Abstract
Biostimulants, particularly single amino acids, can increase plant growth and crop quality, gaining significant attention. This study investigates the effects of 10 amino acids via root/foliar application on the growth, quality, taste, and volatile flavor of mini-watermelons and compares the differences between the application methods. Here, we employed electronic noses, electronic tongues, and gas chromatography–ion mobility spectrometry to investigate these effects. Root application excels in fruit growth and pectin accumulation, while foliar application boosts soluble protein and specific nutrients. Specifically, root application (except for Val) significantly increases fruit weight, with Gly being most effective for longitudinal diameter, while most amino acids (except Val/Lys) promote transverse diameter. Pectin content shows bidirectional regulation: root application of Glu/Gly/Lys/Pro/Trp/Val enhances pectin, whereas foliar application inhibits it. For taste indices, most treatments improve soluble solids (except Glu root/Arg-Leu foliar), and Ala/Asp/Glu/Gly reduce titratable acids, optimizing the sugar–acid ratio. Foliar application is more efficient for soluble protein accumulation (Ala/Glu/Gly/Pro/Leu). For nutritional quality, except for Lys, all treatments increase vitamin C and widely promote total phenolics and lycopene, with only minor exceptions, and only Arg foliar application enhances ORAC. Additionally, the results revealed that root-applied lysine and valine greatly raised the levels of hexanal and 2-nonenal, whereas foliar-applied valine significantly increased n-nonanal and (Z)-6-nonenal. Overall, we found that amino acids can considerably improve mini-watermelon production, quality, taste, and antioxidant capacity, providing theoretical and practical references for their widespread use in agriculture.
1. Introduction
Watermelon (Citrullus lanatus), also known as winter melon and summer melon, is an important annual vine species in the genus Citrullus [1]. It is renowned as the “King of Summer Fruits” and is loved by many for its deliciously juicy flesh and nutritional value. The fruit pulp of watermelon contains abundant vitamins, carbohydrates, and essential minerals, including potassium, calcium, and magnesium, demonstrating both thirst-quenching properties and significant health benefits [2]. Globally, watermelon cultivation consistently ranks among the top agricultural commodities in terms of both planted area and yield, with Asia being particularly prominent in production. As the world’s leading producer, China maintains the largest cultivation area and highest output of watermelon [3].
Chemical fertilizer application has been an essential agricultural practice in watermelon cultivation [4]. However, long-term excessive application of chemical fertilizers often inhibits the richness of microbial communities in the soil and aggravates soil deterioration, which negatively affects plant development and fruit quality [5]. For example, previous researchers experimented with varying watermelon rotation times and discovered that the treated soil where wheat was planted after 6 years of watermelon cultivation had a higher abundance of bacterial and fungal communities than when watermelon was grown continuously for 7 years. This helped promote the land’s long-term protection and health [6]. Additionally, to minimize continuous cropping obstacles, lower production costs, and enhance fruit quality and yield, modern agricultural research has also made it a priority to look into fertilizer use optimization methods.
In recent years, biostimulants, including amino acids, have drawn significant scientific attention. Amino acids are essential components of plant growth and one of the most common types of biostimulants [7,8]. They primarily consist of carbon, hydrogen, oxygen, and nitrogen, which provide organic nitrogen while also regulating plant physiological and biochemical processes [9]. Exogenous amino acids at the optimum concentrations can stimulate plant growth and improve yield and quality. Recently, some studies have demonstrated that exogenous amino acids can be utilized as “nutrients” for plant growth, including in onion, leafy cabbage, etc., to enhance energy and support intricate physiological and biochemical processes and ultimately accomplish the goals of controlling plant growth, enhancing plant quality, and increasing yield [10,11]. Alanine (Ala) contributes to glucose metabolism, regulating stomatal aperture, increasing chlorophyll production, and providing pathogen resistance [12]. Aspartate (Asp) has metal ion chelation properties and acts as a metabolic link between carbohydrate and amino acid pathways [13]. Exogenous application enhances its participation in sugar metabolism through complex metabolic transformations [14]. As a biosynthetic precursor, glutamate (Glu) not only directly contributes to protein synthesis but also transforms diverse amino acids for subsequent protein formation when applied exogenously [15]. Glycine (Gly) can increase the integrity of cell membranes and shield them from peroxidation during normal plant development and/or under stressful environmental conditions [16]. Arginine (Arg) serves as a precursor for polyamines and nitric oxide, playing dual roles in nitrogen storage and protein synthesis. Its immediate uptake by plants influences growth patterns and quality characteristics via complex metabolic processes [17]. For example, Arg treatment positively affected tomato quality, especially lycopene and vitamin C, by improving nitrogen accumulation [18]. Lysine (Lys) catabolites supply essential carbon substrates for the tricarboxylic acid (TCA) cycle [19]. Proline (Pro) enhances pollen viability and plays a pivotal role in plant adaptation to abiotic stresses [20]. Pro application contributes to enhancing growth, yield, and fruit quality, while reducing the percentage of cracked fruits in Manfalouty pomegranates [21]. Tryptophan (Trp) is the biosynthetic origin of auxins and glucosinolates, crucially regulating plant development while stimulating aromatic compound production [22], and Trp at 100 ppm has been shown to increase yield and improve fruit quality, including minimizing fruit cracking in Manfalouty pomegranates [21]. Both soil and foliar applications of Trp at concentrations of 40 mg·kg−1 and 10 mg·L−1, respectively, have been shown to reduce early flower bud drop and enhance the growth and yield of okra [23]. Valine (Val) plays a role in enhancing the flavor profile of plant tissues [24]. Leucine (Leu) acts as a precursor for certain aromatic compounds, and experimental evidence supports its capacity to enhance pollen vitality and germination rates [25].
Leveraging amino acids as biostimulants, we hypothesize that exogenous amino acid application could regulate fruit growth, taste and flavor, and quality-related pathways to enhance the economic value of mini-watermelons. The mini-watermelon (‘Chaoyue Mengxiang’) fruit cycle is about 31 days, which is 15 to 20 days shorter than that of the normal watermelon fruit cycle. This rapid development feature makes it easier to identify the essential physiological nodes involved in fruit quality production. Secondly, the mini-watermelon (‘Chaoyue Mengxiang’) weighs 1 to 1.4 kg per fruit, approximately 1/5 to 1/3 the weight of an average watermelon, making it ideal for physiological sampling at various phases of fruit growth. Meanwhile, its compact plant type (60–80 cm in height) and short vine structure reduce the area requirements for field culture, making it ideal for performing physiological mechanism studies in controlled situations.
The purpose of this study is to investigate the effect of using individual amino acids, as a form of biostimulant, on the growth, quality, taste, and volatile flavor of mini-watermelons. The quality of mini-watermelon fruits was evaluated through field treatments with individual elemental amino acids, assessing fruit yield, fruit appearance, and flavor. The growth and quality of mini-watermelons treated with biostimulants were measured using an electronic nose, electronic tongue, and GC-IMS technologies. Our data provides scientific guidelines for the production of mini-watermelons, along with solid theoretical backing and practical references for the widespread use of elemental amino acids in agriculture.
2. Materials and Methods
2.1. Experimental Materials and Cultivation Conditions
Experimental material: The mini-watermelon cultivar ‘Chaoyue Mengxiang’ (registered under the approval number Jingshengua 2012001) was developed by the Beijing Agricultural Technology Extension Station. The experimental material was the mini-watermelon cultivar ‘Chaoyue Mengxiang’, and the effects of individual amino acids (Ala, Asp, Glu, Gly, Arg, Lys, Pro, Trp, Val, Leu) on mini-watermelon ‘Chaoyue Mengxiang’ quality and yield were tested. The 10 individual amino acids used were commercial reagents purchased from Hebei Changhao Biotechnology Co., Ltd. (Hengshui, China), with a purity of ≥99% and meeting agricultural test reagent standards.
Cultivation conditions: The mini-watermelons were cultivated in the experimental field of Beijing Lichun Agricultural Technology Co., Ltd. (Beijing, China), under open-field cultivation conditions, transplanted on 25 March 2023, and harvested on 4 June 2023. Ambient temperature conditions in Beijing during the trial were as follows: mean daily temperature of 8.5 ± 2.3 °C in late March at planting; 16.2 ± 3.1 °C in April with a diurnal temperature range of 10.5 ± 1.8 °C; 23.4 ± 2.7 °C in May; and 26.8 ± 1.9 °C in early June at harvest, satisfying the temperature requirements for mini-watermelon growth (optimum mean daily temperature 22–28 °C).
The soil type of the experimental field is cinnamonic soil with a pH of 7.2 ± 0.3, organic matter content of 1.8 ± 0.2%, satisfying the soil fertility requirements for mini-watermelon cultivation. Vertical trellis cultivation with double-vine training was used, with a planting density of 1600–1800 plants/mu (24,000–27,000 plants/ha). Basal fertilizers and sufficient water were applied prior to transplanting. The second or third female flower was selected for fruit setting, assisted by artificial pollination to ensure uniform fruiting. Meanwhile, yellow sticky traps (40 cm × 25 cm) were installed at 30 traps/ha, 30–50 cm above the plants, to trap adult aphids and whiteflies.
Treatment methods: Two different methods were applied: 1 g·L−1 root application or 1 g·L−1 foliar spraying with 10 individual amino acids. Treatments began 7 days after pollination and were repeated every 10 days, with 200 mL root treatment per plant and foliar spraying until leaf surface runoff. A blank control (CK) group was set up, in which equal volumes of water (the same as the amino acid treatments) were applied via root or foliar application, with all other cultivation management measures identical to those of the amino acid treatment groups. The CK group strictly followed the same operational procedures (such as application frequency, site, and solution volume) as the amino acid treatments during each period to eliminate interference from water and operational factors in the results.
2.2. Growth Parameter Measurement
Plant growth parameters were measured on 10 randomly selected plants per plot, excluding border plants. Vertical and transverse diameters were determined using graduated rulers. The melon weights were determined using electronic balances (A&D, FX-5000I, Tokyo, Japan).
2.3. Flavor Quality Measurement
The soluble solids content was measured using a refractometer (ATAGO, PAL-α, Tokyo, Japan) with triplicate measurements averaged for each sample. The mini-watermelon’s edible portion was diced and thoroughly mixed. After the appropriate amount of sample had been taken, it was homogenized using a handheld mixer before the juice was extracted using four layers of gauze. One or two drops of clear juice were placed on the prism after wiping it down with a soft flannel cloth at 25 °C. The prism was cleaned with distilled water and dried before use in the refractometer. Distilled water was used to calibrate the refractometer (0 °Brix). A pH meter was used to measure the mini-watermelon juice’s pH value [26].
Titratable acidity was measured using a single-sample titrimeter (EasypH Mettler Toledo, Greifensee, Switzerland). An aliquot of juice was diluted to prepare a 1:25 solution by mixing 2 mL of juice with 48 mL of distilled deionized water. The resulting solution was thoroughly mixed. A 0.2 to 0.5 mL aliquot of the prepared solution was carefully placed on a refractometer, which had been pre-calibrated using a 0.04% citric acid solution. The remaining solution was titrated to a pH endpoint of 8.2 using 0.1 N sodium hydroxide, with precise measurements recorded using titrimeters. Titratable acidity was subsequently calculated as the percentage of malic acid equivalents. Titrimeter readings were plotted against PAM readings, and the linear least squares method was applied to fit the data to an equation, with the coefficient of determination (R2) calculated [27].
The sugar–acid ratio was calculated as the ratio of soluble solid content to titratable acidity values [28].
The soluble protein concentration of the samples was measured using a Bradford Protein Assay Kit (T9310A, Takara, Shiga, Japan) following the instruction manual [29].
2.4. Nutritional Quality Measurement
Ascorbic acid content was determined using a commercial kit (Cat. BC1230, Solarbio, Beijing, China) [30]. Total phenolic content was calculated using a spectrophotometer (MAPADA, M5, CHINA) at 760 nm, with a commercial kit (Cat. BC1340, Solarbio, Beijing, China) [31]. Then, 1 mg of standard lycopene (Cat. SL8700, Solarbio, Beijing, China) was dissolved in 20 mL extraction solution (n-hexane: acetone: anhydrous ethanol = 50:25:25), and then the lycopene standard solution was prepared with a concentration of 50 μg/mL. This was subsequently diluted to 2.5, 5, 7.5, 10 L, 12.5, and 25 ug/mL lycopene standard solutions. Then, 0.1 mL of the standard solution was transferred into a test tube, followed by the addition of 5 mL of the extraction solution, 1 mL of distilled water, and thorough mixing. Oscillatory stratification was performed, and only the colored organic phase from the upper layer was taken for measurement. Absorbance was measured at the maximum absorption wavelength 503 nm and a standard curve was established. Then, 0.1 g of mini-watermelon juice was taken as the sample, and 5 mL of extraction liquid was added, followed by 1 mL of distilled water. Thorough mixing was performed, followed by oscillatory stratification. Finally, only the upper colored organic phase was taken for determination, and the absorbance value was measured at the maximum absorption wavelength of 503 nm [32].
The oxygen radical absorbance capacity (ORAC) assay was performed using a modified protocol based on established procedures. Briefly, sample extracts were prepared by homogenizing 1 g of freeze-dried mini-watermelon tissue in 10 mL of 70% aqueous acetone (pH 2.0) and centrifuging at 10,000× g for 15 min. The supernatant was filtered through a 0.45 μm syringe filter before analysis. The assay mixture contained 70 nM fluorescein sodium salt (Sigma-Aldrich, Shanghai, China) as the fluorescent probe and 153 mM 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH, Sigma-Aldrich) as the peroxyl radical generator, dissolved in 75 mM phosphate buffer (pH 7.4). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Sigma-Aldrich) was used to generate a standard curve ranging from 0 to 1000 μM. Reaction mixtures were incubated at 37 °C in a fluorescence microplate reader (BioTek Synergy H1) with continuous shaking. Fluorescence was measured every minute for 60 min at an excitation wavelength of 485 nm and an emission wavelength of 538 nm. The ORAC value was calculated as the area under the fluorescence decay curve (AUC) using the corresponding formula [33].
2.5. Sensory Evaluation
The electronic tongue system (TS-5000Z, INSENT, Tokyo, Japan) quantified basic taste profiles (umami, salty, sour, bitter, astringent, sweet) based on potentiometric responses of the sensor array to mini-watermelon juice [34]. Mini-watermelon juice was diluted with distilled water at a 1:1 ratio. To ensure the pH value of the sample is between 4 and 7 and the soluble solid content below 5%, the diluted sample was centrifuged at room temperature at 8000 rpm for 15 min. The supernatant was then filtered through double-layer gauze to obtain a clarified solution. The clarified supernatant was used for electronic tongue analysis. The sensor was cleaned for 5 min, followed by 30 s for sample detection and 30 s for aftertaste evaluation. The sensor response intensity initially fluctuated but stabilized after 1–2 measurements. Therefore, each sample was measured 4 to 5 times in parallel, and the data from the last three replicates were used for analysis. Table 1 shows the taste attributes corresponding to each electronic tongue sensor.

Table 1.
Electronic tongue sensors and related taste.
Flavor characteristics were evaluated using a PEN3 electronic nose (AIRSENSE, Schwerin, Germany) with ten metal oxide semiconductor sensors for profiling volatile organic compounds. The differential sensitivities of the sensor arrays to various compounds are annotated in Table 2.

Table 2.
Electronic nose sensors and sensitivity to substances.
2.6. Volatile Compound Analysis
Volatile profiling was performed using gas chromatography–ion mobility spectrometry (GC-IMS, FlavourSpec®, G.A.S., Coventry, UK) with an automated thermal desorption unit. Here, 2 g of samples homogenized with 1 g NaCl (20% w/v) was transferred to 20 mL headspace vials and incubated at 40 °C for 20 min at 500 rpm prior to automated injection. The headspace syringe was maintained at 105 °C with an injection volume of 1 mL in splitless mode. Purge duration was set to 300 s using high-purity nitrogen carrier gas (≥99.999%). The column temperature was maintained at 45 °C.
2.7. Data Analysis
Experimental data were recorded and organized using Microsoft Excel 2019 for graphical presentation. Data were subjected to one-way analysis of variance (ANOVA) using SPSS 26.0. Significance of differences between treatment groups was determined using Tukey’s honestly significant difference (HSD) test at a significance level of p < 0.05. Means labeled with different letters within the same column indicate significant differences according to Tukey’s test. Principal component analysis (PCA) of electronic tongue/nose data was conducted using OriginPro 9.1 (Learning Edition, Northampton, MA, USA). GC-IMS spectral interpretation utilized the embedded LAV software (v2.3.1) with three-dimensional topographic visualization capabilities. Qualitative analysis of volatile organic compounds (VOCs) was achieved through dual database matching against NIST 20 (similarity index > 85%) and IMS drift time libraries (tolerance ± 0.3 ms).
3. Results
3.1. Root/Foliar-Applied Amino Acids Regulate Pectin and Fruit Traits in Mini-Watermelon
For pectin content, compared to the control (root application: 7.3 μmol/g, foliar application: 7.26 μmol/g), Trp applied to roots increased pectin by 45.48%, which was the highest and significantly greater than other treatments. Asp had no significant difference from the control, while Leu, Arg, and Ala reduced pectin by 15.21 to 29.45%. Foliar-applied Leu resulted in the highest increase (18.46% increase, significantly greater than others), Arg increased pectin by 7.85% (significantly above the control), whereas Trp, Val, Pro, Gly, Asp, Lys, Ala, and Glu decreased pectin by 6.75 to 42.56% (Figure 1A, Table S1A).
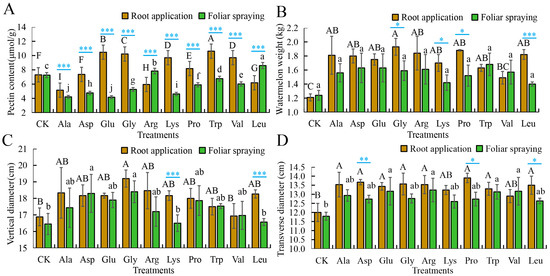
Figure 1.
The application of single amino acids in root application or foliar spraying affects the growth indicators of mini-watermelon. (A) The pectin content after the application of different amino acids. (B) The mini-watermelon weight after the application of different amino acids. (C) The vertical diameter after the application of different amino acids. (D) The transverse diameter after the application of different amino acids. CK is the blank control with root/foliar application of an equal amount of water. Error bars represent standard deviation (SD). The data are the mean ± SD of three biological replicates. Different capital letters indicate that the significance of root application of elemental amino acids reaches the 0.05 level, while lower case letters indicate that the significance of the difference of foliar spray application of elemental amino acids reaches the 0.05 level. * p < 0.05, ** p < 0.01, *** p < 0.001 (Student’s t-test).
Fruit weight mainly reflects the yield of mini-watermelon. Compared to the control (root application: 1.21 kg, foliar application: 1.24 kg), only root applications of individual amino acids significantly increased mini-watermelon weight: root-applied Gly maximized fruit weight (59.5% increase over control), whereas the other nine amino acids raised mini-watermelon weight by 23.14 to 55.37% (no differences among these ten treatments, all significantly over control). Foliar-applied Trp presented the largest weight (35.48% increase), with the other nine amino acids increasing weight by 12.90 to 31.45%, however, none of these increases was statistically significant compared to the control (Figure 1B, Table S1B).
Both root and foliar applications of individual amino acids significantly increased fruit vertical and transverse diameter. For fruit vertical diameter, compared to the control (root application: 16.88 cm, foliar application: 16.45 cm), the highest vertical diameter was observed with root-applied Gly (significantly increased by 13.74% compared with the control), whereas the other nine amino acid treatments raised the vertical diameter by 0.32 to 9.40%, but there was no significant difference from the control. Foliar-applied Gly also showed the longest vertical diameter (11.85% increase), with the other nine amino acids increasing diameter by 0.30 to 11.25%, with no significant differences from the control. (Figure 1C, Table S1C).
For fruit transverse diameter, compared to the control (root application: 12.00 cm, foliar application: 11.79 cm), root-applied Pro resulted in the largest diameter (15.83% increase), with Asp, Gly, Ala, Arg, Leu, Glu, and Trp increasing it by 10.83 to 13.89% (no differences, all significantly over control). Foliar-applied Arg had the widest diameter (12.24% increase), while Glu, Val, and Trp increased diameter by 11.39–11.68%, with no significant differences among them, yet all significantly exceeded the control (Figure 1D, Table S1D).
3.2. Root/Foliar-Applied Amino Acids Regulate the Taste Indices of Mini-Watermelon
For soluble solids content, compared to the control (root application: 10.90 °Brix, foliar application: 10.80 °Brix), root-applied Arg resulted in the highest soluble solids content (11.62% increase over the control), with Trp, Asp, Gly, Ala, Lys, Val, Pro, and Leu increasing it by 1.83 to 10.70% (all significantly higher than the control), while Glu had no difference from the control. Foliar-applied Trp yielded the highest soluble solids content (13.89% increase), with Gly, Pro, Glu, Ala, Asp, Lys, and Val increasing it by 5.56 to 12.96% (all significantly above control), while Arg had no difference from the control, and Leu decreased soluble solids by 8.95% compared to the control (Figure 2A, Table S2A). For titratable acid, compared to the control (root application: 1.01%, foliar application: 1.03%), root-applied Lys maximized titratable acid (17.82% increase over the control), with Glu, Asp, Gly, Arg, and Pro increasing it by 12.87 to 14.85% (all significantly over the control). Foliar-applied Arg and Trp dramatically increased titratable acid by 27.18% and 14.56%, respectively, while Lys and Val increased it by 0.97 to 3.88% (no differences from control) and Leu, Gly, Glu, Asp, and Ala decreased titratable acid by 3.88 to 19.42% versus the control (Figure 2B, Table S2B). For sugar–acid ratio, compared with control (root application: 10.75, foliar application: 10.61), compared with the control, root application of Ala, Asp, Gly, Arg, Trp, Val, Leu, and Pro resulted in sugar–acid ratio changes ranging from −8.00 to 5.95%, but none of these treatments showed significant differences from the control group. Meanwhile, the other two amino acids (Glu, Lys) decreased the ratio by 11.16–12.56% versus the control. Foliar-applied Ala also had the highest ratio (34.40% increase), while Asp, Glu, Gly, and Pro increased it by 16.49 to 32.52% (all significantly over the control); Trp, Leu, and Arg decreased the ratio by 1.13 to 21.39% (Figure 2C, Table S2C). For soluble protein, compared with control (root application: 0.88 mg/g, foliar application: 0.88 mg/g), root-applied Asp maximized soluble protein (38.64% increase over control), with Gly, Leu, Lys, Pro, Arg, Glu, Trp, Val, and Ala increasing it by −13.64 to 17.05% (no differences among these nine treatments). Foliar-applied Ala had the highest soluble protein (31.82% increase), with Gly, Leu, Glu, and Pro increasing it by 20.45 to 30.68% (all significantly above control); Asp, Lys, Trp, Arg, and Val increased it by −3.41 to 11.36%, with no significant differences compared to the control (Figure 2D, Table S2D).
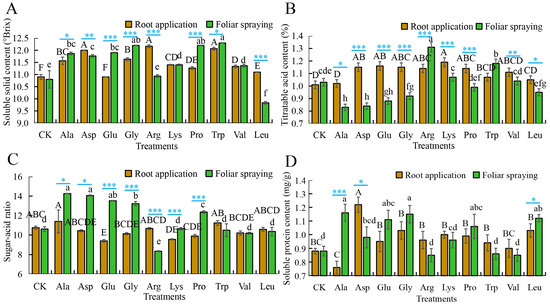
Figure 2.
The application of single amino acids in root application or foliar spraying affects the taste index of mini-watermelon. (A) The soluble solids content (%) after the application of different amino acids. (B) The titratable acid after the application of different amino acids. (C) The sugar–acid ratio after the application of different amino acids. (D) The soluble protein after the application of different amino acids. CK is the blank control with root/foliar application of an equal amount of water. Error bars represent standard deviation (SD). The data are the mean ± SD of three biological replicates. Different capital letters indicate that the significance of root application of elemental amino acids reaches the 0.05 level, while lower case letters indicate that the significance of foliar spray application of elemental amino acids reaches the 0.05 level. * p < 0.05, ** p < 0.01, *** p < 0.001 (Student’s t-test).
Electronic tongue analysis of root-applied amino acids on mini-watermelon taste showed significant differences in seven sensor responses: CA0 (sourness), C00 (bitterness), AE1 (astringency), AAE (umami), AAE-1 (umami aftertaste), CT0 (saltiness), and GL1 (sweetness) [35]. Positive responses were detected for C00, AAE, AAE-1, and GL1, while negative responses were detected for CA0, AE1, C00-1 (bitter aftertaste), AE1-1 (astringent aftertaste), and CT0. Among them, CA0 showed the greatest differentiation between treatments and the control (Figure 3A). Principal component analysis (PCA) of all nine sensors revealed that PC1 (49.30%) and PC2 (24.20%) contribute 73.50% cumulatively. The Asp treatment was the most distinct from the control, whereas Ala was closest. Glu, Gly, and four other amino acids clustered with Trp and Val, indicating similar taste profiles (Figure 3B). Focusing on seven key sensors, PCA showed PC1 (48.80%) and PC2 (28.30%) contributing 77.10%, consistent with nine-sensor analysis, verifying CA0, C00, and AE1 as primary taste-differentiating sensors. AE1-1, GL1, C00-1, CA0, and AE1 positively influenced PC1, while AAE-1, CT0, and AAE showed negative contributions, indicating these parameters collectively dominate taste effects. Asp treatment significantly altered fruit taste, providing experimental evidence for amino-acid-mediated flavor modulation (Figure 3B). Electronic tongue analysis revealed significant effects of foliar-applied amino acids on mini-watermelon taste, with positive responses in C00, AAE, AAE-1, and GL1 and negative responses in CA0, AE1, C00-1, AE1-1, and CT0. Pronounced differences were observed in CA0, C00, AE1, AAE, AAE-1, CT0, and GL1, while C00-1 and AE1-1 showed minimal variation (Figure 3C). PCA based on nine sensors (PC1 = 50.20%, PC2 = 22.50%, cumulative 72.70%) indicated that Leu and Val treatments exhibited similar taste profiles, with Pro treatment furthest from the control (most distinct taste); AE1-1, CA0, AE1, C00-1, and GL1 were key differentiators. Focusing on seven sensors with high response variation (PC1 = 51.50%, PC2 = 25.60%, cumulative 77.10%), reversed PC1 contributions altered sample distribution direction, but inter-sample distances remained similar, suggesting shared taste-modulating effects, for example, Leu/Val and Trp/Asp treatments exhibited similar taste profiles, highlighting the consistent influence of foliar-applied amino acids on mini-watermelon flavor (Figure 3D).
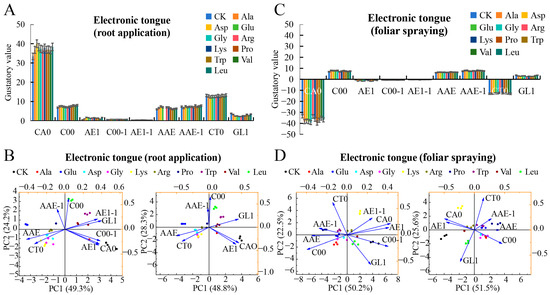
Figure 3.
The effects of root application and foliar spraying of single amino acids on the taste index of mini-watermelon were determined with the assistance of an electronic tongue. (A) The response value of root-applied amino acids on the electronic tongue sensor. (B) Principal component analysis (PCA) of the taste index of mini-watermelon with root-applied amino acids. (C) The response value of foliar-applied amino acids on the electronic tongue sensor. (D) PCA of the taste index of mini-watermelon with foliar-applied amino acids. Error bars represent standard deviation (SD). The data are the mean ± SD of three biological replicates.
3.3. Effect of Application Mode and Type of 10 Individual Amino Acids on the Flavor of Mini-Watermelon
Electronic nose analysis revealed significant effects of root-applied amino acids on mini-watermelon flavor, with distinct responses in W5S, W1S, W1W, and W2S sensors [36] (Figure 4A). PCA showed PC1 and PC2 accounted for 84.70% and 6.87% of the variance, respectively (cumulative 91.57%), with most flavor differences distributed along PC1. Leu clustered closely with the control, Val grouped with Gly, and Glu, Asp, Ala, Arg, and Pro formed another cluster, indicating similar flavor profiles within each group. Sensor contributions indicated negative correlations for W3C, W1C, and W5C with PC1, while W6S, W2W, W3S, W5S, W2S, W1S, and W1W were positively correlated, suggesting these seven sensors were the primary drivers of flavor differentiation. Focusing on W5S, W1S, W1W, and W2S, PCA showed PC1 (88.90%) and PC2 (6.86%) contributions (cumulative 95.76%), with all four sensors positively correlated with PC1, though W1W reversed its contribution direction in PC2. These results identify W5S, W1S, W1W, and W2S as key sensors mediating the flavor effects of root-applied amino acids (Figure 4B). Electronic nose analysis revealed significant effects of foliar-applied amino acids on the flavor profile of mini-watermelons, with pronounced differences in W5S, W1S, W1W, and W2S sensors (Figure 4C). PCA (PC1 = 71.30%, PC2 = 17.40%, cumulative 88.70%) showed flavor variation primarily along PC1. Glu clustered with the control, Asp and Trp formed a distinct group, and Gly, Lys, Ala, and Leu clustered closely, suggesting similar profiles. Ala, Leu, and Val treatments were located near the control, while Pro was furthest away, indicating the most distinct flavor. Sensor contributions indicated negative PC1 correlations for W3C, W1C, and W5C and positive correlations for W6S, W2W, W3S, W5S, W2S, W1S, and W1W—with W5S, W2S, W1W, W1S, and W2W being key drivers. Focusing on four sensors (W5S, W1S, W1W, W2S, cumulative 96.50% variance), all positively correlated with PC1, though W1S and W5S reversed their contribution directions in PC2. These results highlight W5S, W1S, W1W, and W2S as critical sensors mediating flavor changes from foliar amino acid applications (Figure 4D).
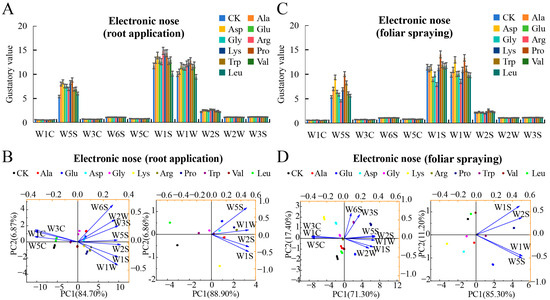
Figure 4.
The effects of root application and foliar spraying of single amino acids on the flavor index of mini-watermelon were determined with the assistance of an electronic nose. (A) The response value of root-applied amino acids on the electronic nose sensor. (B) PCA of the flavor of mini-watermelon with root-applied amino acids. (C) The response value of foliar-applied amino acids on the electronic nose sensor. (D) PCA of the flavor of mini-watermelon with foliar-applied amino acids. Error bars represent standard deviation (SD). The data are the mean ± SD of three biological replicates.
3.4. Correlation Analysis Revealed Interactions Between Taste and Flavor in Mini-Watermelons
Correlation analysis, based on data from two application methods (root and foliar) of ten amino acids, focused on how application mode influences taste–flavor association patterns. Under root application, GL1 showed significant positive correlations with W1C, W3C, and W5C and negative correlations with W2S and W3S. AAE correlated moderately with W6S and W2S, while CT0 positively correlated with W6S. These indicate GL1, AAE, and CT0 are strongly linked to flavor sensors (e.g., W5C, W2S) (Figure 5A). Under foliar spraying, AAE showed significant positive correlations with W5S and W2S (p < 0.01) and negative correlations with W1C and W3C; C00-1 moderately correlated with W1C and W3C; AAE-1 strongly correlated with W5S. These highlight AAE, C00-1, and AAE-1 as key taste–flavor bridges (Figure 5B). Both application methods demonstrated moderate to strong correlations between specific taste parameters (GL1, AAE, CT0) and flavor sensors (W5S, W1C), supporting the presence of an integrated taste–odor interaction in response to amino acid treatments.
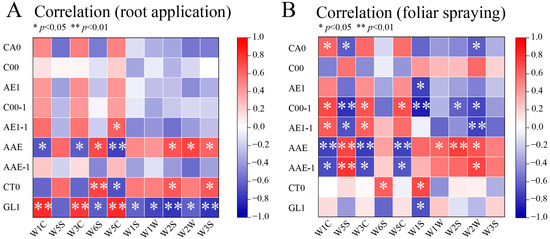
Figure 5.
Pearson correlation heatmap of taste indices and flavor sensor responses in mini-watermelon under different amino acid application methods. (A) Root application. (B) Foliar application.
3.5. Root/Foliar-Applied Amino Acids Regulate Nutritional Quality of Mini-Watermelon
Vitamin C, a potent antioxidant, scavenges free radicals, aids photosynthesis, and enhances disease resistance in watermelon cells [37]. Compared with the control (root application: 10.04 mg/100 g, foliar application: 10.07 mg/100 g), root-applied Gly increased vitamin C content in mini-watermelons by 56.27%, whereas Lys decreased it by 7.57%. The other eight amino acids increased it by 9.36 to 53.49%, with all treatments showing significantly higher levels than the control. Foliar-applied Gly increased vitamin C by 35.95%, with Trp, Ala, Asp, Arg, Val, Lys, and Pro significantly increasing vitamin C by 10.82 to 26.51% (Figure 6A, Table S3A). For total phenolic compounds, under root applications, compared with the control (13.80 mg/100 g), Glu resulted in the highest total phenol content, with a 29.35% increase. Arg, Gly, Val, Trp, Ala, Asp, and Pro significantly increased total phenolic content by 4.49 to 26.09%. Under foliar applications, compared with the control (13.62 mg/100 g), foliar-applied Pro increased total phenol content by 34.21%, Gly, Trp, Arg, Asp, Lys increased total phenol content by 4.99 to 30.76%, whereas Leu, Val, and Glu treatments decreased it by 1.40 to 8.30% (Figure 6B, Table S3B). For lycopene content, compared with the control (11.96 mg/100 g for both root and foliar applications), root-applied Glu resulted in the highest lycopene content in mini-watermelons, with a 40.80% increase. Eight other amino acid treatments significantly increased it by 6.94 to 39.30%, except for the root-applied Ala treatment, which showed no difference from the control. Under foliar spray treatments, Trp led to the highest lycopene content, increasing it by 29.18%, and eight other treatments significantly increased lycopene by 3.18 to 28.34%, except for the foliar-applied Val treatment, which showed no difference from the control (Figure 6C, Table S3C). For oxygen radical absorbance capacity (ORAC), under root application treatments, compared with the control (19.30 uM/100 g), none of the 10 amino acid treatments showed significant differences, indicating that root-applied amino acids did not help increase the ORAC of mini-watermelons. Under foliar spray treatments, compared with the control (19.76 uM/100 g), only Arg treatments significantly increased ORAC by 28.74%, while the other nine treatments showed no significant differences, suggesting that foliar-applied Arg effectively improved the antioxidant capacity of mini-watermelons (Figure 6D, Table S3D).
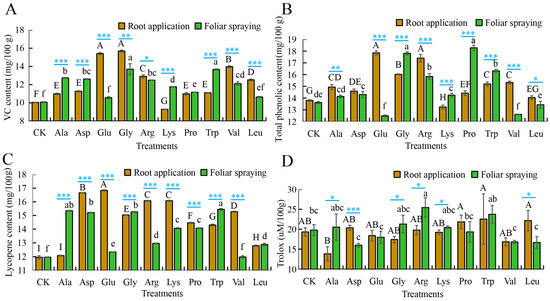
Figure 6.
The application of single amino acids in root application or foliar spraying affects the antioxidant activity of mini-watermelon. (A) The VC content after the application of different amino acids. (B) The total phenolic content after the application of different amino acids. (C) The lycopene content after the application of different amino acids. (D) The Trolox value after the application of different amino acids. CK is the blank control with root/foliar application of an equal amount of water. Error bars represent standard deviation (SD). The data are the mean ± SD of three biological replicates. Different capital letters indicate that the significance of root application of elemental amino acids reaches the 0.05 level, while lower case letters indicate that the significance of foliar spray application of elemental amino acids reaches the 0.05 level. * p < 0.05, ** p < 0.01, *** p < 0.001 (Student’s t-test).
3.6. Effect of Application Mode and Type of 10 Individual Amino Acids on the Volatile Components of Mini-Watermelon
The qualitative analyses of volatile components in mini-watermelons treated with various single amino acids under two application methods are presented in Table S4. A total of 54 volatile compounds were detected, including 16 aldehydes, 13 alcohols, 8 esters, 4 ketones, 4 acids, and 9 compounds from other categories (nitriles, ethers, olefins, alkanes, pyrazines, pyrans, and furans). Under root application, the contents of volatile substances in mini-watermelons treated with Lys, Pro, Trp, Val, and Leu were higher than those of the control (CK) (Figure 7A). Under foliar spraying, the contents of volatile substances in mini-watermelons treated with Ala, Asp, Glu, Gly, and Arg were higher than in the control (Figure 7B).
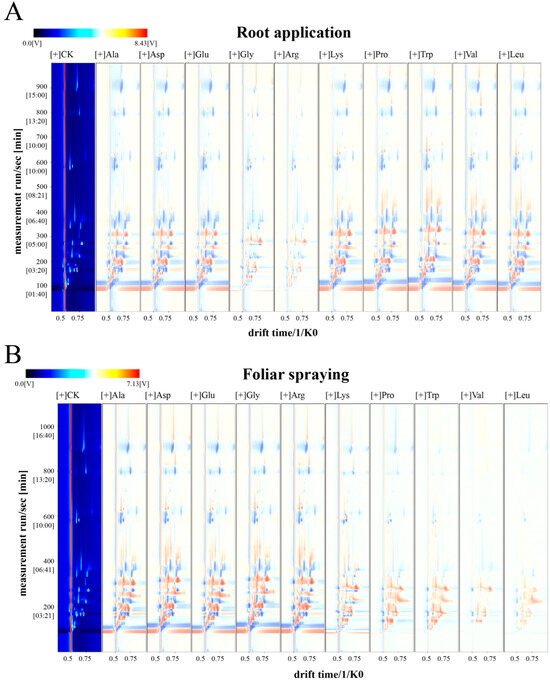
Figure 7.
Difference map of ion mobility spectrum of volatile substances in mini-watermelon treated with different (A) root-applied or (B) foliar-applied amino acids. The red vertical line positioned at 0.5 on the horizontal axis denotes the Reaction Ion Peak (RIP). Each data point located on either side of the RIP corresponds to a specific volatile organic compound (VOC). The color scale reflects the concentration levels of the substances, where white signifies low concentration, red indicates high concentration, and darker shades correspond to progressively higher concentrations.
In mini-watermelons treated with various single amino acids via root application, aldehydes exhibited the highest relative content among volatile compound categories, followed by alcohols, while acids showed the lowest (Table S5). Foliar spray treatments displayed a similar profile: aldehydes were the most abundant, followed by alcohols, with acids being the least abundant and ketones slightly higher (Table S6). Across both application methods, aldehydes consistently represented the dominant volatile component, indicating a general promoting effect of amino acid treatments on aldehyde synthesis. In contrast, acids remained the least abundant, suggesting minimal impact of amino acid regulation on acid production.
Volatile compounds are essential for flavor formation, quality evaluation, and biological interactions of mini-watermelons. Hexanal, n-nonanal, [E]-2-nonenal, and (Z)-6-nonenal are major components of watermelon aroma and have been consistently identified across studies as key constituents of the characteristic watermelon aroma. Hexanal, a typical green leaf volatile, is closely associated with the fresh green flavor of watermelons, and its higher abundance is crucial for forming the fruit’s fresh and crisp flavor characteristics [38]. As an important product of the lipid oxidation, increased [E]-2-nonenal content may be associated with physiological processes such as fruit ripening, flavor development, and stress resistance response mechanisms [39]. Analysis of relative volatile compound contents in mini-watermelons treated with individual amino acids via root and foliar applications revealed that hexanal reached the highest level (4712.9434) under root-applied Lys, indicating that root application of Lys may promote hexanal biosynthesis or accumulation via specific metabolic pathways. [E]-2-nonenal reached the highest abundance (969.78235) under root-applied Val, suggesting that Val application may optimize the supply of synthetic precursors by regulating mini-watermelon physiological status, thereby enhancing its accumulation. As potential flavor-contributing compounds, n-nonanal and (Z)-6-nonenal showed their highest abundances (319.3326 and 513.42596, respectively) under foliar-applied Val. Their simultaneous enhancement under foliar-applied Val suggests that Val may broadly regulate the biosynthetic pathways of lipid oxidation-derived volatiles in mini-watermelons (Figure 8, Tables S7 and S8).
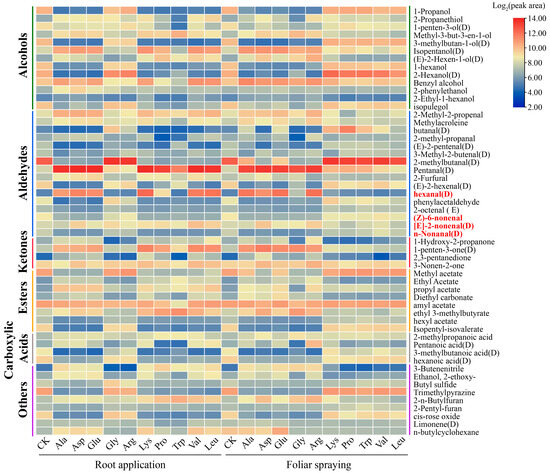
Figure 8.
Relative content of volatile substances (peak area) in mini-watermelon treated with different root-applied or foliar-applied amino acids. Different colors correspond to log2(peak areas), with red denoting a relatively high content and blue denoting a relatively low content. Each column label corresponds to water and 10 amino acids with two application methods, and row labels denote identified volatile compounds. Red-marked Hexanal, n-nonanal, [E]-2-nonenal, and (Z)-6-nonenal are key components of mini-watermelon volatile profiles.
4. Discussion
Amino acids, as a novel form of biofertilizer, have distinct application values and promising future potential, which can not only effectively promote crop growth and development but also significantly increase crop yields and quality, while optimizing the taste characteristics of agricultural products [40]. In this study, we systematically compared the effects of two application methods, foliar spraying and root application of single amino acids, on the growth, quality, and flavor of mini-watermelons, examining the action mechanisms and effect differences of amino acid fertilizers during the mini-watermelon cultivation process. Our findings showed that both root- and foliar-applied amino acids have a substantial influence on mini-watermelon growth and quality. However, there are significant disparities in the impact of these two application strategies. In terms of improving growth-related morphological indicators, such as pectin content, single fruit weight, and fruit transverse diameter, root application of amino acids was more effective than leaf application (Figure 1, Table S1). We believed that the probable cause is that, after being absorbed by the roots, root-applied amino acids could readily reach the plant’s internal circulatory system, supplying nutrients for fruit expansion and secondary metabolite buildup [41]. Although both application methods could increase the content of soluble solids, foliar application of amino acids such as Asp, Glu, Gly, and Ala can reduce the titratable acid of mini-watermelons, indicating that foliar application of amino acids helps to increase the sugar–acid ratio of mini-watermelons and has a greater effect on improving taste. Meanwhile, when it comes to improving soluble protein, foliar treatment outperforms root application. When Ala, Glu, Gly, Pro, and Leu were applied via foliar spraying, soluble protein levels were significantly higher than those of the control, whereas only root-applied Asp resulted in a notable increase in soluble protein content (Figure 2, Table S2). We believed that foliar-sprayed amino acids could directly act on the fruit surface or be rapidly absorbed by the plant through the stomata of leaves, thereby precisely regulating local physiological activities. For example, they could strengthen the cell wall structure to improve fruit firmness or optimize the sugar acid metabolism balance, thus improving fruit quality [9].
In addition, different amino acids had varying effects on mini-watermelon quality, suggesting that differences in the physicochemical properties of amino acids may be an important factor leading to different application effects. In this study, we investigated four indicators related to the growth of mini-watermelons. Pectin, a major primary cell wall component (accounting for approximately 35%), correlates positively with watermelon development and reflects mini-watermelon flesh firmness [42]. Both root application and leaf application of amino acids significantly influenced pectin content. Among them, root application of Glu, Gly, Lys, Pro, Trp, and Val promoted pectin content, whereas foliar application instead inhibited pectin production (Figure 1A, Table S1). In terms of single fruit weight, except for Val, root application of the other nine amino acids could significantly increase the single fruit weight, whereas foliar application of amino acids showed no significant difference from the control. Previous studies have pointed out that Gly is a key intermediate in carbon–nitrogen metabolism, capable of efficiently participating in fruit development pathways when absorbed through the roots [43]. Consistent with these findings, in our data, root-applied Gly performed most outstandingly in increasing fruit weight and vertical diameter (Figure 1B,C, Table S1). Except for Val and Lys, most amino acids significantly increased transverse diameter by root application whereas foliar-applied Val also increased the transverse diameter (Figure 1D, Table S1).
As for the four flavor quality indices, soluble solids content, the term for all substances dissolved in water in liquid food, including sugar, acid, vitamins, minerals, and so on, is one of the most crucial indicators for assessing the quality of fruit [44]. Studies have shown that introducing Arg into apples through vacuum impregnation technology can significantly increase the soluble solid content of the fruit and antioxidant activity [45]. Our study also showed that root-applied Arg and foliar-applied Trp were effective in increasing the soluble solids content of mini-watermelons (Figure 2A, Table S2). Titratable acid (mainly organic acids like citric and malic acid), a key component of fruit flavor, directly influences the sweet–sour balance of taste [46]. Sugar–acid ratio, a core parameter determining fruit palatability, indicates a more balanced flavor and better taste at higher values [47]. In terms of titratable acids, the application of Ala, Asp, Glu, and Gly could assist in lowering the concentration of titratable acids, resulting in some benefits in enhancing the sugar–acid ratio. Soluble protein, a key component of fruit nutritional quality, reflects fruit physiological activity and nutritional richness through its content [48]. Foliar application was more efficient than root application. Foliar application of Ala, Glu, Gly, Pro, and Leu greatly improved the soluble protein content of mini-watermelons.
As for four nutritional quality indices, vitamin C, a potent antioxidant, scavenges free radicals, aids photosynthesis, and enhances disease resistance in watermelon cells [37]. Except for root-applied Lys, all other treatments can considerably increase the vitamin C content of mini-watermelons (Figure 6A, Table S3). Similar to vitamin C, total phenolic compounds exhibit antioxidant capacity, regulating watermelon growth/development and defending against pests and diseases [49]. Except for root application of Lys and Leu, and foliar application of Ala, Glu, Val, and Leu, all other treatments dramatically raised total phenolic content in mini-watermelons (Figure 6B, Table S3). Meanwhile, root-applied Gly and Glu significantly increased the contents of vitamin C and total phenolic compounds in mini-watermelons (Figure 6A,B), which is similar to the conclusion on the impact of foliar-applied Gly and Glu on lettuce growth [50]. Lycopene, the main pigment responsible for the red color in watermelon fruits, exhibits stronger antioxidant capacity than vitamins C and E [51]. Except for foliar application Val, all treatments increased lycopene levels well. The oxygen radical absorbance capacity (ORAC) reflects the comprehensive ability of various antioxidant substances in watermelons to scavenge oxygen free radicals [52]. Consistent with findings by Mohseni et al. (2017), which showed that Arg at concentrations of 250 or 500 μM enhanced strawberry fruit quality compared to the control treatment [53], and foliar-applied Arg significantly increased both lycopene content and ORAC values (Figure 6C,D). Thus, appropriate amino acid types should be precisely selected according to specific production goals.
Research on the volatile flavor of watermelon has progressed significantly in recent years. Beaulieu reported that the primary aroma components in five seedless watermelon varieties were 3-nonen-1-ol, (E,Z)-2,6-nonadienal, 2-nonenal, and 6-nonenal, most of which belonged to C6 and C9 alcohols and aldehydes [54]. Using dynamic headspace purge-and-trap technology, Fredes et al. (2016) identified the key aroma components of watermelon as (Z)-3-nonen-1-ol, β-ionone, (E)-2-heptenal, and (E,Z)-2,6-nonadien-1-ol [55]. Through odor activity value (OAV) and aroma extract dilution analysis (AEDA), Liu determined (Z,Z)-3,6-nonadienal, (Z)-6-nonen-1-ol, (E)-2-hexenal, and (E)-2-nonenal as critical aroma compounds in watermelon [56]. Our study demonstrated that foliar application of Ala and Asp significantly increased the total content of volatile substances in mini-watermelon, thereby contributing to flavor enhancement (Figure 7, Tables S5 and S6). Meanwhile, root application of Lys and Val specifically elevated the contents of hexanal and [E]-2-nonenal, two key volatile components in mini-watermelon (Figure 8, Tables S7 and S8). From a practical perspective, foliar application of Ala and Asp can be adopted to improve the overall flavor of mini-watermelon, thereby enhancing its market competitiveness. Overall, to balance the growth and quality of mini-watermelons, a combined approach of root- and foliar-applied amino acids is recommended in practical production, with targeted use of multiple amino acids to achieve dual improvements. Root-applied Gly and Pro could play a key role in promoting fruit enlargement and increase the yield of mini-watermelon, while foliar-applied Trp and Arg could significantly enhance the flavor and antioxidant capacity of mini-watermelon. We propose prioritizing root application during the fruit growth stage to support development and foliar application during the ripening stage to optimize quality traits. This staged and targeted amino acid application strategy offers a practical and cost-effective approach to improve both yield and quality of mini-watermelon in commercial production.
Additionally, although this study reveals the impacts of single amino acids applied via different methods on mini-watermelon quality, several limitations remain. Future research can focus on three aspects: (1) expanding the scope to include more amino acids and systematically analyzing synergistic effects of combined applications to fully understand their regulatory roles in mini-watermelon quality; (2) considering the influences of growth stages, environmental conditions, and soil fertility to evaluate the universality of findings and develop precise fertilization strategies; (3) deepening the understanding of molecular mechanisms by which amino acids affect quality, integrating genetic engineering to modify crop amino acid metabolic pathways and fundamentally enhance growth potential and quality traits, thus advancing green agricultural innovation.
5. Conclusions
5.1. Fruit Morphology and Pectin Regulation
Root application of amino acids (except Val) significantly increased single fruit weight, with Gly being the most effective in enhancing longitudinal diameter. Transverse diameter was promoted by root application of most amino acids (except Val and Lys), while Val foliar application also showed a promotive effect. Pectin content exhibited bidirectional regulation, root application of Glu, Gly, Lys, Pro, Trp, and Val enhanced pectin accumulation, whereas foliar application universally inhibited it.
5.2. Taste Indices Enhancement
Soluble solids content was significantly increased by most treatments, except for root-applied Glu and foliar-applied Arg and Leu. Titratable acid concentration was decreased by Ala, Asp, Glu, and Gly application, thereby optimizing the sugar–acid ratio. Soluble protein accumulation was more effectively promoted by foliar application, with significant increases observed under Ala, Glu, Gly, Pro, and Leu treatments.
5.3. Nutritional Quality Improvement
Vitamin C content increased under all treatments except for Lys. Both total phenolic and lycopene contents were widely promoted, with only a few exceptions (e.g., root-applied Lys and foliar-applied Val). Notably, only Arg foliar application significantly enhanced oxygen radical absorbance capacity (ORAC).
Overall, root application was more effective in promoting fruit growth and pectin accumulation, whereas foliar application showed better efficacy in promoting soluble protein and specific nutritional compounds. Amino acids such as Gly and Val show pronounced application-mode specificity, providing a theoretical basis for precision amino acid application in mini-watermelon quality regulation. This study employed a single-factor experiment design (10 amino acids × 2 application methods) compatible with traditional fertilization, using inexpensive amino acids (such as glycine) to replace chemical fertilizers, reducing costs while improving quality. It is suitable for commercial mini-watermelon cultivation and does not require special equipment. It is convenient for field promotion and has high repeatability, providing a basis for precise fertilization.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11080877/s1, Table S1: The measurement of single amino acids in root application or foliar spraying affects the growth indicators of mini-watermelon; Table S2: The application of single amino acids in root application or foliar spraying affects the taste index of mini-watermelon; Table S3: The application of single amino acids in root application or foliar spraying affects the antioxidant activity of mini-watermelon; Table S4: Qualitative analysis of volatile components of mini-watermelon treated with various elemental amino acids under two application methods; Table S5: The relative content of volatile types in mini-watermelon treated with various elemental amino acids by root application (peak area); Table S6: The relative content of volatile types in mini-watermelon treated with various elemental amino acids by foliar spraying (peak area); Table S7: The relative content of volatile substances in mini-watermelon treated with various elemental amino acids by root application (peak area); Table S8: The relative content of volatile substances in mini-watermelon treated by foliar spraying of various elemental amino acids (peak area).
Author Contributions
Conceptualization, T.L., J.X., Y.H., and J.Z.; methodology, M.Q., J.H., and J.X.; validation, M.Q., H.W. (Hongxu Wang), and T.L.; investigation, H.W. (Hongxu Wang) and H.W. (Huiyu Wang); data curation, H.W. (Hongxu Wang) and H.W. (Huiyu Wang); writing—original draft preparation, H.W. (Hongxu Wang), Y.H., and H.W. (Huiyu Wang); writing—review and editing, H.W. (Huiyu Wang), Y.H., and T.L.; visualization, H.W. (Hongxu Wang); funding acquisition, T.L. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by construction of cucurbits collaboration and innovation center project, grant number XTCX202301; Beijing Innovation Consortium of Special Crops Research System, grant number BAIC04-2025; Beijing Rural Revitalization Agricultural Science and Technology Project, grant numbers NY2401130424 and NY2401130124.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are thankful to all the lab mates who supported us.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ala | Alanine |
| Asp | Aspartate |
| Glu | Glutamate |
| Gly | Glycine |
| Arg | Arginine |
| Lys | Lysine |
| Pro | Proline |
| Trp | Tryptophan |
| Val | Valine |
| Leu | Leucine |
| TCA | Tricarboxylic acid |
References
- Paris, H.S. Origin and emergence of the sweet dessert watermelon, Citrullus lanatus. Ann. Bot. 2015, 116, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Meghwar, P.; Ghufran Saeed, S.M.; Ullah, A.; Nikolakakis, E.; Panagopoulou, E.; Tsoupras, A.; Smaoui, S.; Mousavi Khaneghah, A. Nutritional benefits of bioactive compounds from watermelon: A comprehensive review. Food Biosci. 2024, 61, 104609. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and melon fruit quality: The genotypic and agro-environmental factors implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Sun, Y.; Yu, L.; Lou, Y.; Fan, X.; Ren, L.; Xu, G. Watermelon responds to organic fertilizer by enhancing root-associated acid phosphatase activity to improve organic phosphorus utilization. J. Plant Physiol. 2022, 279, 153838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, X.; Wang, X.; Xiang, W.; Xiao, M.; Wei, L.; Zhang, Y.; Song, K.; Zhao, Z.; Lv, W.; et al. Effect of fertilization regimes on continuous cropping growth constraints in watermelon is associated with abundance of key ecological clusters in the rhizosphere. Agric. Ecosyst. Environ. 2022, 339, 108135. [Google Scholar] [CrossRef]
- Tian, M.; Liang, J.; Liu, S.; Yu, R.; Zhang, X. Effects of watermelon cropping management on soil bacteria and fungi biodiversity. Agriculture 2023, 13, 1010. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Othman, Y. Effect of foliar application of amino acid biostimulants on growth, macronutrient, total phenol contents and antioxidant activity of soilless grown lettuce cultivars. S. Afr. J. Bot. 2023, 154, 225–231. [Google Scholar] [CrossRef]
- Henderson, B.C.R.; Sanderson, J.M.; Fowles, A. A review of the foliar application of individual amino acids as biostimulants in plants. Discov. Agric. 2025, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Jahanbani, S.; Mumivand, H.; Zahedi, B.; Argento, S. Foliar application of urea and amino acids regulates growth, photosynthesis, pigments, antioxidant activity, and the essential oil content and composition of basil (Ocimum basilicum L.). Agronomy 2024, 14, 2950. [Google Scholar] [CrossRef]
- Haghighi, M.; Barzegar Sadeghabad, A.; Abolghasemi, R. Effect of exogenous amino acids application on the biochemical, antioxidant, and nutritional value of some leafy cabbage cultivars. Sci. Rep. 2022, 12, 17720. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, M.; Voronina, L.; Puchkov, M.; Shcherbakova, N.; Pakina, E.; Zargar, M.; Lyashko, M. Seed priming with exogenous amino acids improves germination rates and enhances photosynthetic pigments of onion seedlings (Allium cepa L.). Horticulturae 2023, 9, 80. [Google Scholar] [CrossRef]
- Sowmya, R.S.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Effect of amino acids on growth, elemental content, functional groups, and essential oils composition on hydroponically cultivated coriander under different conditions. Ind. Crops Prod. 2023, 197, 116577. [Google Scholar] [CrossRef]
- Adelnia, H.; Sirous, F.; Blakey, I.; Ta, H.T. Metal ion chelation of poly(aspartic acid): From scale inhibition to therapeutic potentials. Int. J. Biol. Macromol. 2023, 229, 974–993. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. l-Aspartate: An essential metabolite for plant growth and stress acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.L.; Chung, H.W.; Wu, C.Y.; Wu, H.I.; Lee, Y.T.; Chen, E.C.; Fang, W.; Chang, Y.C. Glutamate stimulates local protein synthesis in the axons of rat cortical neurons by activating α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and metabotropic glutamate Receptors. J. Biol. Chem. 2015, 290, 20748–20760. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipour, N.; Souri, M.K. Beneficial effects of glycine on growth and leaf nutrient concentrations of coriander (Coriandrum sativum) plants. J. Plant Nutr. 2019, 42, 1637–1644. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Q.; Wang, N.; Dai, J.; Lu, Q.; Jia, X.; Lin, L.; Yu, F.; Zuo, Y. Foliar arginine application improves tomato plant growth, yield, and fruit quality via nitrogen accumulation. Plant Growth Regul. 2021, 95, 421–428. [Google Scholar] [CrossRef]
- Xu, J.Z.; Wu, Z.H.; Gao, S.J.; Zhang, W. Rational modification of tricarboxylic acid cycle for improving l-lysine production in Corynebacterium glutamicum. Microb. Cell Factories 2018, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Palombi, N.; Funck, D.; Trovato, M. Proline accumulation in pollen grains as potential target for improved yield stability under salt stress. Front. Plant Sci. 2020, 11, 582877. [Google Scholar] [CrossRef] [PubMed]
- Sayed, O.; Gammal, O.H.M.; Salama, A. Effect of proline and tryptophan amino acids on yield and fruit quality of Manfalouty pomegranate variety. Sci. Hortic. 2014, 169, 1–5. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Hussain, A.; Naveed, M.; Ditta, A.; Nazli, Z.E.H.; Sattar, A. Response of okra (Abelmoschus esculentus L.) to soil and foliar applied L-tryptophan. Soil Environ. 2016, 35, 76–84. [Google Scholar]
- Luo, J.B.; Feng, L.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Physical and flavor characteristics, fatty acid profile, antioxidant status and Nrf2-dependent antioxidant enzyme gene expression changes in young grass carp (Ctenopharyngodon idella) fillets fed dietary valine. PLoS ONE 2017, 12, e0169270. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Dong, X.; Li, Y.; Wang, B. NaCl treatment markedly enhanced pollen viability and pollen preservation time of euhalophyte Suaeda salsa via up regulation of pollen development-related genes. J. Plant Res. 2020, 133, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Workneh, T.; Osthoff, G.; Steyn, M.S.; Coetzer, G.; Pretorius, J. The effect of preharvest treatment, disinfection and storage environment on quality of carrots. J. Food Process. Preserv. 2010, 35, 331–341. [Google Scholar] [CrossRef]
- Patel, H.; Taghavi, T.; Samtani, J.B. Fruit quality of several strawberry cultivars during the harvest season under high tunnel and open field environments. Horticulturae 2023, 9, 1084. [Google Scholar] [CrossRef]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Ren, H.; Zhang, C.; Xiao, D.; Li, Y.; Hou, X.; Liu, T. Regulatory interaction of BcWRKY33A and BcHSFA4A promotes salt tolerance in non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2022, 9, uhac113. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, P.; Xing, Y.; Jia, L.; Zhang, Y.; Jia, T.; Wu, X.; Zhao, B.; Xu, X. Effect of 1α, 25-dihydroxyvitamin D3 on the osteogenic differentiation of human periodontal ligament stem cells and the underlying regulatory mechanism. Int. J. Mol. Med. 2019, 43, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.D.; Zhang, M.; He, M.T.; Gu, M.F.; Lin, M.; Zhang, G. Selection of solvent for extraction of antioxidant components from Cynanchum auriculatum, Cynanchum bungei, and Cynanchum wilfordii roots. Food Sci. Nutr. 2019, 7, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Bowser, T.J.; Weckler, P.; Maness, N.O.; McGlynn, W. Rapid estimation of lycopene concentration in watermelon and tomato puree by fiber optic visible reflectance spectroscopy. Postharvest Biol. Technol. 2009, 52, 103–109. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, G.; Liu, X.; Xiao, Y.; Wanzhang, W. Identification of Xinyang Maojian tea taste using electronic tongue. Sens. Mater. 2019, 31, 2347. [Google Scholar] [CrossRef]
- Jiang, X.; Beibei, Z.; Lei, M.; Zhang, J.; Zhang, J. Analysis of nutrient composition and antioxidant characteristics in the tender shoots of Chinese toon picked under different conditions. LWT 2019, 109, 137–144. [Google Scholar] [CrossRef]
- Sami, A.; Han, S.; Haider, M.; Khizar, R.; Ali, Q.; Shafiq, M.; Tabassum, J.; Khalid, M.; Javed, M.; Sajid, M.; et al. Genetics aspect of vitamin C (Ascorbic Acid) biosynthesis and signaling pathways in fruits and vegetables crops. Funct. Integr. Genom. 2024, 24, 73. [Google Scholar] [CrossRef] [PubMed]
- D’Eusanio, V.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Tassi, L. Volatile aroma compounds of gavina® watermelon (Citrullus lanatus L.) dietary fibers to increase food sustainability. Appl. Chem. 2023, 3, 66–88. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Ma, Y.; Zhao, X.; Zhang, C. Effect of thermal treatments on quality and aroma of watermelon juice. J. Food Qual. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- O’nEill, K.C.; Lee, Y.J. Visualizing genotypic and developmental differences of free amino acids in maize roots with mass spectrometry imaging. Front. Plant Sci. 2020, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent advances in understanding the roles of pectin as an active participant in plant signaling networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.-M. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front. Plant Sci. 2018, 9, 1860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, Z.; Zhang, S.; Chen, F.; Sheng, Z.; Deng, F.; Zeng, Q.; Guo, L. Accurate identification of soluble solid content in citrus by indirect laser-induced breakdown spectroscopy with its leaves. Microchem. J. 2021, 169, 106530. [Google Scholar] [CrossRef]
- Moreno, J.; Echeverria, J.; Silva, A.; Escudero, A.; Petzold, G.; Mella, K.; Escudero, C. Apple snack enriched with L-arginine using vacuum impregnation/ohmic heating technology. Food Sci. Technol. Int. 2017, 23, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Nookaraju, A.; Upadhyaya, C.P.; Pandey, S.K.; Young, K.E.; Hong, S.J.; Park, S.K.; Park, S.W. Molecular approaches for enhancing sweetness in fruits and vegetables. Sci. Hortic. 2010, 127, 1–15. [Google Scholar] [CrossRef]
- Burdulis, D.; Kašėtaitė, A.; Trumbeckaitė, S.; Benetis, R.; Daukšienė, J.; Burdulienė, K.; Raudonė, L. Cultivation of watermelon (Citrullus lanatus (Tunb.)) in a temperate climate: Agronomic strategies and phytochemical composition. Agronomy 2025, 15, 933. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation effects of foliar applied glycine and glutamine amino acids on lettuce growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, F.; Pakkish, Z.; Panahi, B. Arginine impact on yield and fruit qualitative characteristics of strawberry. Agric. Conspec. Sci. 2017, 82, 19–26. [Google Scholar]
- Beaulieu, J.C.; Lea, J.M. Characterization and semiquantitative analysis of volatiles in seedless watermelon varieties using solid-phase microextraction. J. Agric. Food Chem. 2006, 54, 7789–7793. [Google Scholar] [CrossRef] [PubMed]
- Fredes, A.; Sales, C.; Barreda, M.; Valcárcel, M.; Roselló, S.; Beltrán, J. Quantification of prominent volatile compounds responsible for muskmelon and watermelon aroma by purge and trap extraction followed by gas chromatography-mass spectrometry determination. Food Chem. 2016, 190, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, C.; Song, H. Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).