Selenium Nanoparticles Improve Morpho-Physiological and Fruit Quality Parameters of Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Plant Growth and Fruit Yield
2.3. Chlorophyll Content
2.4. Leaf Gas Exchange
2.5. Leaf Osmotic Adjustment
2.6. Fruit Quality Parameters
2.7. Bioactive Compounds
2.8. Statistical Analysis
3. Results
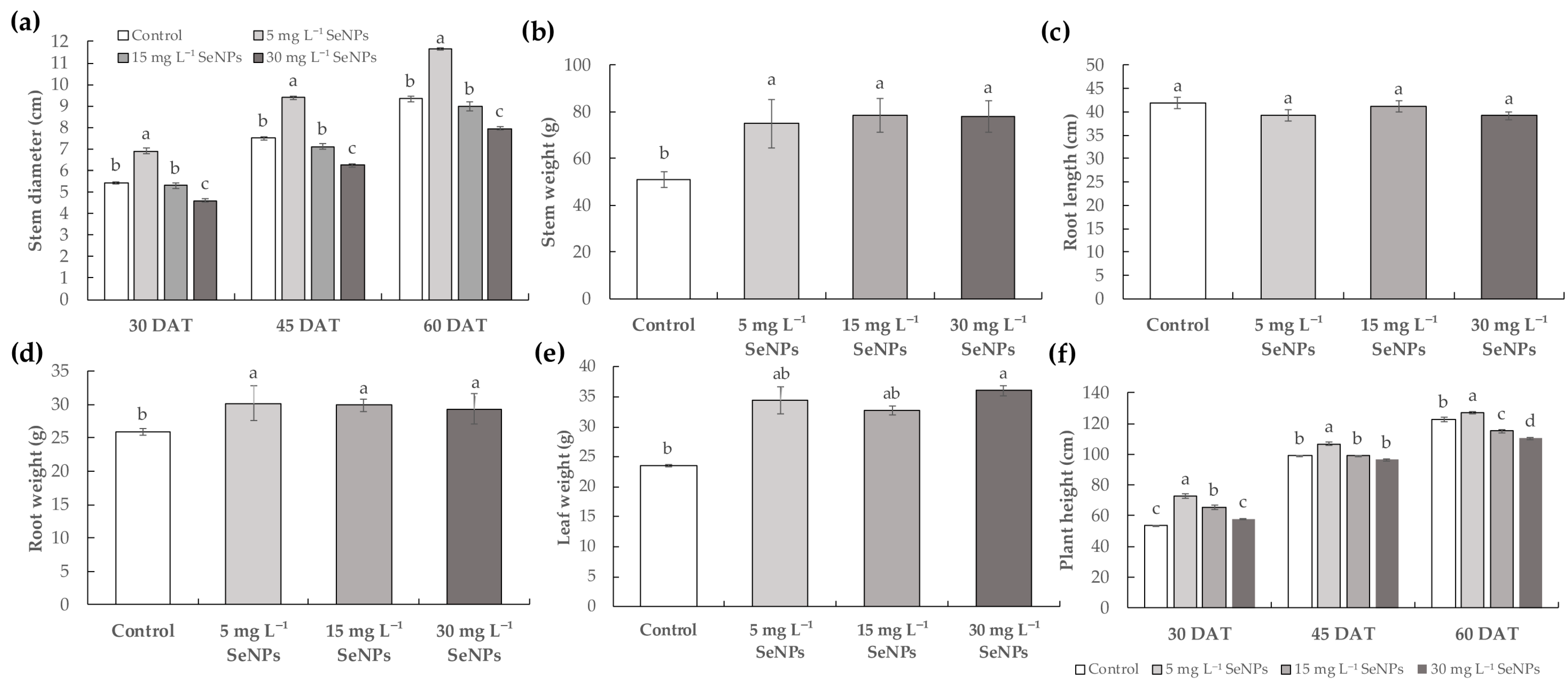
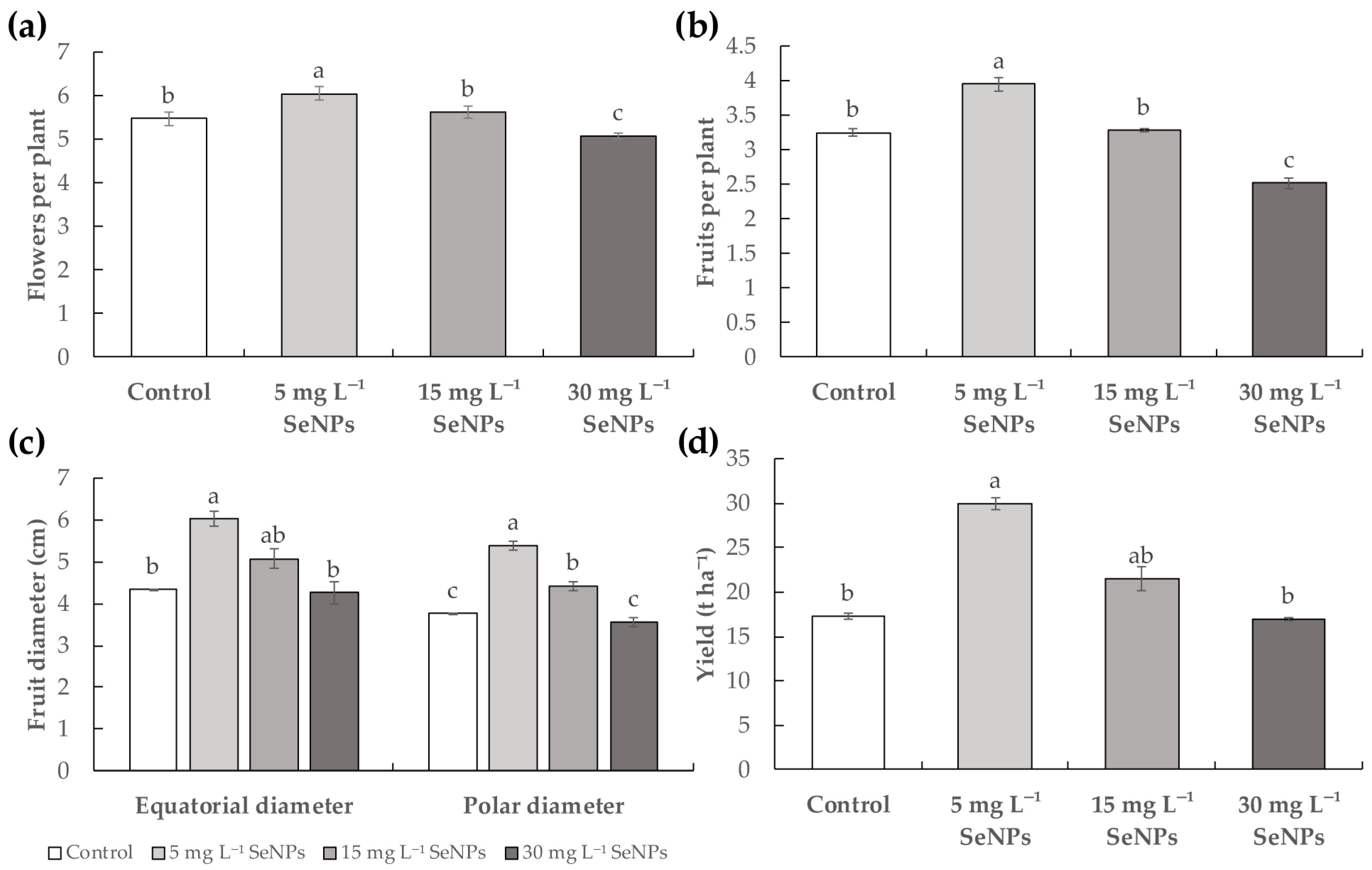
3.1. Plant Growth and Fruit Yield
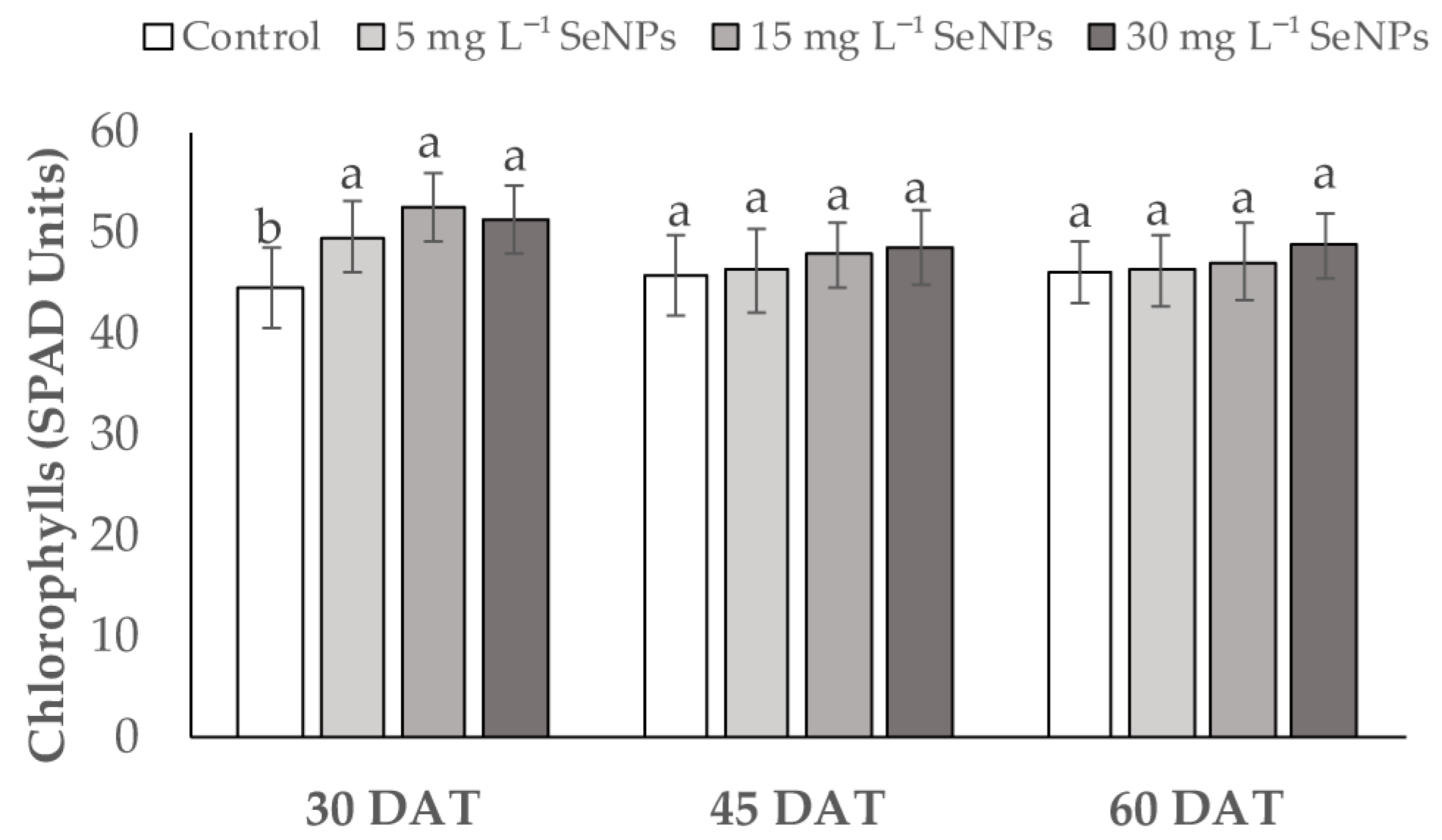
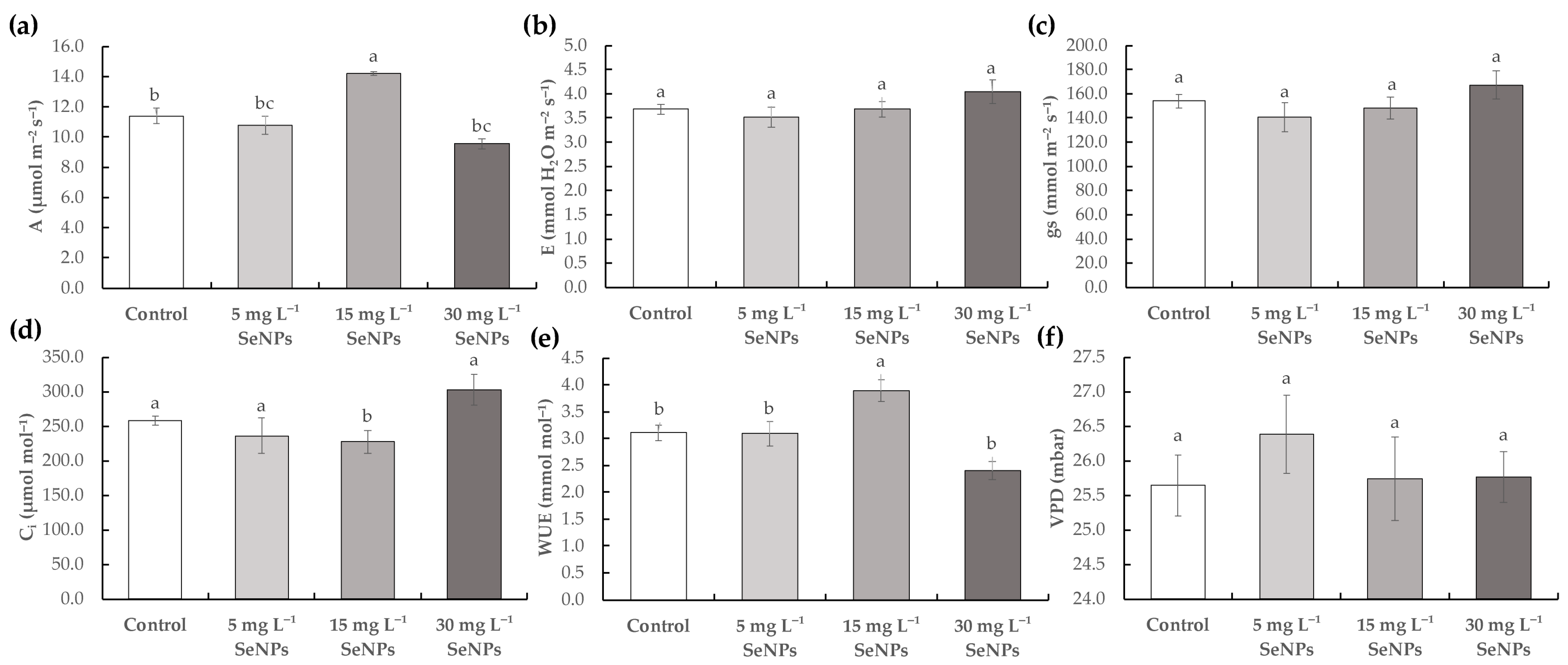
3.2. Physiological Parameters
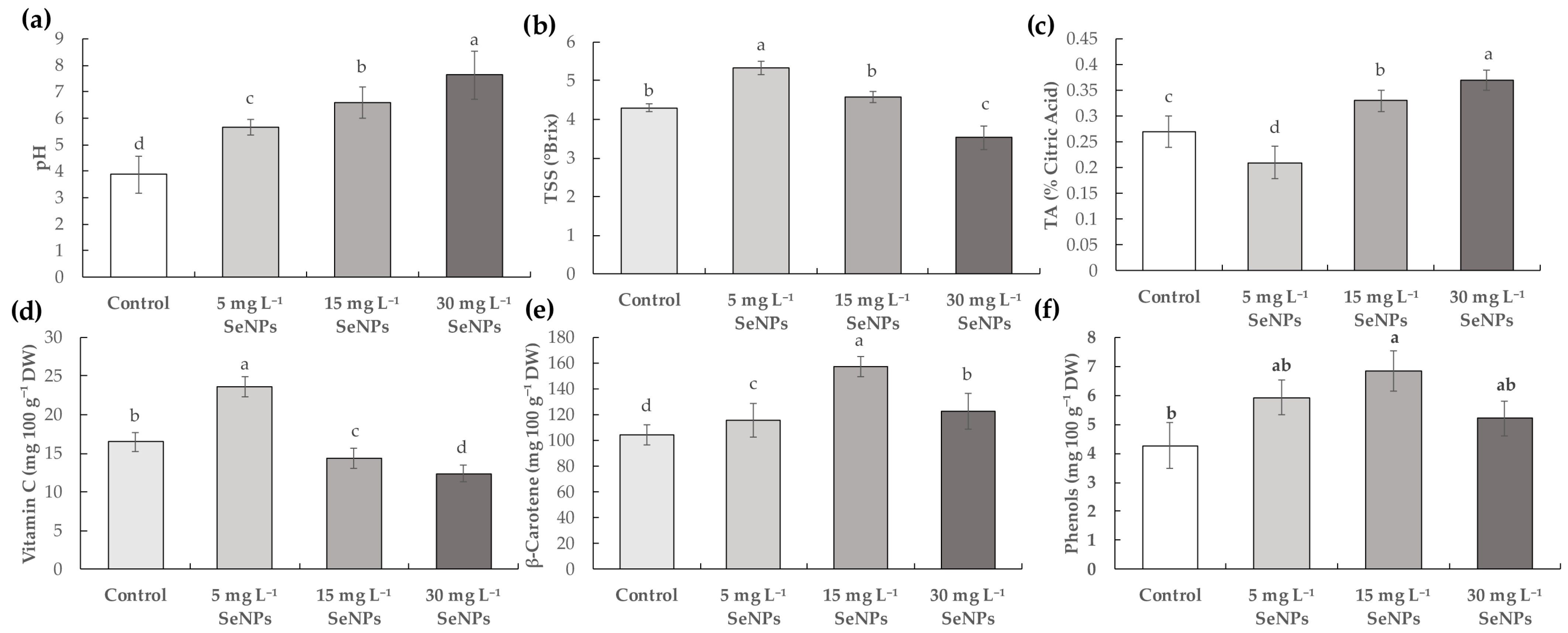
3.3. Fruit Quality and Bioactive Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vats, S.; Bansal, R.; Rana, N.; Kumawat, S.; Bhatt, V.; Jadhav, P.; Kale, V.; Sathe, A.; Sonah, H.; Jugdaohsingh, R.; et al. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 1003–1034. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.L.; Patel, N.; Rani, L.; Kumar, P.; Dutt, I.; Maddodi, B.S.; Chaudhary, V.K. Sustainable options for fertilizer management in agriculture to prevent water contamination: A review. Environ. Dev. Sustain. 2023, 26, 8303–8327. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Abdollahdokht, D.; Gao, Y.; Faramarz, S.; Poustforoosh, A.; Abbasi, M.; Asadikaram, G.; Nematollahi, M.H. Conventional agrochemicals towards nano-biopesticides: An overview on recent advances. Chem. Biol. Technol. Agric. 2022, 9, 13. [Google Scholar] [CrossRef]
- Terán, F.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Facing climate change: Plant stress mitigation strategies in agriculture. Physiol. Plant. 2024, 176, e14484. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pérez, J.J.; Tipán-Torres, H.C.; Llerena-Ramos, L.T.; Hernandez-Montiel, L.G.; Rivas-Garcia, T. Silicon increased the growth, productivity, and nutraceutical quality of tomato (Solanum lycopersicum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13155. [Google Scholar] [CrossRef]
- Shiriaev, A.; Pezzarossa, B.; Rosellini, I.; Malorgio, F.; Lampis, S.; Ippolito, A.; Tonutti, P. Efficacy and Comparison of Different Strategies for Selenium Biofortification of Tomatoes. Horticulturae 2022, 8, 800. [Google Scholar] [CrossRef]
- Gundala, R.R.; Singh, A. What motivates consumers to buy organic foods? Results of an empirical study in the United States. PLoS ONE 2021, 16, e0257288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhu, X.; Zheng, G.; Meng, G.; Dong, Z.; Baek, J.H.; Jeon, C.O.; Yao, Y.; Xuan, Y.H.; Zhang, J.; et al. Development of biofertilizers for sustainable agriculture over four decades (1980–2022). Geogr. Sustain. 2024, 5, 19–28. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Narvaéz-Ortiz, W.A. Selenium and Nano-Selenium as a New Frontier of Plant Biostimulant. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 41–54. [Google Scholar]
- Cheng, B.; Liu, J.; Li, X.; Yue, L.; Cao, X.; Li, J.; Wang, C.; Wang, Z. Bioavailability of selenium nanoparticles in soil and plant: The role of particle size. Environ. Exp. Bot. 2024, 220, 105682. [Google Scholar] [CrossRef]
- Ikram, S.; Li, Y.; Lin, C.; Yi, D.; Heng, W.; Li, Q.; Tao, L.; Hongjun, Y.; Weijie, J. Selenium in plants: A nexus of growth, antioxidants, and phytohormones. J. Plant Physiol. 2024, 296, 154237. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The role of selenium and nano selenium on physiological responses in plant: A review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, M.; Xu, J.; Xu, F.; Zhang, W. The application of organic selenium (SeMet) improve the photosynthetic characteristics, yield and quality of hybrid rice. Plant Physiol. Biochem. 2024, 208, 108457. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.S.; Aslam, M.U.; Valipour, M.; Iqbal, R.; Haider, I.; Mustafa, A.E.Z.M.A.; Elshikh, M.S.; Ali, I.; Roy, R.; Elshamly, A.M.S. Seed priming with selenium improves growth and yield of quinoa plants suffering drought. Sci. Rep. 2024, 14, 886. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Rahmat, S.; Zeinalzadeh, N.; Farsad-Akhtar, N.; Hosseinpour-Feizi, M.A. Senescence is delayed by selenium in oilseed rape plants. J. Trace Elem. Med. Biol. 2019, 55, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Li, Q.; Xian, L.; Yuan, L.; Lin, Z.; Chen, X.; Wang, J.; Li, T. The use of selenium for controlling plant fungal diseases and insect pests. Front. Plant Sci. 2023, 14, 1102594. [Google Scholar] [CrossRef] [PubMed]
- Somagattu, P.; Chinnannan, K.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Selenium dynamics in plants: Uptake, transport, toxicity, and sustainable management strategies. Sci. Total Environ. 2024, 949, 175033. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Nedjimi, B. Selenium as a powerful trace element for mitigation of plant salt stress: A review. J. Trace Elem. Miner. 2024, 8, 100123. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol. Trace Elem. Res. 2022, 200, 2528–2548. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Shanmugam, V.K. Chemopreventive mechanism of action by oxidative stress and toxicity induced surface decorated selenium nanoparticles. J. Trace Elem. Med. Biol. 2020, 62, 126549. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K. Nanoparticle Size Comparison. Available online: https://app.biorender.com/biorender-templates/details/t-65fb245c3cb8c55fa6777d6e-nanoparticle-size-comparison (accessed on 2 June 2025).
- García-Locascio, E.; Valenzuela, E.I.; Cervantes-Avilés, P. Impact of seed priming with Selenium nanoparticles on germination and seedlings growth of tomato. Sci. Rep. 2024, 14, 6726. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; De La Fuente, M.C.; Valdés-Reyna, J.; Juárez-Maldonado, A. Impact of Selenium and Copper Nanoparticles on Yield, Antioxidant System, and Fruit Quality of Tomato Plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Javad, S.; Iqbal, S.; Shahzadi, K.; Gatasheh, M.K.; Javed, T. Alleviation potential of green-synthesized selenium nanoparticles for cadmium stress in Solanum lycopersicum L: Modulation of secondary metabolites and physiochemical attributes. Plant Cell Rep. 2024, 43, 113. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Qin, H.; Wang, G.; Lei, B.; Yang, X.; Zhong, M. Selenium Nanoparticles Mitigate Cadmium Stress in Tomato through Enhanced Accumulation and Transport of Sulfate/Selenite and Polyamines. J. Agric. Food Chem. 2024, 72, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, B.; Liu, Y.; Pan, C.; Zhou, Z.; Diao, J.; Zhang, Y. Selenium nanoparticle alleviates penthiopyrad-induced oxidative stress and restores the development and flavor quality of tomato fruit. J. Food Compos. Anal. 2024, 130, 106142. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- (USDA), U.S.D. of A. United States Standards for Grades of Fresh Tomatoes. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Standard%5B1%5D.pdf (accessed on 22 July 2024).
- Reyes-Pérez, J.J.; Llerena-Ramos, L.T.; Hernández-Montiel, L.G.; Reynel-Chila, V.H.; Tezara, W.; Rivas-García, T. Chitosan improves morpho-physiological, rooting atributes and profitability of two cocoa (Theobroma cacao L.) varieties during vegetative propagation. Trop. Subtrop. Agroecosyst. 2023, 26, 4761. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC; Methods 932.06, 925.09, 985.29, 923.03; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Levine, M.; Katz, A.; Padayatty, S.J.; Wang, Y.; Eck, P.; Kwon, O.; Chen, S.; Lee, J.H. Vitamin C. In Encyclopedia of Dietary Supplements; Marcel Dekker: New York, NY, USA; pp. 745–755. ISBN 0824755030.
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Cumplido-Nájera, C.F.; González-Morales, S.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. 2019, 245, 82–89. [Google Scholar] [CrossRef]
- Shahdadi, F.; Mirzaei, H.O.; Daraei Garmakhany, A. Study of phenolic compound and antioxidant activity of date fruit as a function of ripening stages and drying process. J. Food Sci. Technol. 2015, 52, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gawad, F.A.E.; Ali, E.A.E.; Karunanithi, S.; Yugiani, P.; Srivastav, P.P. Nanotechnology: Current applications and future scope in food packaging systems. Meas. Food 2024, 13, 100131. [Google Scholar] [CrossRef]
- Javaid, A.; Hameed, S.; Li, L.; Zhang, Z.; Zhang, B.; Rahman, M.U. Can nanotechnology and genomics innovations trigger agricultural revolution and sustainable development? Funct. Integr. Genom. 2024, 24, 216. [Google Scholar] [CrossRef] [PubMed]
- El-Moneim, D.A.; Dawood, M.F.A.; Moursi, Y.S.; Farghaly, A.A.; Afifi, M.; Sallam, A. Positive and negative effects of nanoparticles on agricultural crops. Nanotechnol. Environ. Eng. 2021, 6, 21. [Google Scholar] [CrossRef]
- Singh, T.; Singh, A.; Wang, W.; Yadav, D.; Kumar, A.; Singh, P.K. Biosynthesized Nanoparticles and Its Implications in Agriculture. In Biological Synthesis of Nanoparticles and Their Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 257–274. [Google Scholar] [CrossRef]
- Rehmanullah; Muhammad, Z.; Inayat, N.; Majeed, A. Application of nanoparticles in agriculture as fertilizers and pesticides: Challenges and opportunities. In New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; pp. 281–293. [Google Scholar] [CrossRef]
- Ahmad, A.; Javad, S.; Iqbal, S.; Shahid, T.; Naz, S.; Shah, A.A.; Shaffique, S.; Gatasheh, M.K. Efficacy of soil drench and foliar application of iron nanoparticles on the growth and physiology of Solanum lycopersicum L. exposed to cadmium stress. Sci. Rep. 2024, 14, 27920. [Google Scholar] [CrossRef] [PubMed]
- El-Batal, A.I.; Sidkey, N.M.; Ismail, A.A.; Arafa, R.A.; Fathy, R.M. Impact of Silver and Selenium Nanoparticles Synthesized by Gamma Irradiation and Their Physiological Response on Early Blight Disease of Potato. J. Chem. Pharm. Res. 2016, 8, 934–951. [Google Scholar]
- Domokos-Szabolcsy, E.; Marton, L.; Sztrik, A.; Babka, B.; Prokisch, J.; Fari, M. Accumulation of red elemental selenium nanoparticles and their biological effects in Nicotinia tabacum. Plant Growth Regul. 2012, 68, 525–531. [Google Scholar] [CrossRef]
- Sariñana-Navarrete, M.d.l.Á.; Morelos-Moreno, Á.; Sánchez, E.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Preciado-Rangel, P. Selenium Nanoparticles Improve Quality, Bioactive Compounds and Enzymatic Activity in Jalapeño Pepper Fruits. Agronomy 2023, 13, 652. [Google Scholar] [CrossRef]
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in Research on the Toxicological Effects of Selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Shanmugam, R.; Lakshmi, T. A review on plant-mediated selenium nanoparticles and its applications. J. Popul. Ther. Clin. Pharmacol. J. Ther. Popul. Pharmacol. Clin. 2022, 28, e29–e40. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction With Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.Y.; Rao, S.; Huang, X.; Liu, X.; Cheng, S.; Xu, F. Interaction between selenium and essential micronutrient elements in plants: A systematic review. Sci. Total Environ. 2022, 853, 158673. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wei, Y.; Sun, S.; Wang, J.; Wang, W.; Han, D.; Shao, H.; Jia, H.; Fu, Y. Selenium Modulates the Level of Auxin to Alleviate the Toxicity of Cadmium in Tobacco. Int. J. Mol. Sci. 2019, 20, 3772. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; de Paiva Gonçalves, J.; Siqueira, J.A.; Martins, A.O.; Ribeiro, D.M.; Nunes-Nesi, A.; Zsögön, A.; Araújo, W.L. Auxin metabolism and the modulation of plant growth. Environ. Exp. Bot. 2024, 226, 105917. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of Chlorophyll, Carotenoid and SPAD-502 Measurement to Salinity and Nutrient Stress in Wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Zsiros, O.; Nagy, V.; Párducz, Á.; Nagy, G.; Ünnep, R.; El-Ramady, H.; Prokisch, J.; Lisztes-Szabó, Z.; Fári, M.; Csajbók, J.; et al. Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth. Res. 2019, 139, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [PubMed]
- Tryfon, P.; Sperdouli, I.; Moustaka, J.; Adamakis, I.D.S.; Giannousi, K.; Dendrinou-Samara, C.; Moustakas, M. Hormetic Response of Photosystem II Function Induced by Nontoxic Calcium Hydroxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 8350. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt Stress Inhibits Photosynthesis and Destroys Chloroplast Structure by Downregulating Chloroplast Development–Related Genes in Robinia pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, R.; Seyedi, A.; Esmaeilizadeh, M.; Seyedi, N.; Morteza Zahedi, S.; Malekzadeh, M.R. Improving the performance of the photosynthetic apparatus of Citrus sinensis with the use of chitosan-selenium nanocomposite (CS + Se NPs) under salinity stress. BMC Plant Biol. 2024, 24, 745. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Ayala, D.; Tapia, G.; Ferrio, J.P. Leaf Carbon and Water Isotopes Correlate with Leaf Hydraulic Traits in Three Solanum Species (S. peruvianum, S. lycopersicum and S. chilense). Agriculture 2023, 13, 525. [Google Scholar] [CrossRef]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Natl. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Chatelet, D.S.; Pasquet-Kok, J.; Rawls, M.; Donoghue, M.J.; Edwards, E.J.; Sack, L. Hydraulic basis for the evolution of photosynthetic productivity. Nat. Plants 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- McKown, A.D.; Cochard, H.; Sack, L. Decoding leaf hydraulics with a spatially explicit model: Principles of venation architecture and implications for its evolution. Am. Nat. 2010, 175, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Wu, H.; Yuan, Q.; Wang, J.; Cui, J.; Lin, A. Effects of selenium fertilizer application and tomato varieties on tomato fruit quality: A meta-analysis. Sci. Hortic. 2022, 304, 111242. [Google Scholar] [CrossRef]
- Zhu, F.; Wen, W.; Cheng, Y.; Fernie, A.R. The metabolic changes that effect fruit quality during tomato fruit ripening. Mol. Hortic. 2022, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Estrada, Y.; Flores, F.B.; Bolarín, M.C. Improving production and fruit quality of tomato under abiotic stress: Genes for the future of tomato breeding for a sustainable agriculture. Environ. Exp. Bot. 2022, 204, 105086. [Google Scholar] [CrossRef]
- Pinedo-Guerrero, Z.H.; Delia Hernández-Fuentes, A.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Cu Nanoparticles in Hydrogels of Chitosan-PVA Affects the Characteristics of Post-Harvest and Bioactive Compounds of Jalapeño Pepper. Molecules 2017, 22, 926. [Google Scholar] [CrossRef] [PubMed]
- Saffan, M.M.; Koriem, M.A.; El-Henawy, A.; El-Mahdy, S.; El-Ramady, H.; Elbehiry, F.; Omara, A.E.D.; Bayoumi, Y.; Badgar, K.; Prokisch, J. Sustainable Production of Tomato Plants (Solanum lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper. Sustainability 2022, 14, 3236. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, T.; Kuromori, T.; Takeuchi, Y.; Yamaji, N.; Yokosho, K.; Shimazawa, A.; Sugimoto, E.; Omote, H.; Ma, J.F.; Shinozaki, K.; et al. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat. Commun. 2015, 6, 5928. [Google Scholar] [CrossRef] [PubMed]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Pérez, J.J.; Rivas-García, T.; Llerena-Ramos, L.T.; Ramos-Remache, R.A.; Vásquez Cortez, L.H.; Preciado-Rangel, P.; Martínez-Camacho, R.A. Selenium Nanoparticles Improve Morpho-Physiological and Fruit Quality Parameters of Tomato. Horticulturae 2025, 11, 876. https://doi.org/10.3390/horticulturae11080876
Reyes-Pérez JJ, Rivas-García T, Llerena-Ramos LT, Ramos-Remache RA, Vásquez Cortez LH, Preciado-Rangel P, Martínez-Camacho RA. Selenium Nanoparticles Improve Morpho-Physiological and Fruit Quality Parameters of Tomato. Horticulturae. 2025; 11(8):876. https://doi.org/10.3390/horticulturae11080876
Chicago/Turabian StyleReyes-Pérez, Juan José, Tomás Rivas-García, Luis Tarquino Llerena-Ramos, Rommel Arturo Ramos-Remache, Luis Humberto Vásquez Cortez, Pablo Preciado-Rangel, and Rubí A. Martínez-Camacho. 2025. "Selenium Nanoparticles Improve Morpho-Physiological and Fruit Quality Parameters of Tomato" Horticulturae 11, no. 8: 876. https://doi.org/10.3390/horticulturae11080876
APA StyleReyes-Pérez, J. J., Rivas-García, T., Llerena-Ramos, L. T., Ramos-Remache, R. A., Vásquez Cortez, L. H., Preciado-Rangel, P., & Martínez-Camacho, R. A. (2025). Selenium Nanoparticles Improve Morpho-Physiological and Fruit Quality Parameters of Tomato. Horticulturae, 11(8), 876. https://doi.org/10.3390/horticulturae11080876







