Temporary Immersion Bioreactor for In Vitro Multiplication of Raspberry (Rubus idaeus L.)
Abstract
1. Introduction
2. Materials and Methods
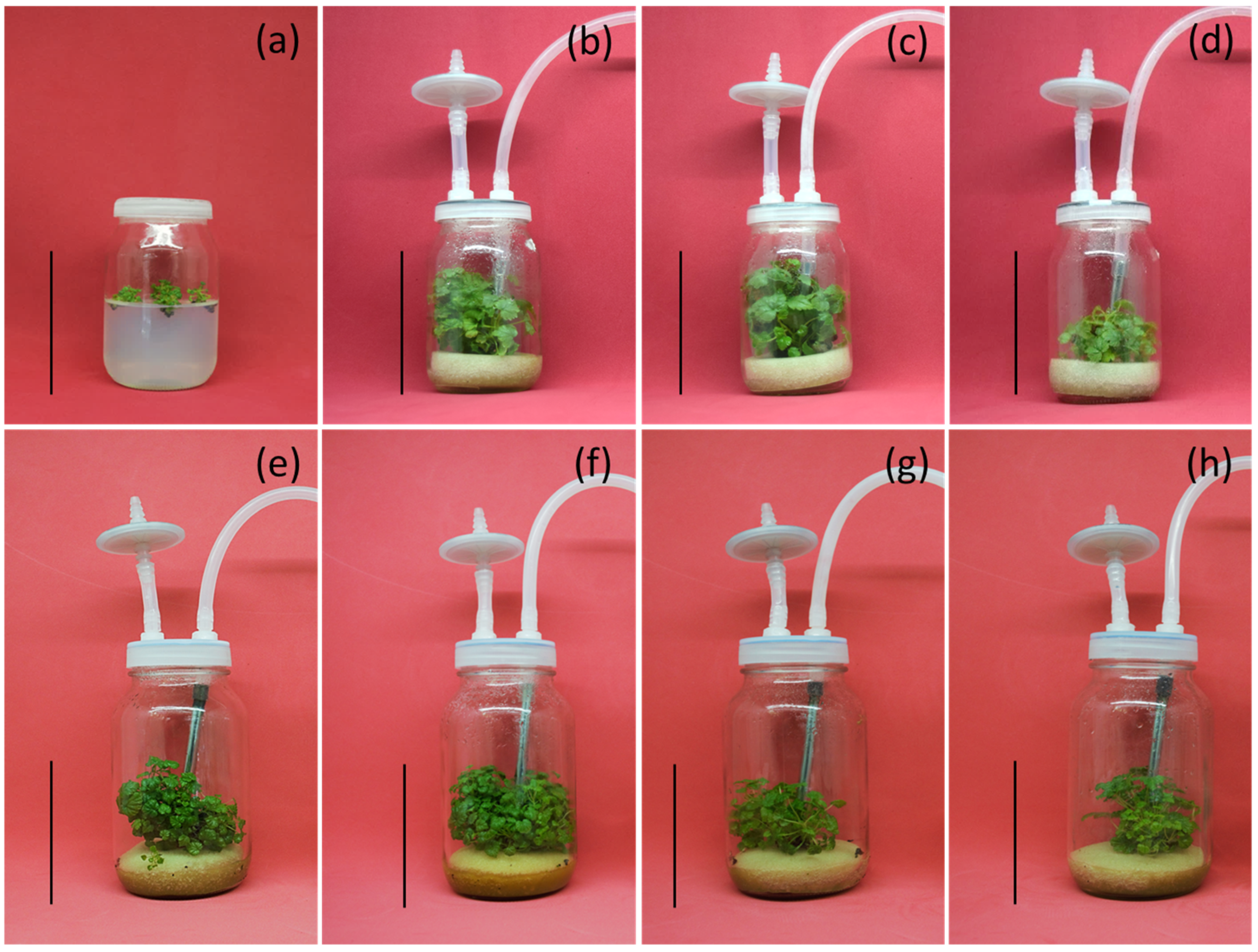
2.1. Plant Material, Establishment and In Vitro Culture Conditions
2.2. Evaluation of the Different Cultivation Methods
2.3. Evaluation of Different Immersion Frequencies in Temporary Immersion
2.4. Evaluation of Different Medium Volumes per Explant in Temporary Immersion
2.5. Chlorophyll and Carotenoid Determination
2.6. Determination of Hyperhydricity Rate
2.7. Ex Vitro Acclimatization
2.8. Data Analysis
3. Results
3.1. Effect of Cultivation Method on In Vitro Multiplication
3.2. Effect of Immersion Frequency on Shoot Regeneration
3.3. Effect of Medium Volume per Explant on Shoot Regeneration
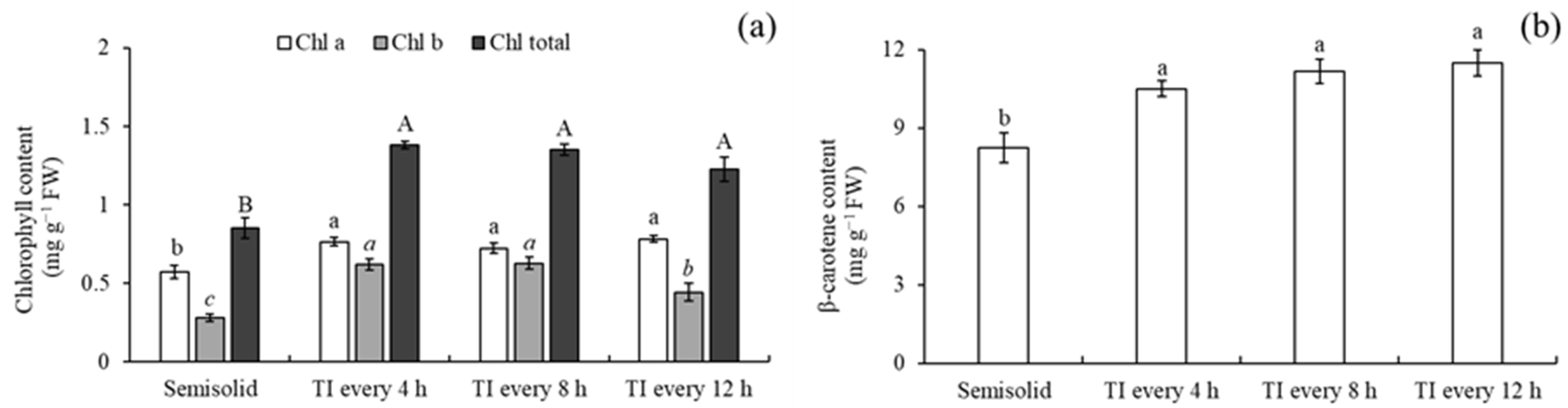
3.4. Chlorophyll and Carotenoid Determination During Different Cultivation Methods and Immersion Frequency
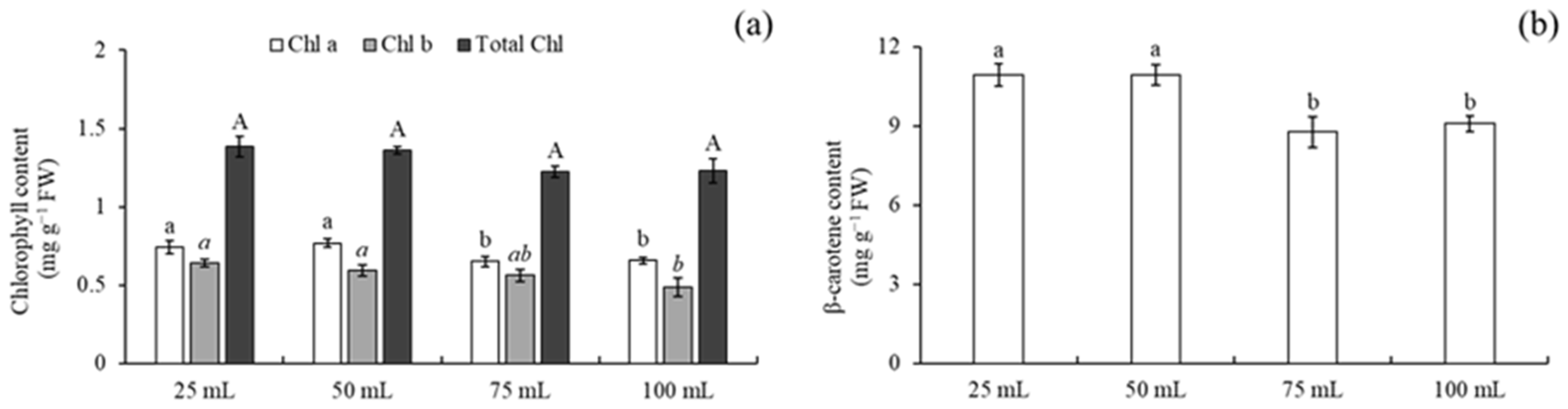
3.5. Chlorophyll and Carotenoid Determination in Medium Volume
3.6. Acclimatization
4. Discussion
4.1. Effect of Cultivation Method on In Vitro Multiplication
4.2. Effect of Immersion Frequency on Shoot Multiplication
4.3. Effect of Medium Volumes on Shoot Multiplication
4.4. Chlorophyll and Carotenoid Determination
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morcillo-Martín, R.; Rabasco-Vílchez, L.; Espinosa, E.; Pérez-Rodríguez, F.; Rodríguez, A. Raspberry (Rubus idaeus L.) waste-derived nanocellulose for circular application in edible films and coatings. LWT 2023, 188, 115438. [Google Scholar] [CrossRef]
- La Torre, C.; Loizzo, M.R.; Frattaruolo, L.; Plastina, P.; Grisolia, A.; Armentano, B.; Cappello, M.S.; Cappello, A.R.; Tundis, R. Chemical Profile and Bioactivity of Rubus idaeus L. Fruits Grown in Conventional and Aeroponic Systems. Plants 2024, 13, 1115. [Google Scholar] [CrossRef]
- Zhang, W.; Lao, F.; Bi, S.; Pan, X.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Insights into the major aroma-active compounds in clear red raspberry juice (Rubus idaeus L. cv. Heritage) by molecular sensory science approaches. Food Chem. 2021, 336, 127721. [Google Scholar] [CrossRef]
- Garjonyte, R.; Budiene, J.; Labanauskas, L.; Judzentiene, A. In vitro antioxidant and prooxidant activities of red raspberry (Rubus idaeus L.) stem extracts. Molecules 2022, 27, 4073. [Google Scholar] [CrossRef]
- Fuentealba, C.; Álvarez, F.; Ponce, E.; Veas, S.; Salazar, M.; Romero, D.; Ayala-Raso, A.; Álvaro, J.E.; Valdenegro, M.; Figueroa, C.R.; et al. Differences in primary metabolism related to quality of raspberry (Rubus idaeus L.) fruit under open field and protected soilless culture growing conditions. Front. Plant Sci. 2024, 14, 1324066. [Google Scholar] [CrossRef] [PubMed]
- García-Merino, G.F.; Ramírez-Mosqueda, M.A.; Aguilar-Rivera, N.; Medorio-García, H.P.; Reyes-Tomas, G.A.; Rodríguez-Deméneghi, M.V. Sugarcane morphological responses enhancement via transverse thin cell layer with cytokinin’s and photoperiods treatments. Braz. J. Bot. 2024, 47, 577–583. [Google Scholar] [CrossRef]
- Mancilla-Álvarez, E.; Spinoso-Castillo, J.L.; Muñoz-Márquez, T.R.A.; Palacios-Pardo, K.F.; Bello-Bello, J.J. Temporary immersion bioreactor as an efficient method for in vitro propagation of Agave marmorata. S. Afr. J. Bot. 2024, 169, 6–11. [Google Scholar] [CrossRef]
- Martínez-Arroyo, M.C.; Mancilla-Álvarez, E.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Evaluation of the effect of different culture systems on photomixotrophic capacity during in vitro multiplication of pitahaya (Hylocereus undatus). S. Afr. J. Bot. 2023, 159, 396–404. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Mancilla-Álvarez, E.; Spinoso-Castillo, J.L. Scaling-up procedures and factors for mass micropropagation using temporary immersion systems. In Vitr. Cell. Dev. Biol.-Plant. 2025, 61, 321–332. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). In Vitr. Cell. Dev. Biol.-Plant. 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Debnath, S.C. A two-step procedure for in vitro multiplication of cloudberry (Rubus chamaemorus L.) shoots using bioreactor. Plant Cell Tissue Organ Cult. 2007, 88, 185–191. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Vergara, C.; Quiroz, K.; Carrasco, B.; García-Gonzales, R. Establishment of photomixotrophic cultures for raspberry micropropagation in temporary immersion bioreactors (TIBs). Sci. Hortic. 2013, 160, 49–53. [Google Scholar] [CrossRef]
- Jones-Castro, F.; Flores-Mora, D.M. Establecimiento in vitro y pruebas preliminares de micropropagación en medio semisólido y líquido de frambuesa (Rubus idaeus L.). Tecnol. Marcha. 2007, 20, 46–54. [Google Scholar]
- Debnath, S.C. Bioreactor-induced adventitious shoot regeneration affects genotype-dependent morphology but maintains clonal fidelity in red raspberry. In Vitr. Cell. Dev. Biol.-Plant. 2014, 50, 777–788. [Google Scholar] [CrossRef]
- Georgieva, L.; Tsvetkov, I.; Georgieva, M.; Kondakova, V. New protocol for in vitro propagation of berry plants using TIS bioreactor. Bulg. J. Agric. Sci. 2016, 22, 745–751. [Google Scholar]
- Bošnjak, D.; Marković, M.; Agić, D.; Vinković, T.; Tkalec Kojić, M.; Ravnjak, B.; Stanisavljević, A. The influence of nutrient media modification on the morphological parameters in raspberry (Rubus idaeus L.) micropropagation in liquid and semi-solid media. Poljoprivreda 2021, 27, 22–29. [Google Scholar] [CrossRef]
- Welander, M.; Persson, J.; Asp, H.; Zhu, L.H. Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar] [CrossRef]
- Clapa, D.; Nemeș, S.A.; Ranga, F.; Hârța, M.; Vodnar, D.C.; Călinoiu, L.F. Micropropagation of Vaccinium corymbosum L.: An alternative procedure for the production of secondary metabolites. Horticulturae 2022, 8, 480. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr.) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Sambolín-Pérez, C.A.; Aybar-Batista, R.; Morales-Marrero, S.; Andino-Santiago, D.; Reyes-Colón, A.; Negrón-Berríos, J.A.; Núñez-Marrero, A.; Arun, A. Biochemical and molecular characterization of Musa sp. cultured in temporary immersion bioreactor. Plants 2023, 12, 3770. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Harborne, J.B. Nitrogen Compounds. In Phytochemical Methods; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1973; pp. 166–211. [Google Scholar]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef] [PubMed]
- Elazab, D.; Capuana, M.; Ozudogru, E.A.; Anichini, M.; Lambardi, M. Use of Liquid Culture with the ElecTIS Bioreactor for Faster Recovery of Blackberry (Rubus fruticosus L.) Shoots from Conservation at 4 °C. Horticulturae 2023, 9, 680. [Google Scholar] [CrossRef]
- San José, M.C.; Blázquez, N.; Cernadas, M.J.; Janeiro, L.V.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult. 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Gailis, A.; Samsone, I.; Šēnhofa, S.; Girgžde, E.; Kāpostiņš, R.; Jansons, Ā. Silver birch (Betula pendula Roth.) culture initiation in vitro and genotype-determined differences in micropropagation. New For. 2021, 52, 791–806. [Google Scholar] [CrossRef]
- Ivanova, M.; Van Staden, J. Natural ventilation effectively reduces hyperhydricity in shoot cultures of Aloe polyphylla Schönland ex Pillans. Plant Growth Regul. 2010, 60, 143–150. [Google Scholar] [CrossRef]
- Pérez-Bueno, M.L.; Pineda, M.; Barón, M. Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front. Plant Sci. 2019, 10, 1135. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Barrera, G.P.; Freire-Seijo, M.; Rodríguez, R.B.; Garcia, S.T. Anatomical and biochemical changes of shoots of Bambusa vulgaris Schrad. ex Wendl under different in vitro shoot culture systems. Braz. J. Bot. 2023, 46, 815–822. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Martín, R.; Capote, A.; Pérez, A.; Hernández-Díaz, E.K.; Rojas, L.; Jiménez, E.; Quiala, E.; Angenon, G.; Garcia-Gonzales, R.; et al. Efficient direct shoot organogenesis, genetic stability and secondary metabolite production of micropropagated Digitalis purpurea L. Ind. Crops Prod. 2018, 116, 25. [Google Scholar] [CrossRef]
- López-Pérez, M.; Seidu, Z. Establishing and Maintaining In Vitro Cultures of Asexual Blood Stages of Plasmodium falciparum. In Malaria Immunology; Jensen, A.T.R., Hviid, L., Eds.; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2470, pp. 37–49. ISBN 978-1-0716-2188-2. [Google Scholar] [CrossRef]
- Majada, J.P.; Sánchez-Tamés, R.; Revilla, M.A.; Casares, A. Micropropagation of Ilex aquifolium L. In Vitr. Cell. Dev. Biol.-Plant. 2000, 36, 521–526. [Google Scholar] [CrossRef]
- Saleta, R.; Garrido, J.; Sánchez, C.; Ferreiro-Vera, C.; Codesido, V.; Vidal, N. A temporary immersion system to improve Cannabis sativa micropropagation. Front. Plant Sci. 2022, 13, 895971. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, W.A.; Xu, J.; Beleski, D.G. Micropropagation of Brassavola nodosa L. Lindl. using SETIS™ bioreactor. Plant Cell Tissue Organ Cult. 2023, 153, 67–76. [Google Scholar] [CrossRef]
- Ayub, R.A.; Pereira, A.B. Brassinosteroid combined with indolebutyric acid in blueberry micropropagation. J. Agric. Sci. 2020, 14, 59–65. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Schettino-Salomón, S.; Ortega-Espinoza, J.; Spinoso-Castillo, J.L. A temporary immersion system for mass micropropagation of pitahaya (Hylocereus undatus). 3 Biotech 2021, 11, 437. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Debnath, S.C. A Scale-up System for Lowbush Blueberry Micropropagation Using a Bioreactor. HortScience 2009, 44, 1962–1966. [Google Scholar] [CrossRef]
- Ayub, R.A.; Pereira, A.B.; Santos, J.N.D.; Silva, D.M.D.; Pessenti, I.L. Sucrose concentration and blueberry plant density in temporary immersion systems (TIS). Rev. Bras. Frutic. 2021, 43, e-166. [Google Scholar] [CrossRef]
- Kryukov, L.A.; Vodolazhsky, D.I.; Kamenetsky-Goldstein, R. Micropropagation of grapevine and strawberry from South Russia: Rapid production and genetic uniformity. Agronomy 2022, 12, 308. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Effects of supporting materials in in vitro acclimatization stage on ex vitro growth of wasabi plants. Sci. Hortic. 2020, 261, 109042. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Bello-Bello, J.J. CO2-enriched air in a temporary immersion system induces photomixotrophism during in vitro multiplication in vanilla. Plant Cell Tissue Organ Cult. 2023, 155, 29–39. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Carle, R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830. [Google Scholar] [CrossRef]
- Arigundam, U.; Variyath, A.M.; Siow, Y.L.; Marshall, D.; Debnath, S.C. Liquid culture for efficient in vitro propagation of adventitious shoots in wild Vaccinium vitis-idaea ssp. minus (lingonberry) using temporary immersion and stationary bioreactors. Sci. Hortic. 2020, 264, 109199. [Google Scholar] [CrossRef]

| Treatments | Number of Shoots Per Explant | Shoot Length (cm) | Number of Leaves Per Shoot | Hyperhydricity (%) | Survival (%) |
|---|---|---|---|---|---|
| (I) Cultivation method | |||||
| Semisolid | 2.45 ± 0.15 b | 1.04 ± 0.09 b | 5.72 ± 0.23 b | 1.00 ± 0.00 b | 96.33 ± 2.00 a |
| Temporary immersion | 5.76 ± 0.27 a | 3.38 ± 0.10 a | 7.47 ± 0.31 a | 17.20 ± 2.15 a | 98.33 ± 1.20 a |
| (II) Immersion frequency (2 min immersion) | |||||
| Frequency every 4 h | 5.81 ± 0.16 a | 2.36 ± 0.08 b | 6.54 ± 0.19 bc | 54.00 ± 4.72 a | 98.00 ± 1.00 a |
| Frequency every 8 h | 5.76 ± 0.27 a | 3.38 ± 0.10 a | 7.47 ± 0.31 ab | 17.20 ± 2.15 b | 98.33 ± 1.20 a |
| Frequency every 12 h | 4.15 ± 0.22 b | 2.38 ± 0.12 b | 7.69 ± 0.36 a | 14.00 ± 1.73 b | 97.00 ± 1.52 a |
| (III) Culture medium volume per explant (mL) * | |||||
| 25 | 5.76 ± 0.18 a | 3.44 ± 0.07 b | 6.46 ± 0.19 c | 2.00 ± 0.64 c | 99.00 ± 1.00 a |
| 50 | 5.75 ± 0.16 a | 3.40 ± 0.06 b | 7.34 ± 0.14 b | 17.55 ± 1.50 b | 99.00 ± 1.00 a |
| 71 | 3.81 ± 0.15 b | 3.70 ± 0.19 ab | 8.13 ± 0.23 a | 34.00 ± 4.05 a | 97.00 ± 1.52 a |
| 100 | 4.17 ± 0.23 b | 3.90 ± 0.08 a | 8.11 ± 0.26 a | 39.60 ± 3.38 a | 97.00 ± 1.52 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Beristain, B.; Mancilla-Álvarez, E.; López-Buenfil, J.A.; Bello-Bello, J.J. Temporary Immersion Bioreactor for In Vitro Multiplication of Raspberry (Rubus idaeus L.). Horticulturae 2025, 11, 842. https://doi.org/10.3390/horticulturae11070842
Reyes-Beristain B, Mancilla-Álvarez E, López-Buenfil JA, Bello-Bello JJ. Temporary Immersion Bioreactor for In Vitro Multiplication of Raspberry (Rubus idaeus L.). Horticulturae. 2025; 11(7):842. https://doi.org/10.3390/horticulturae11070842
Chicago/Turabian StyleReyes-Beristain, Bruno, Eucario Mancilla-Álvarez, José Abel López-Buenfil, and Jericó Jabín Bello-Bello. 2025. "Temporary Immersion Bioreactor for In Vitro Multiplication of Raspberry (Rubus idaeus L.)" Horticulturae 11, no. 7: 842. https://doi.org/10.3390/horticulturae11070842
APA StyleReyes-Beristain, B., Mancilla-Álvarez, E., López-Buenfil, J. A., & Bello-Bello, J. J. (2025). Temporary Immersion Bioreactor for In Vitro Multiplication of Raspberry (Rubus idaeus L.). Horticulturae, 11(7), 842. https://doi.org/10.3390/horticulturae11070842







