1. Introduction
Aronia melanocarpa, commonly known as black chokeberry, represents a shrub species of significant economic and pharmacological importance, indigenous to North America and widely cultivated throughout Europe and Asia [
1]. The fruits of
A. melanocarpa are notably esteemed for their exceptionally high concentrations of polyphenolic compounds, including anthocyanins, flavonoids, and proanthocyanidins, which contribute to their robust antioxidant activity and therapeutic potential. A multitude of studies have correlated these phytochemicals with a range of beneficial health effects, including anti-inflammatory, antidiabetic, hepatoprotective, and anticancer properties, thereby positioning black chokeberry as a promising candidate for the development of functional food and nutraceutical products [
2,
3,
4].
Beyond its medicinal uses,
A. melanocarpa interests horticulturists due to its ability to thrive in diverse environmental conditions, including poor soils, drought, and frost. However, traditional propagation methods, including seed and stem cuttings, face several challenges [
1]. Seed propagation tends to lead to genetic diversity and delays in flowering and fruiting, while vegetative propagation can be restricted by seasonal factors and a relatively low rate of multiplication [
1,
5]. These challenges have prompted the need for improved propagation methods, particularly in vitro micropropagation, which enables rapid multiplication, year-round cultivation, and the production of uniform, disease-free planting material.
Numerous micropropagation protocols have been developed for
A. melanocarpa, examining various combinations of basal media, plant growth regulators (PGRs), and environmental conditions [
6]. Initial investigations concentrated on utilizing standard media, including Murashige and Skoog (MS) and Woody Plant Medium (WPM), augmented with cytokinins such as benzylaminopurine (BAP), kinetin (KIN), and thidiazuron (TDZ), which have demonstrated differential effects on shoot initiation and multiplication [
7]. Nevertheless, the responses to these treatments were frequently genotype-dependent, with specific cultivars, including “Nero” and “Viking,” displaying superior regeneration efficiency compared to others [
8,
9].
Recent research has addressed these challenges, including genotype-dependent responses, low multiplication rates, and environmental constraints, by optimizing culture media and modifying conditions such as light intensity, vessel type, and carbohydrate sources. Higher BAP concentrations (5 mg/L) were shown to maximize shoot proliferation in
A. melanocarpa [
10]. Kaya and Sarıyer [
11] reported that selecting appropriate vessel types helped minimize hyperhydricity while enhancing shoot elongation during the micropropagation of
A. melanocarpa “Viking”, emphasizing how vessel choice can directly affect shoot quality. These results suggest that even minor adjustments to the in vitro culture environment can significantly enhance plantlet development. Griffis et al. [
12] analyzed the effectiveness of semi-solid medium systems in conjunction with Plantform
TM bioreactor systems for the micropropagation of
A. melanocarpa “Viking”. They found that bioreactor systems yielded higher shoot multiplication rates and enhanced overall plant quality. This study highlights the promise of temporary immersion systems (TIS) and other advanced in vitro technologies for the large-scale propagation of woody shrubs. However, the complexity and cost of these systems may hinder their use in smaller operations, underscoring the need for more straightforward yet effective alternatives.
The investigation into new culture media designed explicitly for woody and shrubby species is gaining traction. Palaz et al. [
13] originally formulated the Shrub Plant Medium (SPM), which has demonstrated greater effectiveness than traditional MS and WPM media in promoting shoot proliferation and rooting across various deciduous shrub taxa, such as sumac (
Rhus coriaria). While its use with
A. melanocarpa is still largely unexplored, initial findings suggest that SPM may provide improved nutrient availability and hormonal responses tailored to the needs of woody plants. This improved performance is attributed to adjustments in nitrogen balance, enhanced calcium and magnesium content, optimized iron chelation, and an enriched vitamin profile, all of which contribute to better morphogenic stability in woody taxa. No plant growth regulators (PGRs) were pre-added to the basal medium, allowing for tailored hormonal supplementation in downstream applications. This composition was optimized to provide enhanced nutrient availability, reduced oxidative stress, and improved hormonal responsiveness in woody species, distinguishing it from standard MS or WPM media. This presents fresh opportunities for enhancing micropropagation techniques for underappreciated yet valuable species, such as chokeberry.
Recently, machine learning (ML) has become a revolutionary tool in plant tissue culture, facilitating data-driven optimization of intricate biological systems. ML models have proven effective in predicting and improving various in vitro outcomes, such as shoot regeneration, root induction, and the optimization of growth media [
14,
15,
16]. The application of ML in species such as orchids, olive, cannabis, and lavender demonstrates its ability to manage non-linear interactions among multiple input parameters, thereby aiding in the development of protocols for enhanced morphological and physiological responses [
17,
18]. Additionally, research has investigated the use of ML in designing sterilization protocols, predicting stress responses, and modeling growth performance under abiotic stressors [
19].
This study aims to establish a reliable and reproducible in vitro propagation protocol for A. melanocarpa, a cultivar renowned for its high polyphenolic content and horticultural durability. We focus on how SPM-based hormonal treatments and container size affect shoot induction while assessing the rooting success of various auxin combinations in a half-strength SPM formulation. Additionally, we apply supervised machine learning techniques to model and predict plantlet responses, improving the protocol’s reproducibility, scalability, and biological significance. By integrating detailed morphological assessments with machine learning models that accurately predict shoot number and root length (R2 > 0.95), this study provides a data-driven framework for optimizing micropropagation protocols in woody shrubs. Such predictive tools can streamline protocol adjustments, reduce empirical trial iterations, and ultimately facilitate more cost-effective and reliable commercial-scale production of black chokeberry.
2. Materials and Methods
2.1. Plant Material and Reagents
This study was conducted at the Tissue Culture Laboratories of the East Mediterranean Transitional Zone Agricultural Research Institute to establish a reliable micropropagation protocol for A. melanocarpa var. Nero (black chokeberry). Actively growing axillary buds were carefully collected from 3- to 4-year-old donor plants maintained under controlled greenhouse conditions in the institute’s experimental orchard. Using sterile instruments, the buds were precisely excised from lateral branches and immediately transferred to the laboratory to initiate the in vitro culture process.
2.2. Surface Sterilization Procedure
A multi-step surface disinfection protocol was established to maintain aseptic culture conditions. Initially, the collected nodal segments, each containing an axillary bud, underwent a cleansing process before in vitro culture. This process involved immersing and agitating them in a beaker containing a mixture of liquid detergent and tap water for 30 min to remove surface debris. The explants were then moved to a laminar airflow cabinet for additional disinfection treatment. The initial step involved immersing the explants in 70% ethanol for 30 s to facilitate surface sterilization. Three rinses with sterile distilled water followed this to reduce ethanol phytotoxicity. Next, the explants were incubated for 20 min in a 30% (v/v) solution of commercial bleach (equivalent to 2.5% sodium hypochlorite), supplemented with 1–2 drops of Tween-80 (Sigma-Aldrich, St. Louis, MO, USA) as a surfactant to enhance contact with the explant surface. Finally, three additional rinses with sterile distilled water were conducted to remove any residual disinfectant. Aseptic conditions, damaged or necrotic tissues were meticulously removed with a sterile scalpel. The healthy apical sections, measuring approximately 1.5 to 2.0 cm each and containing a single axillary bud, were prepared for culture initiation and placed on the specified basal medium for shoot induction.
All reagents and culture media components used in this study were of analytical grade. Plant growth regulators, including IAA (Indole-3-acetic acid), IBA (Indole-3-butyric acid), and NAA (1-Naphthaleneacetic acid), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Macronutrients and micronutrients used in the SPM formulation were also obtained from Sigma-Aldrich (St. Louis, MO, USA). Vitamins (thiamine HCl, nicotinic acid, pyridoxine HCl, and myo-inositol) and gelling agents (agar) were sourced from Duchefa Biochemie (Haarlem, The Netherlands). Ethanol (≥96%) and sodium hypochlorite were acquired from Sigma-Aldrich (St. Louis, MO, USA). All solutions were prepared using sterile distilled water, and pH was adjusted using 1 N NaOH or HCl as needed.
2.3. Shoot Induction Conditions
Induction experiments for shoots were carried out using a newly formulated Shrub Plant Medium (SPM) (
Supplementary Table S1 for SPM composition), supplemented with 3% (
w/
v) sucrose and 0.8% (
w/
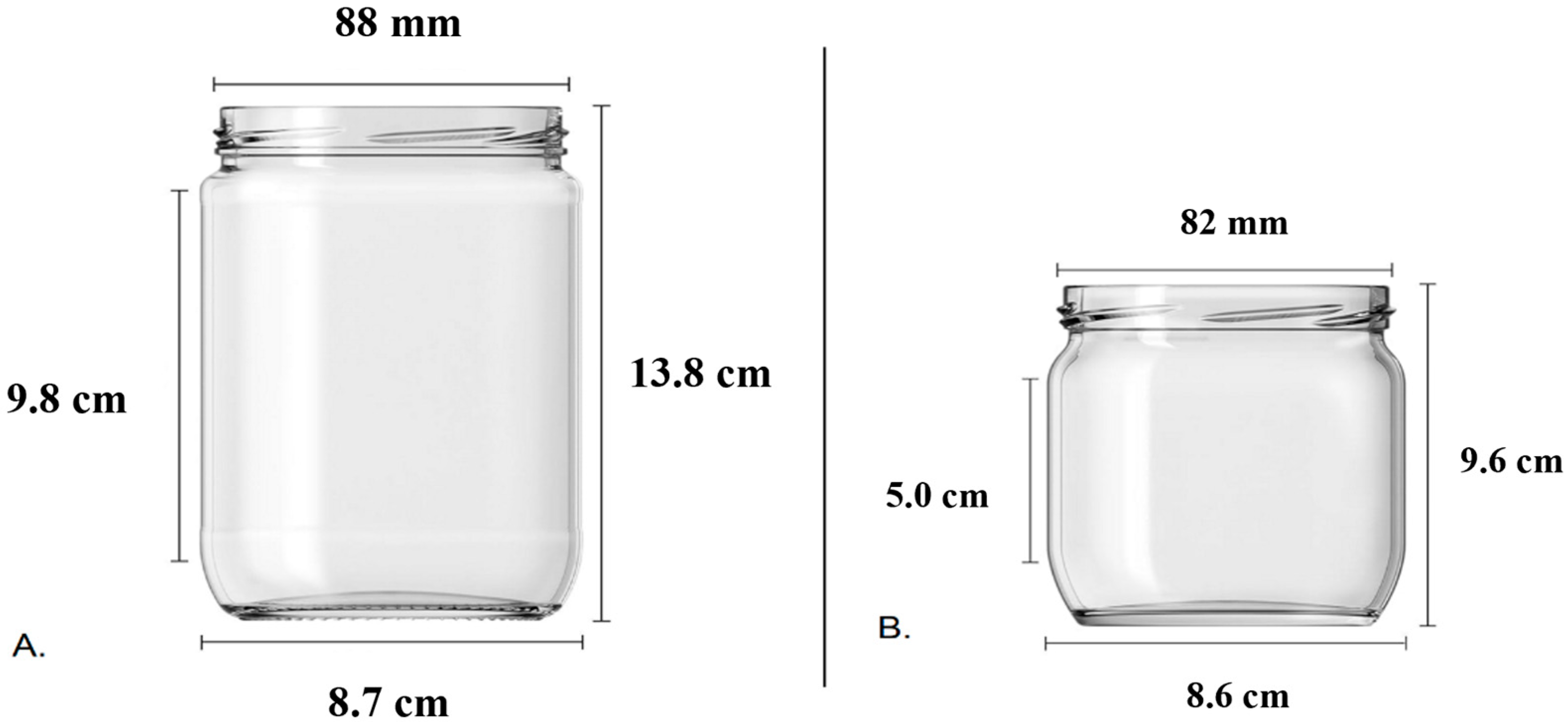
v) agar. The pH was adjusted to a range of 5.6 to 5.8 before autoclaving at 121 °C for 15 min. To ensure uniform dissolution of agar and other medium components, the medium was briefly boiled before autoclaving and dispensing into culture vessels. This step promoted consistent gelling upon cooling after sterilization. A total of 70 mL of the prepared medium was distributed into two types of culture jars to investigate the effect of vessel size on shoot development: small jars with a 425 mL capacity and large jars with a 660 mL capacity (
Figure 1). Three hormonal treatments were tested: hormone-free SPM (control), SPM enriched with 1 mg/L BAP, and SPM enriched with 5 mg/L BAP. Explants were cultured vertically in the selected jar types and media combinations. All cultures were maintained in a controlled growth chamber under a 16/8 h light/dark cycle, with a light intensity of 40 μmol m
2s
1 and a stable temperature of 25 ± 2 °C. After 60 days of culture, morphological parameters, including the number of shoots, shoot length, shoot diameter, number of leaves, leaf length, and leaf diameter, were assessed to determine the response to shoot induction. The morphological appearance of plantlets under these control conditions without PGR is shown in
Figure S1.
2.4. Root Induction Condition
For root induction, explants were sourced from shoots previously cultivated in SPM-based media within 425 mL or 660 mL culture jars and then moved to rooting conditions. This stage employed a half-strength version of the Shrub Plant Medium (½ SPM). The medium consisted of vital ½ strength SPM, 3% (
w/
v) sucrose, and 0.8% (
w/
v) agar. The pH was fine-tuned to 5.6–5.8 before autoclaving at 121 °C and 1 atm pressure for 15 min. Following sterilization, 70 mL of the medium was placed into standard culture jars and permitted to solidify under aseptic conditions. A total of ten auxin treatments were assessed for their effect on root induction efficiency. These treatments included a hormone-free control and media enriched with 0.5 mg/L of IAA, IBA, or NAA, applied individually or in combination with 0.25 mg/L of another auxin. The specific combinations examined were: 0.5 mg/L IAA, 0.5 mg/L IAA + 0.25 mg/L IBA, 0.5 mg/L IAA + 0.25 mg/L NAA, 0.5 mg/L IBA, 0.5 mg/L IBA + 0.25 mg/L IAA, 0.5 mg/L IBA + 0.25 mg/L NAA, 0.5 mg/L NAA, 0.5 mg/L NAA + 0.25 mg/L IAA, and 0.5 mg/L NAA + 0.25 mg/L IBA. These combinations were selected based on previous reports demonstrating the synergistic effects of auxins such as IBA, NAA, and IAA on root initiation and elongation in woody shrubs, including
A. melanocarpa and related taxa (
Rhus coriaria,
Rubus spp.) [
20]. IBA is often associated with promoting root elongation, whereas NAA enhances root number and suppresses callus formation. Binary combinations were therefore included to evaluate their interactive effects and optimize rooting efficiency. All cultures were kept in a growth chamber at 25 ± 2 °C under a 16/8 h light/dark cycle with a 40 μmol m
−2 s
−1 light intensity. After a 60-day incubation period, various morphological parameters related to rooting were evaluated, including rooting rate (%), number of roots, root length (mm), root thickness (mm), and shoot length measured during the rooting phase.
2.5. Acclimatization
Upon completion of the rooting phase, acclimatization was initiated to facilitate the transition of in vitro-grown plantlets to ex vitro conditions. Initially, the lids of the culture vessels were loosened overnight to allow gradual humidity exchange. The following day, plantlets were gently removed from the medium and rinsed with sterile distilled water to eliminate residual agar. The cleaned plantlets were first transferred into transparent plastic containers and placed in an artificial light acclimatization chamber maintained at 25 ± 2 °C. After one month under these semi-closed conditions, they were moved into plug trays and subsequently transplanted into pots containing a 1:1 (
v/
v) mixture of autoclaved peat and perlite. The potted plants were maintained for an additional month in a shaded and protected greenhouse area with standard cultural care. At the end of the two-month acclimatization period, plantlets were successfully transitioned to outdoor conditions. This stepwise acclimatization ensured high survival rates and healthy establishment of the micropropagated plants (
Figure 2).
2.6. Statistical Analysis
All experimental treatments were structured factorially within a completely randomized design (CRD). Each treatment included three biological replicates. For each replicate, five culture jars were used, and 5 explants were placed per jar, resulting in 15 explants per replicate and 45 explants per treatment in total. This design ensured robust evaluation of treatment effects across independently replicated experimental units. Data collected on shoot and root development parameters were analyzed using analysis of variance (ANOVA) to evaluate the impacts of different plant growth regulator (PGR) treatments, jar sizes, and their interactions. If significant differences were found (p < 0.05), mean comparisons were carried out with Fisher’s Least Significant Difference (LSD) test to identify which treatment combinations led to the observed variations. Statistical computations were conducted using R software (version 4.3.1). The “agricolae” package facilitated the execution of ANOVA and post hoc LSD tests. Pearson’s correlation coefficients were computed and visualized with the “corrplot” package to examine the relationships between morphological traits. This approach allowed for identifying positive and negative interrelationships among essential growth parameters. Additionally, Principal Component Analysis (PCA) was performed using the “factoextra” package to reduce dimensionality and identify the key components that account for the variance in the dataset. Before PCA, the data were standardized by centering at zero mean and scaling to unit variance (z-scores) using the prcomp function in R (center = TRUE, scale = TRUE) to ensure balanced contributions from all variables, regardless of their original scales.
The findings from PCA reinforced the understanding of correlation patterns by emphasizing the traits that had the most significant impact on treatment clustering and morphological responses. These statistical techniques established a solid foundation for evaluating treatment efficacy and identifying hidden patterns in the complex trait data collected during the study.
2.7. Predictive Modeling Procedure
This study focused on developing machine learning models to predict in vitro shoot and root morphogenesis responses in
A. melanocarpa, utilizing the R programming environment (version 4.3.1). The modeling workflow employed the “caret” package, which facilitated the process of model training, validation, and performance evaluation. To ensure a dependable and unbiased assessment, a Leave-One-Out Cross-Validation (LOOCV) strategy was applied [
21]. This technique involves training the model on all but one data point and testing it against the omitted observation, a procedure repeated for each instance. Such a method is especially beneficial for moderately sized biological datasets, as it optimizes the utilization of available data while reducing the risk of overfitting. Distinct predictive models were developed for the shoot and root datasets due to their unique experimental designs and outcome variables. In the shoot dataset, the input features comprised the jar size (425 mL or 660 mL) and the concentration of 6-Benzylaminopurine (BAP) in the medium (0, 1, or 5 mg/L). The predicted output traits included the number of shoots, shoot length, shoot diameter, number of leaves, leaf length, and leaf diameter. Conversely, the root dataset focused on the type and concentration of auxins used in ½ strength Shrub Plant Medium (SPM). These auxins, indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), and 1-naphthaleneacetic acid (NAA), were evaluated both individually and in pairs. The variables predicted during the rooting phase encompassed rooting rate (%), number of roots, root length, root thickness, and shoot length during rooting. The dataset used for machine learning comprised a total of 160 samples, each representing an individual replicate under specific combinations of PGR treatments and container volumes. These data were directly derived from our in vitro experiments. Before modeling, all numerical input features were standardized to have a mean of zero and a variance of one (z-scores) to eliminate scale effects and ensure that each variable contributed equally during model training. This preprocessing step is crucial for algorithms such as SVM and MLP, which are sensitive to differences in data scale.
We utilized four machine learning algorithms to assess their performance thoroughly: Random Forest (RF) [
16,
22,
23], Gradient Boosting Machine (GBM) [
24,
25], Multilayer Perceptron (MLP) [
17,
20], and Support Vector Machine (SVM) [
26]. These models were carefully selected due to their effectiveness in handling non-linear, multivariate biological data, especially in the exciting fields of plant biotechnology and predictive phenotyping research. To evaluate model performance, we applied three key statistical metrics: the coefficient of determination (R
2), which shows how much variance is explained by the model (Equation (1)); the root mean square error (RMSE), reflecting the average size of prediction errors (Equation (2)); and the mean absolute error (MAE), which gives a clear look at the average difference between predicted and actual values (Equation (3)). By bringing these metrics together, we thoroughly compared the algorithms’ performance, helping us pinpoint the models that are ideally suited for accurately predicting shoot and root development traits across various in vitro culture conditions.
While = actual value, = predicted value, = mean o the actual values, and n = sample count
3. Results
3.1. Effects of Ausxin Combinations on Root Induction and Development
The application of various plant growth regulators (PGRs) significantly influenced the rooting parameters of
Aronia spp., as shown in
Table 1. All measured traits, including rooting rate, root length, number of roots, root thickness, and shoot length, varied considerably in response to the different PGR treatments. Differences were confirmed by LSD analysis at a highly significant level (
p < 0.0001) for all variables.
The highest rooting rate (100%) was observed in the treatment combining 0.5 mg/L NAA + 0.25 mg/L IBA, significantly outperforming all other treatments, including the control (25%). This combination also resulted in the highest number of roots per explant (7.6 ± 0.5), indicating a synergistic effect of auxins on root initiation and proliferation. In terms of root length, the longest roots were produced in the 0.5 IBA + 0.25 NAA treatment (62.6 ± 2.1 mm), followed by 0.5 IBA + 0.25 IAA (50 ± 3.5 mm) (
Figure 3).
Interestingly, while root thickness showed high variability, the thickest roots were observed in the 0.5 IBA treatment alone (8.7 ± 8.7 mm), although with a high standard deviation that reflects inconsistent performance in this trait. Other treatments, including combinations of PGRs, yielded more moderate and consistent root thickness (ranging from 1.3 to 2.3 mm), which may be more desirable for uniform root system development. Concerning shoot elongation in the rooting media, the 0.5 IBA + 0.25 NAA and 0.5 IBA + 0.25 IAA treatments significantly improved shoot lengths (66.4 ± 5.8 mm and 60.2 ± 3.6 mm, respectively) compared to the control and single PGR applications. Notably, control explants exhibited the lowest values across nearly all parameters, underscoring the necessity of exogenous PGRs for effective root induction and development in
Aronia.
Supplementary Figure S15 illustrates the limited rooting and shoot development observed in the control treatment. Detailed morphological views of plantlets under these various auxin treatments are shown in
Supplementary Figures S4–S14.
These findings establish a strong basis for optimizing in vitro propagation protocols for this species and potentially enhancing its large-scale clonal multiplication efficiency.
3.2. Influence of BAP Concentration and Jar Size on Shoot Multiplication
As shown in
Table 2, the impact of varying BAP concentrations in newly developed Shrub Plants Medium (SPM) and container sizes on a range of shoot growth parameters in
Aronia cultures. All growth traits displayed statistically significant differences based on BAP concentration, jar size, and their interactions, as highlighted by the LSD values (
p < 0.0001 or
p < 0.01), except for shoot diameter, which remained unaffected by jar size.
The highest number of shoots per explant was observed in large jars with SPM + 5 mg/L BAP (27.1 ± 2.0), which was significantly higher than all other treatment combinations. Elevating the BAP concentration greatly enhanced shoot multiplication, especially in larger containers. The average shoot count was nearly three times greater in the large jar + 5 mg/L BAP treatment than in small jars at the same BAP level. Shoot elongation peaked in large jars with 5 mg/L BAP (75.6 ± 3.6 mm). Moderate shoot lengths were noted even at 0 mg/L and 1 mg/L BAP in small jars; however, BAP at 5 mg/L significantly enhanced elongation, particularly in larger vessels. Small jars containing 1 mg/L BAP exhibited the shortest shoot lengths.
The highest leaf production was observed in large jars containing 5 mg/L BAP (20.8 ± 1.6), followed by small jars at the same concentration (15.9 ± 1.3). This highlights the effects of cytokinin concentration and container size on plant growth. Leaf numbers increased significantly with higher BAP concentrations for both jar sizes, with notable interaction effects recorded. Meanwhile, leaf diameter peaked in small jars with 1 mg/L BAP (8.0 ± 0.81 mm) and was least in large jars with 5 mg/L BAP (4.55 ± 0.5 mm). In contrast, leaf length was most significant in jars with 0 or 1 mg/L BAP, particularly in smaller containers, indicating that increased BAP concentrations and larger jars might limit individual leaf elongation even while promoting proliferation. These conflicting trends reflect a trade-off between proliferation and the development of particular organs. Shoot thickness showed no significant variation due to jar size alone; however, it was influenced by the treatment. The thickest shoots were found in the SPM + 1 mg/L BAP group (1.2 ± 0.2 mm), whereas the thinnest were in the 5 mg/L BAP group (0.97 ± 0.2 mm).
Although 5 mg/L BAP in large jars enhances shoot proliferation, it might adversely affect the size of individual shoots and leaves, likely due to hormonal overstimulation or nutrient competition. Therefore, using 1 mg/L BAP in large jars could provide an optimal balance for achieving moderate proliferation while preserving strong shoot architecture. These results provide valuable insights for enhancing in vitro micropropagation protocols for
Aronia spp., particularly when tailoring multiplication strategies to specific downstream objectives, such as ex vitro acclimatization or secondary metabolite production. Additional morphological differences across BAP concentrations and container sizes are provided in
Supplementary Figures S2 and S3.
Significant visual differences in shoot multiplication were observed across BAP concentrations and jar sizes, as illustrated in
Figure 4. Cultures grown in large jars with 5 mg/L BAP exhibited dense shoot clusters with elongated shoots and high leaf numbers, supporting the statistical trends in
Table 2.
3.3. Relationships Among Morphological Traits
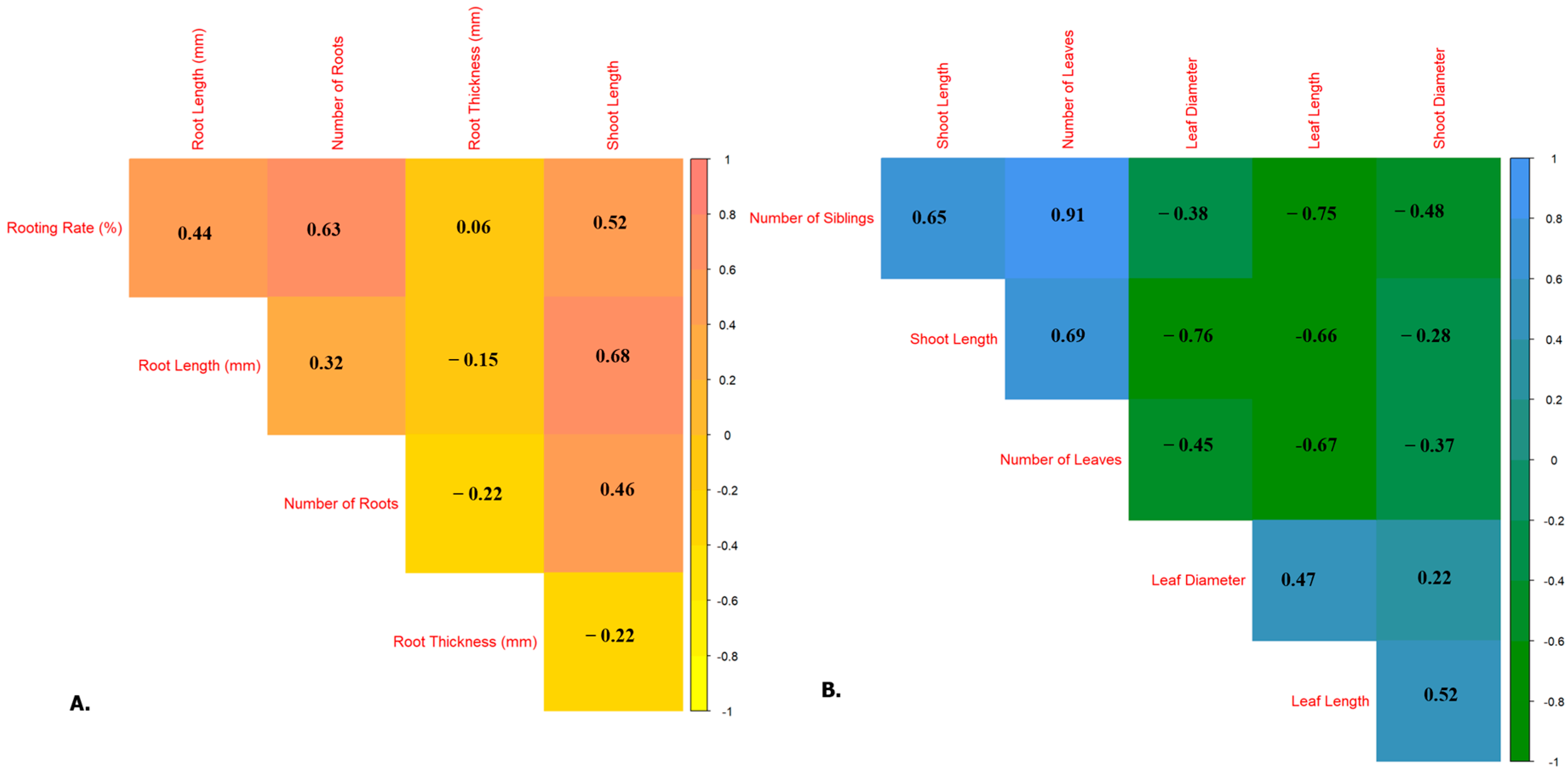
Pearson’s correlation coefficients were calculated for traits assessed during the rooting (
Figure 5A) and shooting (
Figure 5B) phases to clarify the interrelationships between key morphological parameters. The findings indicate distinct patterns of association, offering insights into the developmental dynamics responding to various PGR treatments and jar conditions.
Figure 5A presents the correlation matrix for rooting-related traits: rooting rate (%), root length, number of roots, root thickness, and shoot length during the rooting phase. The most significant positive correlation was found between rooting rate and number of roots (r = 0.63), suggesting that treatments that increase rooting frequency also enhance root initiation per explant. Additionally, rooting rate showed a moderate correlation with root length (r = 0.44) and shoot length in rooting media (r = 0.52), indicating that favorable rooting conditions concurrently promote early shoot development.
A strong positive correlation between root length and shoot length (r = 0.68) implies synchronous development of below- and above-ground organs during early growth. However, root thickness demonstrates negative or weak correlations with other traits, notably showing a negative association with the number of roots (r = −0.22) and shoot length (r = −0.22), suggesting that excessive root girth may be inversely associated with fine root proliferation or shoot vigor under in vitro conditions.
During the shooting stage (
Figure 5B), a strong and statistically significant correlation was observed between the number of shoots and the number of leaves (r = 0.91), as well as between shoot length and the number of leaves (r = 0.65). This emphasizes the interdependent enhancement of vegetative proliferation traits in cytokinin-rich environments. These results reinforce the notion that shoot multiplication closely relates to leaf production and elongation, yielding important insights for improving micropropagation efficiency.
Interestingly, leaf diameter and length were negatively correlated with the number of shoots (r = −0.38 and −0.75, respectively) and shoot length (r = −0.76 and −0.66, respectively). This indicates a trade-off between shoot multiplication and the size of individual leaf organs. This may be due to the release of cytokinin-induced apical dominance, which promotes the initiation of multiple shoots over their elongation and leaf expansion. Similarly, shoot diameter displayed a weak to moderate negative correlation with other proliferation traits, particularly the number of shoots (r = −0.48), suggesting that an increased shoot number may compromise stem robustness. Conversely, a modest positive correlation was observed between leaf diameter and leaf length (r = 0.47) and between leaf length and shoot diameter (r = 0.52), indicating some coordination in organ growth among individual explants.
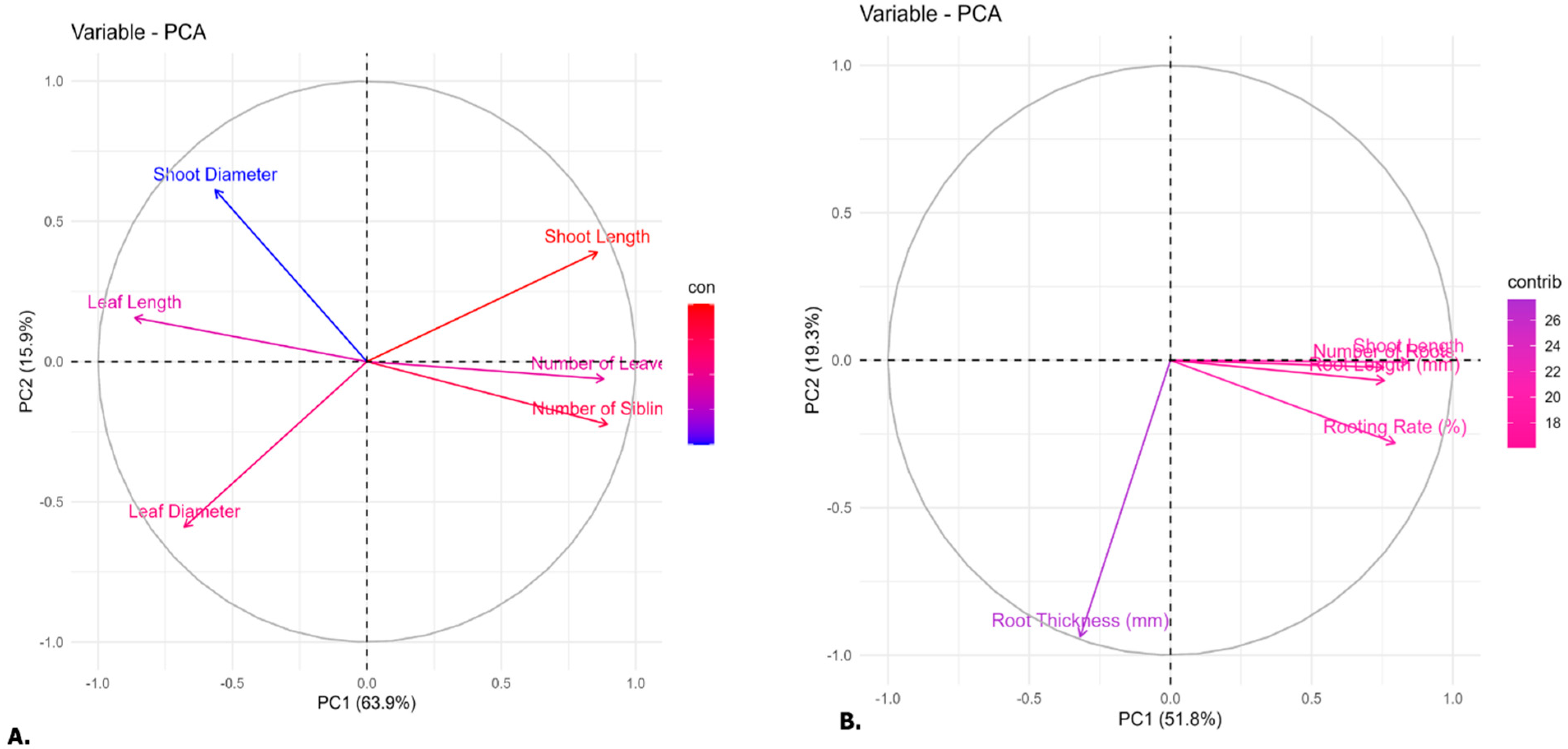
3.4. Multivariate Analysis of Morphogenic Responses
Principal Component Analysis (PCA) was performed to examine the relationships among key morphological traits during the in vitro development of shoots and roots in
Aronia spp., identifying which traits contributed most to the variance observed across treatments (
Figure 6). In the shoot dataset (
Figure 6A), the first two principal components captured 79.8% of the total variance, with PC1 and PC2 explaining 63.9% and 15.9%, respectively. Traits related to shoot proliferation, such as shoot length, leaf quantity, and the number of shoots, were closely clustered along the positive axis of PC1, indicating strong positive correlations and a unified influence on the primary direction of variability. This suggests that treatments encouraging shoot multiplication also foster enhanced overall vegetative development. In contrast, shoot diameter was mainly associated with PC2, displaying a near-orthogonal relationship with shoot number and length, which suggests a certain degree of independence or even a trade-off between proliferation and stem thickness. Likewise, leaf diameter and length contributed less significantly, projecting in opposite directions and indicating potential antagonistic relationships with traits associated with shoot multiplication. These observations align with established effects of cytokinins, where excessive shoot proliferation might lead to reduced size and robustness of individual organs.
In the root dataset (
Figure 6B), the first two principal components account for 71.1% of the total variance, with PC1 explaining 51.8% and PC2 explaining 19.3%. Rooting rate, root length, the number of roots, and shoot length (measured during rooting) were all strongly and positively loaded on PC1, reflecting their close physiological interdependence. Treatments that improved rooting efficiency also promoted greater root elongation and early shoot development, suggesting a coordinated hormonal or nutritional effect. Interestingly, root thickness exhibited a distinct pattern, contributing strongly to PC2 and orienting away from the other traits. This divergence suggests that root thickness may be regulated independently of rooting success, representing an anatomical response to specific treatments or microenvironmental stress.
These findings underscore the importance of a multivariate approach in refining micropropagation methods. Exclusively focusing on increasing shoot numbers or rooting speeds risks overlooking key quality traits, such as organ thickness and uniformity, which are crucial for effective acclimatization and field performance.
3.5. Predictive Modeling of Shoot and Root Traits Using Machine Learning
To evaluate the ability of machine learning algorithms to predict in vitro morphological responses of
Aronia to various treatments, four supervised learning models—Random Forest (RF), Gradient Boosting Machine (GBM), Multilayer Perceptron (MLP), and Support Vector Machine (SVM)—were assessed based on twelve plant growth parameters using three standard metrics: Mean Squared Error (MSE), Root Mean Squared Error (RMSE), and the Coefficient of Determination (R
2) (
Table 3).
Random Forest (RF) and MLP consistently excelled across most traits, achieving high R2 values (≥0.90) and low MSE/RMSE readings. This was especially true for traits related to shoot proliferation, such as shoot number (R2 = 0.98 for RF and 0.97 for MLP) and shoot length (R2 = 0.96 for RF and 0.94 for MLP). SVM also demonstrated strong performance in these areas (R2 = 0.96 and 0.91), whereas GBM underperformed, particularly in terms of RMSE, indicating greater residual variability. When considering leaf morphology, the predictive performance of all models experienced a slight decline, likely due to the presence of more complex or subtle variations in these traits. For instance, the leaf diameter exhibited relatively low R2 values, ranging from 0.41 for GBM to 0.61 for SVM, despite similar MSE values across the models. In contrast, leaf length was predicted more reliably (R2 = 0.71 for RF, MLP, and SVM), indicating that while specific leaf characteristics might be inherently challenging to model due to variability or sensitivity in measurement, others can still benefit from practical machine learning estimation. Root-related parameters showed varying performance based on the model used. The MLP and SVM models performed well in predicting rooting rate (R2 = 0.92 for both) and root length (R2 = 0.92 and 0.91, respectively), whereas the GBM model consistently lagged with lower performance (R2 = 0.67 and 0.47). Importantly, root thickness was the most challenging trait to predict, with R2 values ranging from 0.19 (SVM) to 0.56 (RF). This suggests that more intricate or less easily measurable factors might affect this trait within the tissue culture environment. Notably, shoot length in rooting media was accurately modeled by MLP and SVM (R2 = 0.94), validating the capability of these models to represent integrated root–shoot interaction dynamics. RF emerged as the most consistently accurate model, closely trailed by MLP and SVM. In contrast, GBM showed relatively poorer performance across various traits, especially in predicting shoot diameter and root thickness.
The results reveal that ensemble learning techniques, such as random forests (RF), and neural network methods, particularly multilayer perceptron (MLP), offer outstanding predictive accuracy for most in vitro morphophysiological traits in Aronia. This confirms their effectiveness in predictive modeling for plant tissue culture. By facilitating precise trait predictions, machine learning enables the efficient evaluation of growth regulator treatments and enhances protocol optimization, thereby minimizing the need for trial-and-error testing.
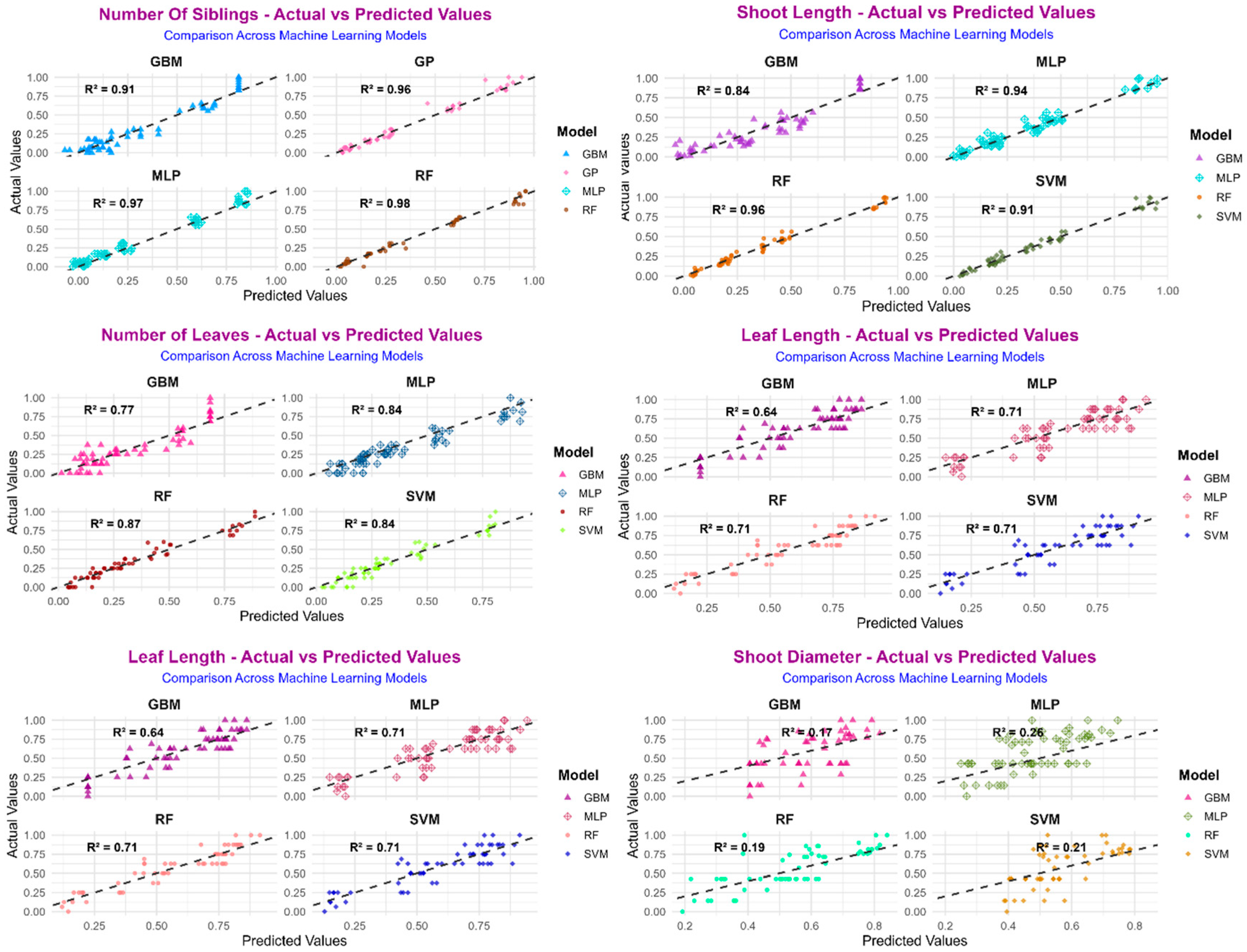
To further confirm the accuracy of the predictive models, scatter plots comparing actual and predicted values were created for each model based on selected morphological traits (
Figure 7 and
Figure 8). These plots illustrate the model’s fit and demonstrate the alignment of predicted outcomes with observed measurements. In every instance, especially for traits like the number of shoots, shoot length, and rooting rate, the predicted values from the Random Forest (RF), Multilayer Perceptron (MLP), and Support Vector Machine (SVM) models showed a nearly linear relationship with the actual values, indicating strong predictive reliability and minimal deviation. The proximity of data points to the 1:1 diagonal line further validates the precision of the models, particularly for traits with higher R
2 values outlined in
Table 3. In contrast, the Gradient Boosting Machine (GBM) model’s predictions displayed greater dispersion, which supports the observed numerical performance trends. These visuals reinforce the effectiveness of RF, MLP, and SVM in modeling complex plant morphogenic responses, highlighting the importance of integrating machine learning techniques into in vitro propagation studies.
3.6. Acclimatization Success
Following the in vitro rooting phase, acclimatization success varied depending on the auxin treatment applied during rooting. The highest survival rate was observed in plantlets derived from the medium supplemented with 0.5 mg/L NAA + 0.25 mg/L IBA, achieving 85% acclimatization success. This was followed by 0.5 mg/L NAA alone (80%) and 0.5 mg/L NAA + 0.25 mg/L IAA (75%), indicating that NAA-based treatments generally promoted superior post-transfer viability. Moderate survival rates were recorded in combinations such as 0.5 mg/L IAA + 0.25 mg/L NAA (72%) and 0.5 mg/L IAA + 0.25 mg/L IBA (70%). Control plantlets showed the lowest acclimatization rate (60%), suggesting that auxin supplementation during rooting contributes significantly to ex vitro adaptation. These findings support the practical applicability of optimized rooting treatments in ensuring the successful transition of A. melanocarpa plantlets to greenhouse conditions.
4. Discussion
The current study aimed to establish an optimized in vitro propagation protocol for
Aronia melanocarpa cv. “Nero”, focusing on the effects of different culture conditions, including hormonal treatments (BAP, IBA, NAA), jar size, and medium type (SPM). The findings demonstrated significant influences of these factors on shoot and root development parameters. Our results showed a notably high rooting rate (100%) with the combination of 0.5 mg/L NAA and 0.25 mg/L IBA, significantly superior to other treatments. This synergistic effect between NAA and IBA on rooting aligns with similar outcomes observed by Rusea et al. [
1], who reported that higher IBA concentrations promoted superior rooting percentages and root numbers, highlighting the critical role auxin combinations play in
Aronia rooting efficiency. Interestingly, rooting parameters like root length and shoot elongation during rooting were optimized with combinations involving IBA, particularly 0.5 mg/L IBA + 0.25 mg/L NAA. These results complement the findings by Polat and Eskimez [
20], who similarly observed enhanced shoot and root development under combined auxin treatments. The superior rooting response observed with the combination of 0.5 mg/L NAA + 0.25 mg/L IBA is likely due to the complementary physiological roles of these two auxins. NAA, being more chemically stable, tends to persist longer in the medium and efficiently triggers the initiation of root primordia.
In contrast, IBA is more readily metabolized within plant tissues, supporting subsequent processes such as root elongation and vascular development. This combined effect provides both an initial stimulus for root induction and sustained support for further root growth and differentiation, resulting in the highest rooting rates and longest roots among the treatments tested. Conversely, our study noted that single auxin treatments such as 0.5 mg/L IBA alone produced inconsistent root thickness, echoing observations by Nas et al. [
27], who indicated variability in root development under singular auxin conditions. Our results suggest that combined auxin treatments, especially those involving NAA and IBA, are more effective than single auxin applications in promoting robust rooting responses in
Aronia.
Shoot induction experiments highlighted the significance of cytokinin (BAP) concentration and culture vessel size. The optimal shoot multiplication (27.1 ± 2.0 shoots per explant) occurred at 5 mg/L BAP in larger culture jars (660 mL). Bayhan and Yücesan [
1] similarly found maximal shoot proliferation at higher BAP concentrations (5 mg/L), although they reported a risk of vitrification at increased hormone levels, a phenomenon not significantly noted in our study. The significant two-way interaction between BAP concentration and jar size indicates that the proliferation response is dependent on hormones and heavily influenced by the microenvironment in the culture vessel. Kaya and Sarıyer [
11] found similar positive responses with moderate cytokinin levels, reporting that balanced hormonal regimes are crucial for sustainable micropropagation without adverse effects such as vitrification or reduced morphological integrity. Our results on acclimatization revealed high success rates using a peat and perlite mixture, aligning well with Rusea et al. [
1], who reported best survival rates in similar substrates, confirming that substrate choice plays a critical role in successful ex vitro establishment. Notably, Bayhan and Yücesan [
10] emphasized that the success of acclimatization significantly depended on the previous in vitro culture conditions, highlighting the interconnected nature of the culture and acclimatization phases. Comparative analyses of stress conditions, such as alkaline stress [
28] and salinity stress [
27], demonstrated that
Aronia melanocarpa exhibits sensitivity to environmental stressors, with growth parameters significantly reduced under adverse conditions. This underlines the need for optimized culture conditions that enhance plant resilience, as evident in our study’s carefully modulated hormonal treatments. This pattern suggests that elevated cytokinin levels, in combination with larger container volumes, enhance shoot proliferation, likely by improving gas exchange and providing more space, while potentially reducing the size of individual organs. Such interactions reflect a balance between breaking apical dominance to favor multiple shoots and limiting elongation or leaf expansion.
Compared to previous studies on
A. melanocarpa, the current protocol achieved notably higher shoot multiplication rates and rooting efficiencies under simplified conditions. For instance, Bayhan and Yücesan [
10] reported a maximum of ~15 shoots per explant under 5 mg/L BAP using MS medium. In contrast, our protocol achieved over 27 shoots per explant using SPM medium in large jars, indicating that the tailored nutrient composition and improved microenvironment significantly enhanced proliferation. Similarly, while Rusea et al. [
1] and Polat and Eskimez [
20] achieved rooting rates of around 80–90% under higher IBA concentrations, our combination of 0.5 mg/L NAA + 0.25 mg/L IBA resulted in 100% rooting without inducing callus formation. Unlike studies relying on complex temporary immersion systems [
12], our approach maintained high efficiency with standard semi-solid media, reducing technical requirements and costs. Additionally, this is the first study, to our knowledge, that integrates machine learning models to predict
Aronia in vitro morphogenesis, providing a data-driven tool to optimize protocols further and reduce experimental iterations.
The significant influence of container volume observed in our study, where 660 mL jars markedly enhanced shoot multiplication, shoot length, and leaf number, underscores an often underappreciated parameter in micropropagation. Similar findings were reported by Malik et al. [
29], who demonstrated that larger culture vessels (470 mL plastic jars) substantially improved growth indices, carbohydrate accumulation, and chlorophyll content in in vitro cultures of Dendrobium Sabin Blue orchids. These improvements were attributed to more favorable microenvironmental conditions, including better gas exchange and reduced accumulation of inhibitory volatiles such as ethylene. Although studies on
Aronia have not previously examined vessel size explicitly, our results highlight container volume as a straightforward yet powerful optimization factor to enhance morphogenic responses in woody shrub tissue culture.
The correlation patterns highlight the complexity of hormonal regulation in vitro during shoot and root morphogenesis. The rooting phase shows more integrated growth among root and shoot traits, while the shooting phase reveals a dichotomy between proliferation and organ expansion. These results highlight the importance of balancing PGR concentrations and culture vessel conditions to optimize proliferation while maintaining morphological quality. Such trait interdependencies are essential for optimizing micropropagation protocols and ensuring the uniformity and quality of regenerated Aronia plants.
The PCA findings indicate that different yet interconnected variation axes impact shoot and root growth in Aronia. While traits related to shoot growth typically co-vary and represent the main source of phenotypic diversity during the shoot phase, traits associated with roots also tend to group, except for root thickness.
Integration of machine learning (ML) models into our study provided valuable insights into optimizing in vitro propagation protocols. Using algorithms such as XGBoost, SVM, RF, MLP, and GRNN, we demonstrated robust predictive capabilities for various culture parameters. Specifically, the XGBoost model showed high predictive accuracy for variables such as shoot length, shoot multiplication, and root parameters [
30]. Similarly, RF and MLP models exhibited strong predictive performances, particularly in predicting physiological responses to hormone treatments and environmental stressors [
23]. Notably, the multilayer perceptron (MLP) model showed superior accuracy in predicting rooting parameters, underscoring its potential in refining tissue culture protocols [
16].
Furthermore, sensitivity analyses revealed the critical role of auxin (IBA, NAA) and cytokinin (BAP) concentrations in determining the outcomes of micropropagation. For instance, studies by Jafari and Daneshvar [
31] and Rezaei et al. [
32] highlighted the explant-dependent and hormone-specific nature of tissue culture responses, reinforcing the complexity and necessity of precise optimization in plant tissue culture systems. The integration of ML with genetic algorithms further optimized hormone combinations, effectively maximizing micropropagation efficiency [
32].
Although the present study utilized a robust factorial design with multiple replicates and employed LOOCV to minimize overfitting risks, it is acknowledged that the machine learning models were developed based on a single experimental dataset. Thus, their predictive capability across different genotypes, environmental conditions, or extended temporal scales remains to be validated. Future research should focus on testing these models using independent datasets to confirm their generalizability and practical utility in diverse in vitro culture scenarios.
Collectively, our findings underscore the significance of specific hormonal combinations, vessel sizes, media formulations, and ML-guided optimization in refining micropropagation protocols for A. melanocarpa. Future research should investigate the detailed physiological responses underlying these effects and further validate ML models to enhance the predictive accuracy and practical application of optimized tissue culture protocols.
Future studies will incorporate systematic hyperparameter tuning and feature-importance extraction, which could elucidate the most influential drivers of morphogenesis. We also plan to apply statistical comparisons across models and assess train–test RMSE ratios to better quantify robustness and mitigate concerns about overfitting.