Evidence of Graft Incompatibility and Rootstock Scion Interactions in Cacao
Abstract
1. Introduction
2. Materials and Methods
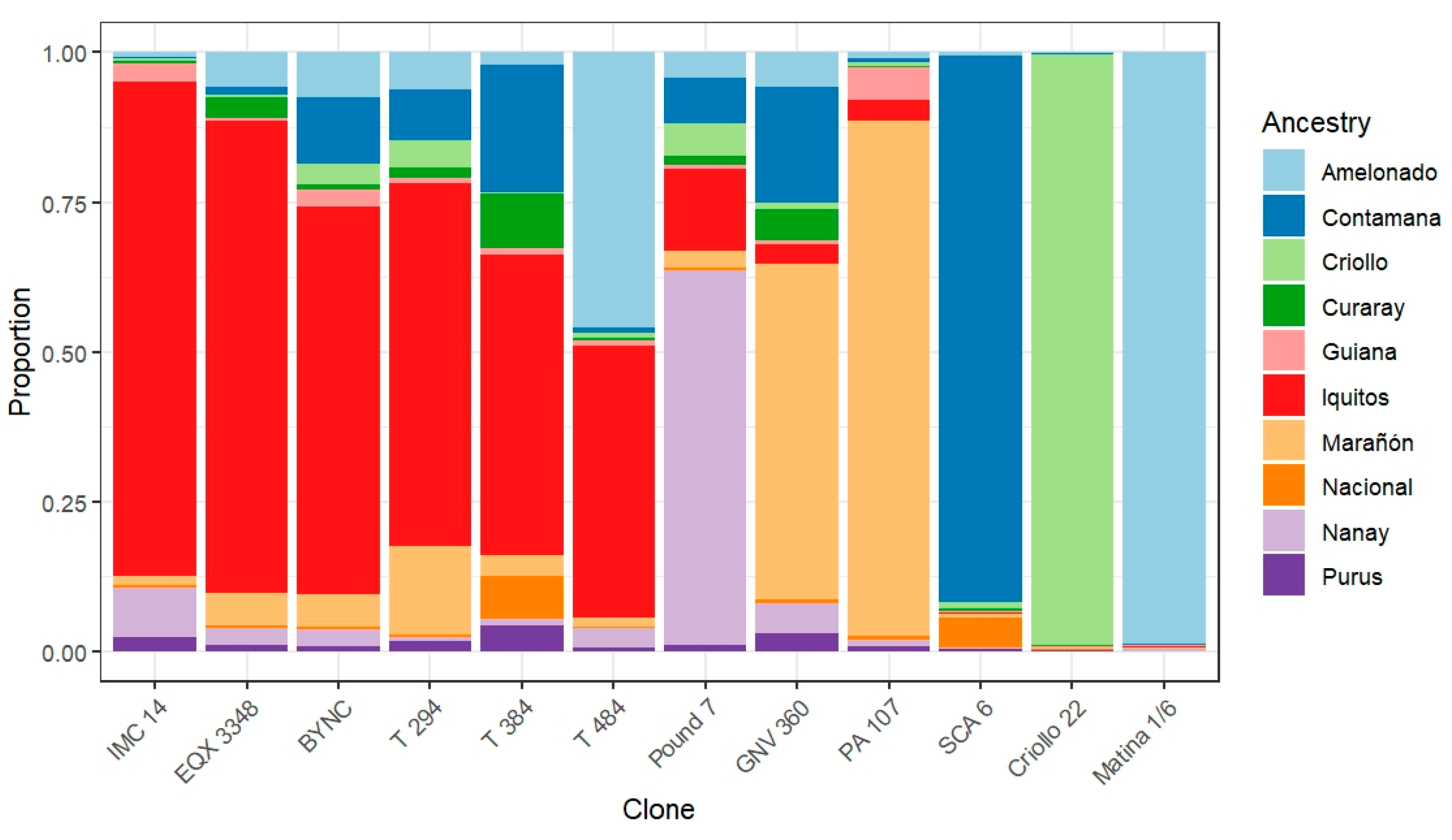
2.1. Planting Materials
2.2. Grafting
2.3. Morphological Measurements
2.4. Graft Junction Imaging
2.5. Nutrient and Carbohydrate Quantification
2.6. Statistical Analysis
- X = Matrix of fixed effect coefficients
- Z = Matrix of random effect coefficients
- β = Scion cultivar
- µ = Rootstock family
2.7. Phytophthora Palmivora Inoculations
2.7.1. Rootstock Inoculations
2.7.2. Scion Inoculations
3. Results
3.1. Graft Incompatibility
3.2. Survival
3.3. Morphological Traits
3.3.1. Rootstock Diameter
3.3.2. Scion Diameter
3.3.3. Flush Length
3.3.4. Plant Biomass
3.3.5. Graft Junction Scans
3.4. Nutrient and Carbohydrate Analysis
3.4.1. Foliar Nutrient Analysis
3.4.2. Starch and Chlorophyll
3.5. Phytophthora palmivora Lesions
3.5.1. Rootstock Lesions
3.5.2. Scion Canker Lesion
4. Discussion
4.1. Graft Incompatibilities Increase with Genetic Distance
4.2. Vigor Is a Complex Trait Reflecting Direct and Interactive Genetic Effects, as Well as the Environment
4.3. Rootstock and Scion Affect Foliar Nutrition in a Nutrient-Specific and Dynamic Manner
4.4. Phytophthora palmivora Resistance
5. Conclusions
- Evidence of both local and translocated graft incompatibilities in cacao is presented for the first time, and genetic distance and phenolic responses at the graft junction are proposed as further steps in understanding the incompatibility response. The accumulation of starches in the rootstock tissue below the graft junction and hypertrophy of rootstock with Criollo 22 scions, also presented for the first time, potentially indicate other complications of graft incompatibility that could impact the resilience or productivity of the scion over time. The role of cacao root systems in the storage and translocation of nonstructural carbohydrates should be further explored.
- This study demonstrated applications of rootstock in driving nutrient use efficiency and limiting heavy metal uptake, particularly in the first 4 months after grafting. The rootstock showed significant effects on potassium, magnesium, calcium, zinc, and copper 4 months after grafting and manganese, iron, and aluminum 24 and 30 months after grafting. Scion identity was significant in explaining foliar sodium at two of the three time points measured. Both rootstock and scion had significant effects on nitrogen, phosphorus, sodium, and boron.
- It was shown that seedling response to Phytophthora palmivora inoculations could be predictive of conferred resistance when used as a rootstock. This could help to expedite the screening of rootstock, but should be validated in field studies and with larger populations.
- Early-stage greenhouse studies of rootstock–scion interactions can be informative, but factors like microclimate and container effects may influence certain traits disproportionately when experiments last for multiple years. Leaf biomass was particularly sensitive to position-related microclimatic variation within the greenhouse. Although certain morphological traits—such as root, leaf, and branch biomass—appeared to converge toward limits potentially imposed by container size, trunk biomass and rootstock diameter continued to exhibit significant cultivar-specific variation throughout the study.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanaud, C.; Vignes, H.; Utge, J.; Valette, G.; Rhoné, B.; Garcia Caputi, M.; Angarita Nieto, S.; Fouet, O.; Gaikward, N.; Zarillo, S.; et al. A Revisited History of Cacao Domestication in Pre Columbian Times Revealed by Archaeo genomic Approaches. Sci. Rep. 2024, 14, 2972. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Variable Date. “FAOSTAT”. Available online: https://www.fao.org/faostat/en/ (accessed on 18 May 2021).
- Phillips Mora, W.; Arciniegas Leal, A.; Mata Quirós, A.; Motamayor Arias, J.C. Catalogue of Cacao Clones Selected by CATIE for Commercial Plantings; Tropical Agricultural Research and Higher Education (CATIE): Turrialba, Costa Rica, 2013. [Google Scholar]
- N’Zi, J.C.; Kahia, J.; Diby, L.; Kouamé, H. Compatibility of Ten Elite Cocoa (Theobroma cacao L.) Clones. Horticulturae 2017, 3, 45. [Google Scholar] [CrossRef]
- Susilo, A.W.; Anita Sari, I.; Setyawan, B. Yield Performance of Promising Cocoa Clones (Theobroma cacao L.) in Dry Climatic Conditions. Pelita Perkeb. 2020, 36, 24–31. [Google Scholar]
- Jaimez, R.E.; Barragan, L.; Fernández Niño, M.; Wessjohann, L.A.; Cedeño Garcia, G.; Cantos, I.S.; Arteaga, F. Theobroma cacao L. Cultivar CCN 51: A Comprehensive Review on Origin, Genetics, Sensory Properties, Production Dynamics, and Physiological Aspects. PeerJ 2020, 10, e12676. [Google Scholar] [CrossRef]
- Supplying New Cocoa Planting Material to Farmers: A Review of Propagation Methodologies; Laliberté, B., End, M., Sena Gomes, A.R., Andrade Sodré, G., Guiltinan, M., Lockwood, R., Maximova, S., Eds.; Bioversity International: Rome, Italy, 2015. [Google Scholar]
- Galvis, D.A.; Jaimes Suárez, Y.Y.; Rojas Molina, J.; Ruiz, R.; León Moreno, C.E.; Carvalho, F.E.L. Unveiling Cacao Root stock Genotypes with Potential Use in the Mitigation of Cadmium Bioaccumulation. Plants 2023, 12, 2941. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.A.R.; Phillips-Mora, W.; Arciniegas-Leal, A.; Mata-Quirós, A.; Haiminen, N.; Mustiga, G.; Iii, D.L.; van Bakel, H.; Kuhn, D.N.; Parida, L.; et al. Application of Genome Wide Association and Genomic Prediction for Improvement of Cacao Productivity and Resistance to Black and Frosty Pod Diseases. Front. Plant Sci. 2017, 8, 1905. [Google Scholar] [CrossRef] [PubMed]
- Jaimez, R.E.; Peña, G.; Barragán, L.; Chica, E.; Arteaga, F.; Cedeño, G. The Effect of Water Deficit on Leaf Stomatal Conductance, Water Relations, Chlorophyll Fluorescence and Growth of Rootstock Scion Combinations of Cacao. Sci. Hortic. 2023, 321, 112335. [Google Scholar] [CrossRef]
- DuVal, A.E. Sources of Tree to Tree Variability and Their Impact on Breeding and Production of Theobroma cacao L. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2019. [Google Scholar]
- Osorio Montoya, A.M.H.; de la Hoz Vasquez, T.; Urrea Trujillo, A.I. Propagation of IMC67 Plants, Universal Cacao (Theobroma cacao L.) Rootstock via Somatic Embryogenesis. Int. J. Fruit Sci. 2022, 22, 78–94. [Google Scholar] [CrossRef]
- Hunter, R.J. The Status of Cocoa (Theobroma cacao, Sterculiaceae) in the Western Hemisphere. Econ. Bot. 1990, 44, 425–439. [Google Scholar] [CrossRef]
- Yin, J.P.T. Rootstock Effects on Cocoa in Sabah, Malaysia. Exp. Agric. 2004, 40, 445–452. [Google Scholar] [CrossRef]
- Silva, S.D.V.M.; Pinto, L.R.M.; Oliveira, B.F.D.; Damaceno, V.O.; Pires, J.L.; Dias, C.T.D.S. Resistência de Progênies de Cacaueiro à Murcha de Ceratocystis. Trop. Plant Pathol. 2012, 37, 191–195. [Google Scholar] [CrossRef]
- Jaimez, R.; Loor, R.; Arteaga, F.; Márquez, V.; Tezara, W. Differential Response of Photosynthetic Activity, Leaf Nutrient Content and Yield to Long Term Drought in Cacao Clones. Acta Agronómica 2021, 70, 274–284. [Google Scholar] [CrossRef]
- Irizarry, H.; Goenaga, R. Clonal Selection in Cacao Based on Early Yield Performance of Grafted Trees. J. Agric. Univ. Puerto Rico 2000, 84, 153–162. [Google Scholar] [CrossRef]
- Irish, B.M.; Goenaga, R.; Zhang, D.; Schnell, R.; Brown, J.S.; Motamayor, J.C. Microsatellite Fingerprinting of the USDA ARS Tropical Agriculture Research Station Cacao (Theobroma cacao L.) Germplasm Collection. Crop Sci. 2010, 50, 656–667. [Google Scholar] [CrossRef]
- Delgadillo-Durán, P.; Soto-Suárez, M.; Rodriguez-Polanco, L.; Carrero-Gutierrez, M.; Torres-Rojas, E.; Yockteng, R. A new method for the inoculation of Phytophthora palmivora (Butler) into cacao seedlings under greenhouse conditions. Plant Methods 2020, 16, 114. [Google Scholar] [CrossRef]
- Sarmiento, S.; Gamboa, J.; Velásquez, J. Desempeño Agronómico de Tres Clones de Cacao en Fase de Vivero en la Amazonía Colombiana. Ing. Amazon. 2011, 4, 39–47. [Google Scholar]
- DuVal, A.; Gezan, S.A.; Mustiga, G.; Stack, C.; Marelli, J.-P.; Chaparro, J.; Livingstone, D.; Royaert, S.; Motamayor, J.C. Genetic Parameters and the Impact of Off Types for Theobroma cacao L. in a Breeding Program in Brazil. Front. Plant Sci. 2017, 8, 2059. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Motamayor, J.C.; Lachenaud, P.; da Silva e Mota, J.W.; Loor, R.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J. Geographic and Genetic Population Differentiation of the Amazonian Chocolate Tree (Theobroma cacao L). PLoS ONE 2008, 3, e3311. [Google Scholar] [CrossRef]
- Schmidt, J.E.; DuVal, A.; Puig, A.; Tempeleu, A.; Crow, T. Interactive and Dynamic Effects of Rootstock and Rhizobiome on Scion Nutrition in Cacao Seedlings. Front. Agron. 2021, 3, 754646. [Google Scholar] [CrossRef]
- Motamayor, J.C.; Mockaitis, K.; Schmutz, J.; Haiminen, N.; Iii, D.L.; Cornejo, O.; Findley, S.D.; Zheng, P.; Utro, F.; Royaert, S.; et al. The genome sequence of the most widely cultivated cacao type and its use to identify candidate genes regulating pod color. Genome Biol. 2013, 14, r53. [Google Scholar] [CrossRef]
- Argout, X.; Salse, J.; Aury, J.M.; Guiltinan, M.J.; Droc, G.; Gouzy, J.; Allegre, M.; Chaparro, C.; Legavre, T.; Maximova, S.N.; et al. The Genome of Theobroma cacao. Nat. Genet. 2011, 43, 101–108. [Google Scholar] [CrossRef]
- Morrissey, J.; Stack, J.C.; Valls, R.; Motamayor, J.C. Low-cost assembly of a cacao crop genome is able to resolve complex heterozygous bubbles. Hortic. Res. 2019, 6, 44. [Google Scholar] [CrossRef]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.S.S.; da Silva, A.S.A.; Ferreira Leitão, V.S.; da Silva Bon, E.P. Amino Acids Interference on the Quan-tification of Reducing Sugars by the 3,5 Dinitrosalicylic Acid Assay Mislead Carbohydrase Activity Measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 4 March 2025).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Endelman, J.B. Ridge Regression and Other Kernels for Genomic Selection with R Package rrBLUP. Plant Genome 2011, 4, 255–258. [Google Scholar] [CrossRef]
- Puig, A.S.; Quintanilla, W.; Matsumoto, T.; Keith, L.; Gutierrez, O.A.; Marelli, J.P. Phytophthora palmivora Causing Disease on Theobroma cacao in Hawaii. Agriculture 2021, 11, 396. [Google Scholar] [CrossRef]
- Puig, A.S.; Keith, L.M.; Matsumoto, T.K.; Gutierrez, O.A.; Marelli, J.P. Virulence Tests of Neofusicoccum parvum, Lasiodiplodia theobromae, and Phytophthora palmivora on Theobroma cacao. Eur. J. Plant Pathol. 2021, 159, 851–862. [Google Scholar] [CrossRef]
- Albrecht, U.; Meyering, B.; Bowman, K.D. Graft Compatibility of New Scion Rootstock Combinations. Citrus Industry, 10–13 November 2021. Available online: https://citrusindustry.net/2021/11/15/graft-compatibility-of-new-scion%e2%88%92rootstock-combinations/ (accessed on 6 June 2025).
- Adhikari, P.B.; Xu, Q.; Notaguchi, M. Compatible Graft Establishment in Fruit Trees and Its Potential Markers. Agronomy 2022, 12, 1981. [Google Scholar] [CrossRef]
- N’zi, J.C.; Koné, I.; M’bo, K.A.A.; Koné, S.; C. Kouamé, C. Successful Grafting Elite Cocoa Clones (Theobroma cacao L.) as a Function of the Age of Rootstock. Heliyon 2023, 9, e18732. [Google Scholar] [CrossRef]
- Gainza, F.; Opazo, I.; Muñoz, C. Graft incompatibility in plants: Metabolic changes during formation and establishment of the rootstock/scion union with emphasis on Prunus species. Chil. J. Agric. Res. 2015, 75, 28–34. [Google Scholar] [CrossRef]
- Loupit, G.; Cookson, S.J. Identifying Molecular Markers of Successful Graft Union Formation and Compatibility. Front. Plant Sci. 2020, 11, 610352. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, E.G.; Arghavan, S.; Fahadan, A.; Zamanipour, M. Possibility of early detection of graft incompatibility in some commercial plum cultivars by phenolic compounds analysis. J. Hortic. Sci. 2022, 17, 488–495. [Google Scholar] [CrossRef]
- Gamba, G.; Cisse, V.; Donno, D.; Razafindrakoto, Z.R.; Beccaro, G.L. Quali-Quantitative Study on Phenol Compounds as Early Predictive Markers of Graft Incompatibility: A Case Study on Chestnut (Castanea spp.). Horticulturae 2021, 8, 32. [Google Scholar] [CrossRef]
- Gamba, G.; Donno, D.; Akyüz, B.; Valera, B.C.; Beccaro, G.L. Insights into chestnut (Castanea spp.) graft incompatibility through the monitoring of chemical and physiological parameters. Planta 2025, 261, 1–14. [Google Scholar] [CrossRef]
- Canas, S.; Assunção, M.; Brazão, J.; Zanol, G.; Eiras Dias, J.E. Phenolic Compounds Involved in Grafting Incompatibility of Vitis spp.: Development and Validation of an Analytical Method for Their Quantification. Phytochem. Anal. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Mensah, P.; Melo, R.R.; Mitchual, S.J.; Govina, J.; Seidu, H. Characterisation and Utilisation of Theobroma cacao Stem Wood. Ghana J. For. 2021, 37, 79–99. [Google Scholar]
- Febres, V.J.; Fadli, A.; Meyering, B.; Yu, F.; Bowman, K.D.; Chaparro, J.X.; Albrecht, U. Dissection of Transcriptional Events in Graft Incompatible Reactions of ‘Bearss’ Lemon (Citrus limon) and ‘Valencia’ Sweet Orange (C. sinensis) on a Novel Citrandarin (C. reticulata × Poncirus trifoliata) Rootstock. Front. Plant Sci. 2024, 15, 1421734. [Google Scholar] [CrossRef]
- Cornejo, O.E.; Yee, M.C.; Dominguez, V.; Andrews, M.; Sockell, A.; Strandberg, E.; Livingstone, D.; Stack, C.; Romero, A.; Umaharan, P.; et al. Population Genomic Analyses of the Chocolate Tree, Theobroma cacao L., Provide Insights into Its Domestication Process. Commun. Biol. 2018, 1, 167. [Google Scholar] [CrossRef]
- Fernández Paz, J.; Cortés, A.J.; Hernández Varela, C.A.; Mejía de Tafur, M.S.; Rodriguez Medina, C.; Baligar, V.C. Root stock Mediated Genetic Variance in Cadmium Uptake by Juvenile Cacao (Theobroma cacao L.) Genotypes, and Its Effect on Growth and Physiology. Front. Plant Sci. 2021, 12, 777842. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Dorman, M.; Ward, A.; Firl, A. Rootstock, Scion, and Microbiome Contributions to Cadmium Mitigation in Five Indonesian Cocoa Cultivars. Pelita Perkeb. Coffee Cocoa Res. J. 2023, 39, 201–215. [Google Scholar] [CrossRef]
- de Almeida, N.M.; de Almeida, A.-A.F.; Santos, N.d.A.; Nascimento, J.L.D.; Neto, C.H.d.C.; Pirovani, C.P.; Ahnert, D.; Baligar, V.C. Scion-rootstock interaction and tolerance to cadmium toxicity in juvenile Theobroma cacao plants. Sci. Hortic. 2022, 300, 111086. [Google Scholar] [CrossRef]
- Resende, M.L.V.; Flood, J.; Ramsden, J.D.; Rowan, M.G.; Beale, M.H.; Cooper, R.M. Novel Phytoalexins Including Elemental Sulphur in the Resistance of Cocoa (Theobroma cocoa L.) to Verticillium Wilt (Verticillium dahliae Kleb.). Physiol. Mol. Plant Pathol. 1996, 48, 347–359. [Google Scholar] [CrossRef]
- Gattward, J.N.; Almeida, A.A.F.; Souza, J.O., Jr.; Gomes, F.P.; Kronzucker, H.J. Sodium–Potassium Synergism in Theobroma cacao: Stimulation of Photosynthesis, Water Use Efficiency and Mineral Nutrition. Physiol. Plant. 2012, 146, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Garg, N.; Garg, S.; Kumar, A. Starch phosphorylase: Role in starch metabolism and biotechnological applications. Crit. Rev. Biotechnol. 2009, 29, 214–224. [Google Scholar] [CrossRef]
- Ritte, G.; Scharf, A.; Eckermann, N.; Haebel, S.; Steup, M. Phosphorylation of Transitory Starch Is Increased during Degradation. Plant Physiol. 2004, 135, 2068–2077. [Google Scholar] [CrossRef]
- Lim, S.T.; Kasemsuwan, T.; Jane, J. Characterization of Phosphorous in Starch by 31P Nuclear Magnetic Resonance Spectroscopy. Cereal Chem. 1994, 71, 488–493. [Google Scholar]









| Trait | Variables | ChiSq | DF | Pr (>ChiSq) |
|---|---|---|---|---|
| Graft Take | Rootstock | 10.92 | 8 | 0.21 |
| Scion | 7.79 | 2 | 0.02 * | |
| Rootstock × Scion | 28.19 | 16 | 0.03 * | |
| Survival | Rootstock | 19.39 | 8 | 0.01 * |
| Scion | 11.8 | 2 | 0.003 ** | |
| Rootstock × Scion | 14.99 | 16 | 0.525 |
| Rootstock | Scion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Diameter below graft, 4 MAG | 8 | 251.6 | 31.5 | 5.9 | 3.96 × 10−6 *** | 2 | 23.1 | 11.6 | 1.6 | 0.208 |
| Diameter below graft, 16 MAG | 8 | 139.8 | 17.4 | 3.1 | 0.00459 ** | 2 | 15.7 | 7.8 | 1.2 | 0.318 |
| Diameter below graft, 30 MAG | 8 | 100.4 | 12.6 | 1.9 | 0.0925 | 2 | 32.8 | 16.4 | 2.2 | 0.117 |
| Δ Diameter below graft, 4–30 MAG | 8 | 72.56 | 9.1 | 1.5 | 0.189 | 2 | 77.5 | 38.7 | 7.5 | 0.00152 ** |
| Basal diameter, 16 MAG | 8 | 288.9 | 36.1 | 5.0 | 0.0036 ** | 2 | 14.4 | 7.2 | 0.7 | 0.49 |
| Basal Diameter, 30 MAG | 8 | 227.5 | 28.4 | 2.4 | 0.0322 * | 2 | 16.3 | 8.2 | 0.6 | 0.579 |
| Δ Basal diameter, 16–30 MAG | 8 | 192.3 | 24.0 | 2.6 | 0.0214 * | 2 | 36.5 | 18.23 | 1.6 | 0.213 |
| Scion diameter, 16 MAG | 8 | 79.8 | 10 | 1.3 | 0.25 | 2 | 20.3 | 10.2 | 1.3 | 0.276 |
| Scion diameter, 30 MAG | 8 | 36.5 | 4.6 | 0.5 | 0.823 | 2 | 21.4 | 10.7 | 1.4 | 0.264 |
| Δ Scion diameter, 16–30 MAG | 8 | 77.6 | 9.704 | 1.2 | 0.328 | 2 | 26.8 | 13.4 | 1.6 | 0.206 |
| Flush length, 30 MAG | 8 | 80 | 10 | 0.7 | 0.692 | 2 | 79.1 | 39.5 | 2.9 | 0.0593 |
| Leaf dry weight, 30 MAG | 8 | 5147.9 | 643.5 | 1.2 | 0.302 | 2 | 114 | 56.9 | 0.1 | 0.904 |
| Branch dry weight, 30 MAG | 8 | 7816.7 | 977.1 | 0.7 | 0.696 | 2 | 5648 | 2824 | 2.2 | 0.122 |
| Trunk dry weight 1, 30 MAG | 8 | 39,421.8 | 4927.7 | 2.3 | 0.0373 * | 2 | 1395 | 697.7 | 0.3 | 0.769 |
| Root dry weight, 30 MAG | 8 | 61,920 | 7740 | 1.3 | 0.255 | 2 | 14,814 | 7407 | 1.2 | 0.304 |
| Whole plant dry weight, 30 MAG | 8 | 233,617.4 | 29,202.2 | 1.1 | 0.397 | 2 | 28,546 | 14,273 | 0.5 | 0.603 |
| Rootstock | Scion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Nitrogen %—4 MAG | 8 | 4706 | 588.3 | 2.89 | 0.00628 ** | 2 | 1538 | 768.9 | 3.5 | 0.0354 * |
| Nitrogen %—24 MAG | 8 | 0.835 | 0.10435 | 1.089 | 0.383 | 2 | 0.041 | 0.02 | 0.204 | 0.816 |
| Nitrogen %—30 MAG | 7 | 1.192 | 0.1703 | 1.11 | 0.372 | 2 | 0.461 | 0.23 | 1.514 | 0.23 |
| Sulfur %—4 MAG | 8 | 0.0307 | 0.0038 | 1.563 | 0.154 | 2 | 0.00812 | 0.004 | 1.582 | 0.213 |
| Sulfur %—24 MAG | 8 | 0.1309 | 0.0163 | 0.922 | 0.505 | 2 | 0.0214 | 0.01068 | 0.6 | 0.552 |
| Sulfur %—30 MAG | 7 | 0.02021 | 0.0028 | 0.958 | 0.474 | 2 | 0.0169 | 0.008469 | 3.058 | 0.0562. |
| Phosphorus %—4 MAG | 8 | 0.02715 | 0.003394 | 3.064 | 0.00553 ** | 2 | 0.00852 | 0.00426 | 3.332 | 0.0413 * |
| Phosphorus %—24 MAG | 8 | 0.01712 | 0.002139 | 0.757 | 0.641 | 2 | 0.00228 | 0.001138 | 0.407 | 0.667 |
| Phosphorus %—30 MAG | 7 | 0.01397 | 0.001996 | 0.955 | 0.475 | 2 | 0.01137 | 0.005687 | 2.953 | 0.0617. |
| Potassium %—4 MAG | 8 | 1.93 | 0.2418 | 2.459 | 0.0218 * | 2 | 0.043 | 0.0214 | 0.183 | 0.833 |
| Potassium %—24 MAG | 8 | 3.438 | 0.4298 | 1.531 | 0.165 | 2 | 0.195 | 0.0974 | 0.32 | 0.727 |
| Potassium %—30 MAG | 7 | 0.37 | 0.05290 | 0.609 | 0.746 | 2 | 0.229 | 0.1145 | 1.417 | 0.252 |
| Magnesium %—4 MAG | 8 | 0.6075 | 0.07593 | 2.87 | 0.00991 ** | 2 | 0.0855 | 0.04277 | 1.329 | 0.271 |
| Magnesium %—24 MAG | 8 | 0.315 | 0.03938 | 1.335 | 0.244 | 2 | 0.0384 | 0.01922 | 0.62 | 0.541 |
| Magnesium %—30 MAG | 7 | 0.2792 | 0.03989 | 1.591 | 0.164 | 2 | 0.0459 | 0.02293 | 0.839 | 0.438 |
| Calcium %—4 MAG | 8 | 2.310 | 0.28874 | 5.023 | 7.36 × 10−5 *** | 2 | 0.086 | 0.04295 | 0.509 | 0.603 |
| Calcium %—24 MAG | 8 | 1.741 | 0.2177 | 1.753 | 0.104 | 2 | 0.268 | 0.134 | 0.992 | 0.376 |
| Calcium %—30 MAG | 7 | 0.635 | 0.09067 | 1.025 | 0.428 | 2 | 0.14 | 0.07003 | 0.782 | 0.463 |
| Sodium %—4 MAG | 8 | 0.000176 | 2.2 × 10−5 | 0.631 | 0.749 | 2 | 0.00033 | 0.0001682 | 5.685 | 0.00516 ** |
| Sodium %—24 MAG | 8 | 0.01689 | 0.002 | 2.34 | 0.0291 | 2 | 0.00123 | 0.0006147 | 0.582 | 0.561 |
| Sodium %—30 MAG | 7 | 0.001799 | 0.000257 | 1.49 | 0.196 | 2 | 0.001466 | 0.0007329 | 4.539 | 0.0156 * |
| Boron PPM—4 MAG | 8 | 8049 | 1006.1 | 8.227 | 1.38 × 10−7 *** | 2 | 1683 | 841.3 | 4.149 | 0.0198 * |
| Boron PPM—24 MAG | 8 | 845 | 105.6 | 0.945 | 0.487 | 2 | 46 | 22.85 | 0.201 | 0.818 |
| Boron PPM—30 MAG | 7 | 1380 | 197.1 | 1.196 | 0.326 | 2 | 447 | 223.3 | 1.336 | 0.272 |
| Zinc PPM—4 MAG | 8 | 82,281 | 10,285 | 6.567 | 3.15 × 10−6 *** | 2 | 5008 | 2504 | 0.987 | 0.378 |
| Zinc PPM—24 MAG | 8 | 9478 | 1184.7 | 1.81 | 0.0924 | 2 | 1164 | 582 | 0.808 | 0.45 |
| Zinc PPM—30 MAG | 7 | 3466 | 495.1 | 0.667 | 0.699 | 2 | 193 | 96.5 | 0.132 | 0.877 |
| Manganese PPM—4 MAG | 8 | 474,670 | 59,334 | 3.497 | 0.00208 ** | 2 | 18,489 | 9244 | 0.42 | 0.659 |
| Manganese PPM—24 MAG | 8 | 572,322 | 71,540 | 2.286 | 0.0327 * | 2 | 38,372 | 19,186 | 0.526 | 0.593 |
| Manganese PPM—30 MAG | 7 | 38,477 | 5497 | 0.726 | 0.651 | 2 | 10,075 | 5038 | 0.638 | 0.51 |
| Iron PPM—4 MAG | 8 | 190,087 | 23,761 | 17.85 | 1.13 × 10−13 *** | 2 | 3561 | 1781 | 0.459 | 0.634 |
| Iron PPM—24 MAG | 8 | 169,223 | 21,153 | 4.513 | 0.000241 *** | 2 | 8802 | 4401 | 0.661 | 0.52 |
| Iron PPM—30 MAG | 7 | 26,433 | 3776 | 1.646 | 0.148 | 2 | 2351 | 1176 | 0.46 | 0.634 |
| Copper PPM—4 MAG | 8 | 30.07 | 3.759 | 1.906 | 0.0744 | 2 | 10.31 | 5.155 | 2.471 | 0.0918 |
| Copper PPM—24 MAG | 8 | 188.6 | 23.580 | 3.933 | 0.000841 *** | 2 | 1.9 | 0.962 | 0.117 | 0.89 |
| Copper PPM—30 MAG | 7 | 39.71 | 5.672 | 0.869 | 0.539 | 2 | 13.58 | 6.79 | 1.062 | 0.354 |
| Aluminum PPM—4 MAG | 8 | 1727 | 215.87 | 4.439 | 0.000258 *** | 2 | 49 | 24.65 | 0.36 | 0.699 |
| Aluminum PPM—24 MAG | 8 | 1621 | 202.6 | 0634 | 0.746 | 2 | 290 | 144.9 | 0.466 | 0.629 |
| Aluminum PPM—30 MAG | 7 | 2728 | 389.7 | 7.947 | 3.62 × 10−6 *** | 2 | 31 | 15.3 | 0.153 | 0.859 |
| Chlorophyll—30 MAG | 8 | 232,528 | 29,066 | 1.7 | 0.103 | 2 | 70,207 | 35,104 | 2.008 | 0.138 |
| Starch—30 MAG | 8 | 299.3 | 37.41 | 0.68 | 0.707 | 2 | 34.1 | 17.07 | 0.318 | 0.729 |
| Population | N | Lesion Area (cm2) | Std Error |
|---|---|---|---|
| BYNC | 3 | 1.8 | 0.74 |
| PA 107 | 5 | 2.18 | 0.9 |
| GNV 360 | 6 | 4.76 | 2.42 |
| T 294 | 9 | 6.04 | 2.06 |
| SCA 6 | 16 | 7.85 | 6.17 |
| T 484 | 7 | 12.1 | 3.29 |
| EQX 3348 | 8 | 15.77 | 7.5 |
| T 384 | 5 | 26.4 | 18.98 |
| p = 0.072 |
| Scion | Rootstock | N | Lesion Area (cm2) | Std Error |
|---|---|---|---|---|
| Matina 1/6 | BYNC | 4 | 0.72 a | 0.13 |
| Matina 1/6 | SCA 6 | 3 | 2.12 b | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DuVal, A.E.; Tempeleu, A.; Schmidt, J.E.; Puig, A.; Knollenberg, B.J.; Chaparro, J.X.; Stevens, M.E.; Motamayor, J.C. Evidence of Graft Incompatibility and Rootstock Scion Interactions in Cacao. Horticulturae 2025, 11, 899. https://doi.org/10.3390/horticulturae11080899
DuVal AE, Tempeleu A, Schmidt JE, Puig A, Knollenberg BJ, Chaparro JX, Stevens ME, Motamayor JC. Evidence of Graft Incompatibility and Rootstock Scion Interactions in Cacao. Horticulturae. 2025; 11(8):899. https://doi.org/10.3390/horticulturae11080899
Chicago/Turabian StyleDuVal, Ashley E., Alexandra Tempeleu, Jennifer E. Schmidt, Alina Puig, Benjamin J. Knollenberg, José X. Chaparro, Micah E. Stevens, and Juan Carlos Motamayor. 2025. "Evidence of Graft Incompatibility and Rootstock Scion Interactions in Cacao" Horticulturae 11, no. 8: 899. https://doi.org/10.3390/horticulturae11080899
APA StyleDuVal, A. E., Tempeleu, A., Schmidt, J. E., Puig, A., Knollenberg, B. J., Chaparro, J. X., Stevens, M. E., & Motamayor, J. C. (2025). Evidence of Graft Incompatibility and Rootstock Scion Interactions in Cacao. Horticulturae, 11(8), 899. https://doi.org/10.3390/horticulturae11080899










