1. Introduction
Ethnobotany, the study of the relationship between people and plants, has become an essential field of research in understanding the complex interactions between humans and their natural environment [
1]. Plants are vital to human societies, serving as sources of food, medicine, construction materials, and cultural practices [
2]. One such group of plants, the Bignoniaceae family, has garnered interest for its diverse uses and its presence in various aspects of life in tropical and subtropical regions [
3]. In Thailand, particularly in the northeastern province of Maha Sarakham, members of the Bignoniaceae family play an integral role in the daily lives of local communities [
4]. The province, situated in the heart of the Isan region, is known for its rich cultural heritage, where traditional knowledge of plant species is passed down through generations. Maha Sarakham has a tropical monsoon climate with distinct seasons and generally lower rainfall due to its location in a rain shadow zone. These seasonal changes affect plant growth and availability, influencing their traditional uses [
5].
Despite the ecological and cultural relevance of Bignoniaceae in the region, there is a significant lack of focused ethnobotanical research on this plant family in Thailand. The Bignoniaceae family is a diverse group of plants that includes approximately 80 genera and over 800 species, found mainly in tropical and subtropical regions of the world [
6]. In Thailand, species of the Bignoniaceae family are abundant, and they are commonly used for ornamental, medicinal, food, and practical purposes [
7]. In Maha Sarakham Province, many species of this family are cultivated in home gardens, temples, schools, and other public spaces for various uses [
8]. The unique characteristics of these plants—such as vibrant flowers, large fruits, and strong timber—make them valuable both for their aesthetic and functional qualities [
9]. Understanding the traditional knowledge surrounding these plants provides insight into the role they play in the daily lives of local communities, especially in rural settings where indigenous practices and traditional ecological knowledge remain strong [
10].
However, there exists a notable gap in ethnobotanical research specifically related to the Bignoniaceae family in Thailand. While studies have been conducted on the ethnobotany of various plant families in Southeast Asia [
11], little attention has been given to the specific uses of Bignoniaceae species in Thai communities, especially in the northeastern regions like Maha Sarakham. This research gap is critical, as it threatens the preservation of rich plant knowledge associated with cultural identity and sustainable practices. This gap in research means that the valuable traditional knowledge regarding these plants, including their uses in medicine, food, rituals, and other cultural practices, remains poorly documented. As a result, much of the cultural heritage tied to these plants is at risk of being lost, especially as modern development and urbanization continue to encroach upon rural areas [
12]. Thus, it is critical to document the ethnobotanical significance of these species to preserve both the plant knowledge and the cultural practices associated with them [
13].
To address this need, the present study investigates the ethnobotanical significance of the Bignoniaceae family in Maha Sarakham Province. The objectives are to document the traditional uses of these species, analyze their cultural importance, and explore the ways in which local communities continue to utilize them in daily life. By filling this knowledge gap, the research contributes baseline data essential for both cultural preservation and future conservation planning. This research seeks to contribute meaningful data to the field of ethnobotany and highlight the need for conservation strategies that respect and integrate traditional knowledge.
3. Results
3.1. Diversity of Bignoniaceae in Maha Sarakham Province
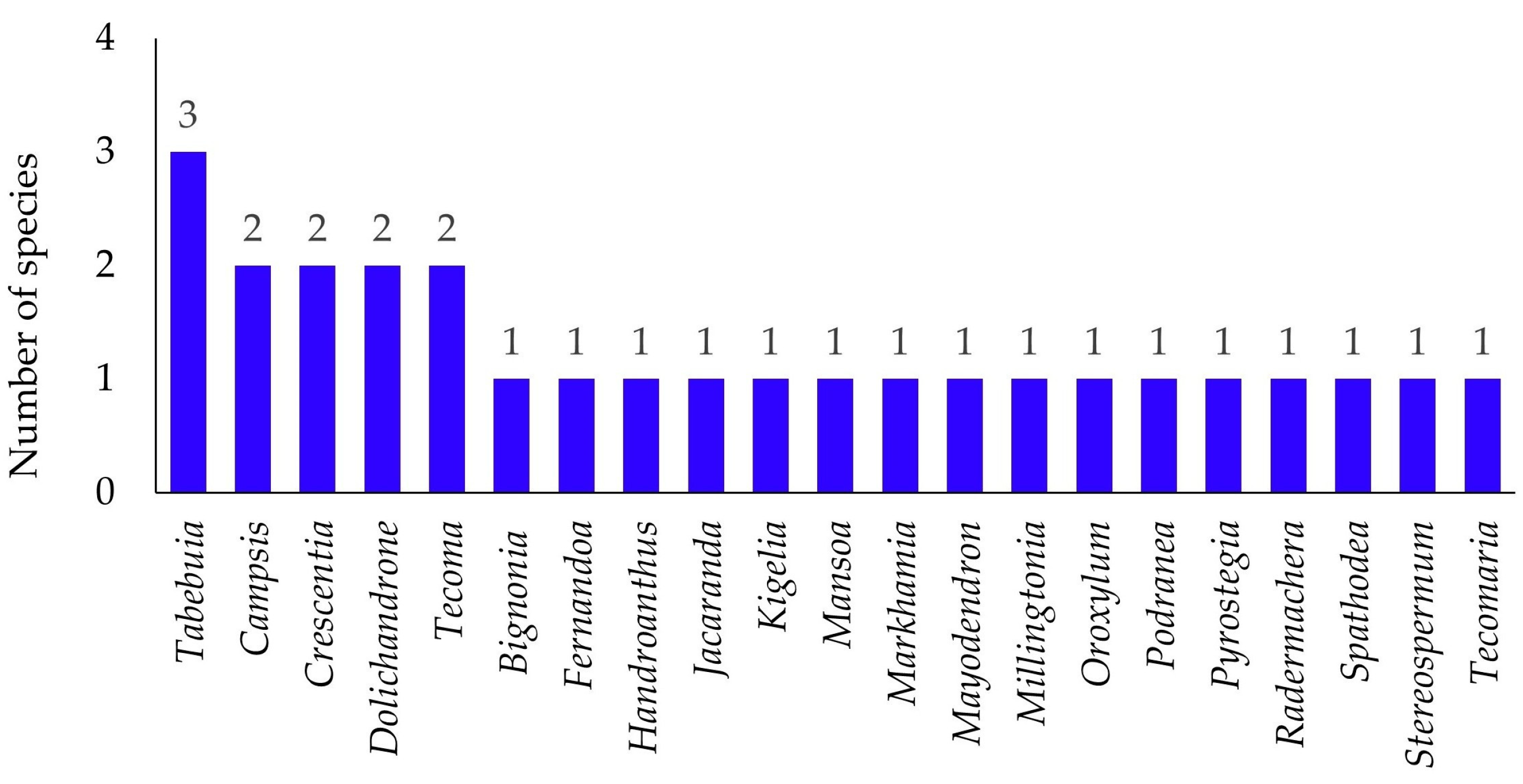
The study found 27 species of Bignoniaceae across 21 genera in Maha Sarakham Province (
Table 1,
Figure 2 and
Figure 3). Most genera are represented by a single species, including
Bignonia,
Fernandoa,
Handroanthus,
Jacaranda,
Kigelia,
Mansoa,
Markhamia,
Mayodendron,
Millingtonia,
Oroxylum,
Podranea,
Pyrostegia,
Radermachera,
Spathodea,
Stereospermum, and
Tecomaria. A few genera have multiple species, with
Tabebuia being the most diverse (3 species), followed by
Campsis,
Crescentia,
Dolichandrone, and
Tecoma (each with 2 species). This pattern shows that while species diversity is spread across many genera, a small number of genera contain the majority of species.
3.2. Phenology of Bignoniaceae in Maha Sarakham Province
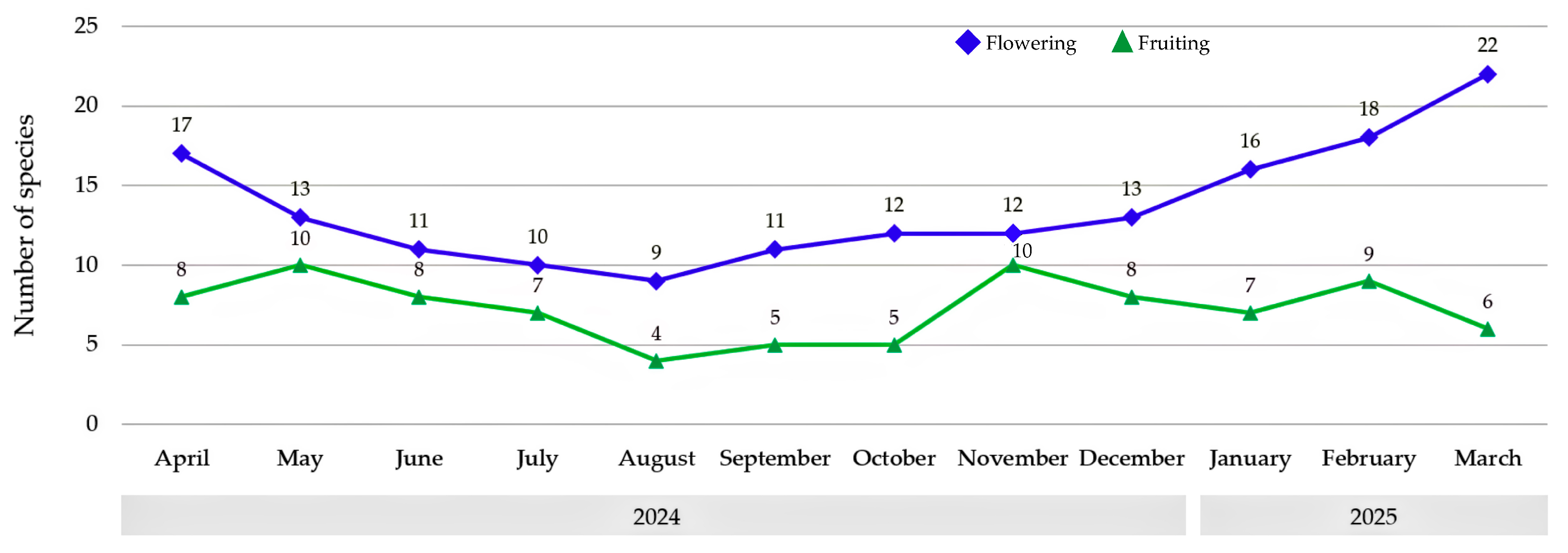
Flowering and fruiting of Bignoniaceae species in Maha Sarakham Province showed clear seasonal patterns (
Figure 4). Flowering occurred year-round, peaking in March (22 species) and reaching its lowest in August (9 species). Fruiting followed a similar but less pronounced trend, with peaks in May and November (10 species each) and a low in August (4 species). Flowering was consistently more frequent than fruiting, indicating a reproductive strategy focused on the dry-to-early rainy season for flowering, with fruiting spread more variably throughout the year.
3.3. Species Use Value (SUV) of Bignoniaceae in Maha Sarakham Province
A detailed list of Bignoniaceae species and their corresponding Species Use Values (SUV) is presented in
Table 1. These values indicate the extent to which each species is utilized across various domains in Maha Sarakham Province, including commercial cultivation, ornamental planting, traditional medicine, firewood, food, handicrafts, dye production, and ritual practices.
To facilitate interpretation, SUVs were grouped into three categories: high (SUV ≥ 0.30), medium (SUV 0.15–0.29), and low (SUV < 0.15). These categories were defined based on the range and distribution of SUV scores in this study and are used here as a heuristic framework to represent varying degrees of cultural and practical importance among species.
The high-use value category (SUV ≥ 0.30) comprises species that are widely utilized within local communities and serve multiple purposes. Among these, Dolichandrone serrulata (SUV = 0.381), D. spathacea (SUV = 0.365), and Oroxylum indicum (SUV = 0.358) are especially valued for their applications in traditional medicine, ritual practices, and, in some cases, ornamental planting. Millingtonia hortensis (SUV = 0.350) is commonly grown for ornamental and ritual purposes, and it is also popular in commercial cultivation due to its fragrant flowers. Tecoma stans cv. ‘Thong Urai Lueang’ (SUV = 0.342) is another high-SUV species, primarily cultivated as an ornamental plant, with additional value in medicinal uses.
The medium-use value category (SUV 0.15–0.29) includes species that are moderately utilized in the region. Fernandoa adenophylla (SUV = 0.254), Tabebuia rosea (SUV = 0.235), and Spathodea campanulata (SUV = 0.212) are mostly grown for ornamental purposes, though they are occasionally referenced in medicinal practices. Stereospermum neuranthum (SUV = 0.204) and Tabebuia aurea (SUV = 0.188) are sometimes used as firewood and also serve decorative functions. Other species in this category, such as Pyrostegia venusta (SUV = 0.177) and Tecomaria capensis ‘Aurea’ (SUV = 0.177), are mainly appreciated for their aesthetic appeal. Crescentia alata and Podranea ricasoliana (SUV = 0.154 each) are likewise cultivated primarily for ornamental purposes.
The low-use value category (SUV < 0.15) includes species with limited use or more restricted cultural relevance. Markhamia stipulata (SUV = 0.073), Crescentia cujete (SUV = 0.077), and Kigelia africana (SUV = 0.085) are occasionally used for firewood or ornamental purposes. Campsis radicans (SUV = 0.104), Mansoa alliacea (SUV = 0.112), and Jacaranda mimosifolia (SUV = 0.123) are primarily grown as ornamentals, with minor additional uses. Similarly, Tabebuia pallida and Handroanthus chrysanthus (SUV = 0.127 each), along with Mayodendron igneum (SUV = 0.123), are cultivated for their aesthetic value. Bignonia magnifica (SUV = 0.146), Campsis grandiflora, and Radermachera yunnanensis (SUV = 0.142 each) are also mainly valued for ornamental planting.
3.4. Genera Use Value (GUV) of Bignoniaceae in Maha Sarakham Province
Table 2 presents the Genera Use Values (GUVs) of Bignoniaceae recorded in Maha Sarakham Province. These values represent the average use of all species within each genus, offering insight into the overall significance of each genus based on local knowledge and utilization.
To facilitate interpretation, GUVs were categorized into three groups based on the range of values observed in this study: high (≥0.30), medium (0.15–0.29), and low (<0.15). This classification was used as an interpretive tool to help differentiate the relative cultural and practical importance of each genus.
The high-use value genera (GUV ≥ 0.30) include Dolichandrone (GUV = 0.373), Millingtonia (GUV = 0.350), and Oroxylum (GUV = 0.358). These genera are among the most valued in the province, with species widely used for medicinal, ornamental, and ritual purposes, and often involved in commercial cultivation. Their consistent presence across various uses underscores their ecological and cultural relevance in the region.
Genera with a medium-use value (GUV 0.15–0.29) include Tecoma (GUV = 0.262), Fernandoa (GUV = 0.254), Spathodea (GUV = 0.212), Stereospermum (GUV = 0.204), Tabebuia (GUV = 0.183), Pyrostegia and Tecomaria (GUV = 0.177 each), and Podranea (GUV = 0.154). These genera include species used for ornamental planting, medicinal applications, and occasionally for firewood or ritual purposes. Though not as dominant as the high-GUV group, they still hold moderate significance in local contexts.
Genera with low-use value (GUV < 0.15) include Bignonia (GUV = 0.146), Radermachera (GUV = 0.142), Campsis and Mayodendron (GUV = 0.123 each), Jacaranda (GUV = 0.123), Handroanthus (GUV = 0.127), Mansoa (GUV = 0.112), Crescentia (GUV = 0.115), Kigelia (GUV = 0.085), and Markhamia (GUV = 0.073). These genera are primarily used for ornamentals, with limited or specialized use in other domains such as rituals or firewood. While their aesthetic value is acknowledged, their broader utility remains relatively low compared to more frequently utilized genera.
3.5. Relative Frequency of Citation (RFC) of Bignoniaceae in Maha Sarakham Province
A comparative assessment between the Relative Frequency of Citation (RFC) and Species Use Value (SUV) shows a tendency to move in the same direction, indicating that species frequently cited by informants tend to have higher utility (
Table 3). However, several discrepancies between RFC and SUVs among species suggest that citation frequency does not always equate to greater diversity of use.
For instance, Dolichandrone serrulata, D. spathacea, and Oroxylum indicum not only have the highest RFC values (0.365, 0.362, and 0.350, respectively) but also rank highest in SUV (0.381, 0.365, and 0.358). This reflects both widespread knowledge and a broad range of uses within the local communities.
Conversely, certain species such as Tecoma stans (RFC = 0.308; SUV = 0.342) and Tecomaria capensis ‘Aurea’ (RFC = 0.154; SUV = 0.177) show higher use values relative to their citation frequencies. This suggests that while these species are known to fewer people, they are highly valued by those who do use them, possibly due to their specialized or multifunctional roles.
In contrast, some species with relatively moderate RFCs exhibit lower SUVs, such as Tabebuia rosea (RFC = 0.250; SUV = 0.235), indicating that although they are widely known, they may serve limited or singular uses.
The lowest RFC and SUVs were observed in species such as Markhamia stipulata (RFC = 0.062; SUV = 0.073) and Kigelia africana (RFC = 0.077; SUV = 0.085), highlighting their limited ethnobotanical recognition and application in the study area.
3.6. Cultural Significance Value (CI) of Bignoniaceae in Maha Sarakham Province
The Cultural Importance (CI) values of Bignoniaceae species in Maha Sarakham Province vary considerably, reflecting different levels of cultural significance (
Table 1).
Oroxylum indicum (0.415) holds the highest CI value, followed closely by
Millingtonia hortensis (0.412) and
Dolichandrone serrulata (0.400), highlighting their strong cultural relevance.
Other species such as Dolichandrone spathacea (0.388) and Tecoma stans cv. ‘Thong Urai Lueang’ (0.369) also demonstrate notable cultural importance. Fernandoa adenophylla (0.281) and Spathodea campanulata (0.258) exhibit moderate CI values, indicating a consistent cultural presence.
Species like Tabebuia rosea (0.258), Tecomaria capensis ‘Aurea’ (0.215), and Stereospermum neuranthum (0.223) show moderate to low levels of cultural significance. Meanwhile, species such as Pyrostegia venusta (0.204) and Tabebuia aurea (0.196) have a lesser but still relevant cultural role.
Several species, including Tecoma stans cv. ‘Thong Urai Micky Mouse’ (0.192), Jacaranda mimosifolia (0.173), and Crescentia alata (0.169), hold relatively low CI values, reflecting their more limited cultural use. Campsis grandiflora (0.162), Bignonia magnifica (0.154), and Handroanthus chrysanthus (0.154) also show lower CI values, indicating a relatively minor cultural importance.
Finally, species like Kigelia africana (0.092), Crescentia cujete (0.081), and Markhamia stipulata (0.081) have the lowest CI values, suggesting their minimal yet still present role in the region’s cultural practices. These varying CI values reflect the diversity in cultural importance among Bignoniaceae species in Maha Sarakham Province.
The Cultural Importance (CI) values for the utilization of Bignoniaceae species in Maha Sarakham Province reveal varying levels of significance across different categories (
Table 4). Ornamentals have the highest CI value (2.34), indicating their major role in the cultural and aesthetic landscape of the region.
Commercial cultivation follows with a CI value of 1.39, highlighting the economic importance of these species. The Food category also has a notable CI value of 0.70, showing that Bignoniaceae species are utilized as a food source within the community.
Uses such as medicines (0.53) and firewood (0.41) hold moderate CI values, indicating a practical role in local life. On the other hand, dyes (0.13) and ritual uses (0.15) have relatively lower CI values, suggesting their limited but still recognized cultural importance. Handicrafts show the lowest CI value (0.08), reflecting a more minimal involvement in cultural practices.
3.7. Cultural Food Significance Index (CFSI) of Bignoniaceae in Maha Sarakham Province
A total of 11 species from the Bignoniaceae family were evaluated for their cultural food significance in Maha Sarakham Province using the Cultural Food Significance Index (CFSI) (
Table 5). The CFSI values varied widely, reflecting differences in cultural familiarity, usage frequency, and food-medicinal roles of the species.
The species with the highest CFSI was Dolichandrone serrulata, with a value of 869.400. This exceptionally high score was driven by its frequent citation (QI = 35), high frequency of use (FUI = 4), diversity of plant parts used (PUI = 5.75), and high appreciation in taste (TSAI = 9.0). This suggests that D. serrulata holds both culinary and medicinal significance in the region.
Dolichandrone spathacea ranked second, with a CFSI of 486.000, followed by Oroxylum indicum (140.400), Millingtonia hortensis (128.250), and Fernandoa adenophylla (86.873). These species showed a combination of moderate to high availability, multifunctional uses, and taste appreciation, making them culturally valuable food-medicinal plants.
Species with lower CFSI values included Spathodea campanulata (4.950), Stereospermum neuranthum (2.340), and Tabebuia rosea (13.365), indicating limited food-related use despite their presence in the local flora.
Interestingly, Crescentia alata (23.760) and C. cujete (16.830), though less cited, still contributed to local food traditions due to their distinct roles and availability.
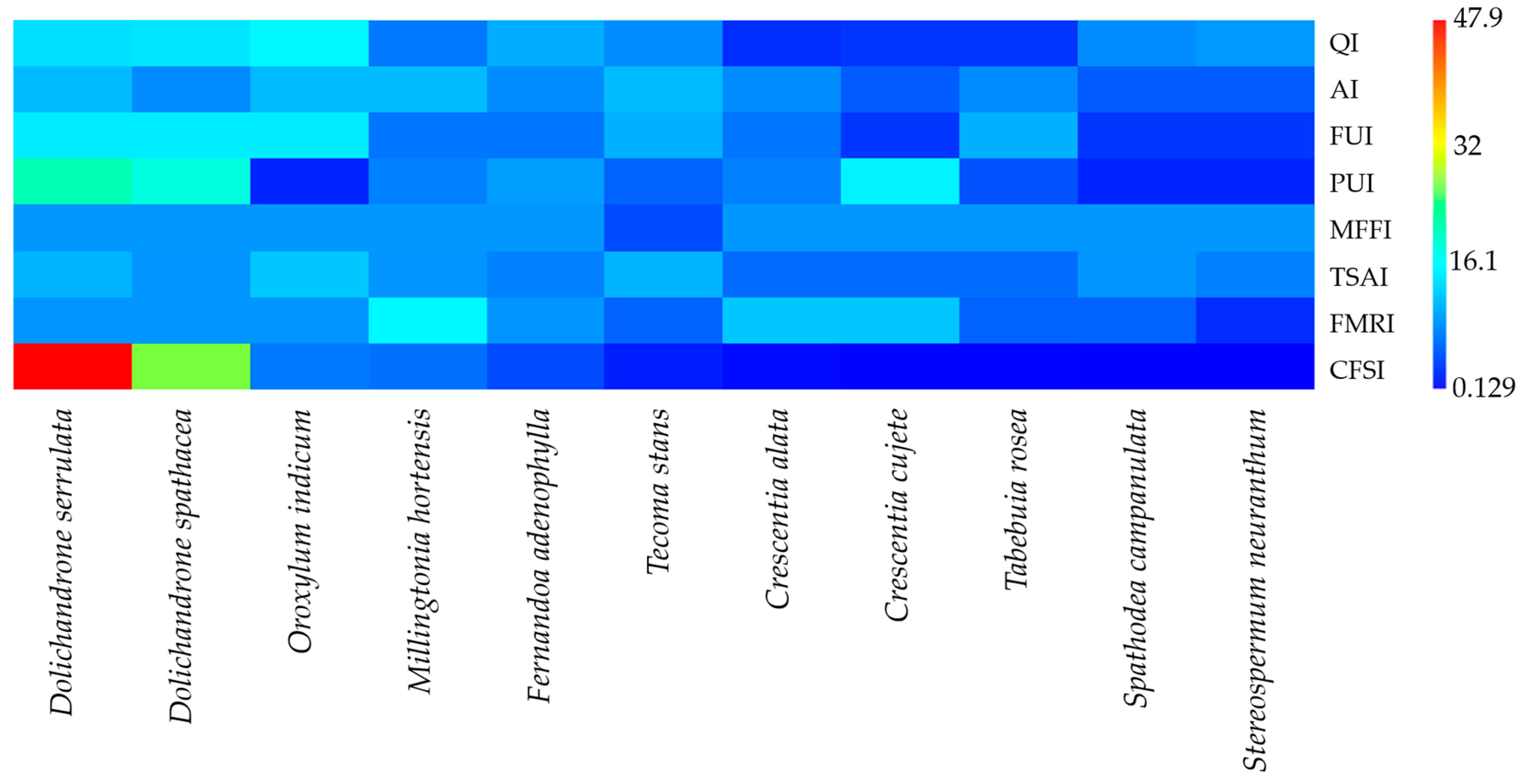
The heatmap analysis (
Figure 5) revealed notable variation in CFSI component scores across species.
Dolichandrone serrulata exhibited the highest cultural food significance, reflected by its top-ranking QI value, indicating frequent mention by informants. It also scored highly on AI and FUI, suggesting both wide availability and frequent consumption.
Dolichandrone spathacea also demonstrated strong cultural relevance, particularly through high AI and TSAI scores, implying its common presence in the wild and favorable taste perception.
Conversely, species such as Spathodea campanulata and Stereospermum neuranthum consistently received low scores across all indices, suggesting limited cultural integration as food sources. Most other species displayed moderate values across the indices, indicating variable but generally lesser roles in local food traditions.
Notably, species with higher PUI and MFFI scores (e.g., Millingtonia hortensis and Oroxylum indicum) were those consumed using multiple plant parts and through versatile methods (e.g., raw or cooked as a main component), underlining their broader culinary utility. In contrast, species with low FMRI values were seldom associated with medicinal uses, potentially reflecting a narrower ethnobotanical relevance.
The integration of these ethnobotanical indices through the CFSI framework enabled the identification of species with both high cultural value and potential for further development as nutritionally and culturally significant food resources.
3.8. Utilization of Bignoniaceae in Maha Sarakham Province
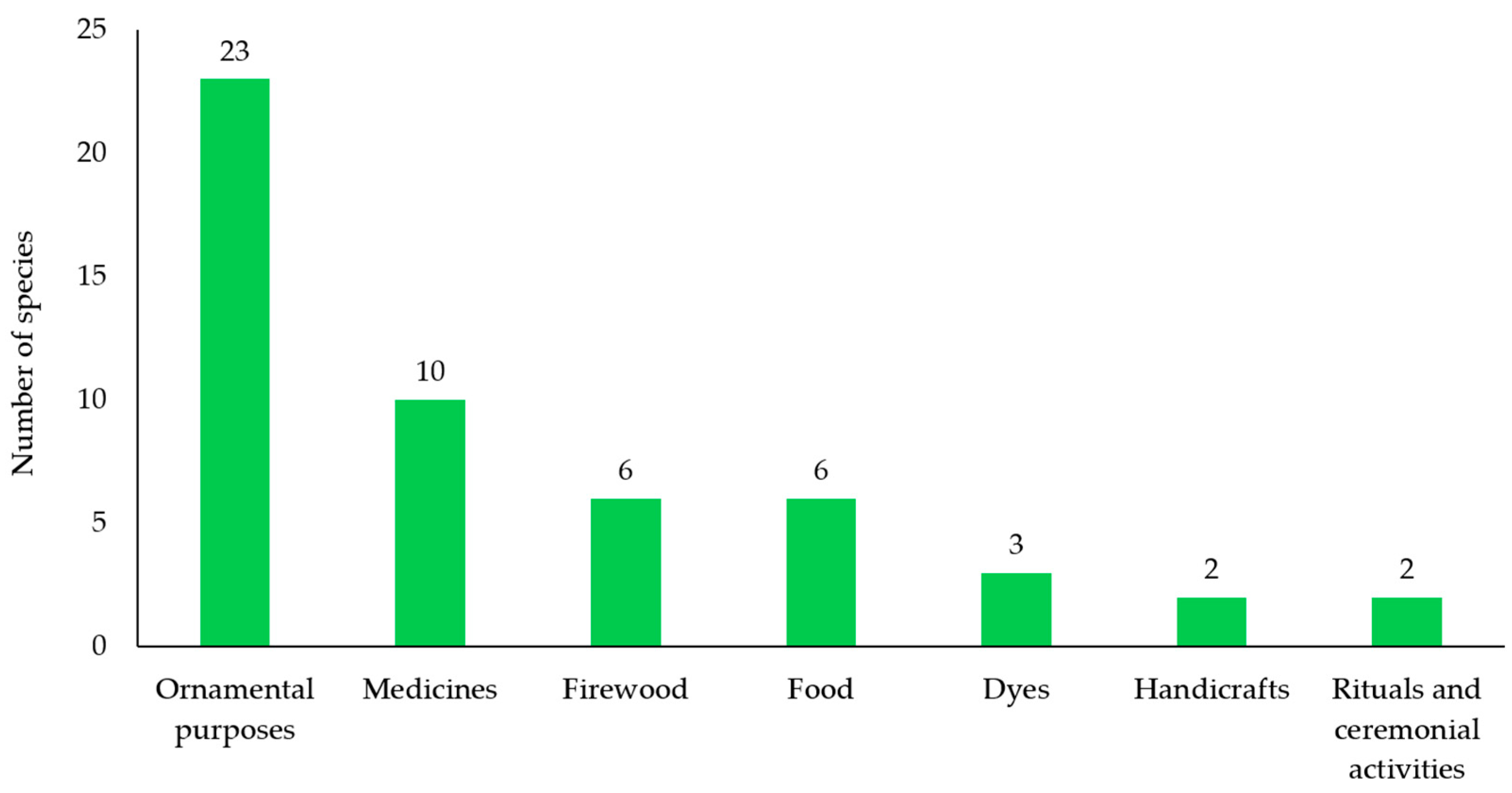
The most prevalent use of the surveyed Bignoniaceae species in Maha Sarakham Province (
Table 5) was for ornamental purposes, accounting for 23 species (44.23%), followed by traditional medicine (10 species, 19.23%), and both firewood and food (each 6 species, 11.54%). Fewer species were used for dyes (3 species, 5.77%), handicrafts (2 species, 3.85%), and rituals or ceremonial activities (2 species, 3.85%) (
Figure 6).
3.8.1. Bignoniaceae Used as Dyes
The surveyed Bignoniaceae plants, namely
Dolichandrone serrulata,
Millingtonia hortensis, and
Oroxylum indicum, were reported to be used as dyes in the region (
Table 1). The bark of
D. serrulata is commonly used to dye silk, imparting a brown tone. Similarly, the bark of
M. hortensis is used to dye silk, giving it a yellow tone. Both the bark and leaves of
O. indicum are used to dye silk and cotton, producing a greenish-yellow tone. The informants highlighted the cultural significance of these plants in dyeing, emphasizing their practical use and importance in the region (
Table 6).
3.8.2. Bignoniaceae Used as Firewood
The surveyed Bignoniaceae species, including
Dolichandrone serrulata,
D. spathacea,
Fernandoa adenophylla,
Markhamia stipulata,
Oroxylum indicum, and
Stereospermum neuranthum, were reported to be used as firewood in the region (
Table 1). These species serve as important fuel sources for the local community, playing a crucial role in meeting cooking and heating needs. The use of these plants emphasizes the practical application of readily available plant materials for daily energy requirements.
3.8.3. Bignoniaceae Used as Food
The surveyed Bignoniaceae species, including
Dolichandrone serrulata,
D. spathacea,
Fernandoa adenophylla,
Oroxylum indicum,
Spathodea campanulata, and
Stereospermum neuranthum, were reported to be used as food sources in the region (
Table 1). These species provide valuable edible parts, such as fruits, flowers, and leaves, which are incorporated into various traditional dishes and local culinary practices. For example, the fruit and young leaves of
O. indicum are commonly consumed for their nutritional value, while the edible flowers of
Spathodea campanulata,
D. serrulata, and
D. spathacea are used in local cuisine. The use of these plants for food highlights their significance in local diets, contributing to both the nutritional and cultural practices of the community (
Table 7).
3.8.4. Bignoniaceae Used as Handicrafts
The survey found that
Stereospermum neuranthum and
Tabebuia rosea, both from the Bignoniaceae family, are used for handicrafts in the region (
Table 1). These plants are valued for their materials, with the wood of
S. neuranthum being used to make poles, floorboards, and house walls. Additionally, the trunk of
T. rosea, in addition to being utilized as firewood, is also used as pulp to make paper. The use of these plants highlights their cultural significance in local handicrafts and underscores their contribution to the handicraft traditions of the region.
3.8.5. Bignoniaceae Used for Ornamental Purposes
The surveyed Bignoniaceae species, including
Bignonia magnifica,
Campsis grandiflora,
C. radicans,
Crescentia alata,
Dolichandrone serrulata,
D. spathacea,
Fernandoa adenophylla,
Handroanthus chrysanthus,
Jacaranda mimosifolia,
Kigelia africana,
Mansoa alliacea,
Mayodendron igneum,
Millingtonia hortensis,
Podranea ricasoliana,
Pyrostegia venusta,
Radermachera yunnanensis,
Spathodea campanulata,
Tabebuia aurea,
T. pallida,
T. rosea,
Tecoma stans,
T. stans cv. ‘Thong Urai Micky Mouse’ and
Tecomaria capensis ‘Aurea’, are widely cultivated for ornamental purposes in the region (
Table 1). These species are valued for their striking flowers, vibrant colors, and attractive growth forms, making them popular choices for landscaping, urban beautification, and private gardens.
These plants can be found in a variety of public and private spaces, including home gardens, temples, educational institutions, government offices, hotels, and resorts. For example, species such as Jacaranda mimosifolia, Tabebuia rosea, and Handroanthus chrysanthus are often planted in public parks, along roadways, and in hotel or resort gardens to enhance the aesthetic appeal of these spaces. Campsis grandiflora and Tecoma stans are frequently used in educational institutions landscapes, while Spathodea campanulata and Radermachera yunnanensis are often seen around government offices and temples, where they provide shade and ornamental value. The cultivation of these species for ornamental use not only enhances the beauty of these locations but also contributes significantly to the local horticultural economy, with many plants being sold in local markets and nurseries. Their widespread use underscores their importance as decorative plants and valuable economic assets for local communities.
3.8.6. Bignoniaceae Used as Medicines
The survey found that several Bignoniaceae species are used as medicines in the region (
Table 1). These include
Crescentia alata,
C. cujete,
Dolichandrone serrulata,
D. spathacea,
Fernandoa adenophylla,
Millingtonia hortensis,
Oroxylum indicum,
Spathodea campanulata,
Tabebuia rosea, and
Tecoma stans. These species are utilized in traditional medicine for treating a variety of ailments, highlighting their significant role in the local healthcare practices and their cultural importance in the region.
3.8.7. Bignoniaceae Used in Rituals and Ceremonial Activities
The study found that two Bignoniaceae species,
Millingtonia hortensis and
Tecoma stans, were widely used in rituals and various activities (
Table 1). The flowers of these plants were commonly offered to Buddha and used as ornamental plants in homes, as they were believed to bring prosperity and wealth.
3.9. Informant Consensus Factor (Fic) of Bignoniaceae in Maha Sarakham Province
The Informant Consensus Factor (F
ic) was calculated to assess the degree of agreement among informants regarding the medicinal use of Bignoniaceae species in Maha Sarakham Province (
Table 8). To categorize the medicinal uses of Bignoniaceae species reported by informants, we grouped ailments into major physiological and ethnomedicinal categories based on previous ethnobotanical literature and the World Health Organization’s International Classification of Diseases (ICD-11) [
25]. The categories identified in our study included the following: (1) skin conditions, (2) infections, (3) obstetrics, gynecology, and urinary tract disorders, (4) central nervous system disorders, (5) gastrointestinal system disorders, (6) respiratory system disorders, and (7) nutrition and blood-related conditions. The F
ic values varied across different ailment categories, ranging from 0.38 to 1.00. The highest consensus (F
ic = 1.00) was observed for the treatment of obstetrics, gynecology, and urinary tract disorders, although this category had the lowest number of use reports (N
ur = 2), indicating a unanimous agreement on a single taxon. The respiratory system category showed a high consensus as well (F
ic = 0.83), followed by the gastrointestinal system (F
ic = 0.72), which also had the highest number of use reports (N
ur = 26), suggesting strong shared knowledge and widespread use in this category. Moderate consensus was found in infections (F
ic = 0.67), the central nervous system (F
ic = 0.50), and nutrition and blood-related conditions (F
ic = 0.57). The lowest consensus was observed in the treatment of skin-related ailments (F
ic = 0.38), reflecting more variability in plant use among informants. It is also noted that the infections category appeared twice in the dataset with slightly differing values, indicating a potential data overlap or classification discrepancy.
3.10. Fidelity Level (FL) of Bignoniaceae in Maha Sarakham Province
The analysis of Fidelity Level (FL) among the documented species within the Bignoniaceae family reveals important insights into the consensus among informants regarding the specific therapeutic uses of these plants. As a key metric in ethnobotanical studies, FL serves to highlight the degree of cultural agreement and the perceived efficacy of plant-based treatments for particular ailments.
In this study, Spathodea campanulata exhibited the highest Fidelity Level (FL = 71.43%), with its bark exclusively cited for the treatment of constipation. This high level of agreement suggests strong cultural validation and therapeutic relevance within the local traditional knowledge system. Similarly, Tabebuia rosea demonstrated a high FL (66.67%) for its use in treating stomach pain and diarrhea, while Fernandoa adenophylla achieved an FL of 66.67% for treating flatulence, particularly with the use of its bark.
Other species with notable FL values include Tecoma stans, with an FL of 75.00% for treating diabetes using bark decoctions, and Crescentia alata, which showed an FL of 60.00% for wound healing and bleeding control when using leaf-based preparations.
Moderate FL values (40–60%) were observed in species such as Crescentia cujete and Dolichandrone serrulata, each used across a range of ailments including gastrointestinal, respiratory, and neurological conditions. These intermediate FL values reflect both a significant level of agreement among informants and the multipurpose nature of these species.
Lower FL values (<40%) were common among species reported for multiple therapeutic uses. For example, Oroxylum indicum was cited for treating wounds, abscesses, and heartburn, resulting in more dispersed informant consensus (FL = 14.29–57.14%). Similarly, Millingtonia hortensis and Dolichandrone spathacea were reported for diverse uses ranging from treating tuberculosis and fever to functioning as hematinic agents, leading to moderate to low FL values.
A detailed summary of the species, used parts, preparation methods, routes of administration, therapeutic applications, and corresponding Fidelity Levels is presented in
Table 9.
3.11. Economic Value of Bignoniaceae in Maha Sarakham Province
A total of 27 Bignoniaceae plant species were recorded as being commercially cultivated in Maha Sarakham Province. The economic data included price ranges per pot, average price per pot, number of pots sold per year, and the calculated economic value (EV) per species (
Table 10).
The highest economic value (EV) was recorded for Dolichandrone serrulata, amounting to 138,000 THB per year, followed by Jacaranda mimosifolia (135,000 THB), Crescentia alata (105,000 THB), and Tecoma stans cv. ‘Thong Urai Micky Mouse’ (96,985 THB). These high EVs were generally the result of either high average prices per pot, substantial sales volume, or a combination of both.
The species with the highest average price per pot was Dolichandrone serrulata at 5750 THB, while the lowest was Stereospermum neuranthum at 72.50 THB. Tecoma stans cv. ‘Thong Urai Lueang’ showed the highest annual volume sold with 175 pots, although its lower average price (85.00 THB/pot) limited its total economic contribution (14,875 THB).
Species with moderate prices and consistent sales, such as Tabebuia aurea (375.00 THB/pot, 140 pots/year), also demonstrated strong economic returns (52,500 THB). Conversely, low-cost species like Fernandoa adenophylla (75.00 THB/pot) and Stereospermum neuranthum (72.50 THB/pot) contributed the least in terms of economic value (4125 THB and 9062.50 THB, respectively).
The data indicate that both price and market demand influence the commercial success of Bignoniaceae species in the region. High-value ornamental species like Jacaranda mimosifolia, Crescentia alata, and Dolichandrone serrulata represent key economic assets in the local nursery trade.
4. Discussion
The present study reveals a moderate to high diversity of Bignoniaceae in Maha Sarakham Province, with 27 species across 21 genera. This diversity reflects the adaptability of Bignoniaceae to local environmental conditions. The dominance of monotypic genera suggests a broad but sparse distribution, likely shaped by habitat specificity, dispersal limitations, and historical biogeography.
In comparison, earlier ethnobotanical studies reported much lower Bignoniaceae representation. Saisor et al. [
8] found only
Millingtonia hortensis among 101 species in a forested area, while Saensouk et al. [
4] recorded only
Oroxylum indicum from local markets. Although valuable, these studies highlight the limited visibility of Bignoniaceae in broader plant surveys.
Our province-wide, family-focused survey, based on interviews with 260 informants and supporting fieldwork, revealed significantly greater diversity. By incorporating ethnobotanical indices such as SUV, RFC, CI, and CFSI, this study not only documents species richness but also emphasizes their cultural and ecological importance. The comparison underscores how thematic and spatial focus can shape research outcomes and affirms the value of targeted taxonomic studies in uncovering the full extent of traditional plant knowledge, particularly for underexplored families like Bignoniaceae.
The distribution of Bignoniaceae genera in Maha Sarakham Province reflects both ecological adaptation and human influence. Genera like
Bignonia,
Jacaranda,
Kigelia, and
Spathodea are notable for their ornamental and ecological roles, often linked to natural occurrence and anthropogenic introduction in urban or cultivated areas. In contrast, genera with higher species richness such as
Tabebuia,
Campsis,
Crescentia,
Dolichandrone, and
Tecoma suggest localized diversification or successful naturalization, likely due to broader ecological tolerance or reproductive strategies favoring establishment across varied habitats [
26,
27]. This uneven species distribution mirrors global Bignoniaceae diversity patterns, where adaptive radiation and widespread cultivation shape genus dominance [
28,
29]. The presence of native genera like
Oroxylum and
Stereospermum further underscores the integration of indigenous flora in the region.
Phenological patterns indicate seasonal reproductive strategies adapted to the tropical monsoon climate. Peak flowering in March coincides with the late dry season, optimizing pollination efficiency through increased pollinator activity and floral visibility [
30,
31]. Reduced flowering during the peak rainy season (August) likely results from environmental stresses such as heavy rainfall and humidity limiting reproductive success [
32]. The bimodal flowering observed towards year-end reflects phenological flexibility in response to climatic variability [
33]. Fruit production follows a more dispersed temporal pattern, with staggered peaks in May and November that may reduce competition for dispersers and predation risk [
34,
35]. The consistent predominance of flowering over fruiting highlights additional biotic and abiotic constraints influencing successful fruit development, including pollinator availability and environmental conditions [
36,
37,
38,
39].
The reproductive phenology of Bignoniaceae in northeastern Thailand is strongly influenced by seasonal cues, reflecting adaptive strategies to the region’s environmental conditions. Understanding these patterns is crucial for effective management of flowering tree resources in landscaping, reforestation, and conservation efforts [
40].
Species Use Values (SUV) reveal the cultural, economic, and ecological significance of Bignoniaceae species to local communities. High-use species such as
Dolichandrone serrulata,
D. spathacea,
Oroxylum indicum,
Millingtonia hortensis, and
Tecoma stans are deeply embedded in traditional medicine, rituals, and ornamental cultivation, highlighting their multifunctional roles and adaptability to local landscapes [
5,
7,
8,
41,
42,
43,
44,
45]. Medium-use species contribute primarily as ornamentals and occasional sources of firewood or medicine, illustrating the varied utility even among less prominent taxa [
46,
47]. Low-use species, while mostly ornamental, may possess untapped ethnobotanical potential due to limited knowledge or recent introduction, suggesting avenues for future exploration [
46].
At the genus level, Genera Use Values (GUVs) emphasize the prominence of
Dolichandrone,
Millingtonia, and
Oroxylum as culturally and ecologically important groups, warranting attention in conservation and sustainable use initiatives [
18,
48]. Medium-use genera like
Tecoma,
Fernandoa, and
Spathodea maintain important roles mainly in ornamentals and traditional uses, while low-use genera such as
Markhamia,
Kigelia, and
Crescentia—primarily valued ornamentally—present opportunities for further ethnobotanical research and community engagement to enhance local biodiversity and livelihoods [
4,
49,
50,
51,
52,
53].
The positive correlation between Relative Frequency of Citation (RFC) and Species Use Value (SUV) indicates that widely known Bignoniaceae species in Maha Sarakham Province, such as
Dolichandrone serrulata,
D. spathacea, and
Oroxylum indicum, hold multifaceted roles in medicine, ornamentation, and rituals, reflecting their integral place in local cultural and economic systems [
4,
19]. However, discrepancies between RFC and SUV—exemplified by
Tecoma stans and
Tecomaria capensis ‘Aurea’—highlight how localized knowledge enhances species’ multifunctionality despite lower citation rates, emphasizing the value of specialized uses within communities [
46,
49]. Conversely, species like
Tabebuia rosea exhibit broad recognition but narrower functional roles, predominantly ornamental [
54], while species with low RFC and SUV, such as
Markhamia stipulata and
Kigelia africana, currently possess limited ethnobotanical relevance but remain important for biodiversity and potential future uses [
55].
Cultural Significance Index (CI) values further underscore the prominence of
Oroxylum indicum,
Millingtonia hortensis, and
Dolichandrone serrulata as keystone species in cultural practices, including rituals, medicine, and commercial cultivation, reflecting their multifaceted contributions to the region’s socio-ecological landscape [
8,
18,
41]. Species with moderate CI, such as
Tecoma stans and
Tabebuia rosea, illustrate more specialized cultural roles, primarily aesthetic, which points to niche contributions within local communities [
56]. The concentration of cultural importance in ornamentals (CI = 2.34), followed by commercial cultivation and food uses, highlights the significant ecological and economic roles these species fulfill, while lower CI values in medicine, firewood, dyes, rituals, and handicrafts suggest more specialized or less frequent applications possibly constrained by availability or knowledge [
57,
58,
59].
The Cultural Food Significance Index (CFSI) reveals that
Dolichandrone serrulata and
D. spathacea are central to local food and medicinal traditions, with high citation, use frequency, and versatility contributing to their cultural prominence [
7,
8,
60,
61,
62,
63,
64]. Other species like
Oroxylum indicum and
Millingtonia hortensis also play important, though more specialized, roles in culinary practices, reflecting diverse but focused contributions to local diets [
4,
8,
41]. In contrast, species such as
Spathodea campanulata and
Stereospermum neuranthum show limited involvement in food culture, highlighting variation in species’ utilitarian significance [
7,
50]. Moderate CFSI values for
Tabebuia rosea and
Crescentia species indicate their distinct but less widespread culinary uses, demonstrating the heterogeneity of food-related ethnobotanical knowledge [
4,
8,
41].
These findings highlight the need to document and safeguard culturally significant species and practices that may be at risk due to urbanization, generational knowledge loss, or shifting land use. Future research should explore community-led conservation strategies and support educational initiatives to revitalize ethnobotanical knowledge systems, ensuring their transmission and integration into regional development and biodiversity planning.
The combined indices emphasize that while some Bignoniaceae species are culturally and economically central due to their multifunctionality and broad recognition, others contribute through specialized or emerging roles, underscoring the importance of preserving ethnobotanical knowledge and exploring underutilized species to support local biodiversity, cultural heritage, and sustainable livelihoods [
4,
18,
46,
49,
57,
65,
66,
67].
The diverse utilization of Bignoniaceae species in Maha Sarakham Province underscores their integral role in local culture, economy, and ecology. The predominance of ornamental uses (44.23%) highlights their cultural importance in beautifying urban and rural landscapes, supporting horticultural economies, and contributing to regional aesthetics [
4,
8,
16,
18,
41,
53,
68,
69]. Additionally, the use of these species for traditional dyes, firewood, food, handicrafts, and spiritual practices illustrates their multifunctional value across livelihoods and cultural expressions [
4,
8,
41,
51,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79].
Medicinal applications (19.23%) further demonstrate the significance of Bignoniaceae in traditional healthcare, where species address various common ailments, reinforcing their enduring relevance in local health systems [
46,
49,
60,
79,
80]. Together, these uses reflect a deep cultural and ecological connection, emphasizing the importance of conserving these species to sustain both biodiversity and cultural heritage [
4,
8,
16,
18,
41].
Interviews revealed that local people acquire Bignoniaceae species for medicinal purposes through two main ways: harvesting plants directly from their home gardens or nearby natural habitats, and purchasing from local herbalists or markets. Species that are commonly cultivated and readily available are often collected from personal gardens, while less accessible species or fresh materials are typically bought from herbal vendors. This dual approach reflects the flexibility and practicality of traditional plant use in the community.
Economically, Bignoniaceae species contribute substantially to the local horticultural market, with commercial cultivation driven by both high-value species like
Dolichandrone serrulata and popular, affordable species such as
Tecoma stans cv. ‘Thong Urai Lueang’ [
4,
81,
82,
83,
84,
85]. The balance between price and demand shapes market dynamics, supporting diverse consumer needs and livelihoods. Moreover, the adaptability and resilience of many Bignoniaceae species to local climatic conditions enhance their horticultural value and suitability for sustainable landscaping in Northeastern Thailand [
4,
16,
57].
These findings highlight the multifaceted ecological, cultural, and economic significance of Bignoniaceae in Maha Sarakham Province, underscoring their vital role in supporting local communities and promoting sustainable development.
Compared to major botanical families in Thailand such as Asteraceae, Convolvulaceae, Leguminosae, Piperaceae, Smilacaceae, and Zingiberaceae [
16,
18,
23,
53,
86,
87,
88,
89,
90,
91,
92], the Bignoniaceae family occupies a more specialized yet culturally significant niche. Its prominence is particularly noted in ornamental, medicinal, and ritual uses. This pattern of culturally meaningful, though relatively limited, utilization is also reflected across Southeast Asia and South America. Despite having fewer species, Bignoniaceae species in these regions hold substantial ecological and ethnobotanical importance [
27,
28,
31,
46,
48,
51,
54,
57,
80].
Within Maha Sarakham Province and more broadly in Thailand, several Bignoniaceae species such as Markhamia stipulata and Kigelia africana remain largely underutilized in ethnobotanical contexts. These species represent untapped potential for further research, conservation efforts, and sustainable community use. Their presence underscores a gap in both biodiversity knowledge and cultural application that could be addressed in future studies.
Regarding non-native Bignoniaceae species introduced to Thailand, many retain traditional uses similar to those documented in their regions of origin. For example,
Tecoma stans and
Spathodea campanulata are used medicinally and ornamentally in Thailand much like in the Americas, their native range [
31,
51]. Understanding the approximate introduction timelines of such species and how their uses align with ancestral knowledge can shed light on processes of cultural adaptation and exchange, enriching the ethnobotanical landscape of Thailand.
The high cultural and utilitarian value attributed to
Dolichandrone serrulata,
D. spathacea, and
Oroxylum indicum in Maha Sarakham Province is consistent with reports from other parts of Thailand and Southeast Asia. These species are multifunctional, serving roles in traditional medicine, ornamentation, and ritual practices. For instance,
Oroxylum indicum is widely recognized in India for its medicinal fruits and seeds, and it is commonly used to treat respiratory and digestive ailments [
42]. This corroborates its longstanding cultural significance and medicinal efficacy across Asia.
While ethnobotanical documentation for
Dolichandrone serrulata outside Thailand is limited, pharmacological studies highlight its antioxidant and α-glucosidase inhibitory activities, underscoring its medicinal potential locally [
93]. Similarly,
Dolichandrone spathacea has demonstrated potent bioactivities such as xanthine oxidase inhibition, as reported in recent Vietnamese research [
94]. The combined evidence of widespread use, pharmacological validation, and cultural integration suggests that accessibility, therapeutic efficacy, and deep-rooted cultural knowledge contribute to the prominence of these species both regionally and locally.