Abstract
Melastoma dodecandrum is primarily propagated through stem cuttings, which limits genetic variation and constrains breeding efforts. To overcome this limitation and facilitate molecular breeding, the establishment of a reliable and efficient regeneration system is essential. This study investigated the effects of plant growth regulators (PGRs) and culture media on the in vitro regeneration system of M. dodecandrum. The highest rate of callus induction (96.67%) was achieved when sterile leaf explants were cultured on Murashige and Skoog (MS) basal medium supplemented with 2.00 mg·L−1 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.50 mg·L−1 6-benzylaminopurine (6-BA). For callus differentiation, the optimal formulation of MS + 2.0 mg·L−1 6-BA + 0.5 mg·L−1 naphthylacetic acid (NAA) resulted in a differentiation frequency of 83.33%. The optimal PGR combinations for shoot proliferation was 1.5 mg·L−1 6-BA + 0.1 mg·L−1 NAA. The optimal rooting media was MS medium supplemented with 0.1, 0.2, or 0.5 mg·L−1 indole-3-butyric acid (IBA) or 1/2MS medium supplemented with 0.5 mg·L−1 IBA. Additionally, this study investigated the dynamic changes in endogenous hormones during the regeneration process. The levels and ratios of hormones, including gibberellin (GA3), abscisic acid (ABA), indole-3-acetic acid (IAA), and zeatin (ZT), collectively regulated the regeneration process. Elevated levels of ABA and GA3 may promote callus initiation as well as the growth and development of adventitious roots during the early induction stage. Reduced levels of ABA and IAA favored callus differentiation into shoots, whereas elevated GA3 levels facilitated proliferation of adventitious shoots. Throughout the regeneration process, fluctuations in ZT levels remained relatively stable. This study successfully established an in vitro regeneration system for M. dodecandrum using leaf explants, providing theoretical guidance and technical support for further molecular breeding efforts, genetic transformation, and industrial development.
1. Introduction
Melastoma dodecandrum Lour is a perennial creeping shrub belonging to the Melastomataceae family. Characterized by dense leaves that lie flat on the ground, it forms a smooth and compact ground cover. This species exhibits shade tolerance and resistance to trampling, making it a popular choice for landscaping as ground cover or potted plants [1]. In addition, the whole plant of M. dodecandrum has medicinal value [2,3,4], with demonstrated pharmacological activities such as hypoglycemic, analgesic, and anti-inflammatory effects [5,6,7], indicating broad potential for application in the pharmaceutical field.
Currently, M. dodecandrum is primarily propagated asexually through stem cuttings to enhance propagation efficiency and shorten the cultivation cycle [8]. However, this method facilitates only quantitative multiplication and fails to introduce genetic variation, particularly in flower-color traits, thereby acting as a major bottleneck in its further breeding and utilization [9]. To overcome this limitation, genetic transformation and other modern molecular breeding strategies are required for the improvement of floral and other agronomic traits. A well-established tissue-culture system is essential for successful genetic manipulation, as it provides a stable platform for transformation [10]. Previous studies on the tissue culture of M. dodecandrum have mainly focused on using stem segments or axillary buds as explants to enhance the efficiency of adventitious bud induction, proliferation, and rooting [11,12,13,14]. However, these explants have limited availability and require complex procedures, making them unsuitable for the large-scale regeneration needed in genetic-transformation protocols. In contrast, leaf explants are abundant, easy to disinfect, and possess strong regenerative capacity, making them highly promising candidates for use in tissue culture [15]. Nevertheless, studies on callus induction and differentiation from M. dodecandrum leaf explants remain scarce, and an efficient and reliable regeneration system using leaf tissues has not yet been established.
Plant regenerative capacity is influenced by multiple factors, notably explant type, composition of the culture medium, and the ratios of concentrations of plant growth regulators (PGRs) [16,17,18]. The use of appropriate PGRs further enhances regeneration efficiency, with the combination and concentration of auxins and cytokinins being particularly critical for optimizing regeneration outcomes [19]. Melastomataceae species typically employ 6-BA with NAA to induce adventitious bud differentiation and proliferation [11,12,13]. Using nodal segment explants of M. dodecandrum, Zhang and Xu demonstrated optimal differentiation on MS medium supplemented with 0.5 mg·L−1 6-BA and 0.05 mg·L−1 NAA, achieving an approximate propagation coefficient of 5 [14]. PGRs modulate endogenous hormone levels during regeneration. Using enzyme-linked immunosorbent assays (ELISA) [20], Li et al. quantified endogenous hormones during callus induction in Pyrus betulifolia Bunge, revealing significant interplay between PGRs and endogenous hormones [21]. However, the specific mechanisms by which endogenous hormones regulate regeneration remain poorly understood and warrant further investigation.
This study aimed to establish a complete and efficient callus-mediated regeneration system for M. dodecandrum and to analyze the dynamic changes in endogenous hormone levels during the regeneration process. These findings contribute to elucidating the regulatory roles of endogenous hormones in organogenesis and provide a theoretical foundation for the development of a stable genetic-transformation system in M. dodecandrum.
2. Materials and Methods
2.1. Experimental Materials
Plant specimens were obtained from M. dodecandrum plants in the germplasm resource nursery at Fujian Agriculture and Forestry University (Fujian, China). Sampling was carried out in April using young, newly emerged leaves. After immersion in detergent solution for 10 min, samples were rinsed under running tap water (1 h) and then subjected to three sterile water washes. Final sterilization was performed under laminar airflow by treatment with 2% NaClO for 8 min. After they had been rinsed in sterile water, explants were aseptically trimmed to 1.0 × 1.0 cm segments. Murashige and Skoog (MS) [22] and half-strength MS (1/2MS) salts—including macronutrients, micronutrients, and vitamins—supplemented with 30 g/L sucrose, 7 g/L agar, and the appropriate PGRs composed the basal media. Finally, the pH of the medium was adjusted to 5.8 and the medium was sterilized at 121 °C for 20 min. The control group (CK) consisted of MS medium without any hormones. The tissue-culture room ws maintained at a temperature of 25 ± 1 °C, with a light cycle of 12 h/day and a light intensity of 2000 ± 300 lx. Stock solutions of 2,4-D, 6-BA, NAA, and IBA (1 mg·mL−1) were filter-sterilized and stored at −20 °C. Working media were supplemented with calculated volumes to achieve target concentrations. All chemicals and reagents used in this study were purchased from Solarbio Company (Beijing, China).
2.2. Screening of Callus-Induction Media
Tender leaves of M. dodecandrum served as explants and were cultured on MS basal medium. Nine callus-induction media were prepared by combining 6-BA (0.1, 0.5, and 1.0 mg·L−1) with 2,4-D (1.0, 1.5, and 2.0 mg·L−1). Each treatment consisted of six culture bottles, each containing five explants, for a total of 30 explants per treatment. Three independent replicate tests were performed. Each set of 30 explants was treated as an independent statistical unit in the analysis. The development of calluses was observed weekly, and the induction rates were determined after a 30-day cultivation period using the following equation: callus-induction rate (%) = (number of leaves producing callus/total inoculated leaves) × 100%.
2.3. Screening of Callus-Differentiation Media
The optimal callus was subsequently transferred to differentiation media containing MS medium supplemented with eight specific combinations of PGRs: 6-BA (0.5, 1.0, 1.5, and 2.0 mg·L−1) and NAA (0.3 and 0.5 mg·L−1), resulting in eight formulations of differentiation media. Each treatment consisted of six culture bottles, each containing five explants, for a total of 30 explants per treatment. Three independent replicate tests were performed. Each set of 30 explants was treated as an independent statistical unit in the analysis. Callus-differentiation rates were recorded after 40 days with the following equation: callus-differentiation rate (%) = (number of calluses producing adventitious buds/total inoculated calluses) × 100%.
2.4. Screening of Adventitious Bud Proliferation Media
Uniform and healthy adventitious bud clusters were aseptically transferred to proliferation media containing MS basal medium supplemented with eight combinations of 6-BA (0.5, 1.0, 1.5, and 2.0 mg·L−1) and NAA (0.1 and 0.2 mg·L−1), forming eight proliferation-media treatments. Each treatment consisted of six culture bottles, each containing five explants, for a total of 30 explants per treatment. Three independent replicate tests were performed. Each set of 30 explants was treated as an independent statistical unit in the analysis. After a period of thirty days, the proliferation coefficient of adventitious buds was calculated with the following equation: adventitious bud proliferation coefficient = (weight of adventitious buds per bottle after 30 days/initial weight of adventitious buds per bottle at inoculation.
2.5. Screening of Adventitious Bud Rooting Medium
Healthy plantlets approximately 3 cm in height that had been obtained from this differentiation stage were transferred to rooting media. MS and 1/2MS basal media were supplemented with IBA (0.1, 0.2, and 0.5 mg·L−1), while the control media contained no PGRs. Each treatment consisted of three culture bottles, each containing ten explants, for a total of 30 explants per treatment. Three independent replicate tests were performed. Each set of 30 explants was treated as an independent statistical unit in the analysis. After 30 days, rooting rate, average root number, and average root length were recorded with the following equation: rooting rate (%) = number of rooted plantlets/total inoculated plantlets × 100%; average root number = total number of roots per bottle/number of plantlets per bottle; average root length = total root length per bottle/number of roots per bottle.
2.6. Determination of Plant Hormone Content
Endogenous hormone levels (GA3, IAA, ZT, ABA) were quantified by ELISA during callus induction (0, 7, 14, 21, 28 days), callus differentiation (0, 10, 20, 30, 40 days), adventitious shoot proliferation (0, 7, 14, 21, 28 days), and rooting (0, 7, 14, 21, 28 days). The ELISA kits used for quantification of IAA, GA3, ABA, and ZT were purchased from Xiangzhiyuan Biotechnology Development Co., Ltd. (Fuzhou, Fujian Province, China). The assay sensitivities were 0.1 ng/mL for IAA and ZT and 0.05 ng/mL for GA3 and ABA. Absorbance measurements were conducted using a BioTek Synergy HTX multi-mode microplate reader (BioTek Instruments, Winooski, VT, USA). Standard curves were established using serial dilutions of the standard solutions supplied with each kit, and endogenous hormone concentrations in the samples were calculated based on the linear regression equations derived from the respective standard curves. All concentrations of endogenous hormone were first calculated in ng/g fresh weight, and the ratios (e.g., GA3/ZT, ABA/IAA) were calculated directly based on these untransformed concentration values without further normalization.
2.7. Data Analysis
Data were recorded using Excel 2010. Data were analyzed using one-way ANOVA after verification of normality (Shapiro–Wilk test, p > 0.05) and homogeneity of variances (Levene’s test, p > 0.05). For statistically significant ANOVA results (p ≤ 0.05), post hoc comparisons were performed with Duncan’s multiple range test. The significance threshold was set at α = 0.05. All analyses were conducted at the explant level (n = 30 per treatment). All statistical analyses were performed using IBM SPSS Statistics 27 (IBM Corp, Armonk, NY, USA). Averages and standard deviations were calculated, and graphs were generated using GraphPad Prism 8 software.
3. Results
3.1. Impact of Various Combinations of PGRs on the Induction of Callus Tissue
Callus was induced in media supplemented with varying concentrations and ratios of 2,4-D and 6-BA. The earliest and most robust callus formation was observed in media containing 0.5 mg·L−1 6-BA and 2.0 mg·L−1 2,4-D. In contrast, no callus development was detected in hormone-free medium (control), in which the leaves eventually browned and died (Table 1, Figure 1). These results demonstrate that both the concentration and ratio of 6-BA and 2,4-D are critical determinants of callus induction. In addition, through continuous observation of callus types from different culture media, it was found that the yellowish-green callus type had a dense structure (Figure 1A), vigorous growth, and a high potential for adventitious bud differentiation at later stages. The yellowish-white and reddish-brown callus tissues displayed loose structures and failed to produce adventitious buds subsequently. Some callus tissues also developed red granules, which continue to expand over time. These callus types were more susceptible to browning (Figure 1B) andd eventual necrosis. Yellowish-white callus types, though compact, showed impaired differentiation capacity in bud-induction medium (Figure 1F). Overall, callus cultured on medium containing 2.0 mg·L−1 2,4-D and 0.5 mg·L−1 6-BA exhibited superior morphological characteristics and a greater capacity for differentiation of adventitious buds (Figure 1G).

Table 1.
Effect of PGRs on callus induction.

Figure 1.
Different types of callus. (A): yellow-green, compact (Y7, Y8, Y9); (B): browned; (C): reddish-brown, loose (Y1, Y2, Y3); (D): yellowish-white with red granules, loose (Y4, Y5); (E): yellow-green, whitish, compact callus (Y6); (F): yellow-green whitish, compact (Y6, placed in the differentiation medium after 25 days); (G): yellow-green, compact (Y8, placed in the differentiation medium after 25 days); bar = 1 cm.
3.2. Effects of Different Combinations of PGRs on Callus Differentiation
As shown in Table 2, the addition of 0.50 mg·L−1 NAA to the medium, combined with varying concentrations of 6-BA, resulted in an initial increase in callus differentiation rates, although these rates then declined with increasing 6-BA concentration. The highest differentiation rate was observed in treatment group F7. During the first week of culture, callus tissues on media supplemented with PGR combinations showed minimal morphological changes. Subsequently, the callus expanded and proliferated, with green bud primordia becoming visible (Figure 2A). After 25 days, adventitious buds began to differentiate, showing distinct leaves and stems (Figure 2B). Between 30 and 40 days, the adventitious buds grew rapidly, with elongated stems, darker leaves, and the formation of multiple seedling clusters (Figure 2C). In hormone-free medium, the callus failed to differentiate (Figure 2D) and eventually browned and died. Therefore, the optimal combination of PGRs for callus differentiation was determined to be 2.0 mg·L−1 6-BA and 0.5 mg·L−1 NAA.

Table 2.
Effects of different PGR treatments on callus differentiation.

Figure 2.
Callus differentiation. (A): 20th day of differentiation; (B): 25th day of differentiation; (C): 40th day of differentiation; (D): 20th day after treatment for the control group’s callus; bar = 0.5 cm.
3.3. Effects of Different Combinations of PGRs on Adventitious Bud Proliferation
As shown in Table 3, all treatment groups (except Z8) supplemented with varying concentrations of NAA and 6-BA exhibited significantly higher proliferation coefficients (p < 0.05) and greater adventitious bud mass compared to the control group (CK). The highest proliferation coefficient was observed in treatment Z3, which had been treated with 1.5 mg·L−1 6-BA and 0.1 mg·L−1 NAA. After 30 days, the volumes of adventitious shoots and proliferation coefficients in treatment Z3 (Figure 3B) were noticeably larger than those of the control group (CK) (Figure 3C).

Table 3.
Effect of PGRs on adventitious bud proliferation.

Figure 3.
Adventitious bud proliferation. (A): The 0th day of adventitious bud proliferation; (B): the 30th day of the adventitious buds of Z3; (C): the 30th day of the adventitious buds of CK; (D): the 30th day of the adventitious buds of Z5; bar = 0.5 cm.
Therefore, the optimal combination of PGRs for shoot proliferation was 1.5 mg·L−1 6-BA + 0.1 mg·L−1 NAA.
3.4. Effects of Different Combinations of PGRs on Adventitious Bud Rooting
In MS medium, treatments supplemented with IBA produced significantly more roots, longer root lengths, and higher rooting rates compared to the control group CK1 (p < 0.05), as shown in Table 4 and Table 5. In 1/2 MS medium, the application of 0.5 mg·L−1 IBA resulted in a 100% rooting rate, with significantly number of roots averaging 7.35 cm, compared to CK2 (80.99% rooting rate) (p < 0.05). Additionally, no callus formation was observed at the stem base, and the roots were thicker and more robust (Figure 4 and Figure 5).

Table 4.
Effect of PGRs on adventitious bud rooting.

Table 5.
Effect of PGRs on adventitious bud rooting.

Figure 4.
Adventitious bud rooting in MS basic medium 30 days later; bar = 1 cm. Note: In the table, “CK” is the control group and “S1–S3”represent treatments 1 to 3 with different concentrations of IBA added.
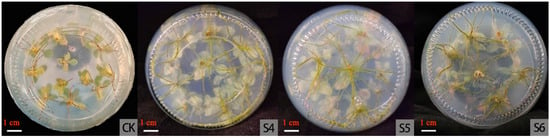
Figure 5.
Adventitious bud rooting in 1/2MS basic medium 30 days later; bar = 1 cm. Note: In the table, “CK” is the control group and “S4–S6”represent treatments 1 to 3 with different concentrations of IBA added.
Therefore, the optimal rooting media were MS medium supplemented with 0.1, 0.2, or 0.5 mg·L−1 IBA and 1/2 MS medium supplemented with 0.5 mg·L−1 IBA.
3.5. Changes of Endogenous Hormones on Callus Induction
As shown in Figure 6A, GA3 levels peaked on days 7 (134.73 ng·g−1) and 21 (127.94 ng·g−1), with both measurements significantly exceeding the baseline level observed on day 0 (p < 0.05), and ABA reached a peak on day 14 (122.78 ng·g−1) that was significantly higher than the baseline level of 89.89 ng·g−1 (p < 0.05). It then gradually declined. IAA exhibited a sustained upward trend, with concentrations significantly exceeding the initial minimum value (14.69 ng·g−1) (p < 0.05). Conversely, ZT maintained consistently low levels without significant variation throughout the induction period (p > 0.05). As shown in Figure 6B, ABA/ZT ratios increased during the early induction stage (0–14 days), and then declined during the mid-to-late stage (14–28 days). Both the ABA/IAA and GA3/IAA ratios exhibited continuous decreases across the entire induction period. The GA3/ZT ratio exhibited distinct phase-dependent fluctuations. It increased from day 0 to day 7, decreased between days 7 and 14, rose again from days 14 to 21, and declined during days 21 to 28.
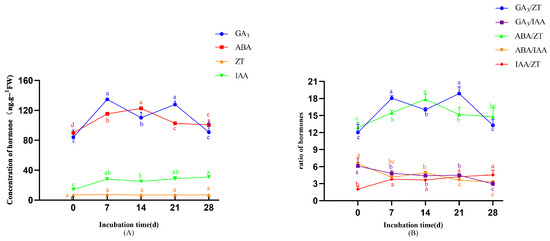
Figure 6.
The changes in endogenous hormones during callus induction. (A) Changes in endogenous hormone during callus induction. (B) Changes in endogenous hormone ratio during callus induction. Note: Different lowercase letters above bars/points indicate significant differences (p < 0.05).
3.6. Changes of Endogenous Hormones on Callus Differentiation
As shown in Figure 7A, GA3 displayed a bimodal pattern characterized by sequential increase–decrease–increase–decrease cycles. ABA content exhibited a significant biphasic trend, with an initial decline followed by a sustained increase starting on day 20—the peak differentiation stage—reaching 94.68 ng·g−1, which was significantly less than in earlier stages (p < 0.05). Concurrently, IAA levels first decreased and then increased, reaching their lowest point on day 20 (29.93 ng·g−1, p < 0.05). In contrast, ZT concentrations remained largely unchanged at basal levels. Figure 7B reveals critical hormonal ratio dynamics: the ratios of the four endogenous (GA3, ABA, IAA, and ZT) hormones exhibited distinct trends during callus differentiation. Both ABA/ZT and IAA/ZT ratios decreased from day 0 to 20 and then increased from day 20 to 40. The GA3/ZT ratio displayed a biphasic pattern, rising initially, then falling, followed by a second increase and subsequent decline. In contrast, GA3/IAA and ABA/IAA ratios showed relatively minor fluctuations throughout the observation period.
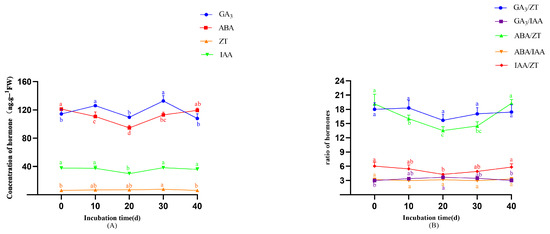
Figure 7.
The changes in levels of endogenous hormones during callus differentiation. (A) Changes in endogenous hormone during callus differentiation. (B) Changes in endogenous hormone ratio during callus differentiation. Note: Different lowercase letters above bars/points indicate significant differences (p < 0.05).
3.7. Changes of Endogenous Hormones on Adventitious Bud Proliferation
During the early proliferation stage (0–14 days), GA3 concentrations increased significantly, peaking on day 14 (147.62 ng·g−1) at a level significantly higher than those at other time points (p < 0.05). ABA displayed a unimodal pattern, reaching its maximum on day 21 (119.33 ng·g−1) (Figure 8A). IAA concentrations showed complex oscillations (decrease-increase-decrease-increase) with an overall downward trend, culminating in lower levels on day 28 (18.92 ng·g−1) compared to day 0 (22.85 ng·g−1) (p < 0.05). In contrast, ZT maintained stable concentrations throughout, without temporal variation (p > 0.05). As shown in Figure 8B, the dynamics of endogenous hormone ratios revealed several distinct trends. GA3/ZT increased during days 0–14, then declined. GA3/IAA exhibited an initial rise followed by progressive reduction. ABA/IAA fluctuated cyclically (increase–decrease–increase–decrease), peaking on day 21. ABA/ZT followed an overall decreasing trajectory. IAA/ZT oscillated in a decrease–increase–decrease–increase pattern.
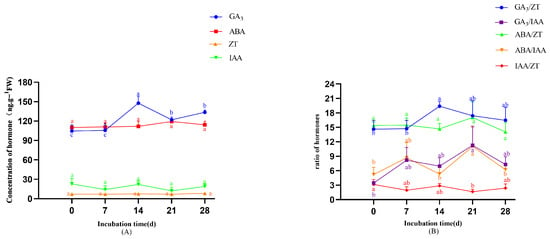
Figure 8.
The changes in endogenous hormones during adventitious bud proliferation. (A) Changes in endogenous hormone during adventitious bud proliferation. (B) Changes in endogenous hormone ratio during adventitious bud proliferation. Note: Different lowercase letters above bars/points indicate significant differences (p < 0.05).
3.8. Changes of Endogenous Hormones on the Rooting of Adventitious Buds
As shown in Figure 9A, ABA concentrations during adventitious root formation followed a decrease-increase-decrease trajectory, reaching a significant minimum on day 14 (97.78 ng·g−1) (p < 0.05 compared to preceding days). GA3 exhibited a unimodal pattern, peaking on day 7 (163.35 ng·g−1), with levels significantly higher than at other time points (p < 0.05). IAA levels were increased significantly on day 28 compared to day 0 (p < 0.05). However, the overall increase was modest and gradual, with similar values observed on day 21. ZT levels displayed biphasic variation during days 0–14 (increase followed by decrease), with day 14 concentrations differing significantly from earlier measurements (p < 0.05). Figure 9B revealed stage-specific ratio dynamics in root development, as follows: the GA3/ZT ratio exhibited an initial increase followed by a decline; the ABA/ZT ratio showed a decreasing trend days on 0–7 and 14–21 but an increased during days 7–14 and 21–28. The IAA/ZT ratio displayed a steady upward trend throughout the rooting process. In contrast, the GA3/IAA and ABA/IAA ratios remained relatively stable, with only minor fluctuations observed.
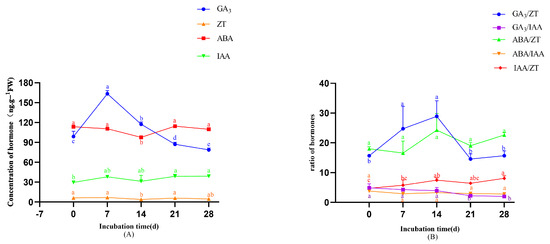
Figure 9.
Changes in endogenous hormones during rooting. (A) Changes in endogenous hormones during rooting. (B) Changes in the ratios of endogenous hormones during rooting. Note: Different lowercase letters above bars/points indicate significant differences (p < 0.05).
4. Discussion
The appropriate concentrations of auxins and cytokinins are critical for efficient callus induction and differentiation. A high cytokinin-to-auxin ratio favors bud formation, whereas a low ratio promotes root development. When these PGRs are properly balanced, callus morphology is maintained [23]. For instance, Yang Wei et al. demonstrated that the optimal PGRs combination for inducing the formation of leaf callus in Hippeastrum ‘Bangkok Rose’ was 2 mg·L−1 2,4-D combined with 0.5 mg·L−1 TDZ, with no callus formation observed in medium lacking 2,4-D [24]. Similarly, Wang Hui et al. found that combining 6-BA and 2,4-D was more effective for callus induction in Polygonatum cyrtonema than a combination of 6-BA and NAA [25]. In this study, the highest callus-induction rate (96.67%) in M. dodecandrum leaf explants was achieved using a combination of 2,4-D and 6-BA. Endogenous hormones, although present in low concentrations, are small-molecule compounds that play pivotal roles in regulating various plant physiological processes [26]. Research indicates that during callus induction, auxin levels progressively increase, with IAA serving as a crucial endogenous hormone for this process. The IAA content exhibited an overall upward trend throughout callus induction, significantly exceeding its level on day 0 (p < 0.05). This suggests that a relatively high IAA concentration is required to sustain callus growth. Studies on Momordica charantia and Actinidia deliciosa also report that elevated endogenous IAA levels facilitate callus induction [27,28]. GA3 levels during callus induction exhibited a biphasic pattern, with peaks at both Day 7 and Day 21, significantly exceeding baseline levels (p < 0.05). This temporal profile suggests elevated GA3 accumulation may be required during the pre-induction and rapid-growth periods of callus growth. Conversely, ABA concentrations followed a unimodal trajectory, peaking on Day 14 at a level representing a significant increase compared to Day 0 (p < 0.05) and indicating potential roles in early callus initiation. Additionally, we observed the emergence and gradual expansion of red granules in some leaf-derived calli during induction. These calli with red granules failed to differentiate adventitious buds. It is hypothesized that the accumulation of red granules such as anthocyanins may impede bud regeneration, although the underlying mechanisms require further investigation. This phenomenon, also observed in Melastoma sanguineum [13], may be linked to hormone ratios or light exposure. In addition, Liu Yalan et al. reported similar red pigmentation in late-stage callus cultures of Vaccinium bracteatum [29], attributing it to the production of red granules (anthocyanins) and other leaf-derived compounds. However, the precise cause of this pigmentation remains an area for further investigation.
This study demonstrated that callus failed to differentiate and eventually died in hormone-free medium. Tian Xuping et al. observed in their research on Dracocephalum rupestre callus-induced adventitious buds that high concentrations of 6-BA and TDZ promoted differentiation but also caused vitrification and morphological abnormalities in regenerated shoots as the concentrations increased [30]. For M. dodecandrum, the optimal PGR combination for callus differentiation was 2.0 mg·L−1 6-BA + 0.5 mg·L−1 NAA, which is consistent with previous findings in Melastoma species, where higher 6-BA and lower NAA concentrations resulted in optimal differentiation efficiency [7,31]. Similarly, this study found that adventitious bud differentiation rates increased with rising 6-BA concentrations when NAA was maintained at a low level (0.3 mg·L−1), aligning with results from D. rupestre (Table 2). Plant callus differentiation is characterized by a gradual decline in endogenous auxin levels and a concurrent rise in cytokinin content [32]. During the differentiation phase in M. dodecandrum, both GA3 and ABA remained elevated, highlighting their critical roles in this process—a trend consistent with studies on callus differentiation in Welsh onion (Allium fistulosum) [33] and moringa (Moringa oleifera) [34]. ABA and IAA concentrations exhibited an initial decline followed by a rebound, reaching their nadir during the peak differentiation period of callus tissue. These minimal levels differed significantly from those in other stages (p < 0.05), indicating that endogenous ABA and IAA serve as key regulators governing the differentiation of callus into adventitious buds.
Adventitious bud proliferation is critical for the mass propagation of tissue-cultured seedlings. Jia Wei et al. reported that in Indigofera tinctoria, increasing NAA concentrations inhibited adventitious bud proliferation. The optimal proliferation medium was MS supplemented with 1.0 mg·L−1 6-BA and 0.1 mg·L−1 NAA [35]. This suggests that appropriately reducing 6-BA and NAA concentrations favors proliferation. In this study, a combination of 1.5 mg·L−1 6-BA + 0.1 mg·L−1 NAA resulted in a higher proliferation coefficient, indicating that lower PGR concentrations effectively enhance proliferation—a trend consistent with findings in Anemone silvestris [36]. Analysis of endogenous hormones revealed asynchronous fluctuations during adventitious bud proliferation in M. dodecandrum. Among the quantified hormones, GA3 exhibited the greatest variation, followed by IAA, indicating their potential regulatory roles in shoot proliferation. Notably, relatively low levels of IAA and ZT were associated with enhanced induction and proliferation of clustered buds, which aligns with findings in Lagerstroemia indica ‘Ziqi’ [37]. In contrast, studies on Lonicera macranthoides demonstrated that higher endogenous GA3 and lower ABA levels promote subculture proliferation and clustered bud differentiation [38]. This partially contrasts with the present findings, in which both the GA3/IAA ratio and the ABA/IAA ratio on dayincreased from day 14 to day 21, peaked on day 21, and then declined from day 21 to day 28. These results indicate that relatively high GA3/IAA and ABA/IAA ratios during the vigorous proliferation phase promote adventitious bud proliferation, whereas lower ratios are required in the later stages of proliferation culture.
Adventitious root formation is a critical step in the success of plant-regeneration systems. Wang Jianzhao observed that IBA was more effective than NAA in inducing the formation of adventitious roots in Camellia japonica. He proposed that IBA more efficiently activates endogenous auxin-related gene expression during root initiation, promoting the accumulation of secondary metabolites conducive to rooting and enhancing adventitious root formation in tissue-cultured seedlings [39]. This finding aligns with the results of Reshi et al., who demonstrated similar effects in Salix tetrasperma root induction [40]. Li et al. reported in Toona sinensis that excessively high or low IBA concentrations negatively affected rooting. Excessive IBA led to the formation of white flocculent structures on stems, poor bud growth, and excessive callus formation at the stem base [41]. Previous studies on Melastoma species identified 1/2MS medium as optimal for rooting [6,7,31]. In this study, the optimal medium combination for M. dodecandrum rooting were MS medium supplemented with 0.1, 0.2, and 0.5 mg·L−1 IBA or 1/2 MS medium supplemented with 0.5 mg·L−1 IBA. Auxins are the primary hormones driving root growth in tissue culture, with exogenous hormones influencing root development by modulating endogenous hormone levels [42]. This study revealed that high levels of GA3, IAA/ZT, and IAA, coupled with low ZT content, favored root growth and development in M. dodecandrum. During root primordia initiation, strong auxin responses were observed in cells and were accompanied by increased endogenous IAA levels to stimulate root elongation. After primordia differentiation, IAA levels rose again, indicating the continued requirement for sustained root growth—consistent with findings in Lagerstroemia indica [43] and Thuja occidentalis [44]. Notably, root primordia induction necessitated elevated GA3 and GA3/ZT levels, which later declined to facilitate root maturation. ABA is typically regarded as an inhibitor of rooting in tissue culture, suppressing cell division and elongation [45]. However, this study found that ABA levels increased during root development. This increase may be associated with post-rooting IAA accumulation, as auxin response factors (e.g., ARF2) may upregulate ABI transcription, potentially activating ABA signaling pathways [46].
5. Conclusions
This study successfully established an efficient in vitro regeneration system for M. dodecandrum through callus induction, achieving a callus-induction rate of 96.67%. Moreover, this study is the first to elucidate the dynamic changes in endogenous hormone levels during the regeneration process of M. dodecandrum and to investigate their regulatory roles in this process. It is proposed that endogenous hormones GA3, ABA and IAA have a significant influence on the plant-regeneration system. In addition, the established regeneration system provides a robust foundation for further research on development, genetic transformation, and molecular breeding of M. dodecandrum.
Author Contributions
Conceptualization, D.P.; methodology, S.W., R.T. and F.W.; software, S.W., Y.P., Y.D. and L.X.; validation, S.W., D.Z. and J.C.; formal analysis, S.W. and D.Z.; investigation, S.W. and R.T.; resources, S.W. and F.W.; data curation, S.W., Y.D. and L.X.; writing—original draft preparation, S.W.; writing—review and editing, S.W.; visualization, D.Z.; supervision, D.P.; project administration, D.P.; funding acquisition, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “The Innovation and Application Engineering Technology Research Center of Ornamental Plant Germplasm Resources in Fujian Province” (PTJH16005), “Straits Flower Industry Science and Technology Innovation Hub” (KJG24054A).
Data Availability Statement
Data are contained within the article.
Acknowledgments
We acknowledge the technical support of laboratory staff during the conduction of laboratory experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cong, L.; Han, D. Ecological characteristics of high quality wild ornamental plants in South China. Isr. J. Ecol. Evol. 2020, 67, 98–105. [Google Scholar] [CrossRef]
- Lai, W.; Wang, Y.; Huang, C.; Xu, H.; Zheng, X.; Li, K.; Wang, J.; Lou, Z. DIREN mitigates DSS-induced colitis in mice and attenuates collagen deposition via inhibiting the Wnt/β-catenin and focal adhesion pathways. Biomed. Pharmacother. 2024, 175, 116671. [Google Scholar] [CrossRef]
- García-Chacón, J.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; González-Miret, M.L.; Osorio, C. Characterization and bioaccessibility assessment of bioactive compounds from camu-camu (Myrciaria dubia) powders and their food applications. Food Res. Int. 2024, 176, 113820. [Google Scholar] [CrossRef]
- Qian, S.; Wang, Z.; Ma, X.; Ming, H.; Liu, J.; Yang, J.; Chen, T.; Liu, L.; Ban, J.; Cuo, J.; et al. Study on the Active Components and Inhibiting Effect of Melastoma dodecandrum Lour. on Human Cervical Cancer Cells Based on Spectrum–Effect Relationship Analysis. Nat. Prod. Commun. 2024, 19, 1934578X231222096. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Liu, Y.; Paul, B.; Xu, Y.; Chen, W. Physicochemical and antioxidant properties of set-type yogurt supplemented by lyophilized water-soluble Melastoma dodecandrum extract-bearded chitosan-coated nutriosomes. Food Hydrocoll. 2024, 146 Pt B, 109311. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, J.; Shao, L.; Liu, S.; Zhou, J.; Mei, M.; Zhang, Z. Screening of active components of Melastoma dodecandrum Lour. against diabetic osteoporosis using cell membrane chromatography-mass spectrometry. Front. Pharmacol. 2024, 15, 1450154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, Y.; Liang, J.; Sun, D.; Li, H.; Chen, L. Polyphenolics and triterpenoids from the whole herbs of Melastoma dodecandrum Lour. and their anti-inflammatory activity. Fitoterapia 2025, 180, 106337. [Google Scholar] [CrossRef]
- Huang, R.; Chen, C.; Ye, M.; Ning, D.; Zhang, S.; Chen, H.; Dong, Q. Research Progress on Cultivation Techniques of Melastoma dodecandrum Lour. Pop. Sci. Technol. 2021, 23, 95–98. [Google Scholar]
- Chen, Y.Y.; Li, D.; Xu, S.P.; Yu, J.Y.; Hu, X.; Long, Y.X.; Zhang, H.Z. Effects of Different Substrates on Rooting of Melastoma dodecandrum Cuttings. Shelter For. Sci. Technol. 2024, 3, 60–63. [Google Scholar] [CrossRef]
- Tong, J.; Fang, L.; Mao, J.; Xu, D.Y.; Dong, Y.F.; Peng, Y.; Zhou, Y. Establishment of a regeneration system for Rhododendron ‘Yanzhimi’. Mol. Plant Breed. 2024, 9, 1–9. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20241118.1934.017.html (accessed on 25 February 2025).
- Hu, X.; Li, D.; Chen, Y.; Pi, H.L.; Yan, S.Z.; Zhang, H.Z. Study on Morphological Differences and Biomass Statistics of Melastoma dodecandrum Under Different Cultivation Substrates. J. Anhui Agric. Sci. 2025, 4, 1–4. Available online: http://kns.cnki.net/kcms/detail/34.1076.S.20250512.1011.002.html (accessed on 12 July 2025).
- He, X.; Yu, Z.; Lin, X.; Huang, C.Y.; Chen, Z.D. Research Progress of Reproduction, Breeding of Common Melastoma. Fujian Sci. Technol. Trop. Crops 2019, 44, 53–57. [Google Scholar]
- Tang, S.; Xu, J.; Jiang, M.; Lin, X.; Zhuo, X.K.; Peng, D.H. Plant regeneration in vitro from leaves of Melastoma sanguineum. J. For. Environ. 2016, 36, 67–72. [Google Scholar]
- Zhang, C.; Xu, G. Tissu Culture and Rapid Propagation of Melastoma dodecandrum. J. Northwest For. Univ. 2004, 3, 75–76. [Google Scholar]
- Wang, J.L.; Yue, K.J.; Liu, H.X.; Tian, X.P. Regeneration of in vitro plants through direct and indirect organogenesis from Dracocephalum rupestre leaf explants. Plant Cell Tissue Organ Cult. (PCTOC) 2025, 161, 7. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Y.; Huang, K.; Wan, X.; Zhang, Z.; Zhu, M.; Wei, C. An Efficient System for Regenerating Adventitious Buds in Stem Segments of Tea Plants. Chin. Bull. Bot. 2023, 58, 308–315. [Google Scholar]
- Yang, S.; Zheng, Z.; Duan, G.; Li, J.L.; Fan, G.H. Screening of Regeneration Medium for Explant of Qingqi No.1. Sci. Technol. Qinghai Agric. For. 2025, 1, 107–112. [Google Scholar] [CrossRef]
- Miao, S.; Wang, L. Establishment of ‘Poinsettia’ Strawberry Regeneration System and Tissue Culture Rapid Propagation System. North. Hortic. 2025, 7, 9–17. [Google Scholar]
- Tian, Y.; Ma, S.; Yang, A.; Han, X.M.; Zhang, C.X. Establishment and Optimization of Regeneration System for Apple Rootstock B9. Acta Hortic. Sin. 2025, 52, 947–958. [Google Scholar] [CrossRef]
- Wu, S.R.; Chen, W.F.; Zhou, X. Enzyme Linked Immunosorbent Assay for Endogenous Plant Hormones. Plant Physiol. Commun. 1988, 5, 53–57. [Google Scholar]
- Li, B. Different Kind and Ratio of Hormones Impact on the Pyrus betulaefolia Bunge Seedlings Callus Production and State. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2010. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Fang, Z.; Li, J.; Ma, J.; Zhang, K.; Ye, C.X. Screening of plant hormone-associated genes during seed dormancy release in Malus sieversii based on transcriptome sequencing. J. Fruit Sci. 2024, 41, 1961–1978. [Google Scholar] [CrossRef]
- Yang, W.; Liu, X.; Zeng, J.; Wu, K.L.; Fang, L.; Wu, S.S.; Zhai, J.W.; Zeng, S.J. Establishment of Callus Regeneration System of Hippeastrum ‘Bangkok Rose’. Chin. J. Trop. Crops 2023, 44, 977–985. [Google Scholar]
- Wang, H.; Xiao, X.; Tang, C.; Ke, X.M.; Zhao, S.S. Establishment of Efficient Regeneration System of Polygonatum cyrtonema Hua. Mol. Plant Breed. 2022, 20, 5386–5393. [Google Scholar]
- Wang, J.; Wang, Q.; Wang, J.; Lu, Y.; Xiao, X.; Gong, W.; Liu, J. Effect of different plant growth regulators on micro-tuber induction and plant regeneration of Pinellia ternate (Thunb) Briet. Physiol. Mol. Biol. Plants 2009, 15, 359–365. [Google Scholar] [CrossRef]
- Song, L.; Gao, F. Changes of Endogenous Hormones in Momordica charantia During in vitro Culture. Chin. Bull. Bot. 2006, 2, 192–196. [Google Scholar]
- Centeno, M.L.; Rodríguez, A.; Feito, I.; Fernández, B. Relationship between endogenous auxin and cytokinin levels and morphogenic responses in Actinidia deliciosa tissue cultures. Plant Cell Rep. 1996, 16, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, T.; Yang, Q.; Jiang, Y.; Shao, X. Callus induction and proliferation of wild Vaccinium bracteatum leaves. Anhui Agric. Sci. Bull. 2023, 29, 83–87. [Google Scholar]
- Tian, X.; Yue, K.; Wang, J.; Liu, H.; Shi, Z.; Kang, H. Callus Induction and Plant Regeneration of Dracocephalum rupestre. Chin. Bull. Bot. 2024, 59, 613–625. [Google Scholar]
- Huang, H. Plant Regeneration and Rapid Propagation of Melastoma intermedium Linn. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2012. [Google Scholar]
- Ai, Y. Effect of Plant Growth Regulator on the Construction of Efficient Regeneration System of Gardenia jasminoides Ellis. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2024. [Google Scholar] [CrossRef]
- Su, H.; Xu, K.; Liu, W. Changes of Endogenous Hormones During the Process of Flower Bud Differentiation of Welsh Onion. Acta Hortic. Sin. 2007, 3, 671–676. [Google Scholar]
- Du, J.; Ruan, M.; Wang, W.; Wang, R.; Huang, S.; Zhang, H.; Zeng, Q. Dynamic changes of endogenous hormones during callus induction and differentiation of Moringa oleifera. Non-Wood For. Res. 2021, 39, 158–167. [Google Scholar] [CrossRef]
- Jia, W.; Yong, J.; Li, A.; Li, C.; Ma, C.; Hu, H. Indigofera bungeana walp: Callus Induction and Adventitious Bud Differentiation Culture. Chin. Agric. Sci. Bull. 2023, 39, 109–117. [Google Scholar]
- Lu, J.; Cao, L.; Tong, G.; Wang, X.; Zhang, L.; Yu, X.; Li, H.; Li, Y. Establishment of a callus induction and regeneration system for Anemone coronaria L. Bull. Bot. 2022, 57, 217–226. [Google Scholar]
- Zhang, Q.; Wang, X.; Chen, L.; Wang, X.; Cai, N. Changes in endogenous hormone contents during tissue culture and proliferation of Lagerstroemia indica ‘Ziqi’. J. Cent. South Univ. For. Technol. 2023, 43, 101–108. [Google Scholar]
- Wang, X.; Zeng, H.; Li, Y.; Wang, X.; Qiao, Z.; Cai, N. Research on changes of endogenous hormone content in the process of subculture of tissue culture of Lonicera macranthoides. Hunan For. Sci. Technol. 2019, 46, 39–43. [Google Scholar]
- Wang, J.; Gao, Y.; Dao, M.; Zhang, H.; Yang, Z.; Chen, L.; Wu, T. Establishment of Regeneration in Vitro System of Camellia japonica and Relationship Between Endogenous Hormones and Adventitious Bud Differentiation. Acta Hortic. Sin. 2024, 51, 1891–1905. [Google Scholar]
- Reshi, Z.A.; Husain, F.M.; Khanam, M.N.; Javed, S.B. Effect of meta-Topolin on morphological, physiochemical, and molecular dynamics during in vitro regeneration of Salix tetrasperma Roxb. BMC Plant Biol. 2025, 25, 121. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Qin, M.; He, Y.; Zhang, J.; Gong, W.; Xiao, X.; Li, P.; Zhou, W. Establishment of in vitro efficient regeneration system for leaves and hypocotyls of Toona sinensis, a multifunctional woody plants. Ind. Crops Prod. 2025, 224, 120328. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Jin, H. Tissue Culture and Plant Regeneration of Melastoma candidum var. albiflorum. North. Hortic. 2017, 11, 119–124. [Google Scholar]
- Huang, F. Study on Tissue Culture and Changes of Endogenous Hormone Content of Lagerstroemia indica ‘Zijingling’. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2022. [Google Scholar] [CrossRef]
- He, C.; Yao, R.; Huang, R.; Mi, X.; Zhang, C.; Tan, Y.; Wang, Y. Effects of K-IBA treatments on adventitious rooting and endogenous hormones contents of shoot cuttings of Thuja occidentals L. J. Cent. South Univ. For. Technol. 2017, 37, 7–12. [Google Scholar]
- Gianinetti, A.; Vernieri, P. On the role of abscisic acid in seed dormancy of red rice. J. Exp. Bot. 2007, 58, 3449–3462. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Z.; Xu, M.L.; Wang, H.J.; Wang, E.; Li, Y.; Wang, L.; Gao, J.; Zhang, J.; Yuan, X.; Zhang, H. Analysis of the transcriptome and related physiological indicators of tree peony (Paeonia suffruticosa Andr.) plantlets before and after rooting in vitro. Plant Cell Tissue Organ Cult. 2021, 147, 529–543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).