra and Pyrus syriaca. However, our analysis showed different relationships, particularly regarding fruit weight and its associations with biochemical properties. Our findings show both similarities and differences with other studies, including those by Rana et al. [88], Heidari et al. [24], and Khadivi et al. [89], which stem from our methodological approach of analyzing individual genotypes rather than location means.
These correlations reveal complex relationships between bioactive components and pomological traits in wild pear genotypes. However, it is important to note that correlation does not imply causation [90]. Since this study did not include molecular genetic analyses or controlled environmental assessments, the underlying causes of these correlations remain unclear. Future studies integrating genotypic data and controlled environmental conditions are necessary to clarify the genetic and environmental contributions to these trait relationships and to inform targeted breeding strategies [91,92]. Both genetic variation and environmental factors likely contribute to the relationships between the traits examined [93,94]. Therefore, interpretations regarding the biological mechanisms behind these correlations should be made cautiously.
The relationships between fruit weight and chemical properties warrant consideration regarding consumer preferences and market demands. Balancing fruit size with bioactive compound content will be critical in breeding programs aiming to meet both nutritional and commercial objectives under evolving climate scenarios [88,89]. In breeding programs, balancing fruit quality with bioactive components is crucial for developing resilient and marketable varieties under changing climate conditions.
4.3. Principal Component and Cluster Analysis
In our study, the first three principal component analyses explained 50.9% of the total variance. This proportion shows similarity to the values reported by Parvin et al. [87] for Pyrus glabra (44.35%) and Pyrus syriaca (44.00%). This distribution of variance indicates a broader and more multidimensional genetic diversity within our studied population.
Furthermore, an examination of the subsequent components provides deeper insight into this diversity. PC3, for instance, was primarily defined by the contrast between fruit weight/pH and titratable acidity, indicating its relation to fruit quality. Similarly, PC4 revealed a strong negative correlation between the total phenolic content and fruit flesh firmness, suggesting a potential trade-off between nutraceutical value and texture. These findings, derived from components beyond the first two, underscore the value of these genetic resources for complex breeding objectives.
PC5 (9.12%) shows a positive relationship with seed weight and a negative relationship with the total flavonoid content. This component may reflect the physiological balance between seed development and secondary metabolite accumulation. Genotypes with larger seeds likely direct their energy resources toward seed development, allocating fewer resources to flavonoid biosynthesis. PC6 (7.60%) and PC7 (6.31%), while contributing less to the total variance, are associated with DPPH activity and vitamin C content, respectively, representing different aspects of antioxidant capacity. The presence of these components emphasizes the multidimensional nature of antioxidant properties in the studied population and suggests that the genetic control of different antioxidant mechanisms may be independent.
Further insights into the population structure were provided by our cluster analysis. This analysis demonstrated a strong relationship between morphological variation and geographical distribution, consistent with observations reported by Voltas et al. [23], Khadivi-Khub and Anjam [95], and Khadivi et al. [89]. The proximity in geography facilitates gene flow [96] and reflects similar climatic conditions, which explains the lower morphological diversity observed among genotypes from nearby locations. This underscores the importance of including genotypes from diverse geographic regions in breeding and conservation programs to maximize genetic diversity and adaptive potential [23,95]. However, the presence of superior genotypes from diverse regions presents significant opportunities for increasing genetic diversity and enhancing resilience in breeding programs, particularly given the lack of interspecific incompatibility barriers within the Pyrus genus [74,97]. This genetic diversity boosts the adaptation potential against climate change and environmental stresses, making it a critical resource for sustainable agriculture and food security.
It is important to note that while PCA and cluster analyses provide valuable insights into phenotypic variation and population structure, these methods do not directly assess underlying genetic mechanisms. Therefore, molecular genetic analyses and controlled environmental studies are essential to validate the genetic basis of observed phenotypic patterns and to disentangle environmental influences [98].
In our study, the first three principal components explained 50.9% of the total variance, consistent with values reported for related Pyrus species [87]. The multidimensional nature of genetic diversity was further highlighted by subsequent components associated with fruit quality and antioxidant traits.
Our hierarchical cluster analysis, based on physicochemical and bioactive properties, grouped wild pear genotypes from Denizli province into two main clusters. Group A, comprising genotypes from Acıpayam, Bozkurt, Çameli, Çardak, and Çivril, was characterized by higher phenolic content and antioxidant activity, whereas Group B included genotypes distinguished by larger fruit size and higher sugar content. Notably, the highest genetic similarity was observed between geographically close locations (Çal and Serinhisar), while the lowest similarity was found between distant locations (Acıpayam and Babadağ). However, some genotypes from distant locations exhibited similar traits, indicating that geographical proximity does not always predict genetic or phenotypic similarity.
These findings align with previous studies demonstrating that gene flow and morphological variation in Pyrus species are influenced by a complex interplay of factors beyond mere geographic distance, including historical dispersal, ecological adaptation, and local selection pressures [23,94]. The observed pattern suggests that while geographic proximity can facilitate gene flow and similar environmental conditions may shape phenotypic traits, other evolutionary and ecological processes contribute significantly to population structure.
5. Conclusions
The comprehensive characterization of wild pear genotypes collected from various locations across Denizli province revealed significant phenotypic variation. High coefficients of variation were particularly noted for the total flavonoid content (26.66%), total sugar (25.91%), and fruit pedicel length (25.43%), underscoring the rich phenotypic diversity among the genotypes sampled within this population. This diversity emphasizes Türkiye’s role as a critical genetic resource in terms of agro-biodiversity, holding substantial potential for the sustainability of local ecosystems and global food security. The results from principal component analysis (PCA) and cluster analysis revealed variation among locations. While these patterns may be influenced by geographical distance and ecological conditions, the current data do not provide sufficient evidence to conclusively support this, highlighting the need for further molecular genetic and controlled environmental studies. The first seven PCA components explained 83.91% of the total variance, with the first two components accounting for 37.91%, highlighting key trait groupings such as seed characteristics and fruit quality parameters. Notably, genotypes from the Çivril, Çal, Pamukkale, and Tavas locations exhibited superior traits in terms of fruit quality and bioactive compounds. These genotypes can be considered priority targets for breeding programs and conservation strategies due to their capacity for climate change adaptation and their potential as functional foods. These findings will contribute to identifying valuable genetic resources for future breeding programs and adaptation studies concerning climate change. The PCA results, in particular, emphasize that important variation is distributed beyond the first two components, underscoring the need to consider multiple traits simultaneously in breeding and conservation programs. To build on these findings, future studies should move beyond phenotypic data to include the molecular characterization of superior genotypes. Furthermore, while this study highlights their functional food potential, targeted in vitro and in vivo analyses are necessary to confirm specific health benefits. Such biological validations will strengthen the scientific basis for developing Pyrus elaeagnifolia-based nutraceuticals and functional food products.
Author Contributions
A.A.: conceptualization, species identification, methodology, investigation, resources, writing—original draft, writing—review and editing, project administration, and supervision. L.K.: methodology, investigation, resources, data curation, formal analysis, statistical analysis, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Bell, R.L.; Quamme, H.A.; Layne, R.E.C.; Skirvin, R.M. Pears. In Fruit Breeding Vol. I Tree and Tropical Fruits; Janick, J., Moore, J.N., Eds.; John Wiley & Sons: New York, NY, USA, 1996; pp. 441–514. [Google Scholar]
- Morgan, J. The Book of Pears: The Definitive History and Guide to over 500 Varieties; Chelsea Green Publishing: Vermont, VT, USA, 2015. [Google Scholar]
- USDA; Agricultural Research Service; National Plant Germplasm System. Germplasm Resources Information Network (GRIN Taxonomy). 2023. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=30491 (accessed on 26 January 2025).
- Aygün, A.; Kırca, L. Ahlat (Pyrus elaeagnifolia). In Minör Meyveler II; Sülüşoğlu Durum, M., Polat, M., Eds.; İKSAD Publishing House: Ankara, Türkiye, 2023; pp. 61–91. [Google Scholar]
- Dumanoğlu, H.; Aygün, A.; Alay, A.; Güneş, N.T.; Özkaya, M.T. Ahlatın (Pyrus elaeagnifolia Pall.) Yeşil Çeliklerinde Köklenme ve Sürme Üzerine Çelik Alma Zamanı IBA ve Putrescine’in Etkileri. Turk. J. Agric. For. 1999, 23, 559–565. [Google Scholar]
- Özçağıran, R.; Ünal, A.; Özeker, E.; İsfendiyaroğlu, M. Ilıman iklim meyve türleri: Yumuşak çekirdekli meyveler; Ege Üniversitesi Yayınları: İzmir, Türkiye, 2014. [Google Scholar]
- Bell, R.L.; Stuart, L.C. Resistance in Eastern European Pyrus germplasm to pear psylla nymphal feeding. HortScience 1990, 25, 789–791. [Google Scholar] [CrossRef]
- Özlük, A. Selection of Wild Pear (Pyrus elaeagnifolia L.) Naturally Grow in Merzifon District. Master’s Thesis, Institute of Science, Tokat, Türkiye, 2015. [Google Scholar]
- Salkić, B.; Salkić, A.; Imširović, E.; Salkić, E.; Salihović, E. Examination of compatibility of autochthonous pear cultivars from the region of northeastern bosnia with vegetative rootstock of the genus Cydonia sp. Int. J. Plant Soil Sci. 2022, 34, 35–39. [Google Scholar] [CrossRef]
- Cansaran, A.; Kaya, Ö.F.; Yıldırım, C. Ovabaşı, Akpınar, Güllüce ve Köseler Köyleri (Gümüşhacıköy/Amasya) Arasında Kalan Bölgede Etnobotanik Bir Araştırma. Fırat Üniversitesi Fen ve Mühendislik Bilimleri Dergisi 2007, 19, 243–257. [Google Scholar]
- Baltas, N. Investigation of a wild pear species (Pyrus elaeagnifolia subsp. elaeagnifolia) from Antalya, Turkey: Polyphenol oxidase properties and anti-xanthine oxidase, antiurease, and antioxidant activity. Int. J. Food Prop. 2017, 20, 585–595. [Google Scholar] [CrossRef]
- Şengül, M.; Topdaş, E.F.; Doğan, H.; Serencam, H. Artvin ilinde geleneksel olarak üretilen farklı marmelat çeşitlerinin bazı fiziksel ve kimyasal özellikleri, antioksidan aktiviteleri ve fenolik profilleri. Akademik Gıda 2018, 16, 51–59. [Google Scholar] [CrossRef]
- Erçetin, H.K.; Güneş, E.; Olcay, G.S. Use of Ahlat Flour in Cookie Production. J. Tour. Gastron. Stud. 2021, 9, 674–686. [Google Scholar] [CrossRef]
- Çakılcıoğlu, U.; Şengün, M.T.; Türkoğlu, D. An ethnobotanical survey of medicinal plants of Yazıkonak and Yurtbaşı districts of Elazığ province, Turkey. J. Med. Plants Res. 2010, 4, 567–572. [Google Scholar]
- Şeker, M.; Yücel, Z.; Nurdan, E. Phenolic compounds and antioxidant activities of local wild pear (Pyrus elaeagrifolia). Int. J. Agric. Biol. 2016, 18, 483–488. [Google Scholar]
- Fidan, M.S.; Oz, M.; Ucuncu, O.; Baltaci, C.; Karatas, S.M. Composition of antimicrobial and antioxidant activities and chemical components of essential oil from flowers and leaves of Pyrus elaeagnifolia Pallas in Turkey. Fresenius Environ. Bull. 2022, 31, E363–E371. [Google Scholar]
- Kayhan, R.; Bulduk, I.; Korcan, S.E.; Abed, A.B.; Ünal, A. Flavonoids, Phenolics and Antioxidant Evaluation of Different Parts of Three Wild Pyrus Species from Turkey. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2024, 94, 903–911. [Google Scholar] [CrossRef]
- Yilmaz, H.; Ercişli, S. Antibacterial and antioxidant activity of fruits of some rose species from Turkey. Rom. Biotechnol. Lett. 2011, 16, 6407–6411. [Google Scholar]
- İlhan, M.; Akkol, E.K.; Taştan, H.; Dereli, F.T.G.; Tümen, İ. Efficacy of Pyrus elaeagnifolia subsp. elaeagnifolia in acetic acid-induced colitis model. Open Chem. 2019, 17, 13–22. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Świderski, G.; Lewandowski, W. Biological activity of new cichoric acid-metal complexes in bacterial strains, yeast-like fungi, and human cell cultures in vitro. Nutrients 2020, 12, 154. [Google Scholar] [CrossRef]
- Peng, B.R.; Tang, X.Y.; Chen, Y.S.; Lai, K.H.; Lee, M.H. Exploring the wound-healing potential and seasonal chemical variability of the Formosan Callery pear Pyrus calleryana: Implications for therapeutic applications. Pharm. Biol. 2024, 62, 621–633. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; dos Santos, A.R.F.; Ramos-Cabrer, A.M.; Sau, F.; Díaz-Hernández, M.B. Morphological variation in local pears from north-western Spain. Sci. Hortic. 2012, 138, 176–182. [Google Scholar] [CrossRef]
- Voltas, J.; Pemán, J.; Fusté, F. Phenotypic diversity and delimitation between wild and cultivated forms of the genus Pyrus in North-eastern Spain based on morphometric analyses. Genet. Resour. Crop Evol. 2007, 54, 1473–1487. [Google Scholar] [CrossRef]
- Gültekin, H.C. Ahlatlar (Pyrus L.). Orman ve Av Dergisi 2014, 91, 49–53. [Google Scholar]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Žlabur, J.Š.; Babojelić, M.S. Traditional, indigenous apple varieties, a fruit with potential for beneficial effects: Their quality traits and bioactive polyphenol contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef]
- Zhang, H.; Tu, K.; Qiu, Z.; Zhuang, W.; Li, Q.; Wen, X. Effects of different rain shelter coverings on volatile organic compounds in mature fruit and postharvest quality of sweet cherry. CyTA J. Food 2021, 19, 465–475. [Google Scholar] [CrossRef]
- Jahufer, M.Z.Z.; Casler, M.D. Application of the Smith-Hazel selection index for improving biomass yield and quality of switchgrass. Crop Sci. 2015, 55, 1212–1222. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M. MGIDI: Towards an effective multivariate selection in biological experiments. Bioinformatics 2020, 37, 1383–1389. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Battisti, D.S.; Naylor, R.L. Historical Warnings of Future Food Insecurity with Unprecedented Seasonal Heat. Science 2009, 323, 240–244. [Google Scholar] [CrossRef]
- Bohra, A.; Kilian, B.; Sivasankar, S.; Caccamo, M.; Mba, C.; McCouch, S.R.; Varshney, R.K. Reap the crop wild relatives for breeding future crops. Trends Biotechnol. 2022, 40, 412–431. [Google Scholar] [CrossRef]
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Müller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Biodiversity for Food and Agriculture. In FAO Commission on Genetic Resources for Food and Agriculture Assessments; Bélanger, J., Pilling, D., Eds.; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Yilmaz, K.U.; Ercisli, S.; Cam, M.; Uzun, A.; Yilmaztekin, M.; Kafkas, E.; Pinar, H. Fruit weight, total phenolics, acidity and sugar content of edible wild pear (Pyrus elaeagnifolia Pall.) fruits. Erwerbs-Obstbau 2015, 57, 179–184. [Google Scholar] [CrossRef]
- Yilmaz, K.U.; Uzun, A.; Cam, M.; Ercisli, S. Some morphological and fruit characteristics of naturally grown Pyrus elaeagrifolia Pall. of Kayseri Province (Central Anatolia, Turkey). Genet. Resour. Crop Evol. 2015, 62, 711–720. [Google Scholar] [CrossRef]
- Murathan, Z.T.; Erbil, N.; Düzgüner, V.; Arslan, M. Şakok Armudunun (Pyrus elaeagnifila Pallas) Antioksidan, Antimikrobiyal ve Mutajenik Özelliklerinin İncelenmesi. Erzincan Univ. J. Sci. Technol. 2019, 12, 447–456. [Google Scholar] [CrossRef]
- Bozhüyük, M.R. Morphological diversity of Pyrus elaeagrifolia Pall. ecotypes in Eastern Anatolia Region. Int. J. Agric. Wildl. Sci. 2021, 7, 368–372. [Google Scholar] [CrossRef]
- Sagbas, H.I.; Ilhan, G.; Ercisli, S.; Anjum, M.A.; Holubec, V. Characterization of Oleaster-Leafed Pear (Pyrus elaeagnifolia Pall. subsp. elaeagnifolia) Fruits in Turkey. Agronomy 2021, 11, 430. [Google Scholar] [CrossRef]
- Uzun, A.; Pinar, H.; Yaman, M.; Yigit, M.A.; Cakiroglu, Y.; Karakaya, A.; Ercisli, S. Identification of genetic diversity in wild pear (Pyrus elaeagnifolia Pall.) Genotypes collected from different regions of Turkey with SSR marker system. Genetika 2022, 54, 109–118. [Google Scholar] [CrossRef]
- Dahlia, F.; Benito, C.; Boussaid, M. Genetic diversity of fruits in wild jujube (Ziziphus lotus L. Desf.) natural populations from Algeria. Poljoprivreda i Sumarstvo 2019, 65, 165–183. [Google Scholar] [CrossRef]
- Eken, B.U.; Kirdök, E.; Velioğlu, E.; Çiftçi, Y.Ö. Assessment of genetic variation of natural populations of wild cherry (Prunus avium L.) via SSR markers. Turk. J. Bot. 2022, 46, 14–25. [Google Scholar] [CrossRef]
- Nishimwe, G.; Augustino, S.; Dahlin, A.S.; Niyitanga, F. Characterization of yield and physico-chemical parameters of selected wild indigenous fruits in Rwanda. Resources 2024, 13, 101. [Google Scholar] [CrossRef]
- Karaçalı, İ. Bahçe Ürünlerinin Muhafaza ve Pazarlanması; Ege Üniversitesi Basımevi: İzmir, Türkiye, 2009; 472p. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Yen, G.; Chen, H. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cemeroğlu, B. Meyve Sebze İşleme Teknolojisi 1. Cilt; Bizim Grup Basımevi: Ankara, Türkiye, 2017; 707p. [Google Scholar]
- Cemeroğlu, B. Gıda Analizleri; Gıda Teknolojisi Derneği Yayınları: Ankara, Türkiye, 2007; pp. 168–171. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.r-project.org/ (accessed on 31 January 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 31 January 2024).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://cran.r-project.org/package=factoextra (accessed on 31 January 2024).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. 2024. Available online: https://cran.r-project.org/package=FactoMineR (accessed on 31 January 2024).
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 31 January 2024).
- Karatas, S.; Ercisli, S. Fruit characteristics of Pyrus elaeagrifolia Pall. genotypes in Eastern Turkey. In Proceedings of the X International Symposium on Agricultural Sciences AgroReS 2021, Sarajevo, Bosnia and Herzegovina, 27–29 May 2021; pp. 30–32. [Google Scholar]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; van Kleunen, M. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Sevindik, E.; Efe, F.; Murathan, Z.T. Molecular genetic diversity and phylogenetic investigation of Pyrus communis L. (Rosaceae) genotypes using cpDNA sequences with RAPD and ISSR analyses. Erwerbs-Obstbau 2023, 65, 231–240. [Google Scholar] [CrossRef]
- Çoban, A.; Değirmenci, F.Ö.; Uluğ, A.; Ateş, M.A.; Yüksel, E.; Eminağaoğlu, Ö.; Kaya, Z. Genetic analysis of village pear (Pyrus communis L.) cultivar populations in northeastern Türkiye. Plant Genet. Resour. 2024, 22, 408–416. [Google Scholar] [CrossRef]
- Gercekcioglu, R.; Ozluk, A.; Atasever, O. Selection of Pyrus elaeagnifolia Pall. from Merzifon district. Bahce 2016, 2, 69–73. [Google Scholar]
- Kececi, L.D. Determination of Some Horticultural Characteristics of Pyrus elaeagrifolia L. Genotypes from Hakkari Region. Master’s Thesis, Adnan Menderes University, Aydın, Türkiye, 2017. [Google Scholar]
- Javadisaber, J.; Dumanoğlu, H.; Şahin, Ö.; Sarıkamış, G.; Ergül, A.; Çakır Aydemir, B. Salt stress tolerance of Pyrus spp. and Cydonia oblonga genotypes assessed by morphological, biochemical and dehydrin gene expression analysis. J. Plant Growth Regul. 2024, 43, 165–177. [Google Scholar] [CrossRef]
- Deligiannidou, E.; Boutsika, A.; Plesias, I.; Xanthopoulou, A.; Moysiadis, T.; Mellidou, I.; Ganopoulos, I. Microsatellite genotyping and genetic diversity of a greek pear (Pyrus communis L.) germplasm collection. Plants 2025, 14, 1816. [Google Scholar] [CrossRef]
- Mertoğlu, K. Investigation of genetic parameters and phytochemical characteristics in plum under altitude change. Genetika 2022, 54, 73–89. [Google Scholar] [CrossRef]
- Wei, T.; Zhong, S.; Huang, B.; Zha, K.; Li, J.; Wen, Q. Influence of Environmental Conditions Associated with Low and High Altitudes on Economic and Quality Characteristics of Fruit Ripening of Camellia chekiangoleosa Hu. Foods 2025, 14, 2266. [Google Scholar] [CrossRef]
- New, A.M.; Cerulus, B.; Govers, S.K.; Perez-Samper, G.; Zhu, B.; Boogmans, S.; Verstrepen, K.J. Different levels of catabolite repression optimize growth in stable and variable environments. PLoS Biol. 2014, 12, e1001764. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, F.; Tranchina, D.; Gresham, D. Fluctuating environments maintain genetic diversity through neutral fitness effects and balancing selection. Mol. Biol. Evol. 2021, 38, 4362–4375. [Google Scholar] [CrossRef]
- Sridhar, V.; Jagan, P.; Rao, M.R.; Saikiran, V.; Kishore, N.; Reddy, M.R.; Kumar, G.P. Development of stable black-gram [Vigna mungo (L.) hepper] genotypes by deciphering genotype × environment interaction using eberhart-russell and ammi models. Electron. J. Plant Breed. 2023, 14, 52–59. [Google Scholar] [CrossRef]
- Islam, S.S.; Sarker, M.B.U.; Rana, M.M.; Hasan, A.K.; Karim, M.; Khomphet, T. Comprehensive assessment of the genotype-environment interaction and yield stability of boro rice genotypes under four environments in Bangladesh using ammi analysis. Scientifica 2024, 2024, 7800747. [Google Scholar] [CrossRef] [PubMed]
- Özay, C.; Pehlivan, E. Bitki sekonder metabolitlerinin biyosentezini ve akümülasyonunu etkileyen faktörler. Ankara Üniversitesi Eczacılık Fakültesi Dergisi 2024, 48, 1248–1263. [Google Scholar] [CrossRef]
- Dibek, E.; Babayeva, A.; Kürkçü, M.S.; Çöl, N.A.; Çöl, B. Bor içeren bazı antibiyotikler. J. Boron 2020, 5, 29–39. [Google Scholar] [CrossRef]
- Vidaković, A.; Poljak, I. Fruit morphological variability and chemical composition in European wild pear (Pyrus pyraster (L.) Burgsd.) natural populations. Genet. Resour. Crop Evol. 2024, 71, 4315–4330. [Google Scholar] [CrossRef]
- Simionca Mărcășan, L.I.; Pop, R.; Somsai, P.A.; Oltean, I.; Popa, S.; Sestras, A.F.; Sestras, R.E. Comparative evaluation of Pyrus species to identify possible resources of interest in pear breeding. Agronomy 2023, 13, 1264. [Google Scholar] [CrossRef]
- Piluzza, G.; Campesi, G.; D’hallewin, G.; Molinu, M.G.; Re, G.A.; Sanna, F.; Sulas, L. Antioxidants in fruit fractions of Mediterranean ancient pear cultivars. Molecules 2023, 28, 3559. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-inflammatory and anticancer effects of anthocyanins in in vitro and in vivo studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef]
- Żbikowska, B.; Kotowska, M.; Gamian, A.; Patek, K.; Matuła, K.; Augustyniak, D.; Sroka, Z. Antimicrobial and Anti-radical Activity of Extracts from Leaves of Various Cultivars of Pyrus communis and Pyrus pyrifolia. Biomolecules 2025, 15, 821. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Yang, L.; Ran, Y.; Hu, Q.; Hong, Y.; Tian, M. Phytochemical analysis, antioxidant, anti-inflammatory and enzyme inhibitory activities of bean pear (Pyrus calleryana fruit). Front. Plant Sci. 2025, 16, 1521990. [Google Scholar] [CrossRef]
- Erbil, N.; Murathan, Z.T.; Arslan, M.; Ilcim, A.; Sayin, B. Antimicrobial, antioxidant, and antimutagenic activities of five Turkish pear cultivars. Erwerbs-Obstbau 2018, 60, 203–209. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupińska, A.; Kruczyńska, D.E. Bioactive compounds and health-promoting properties of pear (Pyrus communis L.) fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef]
- Kırca, L.; Kırca, S.; Aygün, A. Organic acid, phenolic compound and antioxidant contents of fresh and dried fruits of pear (Pyrus communis L.) cultivars. Erwerbs-Obstbau 2023, 65, 677–691. [Google Scholar] [CrossRef]
- Wani, S.G.; Shafi, F.; Jabeen, A.; Malik, M.A. Physicochemical, antioxidant and antimicrobial properties of peel, pulp and seeds of different pear cultivars. Food Humanit. 2025, 4, 100521. [Google Scholar] [CrossRef]
- Demir, T.; Akpınar, Ö.; Kara, H.; Güngör, H. Nar (Punica granatum L.) Kabuğunun İn Vitro Antidiyabetik, Antienflamatuar, Sitotoksik, Antioksidan ve Antimikrobiyal Aktivitesi. Akademik Gıda 2019, 17, 61–71. [Google Scholar] [CrossRef]
- Ruiz-Alcaraz, A.J.; Baquero, L.; Pérez-Munar, P.M.; Oliva-Bolarín, A.; Sánchez-Martínez, M.A.; Ramos-Molina, B.; Moreno, D.A. In vitro study of the differential anti-inflammatory activity of dietary phytochemicals upon human macrophage-like cells as a previous step for dietary intervention. Int. J. Mol. Sci. 2024, 25, 10728. [Google Scholar] [CrossRef] [PubMed]
- Abaci, Z.; Sevindik, E.; Ayvaz, M. Comparative study of bioactive components in pear genotypes from Ardahan/Turkey. Biotechnol. Biotechnol. Equip. 2015, 30, 36–43. [Google Scholar] [CrossRef]
- Azzini, E.; Maiani, G.; Durazzo, A.; Foddai, M.S.; Intorre, F.; Venneria, E.; Forte, V.; Lucchetti, S.; Ambra, R.; Pastore, G.; et al. Giovanni Varieties (Pyrus communis L.): Antioxidant Properties and Phytochemical Characteristics. Oxidative Med. Cell. Longev. 2019, 2019, 6714103. [Google Scholar] [CrossRef]
- Parvin, P.; Gharaghani, A.; Khosravi, A.; Eshghi, S. Phenotypic characterization of Pyrus glabra Boiss. and P. syriaca Boiss.: Implications for conservation and utilization. Trees 2023, 37, 1537–1554. [Google Scholar] [CrossRef]
- Rana, J.C.; Chahota, R.K.; Sharma, V.; Rana, M.; Verma, N.; Verma, B. Genetic diversity and structure of Pyrus accessions of Indian Himalayan region based on morphological and SSR markers. Tree Genet. Genomes 2015, 11, 821. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F.; Moradi, Y.; Paryan, S. Morphological and pomological characterizations of Pyrus syriaca Boiss. germplasm. Sci. Hortic. 2020, 271, 109424. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef]
- Cooper, M.; Messina, C.D.; Podlich, D.; Totir, L.R.; Baumgarten, A.; Hausmann, N.J.; Graham, G. Predicting the future of plant breeding: Complementing empirical evaluation with genetic prediction. Crop Pasture Sci. 2014, 65, 311–336. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Longman: London, UK, 1996. [Google Scholar]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Khadivi-Khub, A.; Anjam, K. Morphological characterization of Prunus scoparia using multivariate analysis. Plant Syst. Evol. 2014, 300, 1361–1372. [Google Scholar] [CrossRef]
- Altekin, H.; Demirbaş, N. Üreticilerin Dip Zeytin Hasat Kararı Üzerinde Etkili Olan Faktörlerin Belirlenmesi: İzmir İli Örneği. ÇOMÜ Ziraat Fak. Derg. 2021, 9, 229–236. [Google Scholar] [CrossRef]
- Montanari, S.; Postman, J.; Bassil, N.V.; Neale, D.B. Reconstruction of the largest pedigree network for pear cultivars and evaluation of the genetic diversity of the USDA-ARS national Pyrus collection. G3 2020, 10, 3285–3297. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T. Principal Component Analysis; Springer Series in Statistics; Springer: New York, NY, USA, 2002. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).