In Vitro Plant Regeneration and Bioactive Metabolite Production of Endangered Medicinal Plant Atractylodes lancea (Thunb.) DC
Abstract
1. Introduction
2. Materials and Methods
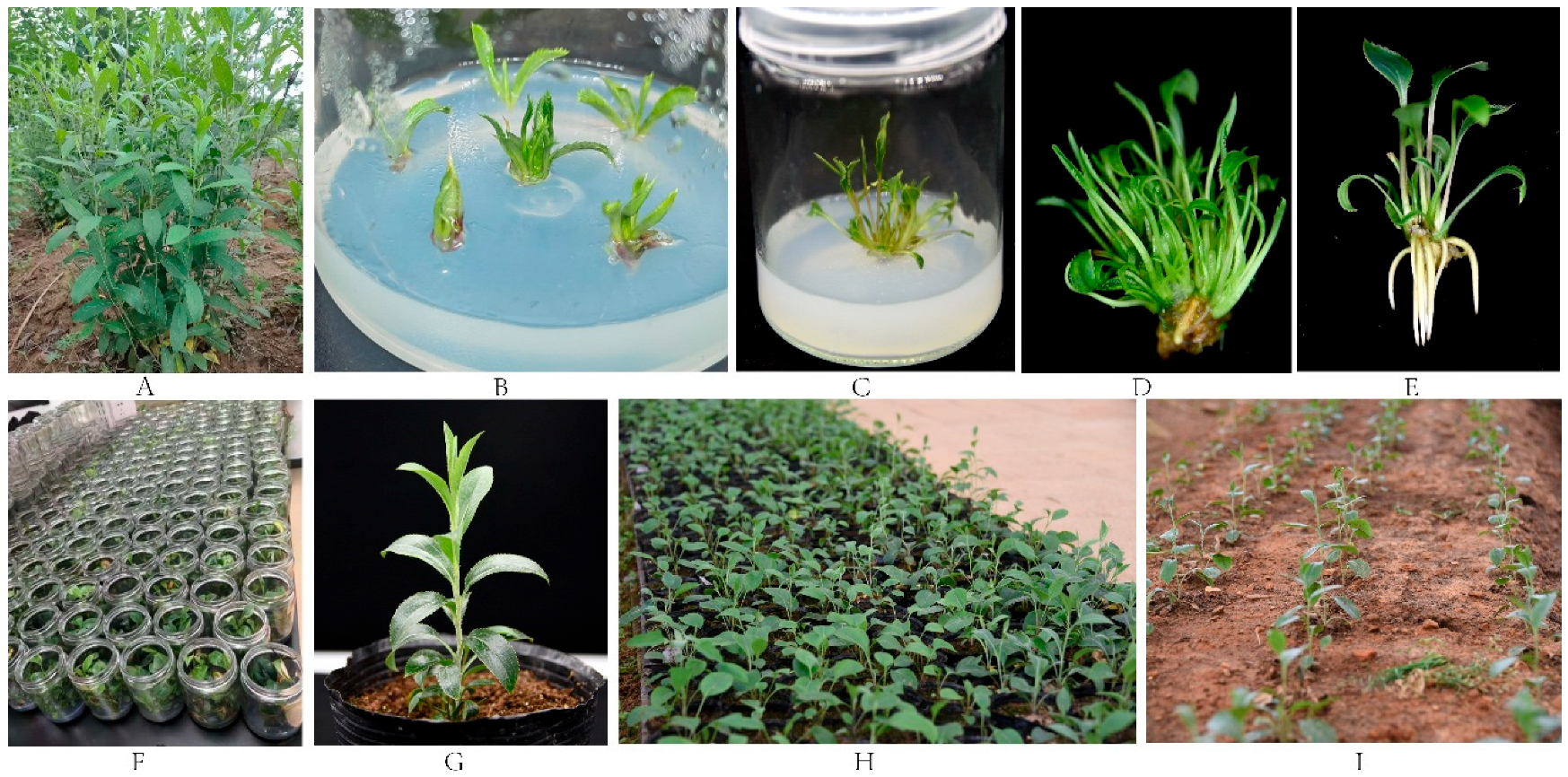
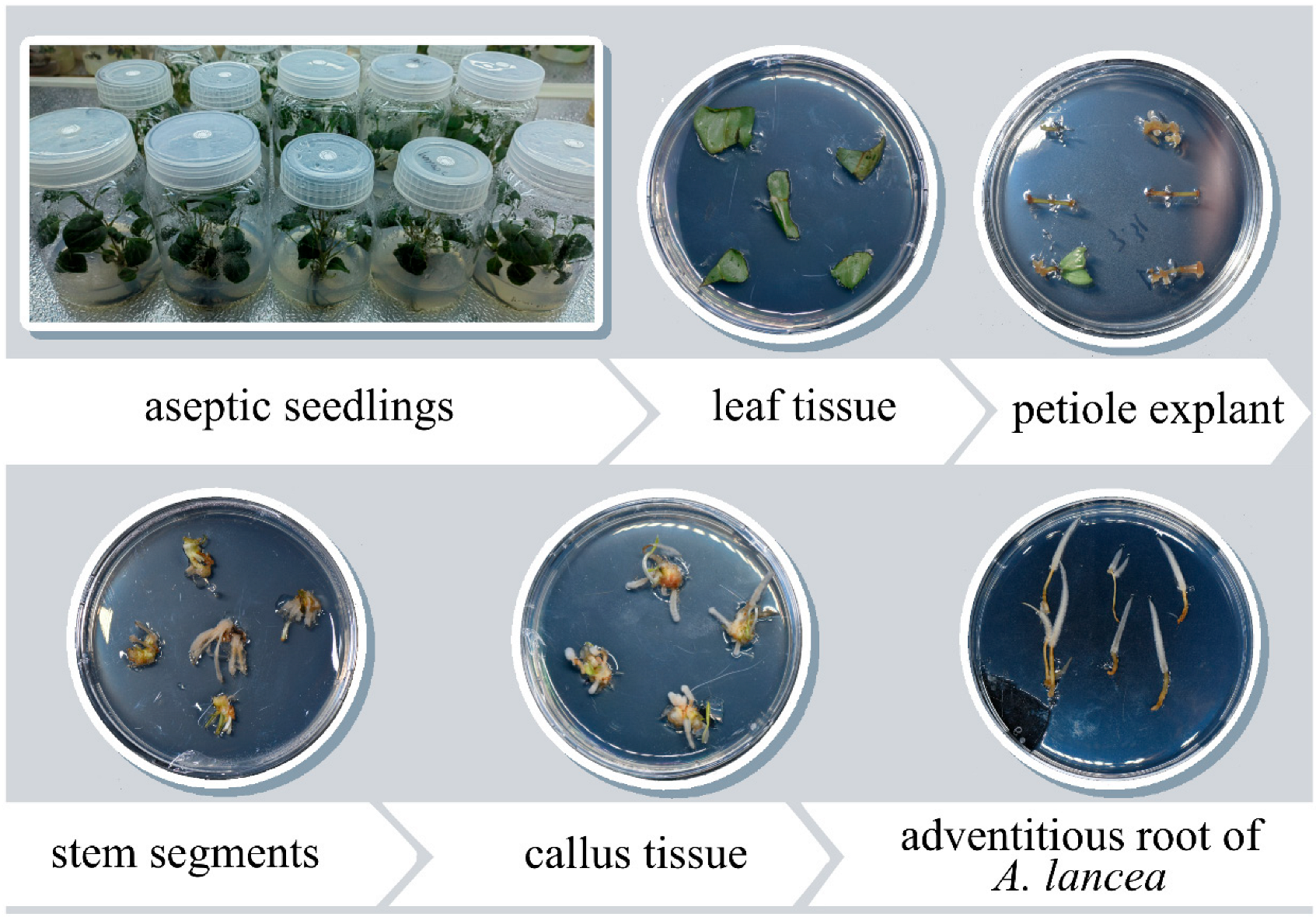
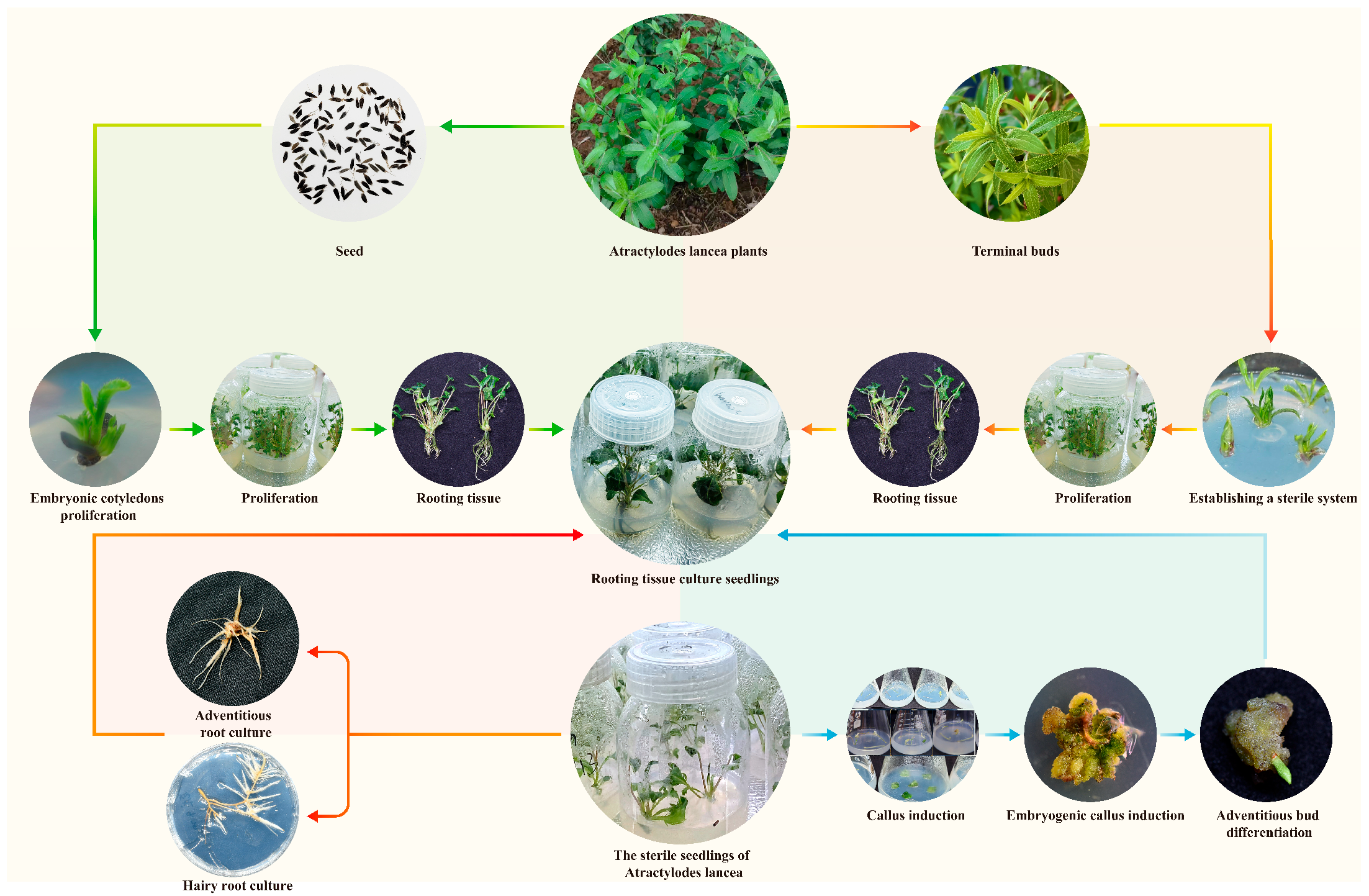
2.1. Plant Materials and Establishment of Aseptic Seedlings
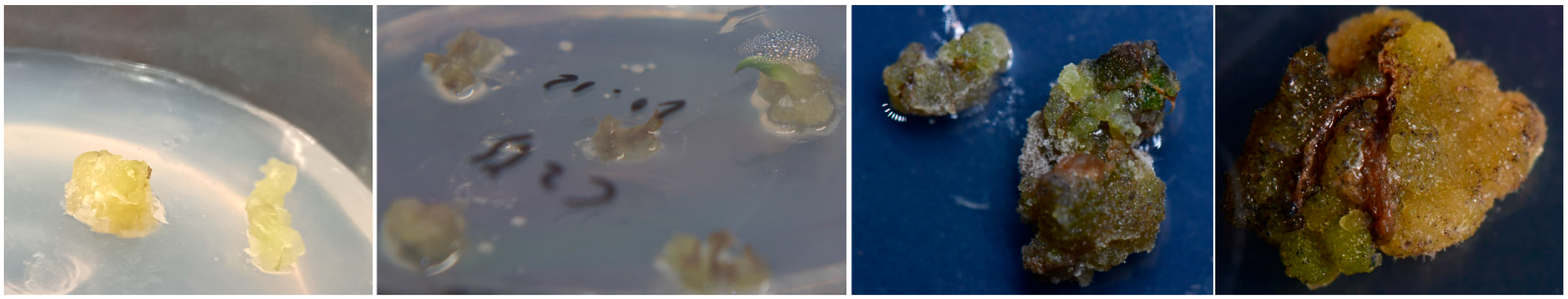
2.2. Effect of Different Exogenous Hormones on the Induction and Proliferation of Calli
2.3. Effect of Different Exogenous Hormones on the Differentiation of Calli
2.4. Effect of Different Conditions on Bulblet and Adventitious Bud Induction
2.5. Effect of Different Conditions on Root Induction
2.6. Establishment of Field Seedlings
2.7. Establishment of Hairy Root and Adventitious Root Cultures of A. lancea
2.8. Determination of Essential Oils
2.8.1. Extraction of Essential Oils
2.8.2. Quantitative Analysis of Embryogenic Calli, Hairy Root, and Adventitious Root Essential Oils
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Exogenous Hormones on the Induction Rate and Differentiation of Calli
3.1.1. Analysis of Variance
3.1.2. Stepwise Regression Analysis
3.2. Effects of Different Exogenous Hormones on the Bud Differentiation Rate
3.3. Effects of Different Hormone Ratios on the Rooting of Seedlings Cultured In Vitro
3.4. Effects of Different Conditions on Adventitious Root Induction in A. lancea
3.5. Comparative Analysis of Essential Oils Content in Embryogenic Calli, Hairy Root, and Adventitious Root of A. lancea
3.6. A Proposed Approach to Produce Bioactive Metabolites and Regenerate Seedlings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Wang, S.; Sun, J.; Li, X.; Wang, H.; Guo, X.; Guo, L. Genome resequencing reveals the genetic basis of population evolution, local adaptation, and rewiring of the rhizome metabolome in Atractylodes lancea. Hortic. Res. 2024, 11, 167. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Lyu, C.; Wang, Y.; Sun, J.; Zhang, Y.; Guo, L. Authenticating the geographic origins of Atractylodes lancea rhizome chemotypes in China through metabolite marker identification. Front. Plant Sci. 2023, 14, 1237800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Zhao, Z.Y.; Chang, L.K.; Cao, Y.; Wang, S.; Kang, C.Z.; Guo, L.P. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021, 266, 113415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lin, H.; Liu, Z.; Qi, K.; Zhang, W.; Wang, H.; Guo, L. Polyacetylenes and sesquiterpenes in Chinese traditional herb Atractylodes lancea: Biomarkers and synergistic effects in red secretory cavities. Mol. Hortic. 2025, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Jiang, D.Q.; Kang, C.Z.; Wang, S.; Lyu, C.G.; Zhang, Y.; Guo, L.P. Research progress and prospect of endophytes from medicinal plant Atractylodes lancea. Chin. J. Chin. Mater. Med. 2021, 46, 19. [Google Scholar] [CrossRef]
- Sun, X.; Guo, J.; Ge, Y.; Xia, B.; Hang, Y.Y. Study of specific random amplification of polymorphic DNA-sequence characterized amplified region (RAPD-SCAR) marker for the endangered Chinese endemic herb Atractylodes lancea. J. Med. Plants Res. 2012, 6, 21. [Google Scholar]
- Shan, T.; Wu, J.; Yu, D.; Xie, J.; Fang, Q.; Zha, L.; Peng, H. Genome survey sequencing of Atractylodes lancea and identification of its SSR markers. Biosci. Rep. 2020, 40, BSR20202709. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wang, S.; Wang, Y.F.; Wang, H.Y.; Qin, M.; Dai, X.Y.; Guo, L.P. Application of tissue culture technology of medicinal plants in sustainable development of Chinese medicinal resources. China J. Chin. Mater. Medica 2023, 48, 1186–1193. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New insights into tissue culture plant-regeneration mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef]
- Huang, T.; Liu, D.; Cui, X.; Li, M.; Jin, L.; Paré, P.W.; Wei, J. In vitro bioactive metabolite production and plant regeneration of medicinal plant Angelica sinensis. Ind. Crops Prod. 2023, 194, 116276. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, C.; Su, H.; Wang, J.; Qi, Y.; Li, M. In vitro plant regeneration and bioactive metabolite production of endangered medicinal plant Fritillaria cirrhosa. Curr. Plant Biol. 2024, 39, 100363. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Combined effects of cytokinin and UV-C light on phenolic pattern in Ceratonia siliqua shoot cultures. Agronomy 2023, 13, 621. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, P.H.; Li, C.H.; Liang, Y.L.; Liu, G.Z.; Yang, S.C.; Zhao, Y. Progress on medicinal plant regeneration and the road ahead. Med. Plant Biology. 2024, 3, e030. [Google Scholar] [CrossRef]
- Zdravković-Korać, S.; Milojević, J.; Belić, M.; Ćalić, D. Tissue culture response of ornamental and medicinal aesculus species—A review. Plants 2022, 11, 277. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, X.; Wang, H.; Dai, X.; Yan, B.; Wang, S.; Guo, L. Induction and metabolomic analysis of hairy roots of Atractylodes lancea. Appl. Microbiol. Biotechnol. 2023, 107, 6655–6670. [Google Scholar] [CrossRef] [PubMed]
- Nwite, P.A.; Eke, C.; Osemwegie, Q.; Ikhajiagbe, B. Assessment of media modification and induction of calli on coconut (Cocos nucifera L.) palm explants. Res. J. Agric. Sci. 2023, 11, 33–39. Available online: https://www.researchgate.net/publication/371609345 (accessed on 21 April 2025).
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef]
- Tahir, S.M.; Victor, K.; Abdulkadir, S. The effect of 2, 4-Dichlorophenoxy acetic acid (2, 4-D) concentration on callus induction in sugarcane (Saccharum officinarum). Niger. J. Basic. Appl. Sci. 2011, 19, 2. [Google Scholar]
- Rocha, V.A.; Aquino, A.M.; Magosso, N.; Souza, P.V.; Justulin, L.A.; Domeniconi, R.F.; Scarano, W.R. 2, 4-dichlorophenoxyacetic acid (2, 4-D) exposure during postnatal development alters the effects of western diet on mouse prostate. Reprod. Toxicol. 2023, 120, 108449. [Google Scholar] [CrossRef]
- Mamdouh, D.; Smetanska, I. Optimization of callus and cell suspension cultures of Lycium schweinfurthii for improved production of phenolics, flavonoids, and antioxidant activity. Horticulturae 2022, 8, 394. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, B.; Luo, X.; Wang, S.; Fu, J.; Zhang, Z.; Hu, G. Development of an Agrobacterium tumefaciens-mediated transformation system for somatic embryos and transcriptome analysis of LcMYB1’s inhibitory effect on somatic embryogenesis in Litchi chinensis. J. Integr. Agri. 2025, 24, 594–609. [Google Scholar]
- Liu, X.; Yang, H.; Guo, B.; Hu, Z. Matabolomic Changes Induced by 6-Benzylaminopurine in Polygonatum cyrtonema. Horticulturae 2024, 10, 327. [Google Scholar] [CrossRef]
- Song, L.; Hu, W.; Wang, C.; Bao, S.; Yan, H.; Luo, H. 6-Benzylaminopurine and Gibberellic Acid Mitigate the Yellowing of Pak Choi (Brassica rapa Subsp. Chinensis) During Storage by Regulating Sugar Scarcity-Induced Chlorophagy. J. Agric. Food Chem. 2025, 73, 7584–7595. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.Q.; Sun, L.; Mei, C.; Wang, H.; Song, Q.; Feng, R. Establishment of a callus regeneration system for potato (Solanum tuberosum L.) cultivar ‘Jinshu 16’. China Seed Ind. 2021, 2, 70–73. [Google Scholar] [CrossRef]
- Guillén, S.; Possas, A.; Valero, A.; Garre, A. Optimal experimental design (OED) for the growth rate of microbial populations. Are they really more “optimal” than uniform designs? Int. J. Food Microbiol. 2024, 413, 110604. [Google Scholar] [CrossRef]
- Wang, J.; Chen, B.; Rao, X.; Huang, J.; Li, M. Optimization of culturing conditions of Porphyridium cruentum using uniform design. World J. Microbiol. Biotechnol. 2007, 23, 1345–1350. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Q.; Cui, G.; Zhang, X.; Lyu, C.; Sun, J.; Guo, L. Recent progress and ongoing challenges in Rhizoma atractylodis research: Biogeography, biosynthesis, quality formation and control. Med. Plant Biol. 2023, 2, 19. [Google Scholar] [CrossRef]
- Samiei, S.; Tajadod, G.; Zarghami, R.; Mirzai, M. A 2, 4-D-free combined direct organogenesis-indirect somatic embryogenesis protocol for mass propagation of date palm (Phoenix dactylifera L.) cv.Barhi. J. Sci. Islam. Repub. Iran. 2022, 33, 125–132. [Google Scholar] [CrossRef]
- Li, S.; Yang, N.; Chen, L. Paraffin section observation of flower bud differentiation of Chimonanthus praecox in Kunming and comparison of the differentiation processes in different regions, China. Hortic. Plant J. 2022, 8, 221–229. [Google Scholar] [CrossRef]
- Guo, Y.; An, L.; Yu, H.; Yang, M. Endogenous hormones and biochemical changes during flower development and florescence in the buds and leaves of Lycium ruthenicum Murr. Forests 2022, 13, 763. [Google Scholar] [CrossRef]
- Li, H.S.; Liu, M.R.; Hou, W.Q.; Wang, F. Preliminary Study on Rapid Propagation and Tissue Culture Techniques of the Medicinal Plant Atractylodes lancea (Thunb). DC. Mol. Plant Breed. 2019, 17, 1611–1615. [Google Scholar] [CrossRef]
- Debnath, S.C.; Ghosh, A. Phenotypic variation and epigenetic insight into tissue culture berry crops. Front. Plant Sci. 2022, 13, 1042726. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, T. Comparative Transcriptomic Insights into the Mechanisms Underlying Maize (Zea mays L.) Embryogenic Callus Differentiation. Agronomy 2024, 14, 1689. [Google Scholar] [CrossRef]
- Li, L.; Deng, X.; Zhang, T.; Tian, Y.; Ma, X.; Wu, P. Propagation methods decide root architecture of Chinese fir: Evidence from tissue culturing, rooted cutting and seed germination. Plants 2022, 11, 2472. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, F.; Qi, C.; Fu, J.; Xiao, Y.; Wu, J. Efficient ex-vitro rooting and acclimatization for tissue culture plantlets of ginger. Plant Cell Tissue Organ Cult. 2022, 150, 451–458. [Google Scholar] [CrossRef]
- Müller-Xing, R.; Xing, Q. The plant stem-cell niche and pluripotency: 15 years of an epigenetic perspective. Front. Plant Sci. 2022, 13, 1018559. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; Ivetić, V. Root system development and field establishment: Effect of seedling quality. New For. 2022, 53, 1021–1067. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Qi, G.; Yao, L.; Li, X.; Paek, K.Y.; Gao, W. Comparison of polysaccharides in ginseng root cultures and cultivated ginseng and establishment of high-content uronic acid plant synthesis system. Ind. Crops Prod. 2022, 186, 115155. [Google Scholar] [CrossRef]
- Hao, N.; Piao, Z.; Zang, J.; Li, H.; Zhou, R. Establishment of adventitious root cultures and assessment of secoiridoid production in the Chinese medicinal plant Gentiana scabra. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 864–873. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, Q.; Zhang, Y.; He, Y.; Yan, B.; Guo, L. Metabolomic profiling and chemical marker identification in medicinal plants of Atractylodes. J. Tradit. Chin. Med. Sci. 2025, 3, 87–95. [Google Scholar] [CrossRef]
- Baque, M.A.; Moh, S.H.; Lee, E.J.; Zhong, J.J.; Paek, K.Y. Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef]
- Gantait, S.; Mukherjee, E. Hairy root culture technology: Applications, constraints and prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Wen, Y.; Wang, L.; Wang, Y.; Liao, C.; Wang, L. Plant hairy roots: Induction, applications, limitations and prospects. Ind. Crops Prod. 2024, 219, 119104. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Harnessing nanoparticle-mediated elicitation in plant tissue culture: A promising approach for secondary metabolite production. Plant Cell Tissue Organ Cult. 2023, 155, 385–402. [Google Scholar] [CrossRef]
- Raza, A. Metabolomics: A systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2022, 41, 741–763. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.; Zhao, C.; Chen, G.Q. Applications of synthetic biology in medical and pharmaceutical fields. Signal Transduct. Target. Ther. 2023, 8, 199. [Google Scholar] [CrossRef]

| Level | Factor | |||
|---|---|---|---|---|
| 2,4-D (A) | NAA (B) | KT (C) | Explant (D) | |
| 1 | 2.0 | 0 | 0.3 | Leaf |
| 2 | 2.0 | 1.0 | 0 | Petiole |
| 3 | 1.5 | 0 | 0.5 | Stem |
| Level | Factor | ||
|---|---|---|---|
| 6-BA (A) | NAA (B) | Medium (C) | |
| 1 | 2.0 | 0.1 | MS |
| 2 | 4.0 | 0.4 | 1/2MS |
| 3 | 6.0 | 0.6 | 1/4MS |
| Level | E: 6-BA (mg/L) | B: NAA (mg/L) |
|---|---|---|
| 1 | 1.00 | 0.10 |
| 2 | 1.50 | 0.20 |
| 3 | 2.00 | 0.30 |
| 4 | 2.50 | 0.50 |
| 5 | 3.00 | 1.00 |
| 6 | 3.50 | 1.50 |
| 7 | 4.00 | 2.00 |
| Level | A: NAA (mg/L) | B: Activated Carbon (g/L) | C: Basic Medium |
|---|---|---|---|
| 1 | 0.1 | 0.2 | 1/2MS |
| 2 | 0.5 | 0.5 | 1/2MS |
| 3 | 1.0 | 1.0 | 1/2MS |
| Test Number | Factor | Induction Rate of Calli/% | |||
|---|---|---|---|---|---|
| 2,4-D (mg/L) | NAA (mg/L) | KT (mg/L) | Explant | ||
| 1 | 2.0 | 0 | 0.3 | 1 | 73.67 ± 3.51 bB |
| 2 | 2.0 | 1.0 | 0 | 2 | 91.00 ± 2.00 aA |
| 3 | 2.0 | 0 | 0.5 | 3 | 64.33 ± 1.53 dC |
| 4 | 2.0 | 0 | 0 | 3 | 34.67 ± 2.52 gF |
| 5 | 2.0 | 1.0 | 0.5 | 1 | 53.00 ± 2.00 eD |
| 6 | 2.0 | 0 | 0.3 | 2 | 72.33 ± 0.58 bcB |
| 7 | 1.5 | 0 | 0.5 | 2 | 67.00 ± 1.00 cdBC |
| 8 | 1.5 | 1.0 | 0.3 | 3 | 45.33 ± 1.53 fE |
| 9 | 1.5 | 0 | 0 | 1 | 18.00 ± 1.00 hG |
| k1 | 43.44 | 55 | 47.89 | 48.22 | |
| k2 | 64.83 | 63.11 | 63.78 | 76.78 | |
| k3 | - | - | 61.44 | 48.11 | |
| R | 21.39 | 8.11 | 15.89 | 28.67 | |
| Level | 6-BA (mg/L) | NAA (mg/L) | Medium | Budding Rate/% |
|---|---|---|---|---|
| 1 | 2.0 | 0.1 | 1.0 | 8.5 |
| 2 | 2.0 | 0.4 | 1.0 | 7.2 |
| 2 | 2.0 | 0.6 | 1.0 | 3.1 |
| 4 | 2.0 | 0.1 | 0.5 | 16.3 |
| 5 | 2.0 | 0.4 | 0.5 | 14.7 |
| 6 | 2.0 | 0.6 | 0.5 | 13.8 |
| 7 | 2.0 | 0.1 | 0.25 | 15.2 |
| 8 | 2.0 | 0.4 | 0.25 | 12.7 |
| 9 | 2.0 | 0.6 | 0.25 | 10.8 |
| 10 | 4.0 | 0.1 | 1.0 | 11.3 |
| 11 | 4.0 | 0.4 | 1.0 | 9.2 |
| 12 | 4.0 | 0.6 | 1.0 | 8.3 |
| 13 | 4.0 | 0.1 | 0.5 | 22.7 |
| 14 | 4.0 | 0.4 | 0.5 | 19.1 |
| 15 | 4.0 | 0.6 | 0.5 | 18.3 |
| 16 | 4.0 | 0.1 | 0.25 | 19.8 |
| 17 | 4.0 | 0.4 | 0.25 | 16.3 |
| 18 | 4.0 | 0.6 | 0.25 | 15.2 |
| 19 | 6.0 | 0.1 | 1.0 | 3.6 |
| 20 | 6.0 | 0.4 | 1.0 | 4.2 |
| 21 | 6.0 | 0.6 | 1.0 | 5.1 |
| 22 | 6.0 | 0.1 | 0.5 | 7.6 |
| 23 | 6.0 | 0.4 | 0.5 | 6.2 |
| 24 | 6.0 | 0.6 | 0.5 | 5.7 |
| 25 | 6.0 | 0.1 | 0.25 | 6.1 |
| 26 | 6.0 | 0.4 | 0.25 | 2.3 |
| 27 | 6.0 | 0.6 | 0.25 | 0 |
| k1 | 11.37 | 12.34 | 10.93 | |
| k2 | 15.58 | 10.21 | 13.82 | |
| k3 | 4.53 | 8.92 | 6.72 | |
| R | 11.05 | 3.42 | 7.1 |
| Test Number | 6-BA (mg/L) | NAA (mg/L) | Proliferation Coefficient |
|---|---|---|---|
| 1 | 1.0 | 1.0 | 5.47 ± 0.25 cCD |
| 2 | 1.5 | 0.2 | 4.10 ± 0.10 dD |
| 3 | 2.0 | 2.0 | 12.00 ± 0.75 aA |
| 4 | 2.5 | 0.3 | 7.57 ± 0.21 bB |
| 5 | 3.0 | 1.5 | 6.17 ± 0.15 cBC |
| 6 | 3.5 | 0.1 | 5.33 ± 0.58 cCD |
| 7 | 4.0 | 0.5 | 2.30 ± 0.26 eE |
| Level | NAA (mg/L) | Activated Carbon (g/L) | Average Number of Roots |
|---|---|---|---|
| 1 | 0.1 | 0.2 | 13.00 ± 1.00 efEF |
| 2 | 0.1 | 0.5 | 9.33 ± 0.58 ghGH |
| 3 | 0.1 | 1 | 8.00 ± 1.00 hH |
| 4 | 0.5 | 0.2 | 17.00 ± 1.00 cdCD |
| 5 | 0.5 | 0.5 | 23.00 ± 1.00 aA |
| 6 | 0.5 | 1 | 21.00 ± 1.00 abAB |
| 7 | 1 | 0.2 | 15.00 ± 1.00 deDEF |
| 8 | 1 | 0.5 | 12.00 ± 1.00 fgFG |
| 9 | 1 | 1 | 7.00 ± 1.00 hH |
| 10 | 0 | 0.2 | 19.00 ± 1.00 bcBC |
| 11 | 0 | 0.5 | 21.00 ± 1.00 abAB |
| 12 | 0 | 1 | 16.00 ± 1.00 dCDE |
| k1 | 18.67 | 16 | |
| k2 | 10.11 | 16.33 | |
| k3 | 20.33 | 13 | |
| k4 | 11.33 | NA | |
| R | 10.22 | 3.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Dai, X.; Li, Q.; Ge, Y.; Kang, C.; Wu, D.; Sun, J.; Wang, Y.; Zhang, Z.; Wang, S. In Vitro Plant Regeneration and Bioactive Metabolite Production of Endangered Medicinal Plant Atractylodes lancea (Thunb.) DC. Horticulturae 2025, 11, 691. https://doi.org/10.3390/horticulturae11060691
Zhang C, Dai X, Li Q, Ge Y, Kang C, Wu D, Sun J, Wang Y, Zhang Z, Wang S. In Vitro Plant Regeneration and Bioactive Metabolite Production of Endangered Medicinal Plant Atractylodes lancea (Thunb.) DC. Horticulturae. 2025; 11(6):691. https://doi.org/10.3390/horticulturae11060691
Chicago/Turabian StyleZhang, Chengcai, Xiaoyu Dai, Qi Li, Yang Ge, Chuanzhi Kang, Dehua Wu, Jiahui Sun, Yiheng Wang, Zekun Zhang, and Sheng Wang. 2025. "In Vitro Plant Regeneration and Bioactive Metabolite Production of Endangered Medicinal Plant Atractylodes lancea (Thunb.) DC" Horticulturae 11, no. 6: 691. https://doi.org/10.3390/horticulturae11060691
APA StyleZhang, C., Dai, X., Li, Q., Ge, Y., Kang, C., Wu, D., Sun, J., Wang, Y., Zhang, Z., & Wang, S. (2025). In Vitro Plant Regeneration and Bioactive Metabolite Production of Endangered Medicinal Plant Atractylodes lancea (Thunb.) DC. Horticulturae, 11(6), 691. https://doi.org/10.3390/horticulturae11060691





