Recent Advances in In Vitro Floral Induction in Tropical Orchids: Progress and Prospects in Vanilla Species
Abstract
1. Introduction
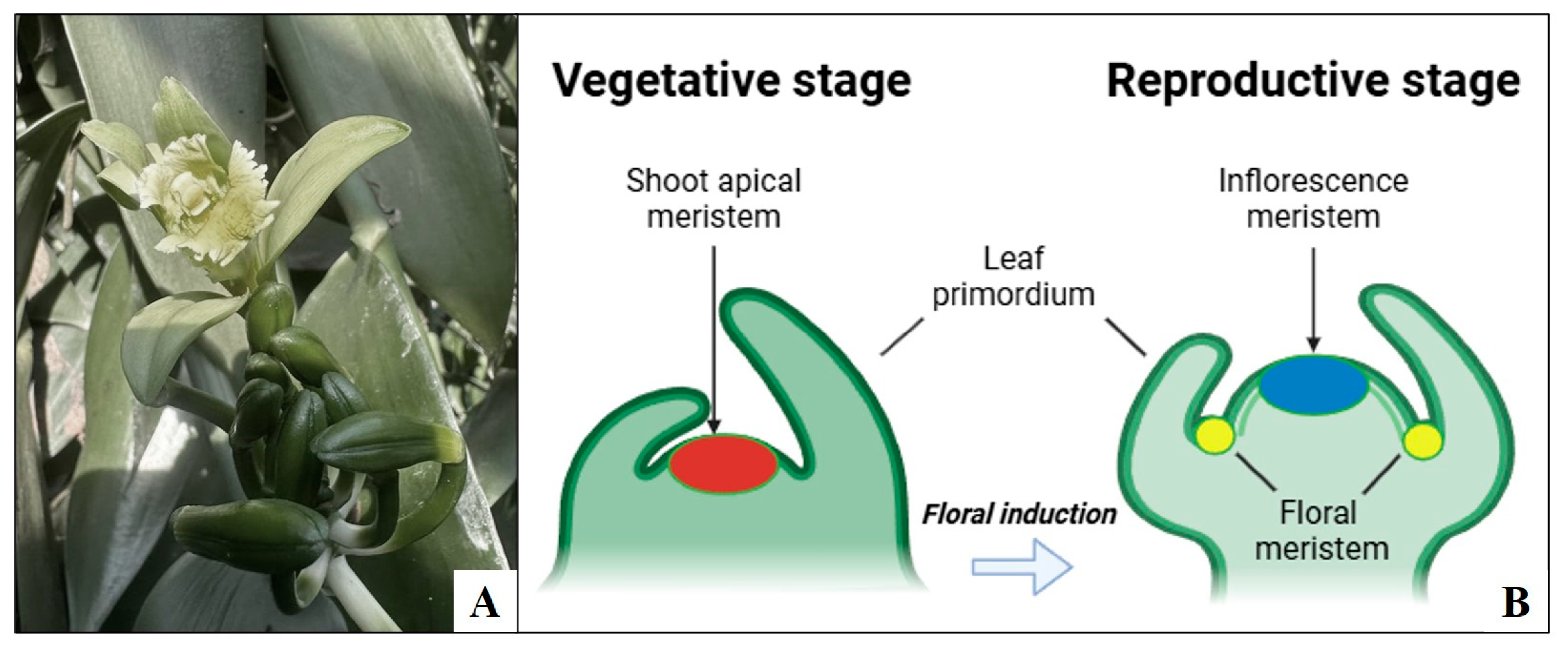
2. Floral Induction and Development in Tropical Orchids
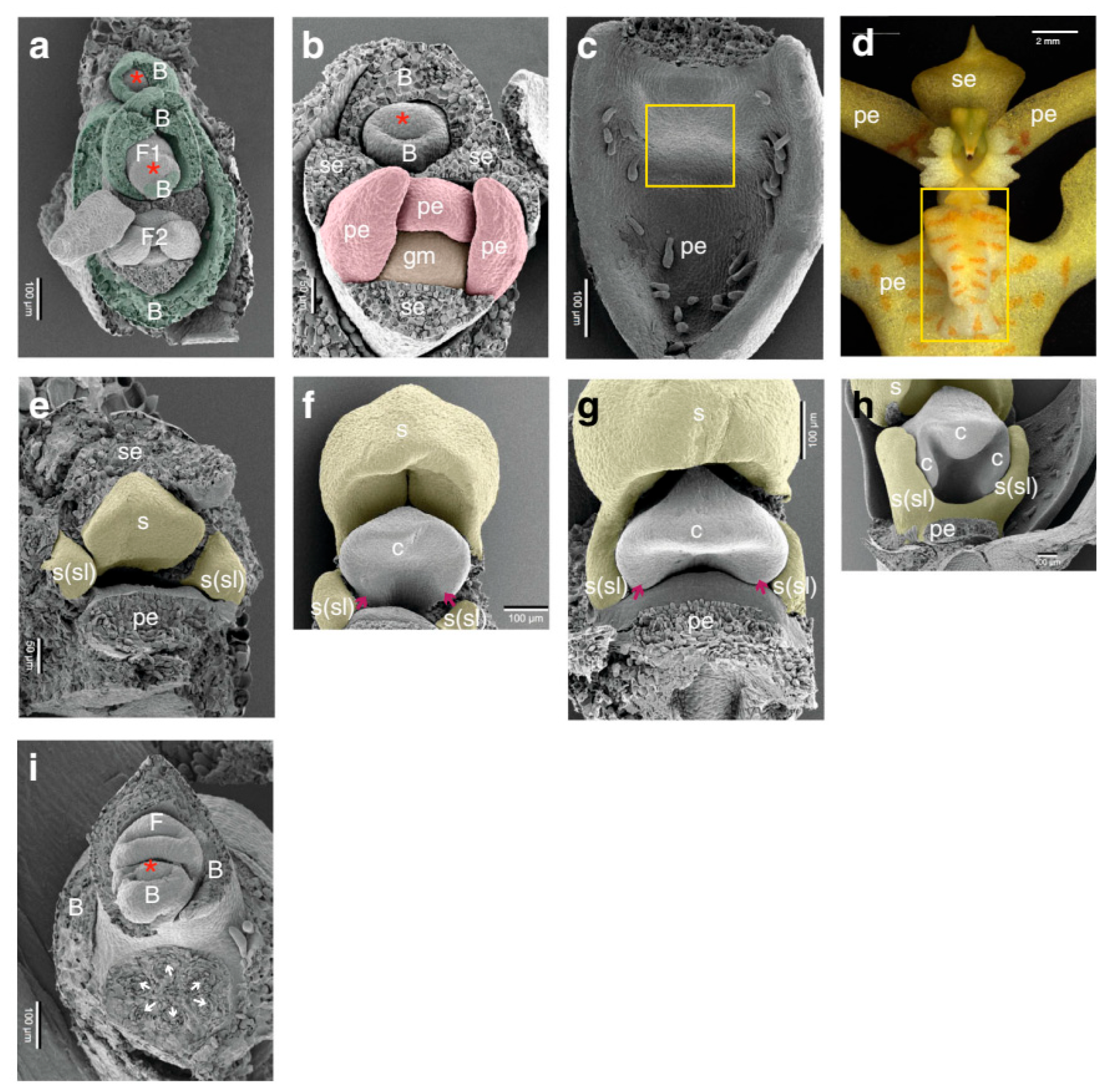
Floral Induction and Development in Vanilla spp.
3. In Vitro Floral Induction in Tropical Orchids
| Species | Explant Type | Culture Conditions (Temperature [°C]/Photoperiod [h]/Irradiance [µmol m−2 s−1]) | Culture Medium | Flower Induction/Formation | Success Rate/Flower Abnormalities | References |
|---|---|---|---|---|---|---|
| Dendrobium candidum Wall. Ex Lindl. | Seed-derived protocorms | 25–27/12/13.5 | Modified MS + PGR (BA, NAA, and ABA) | Induction of floral buds | 27–84% of success/many of the flowers showed abnormalities | [29] |
| Dendrobium candidum Wall. Ex Lindl. | Seed-derived protocorms | 25–27/12/13.5 | Modified MS + PGR (BA, NAA, and ABA) | Flower initiation from protocorms or shoots | 46–83% of success/malformation of petals and lip | [30] |
| Cymbidium niveo-marginatum Mak | Rhizomes and plantlets | 25–26/16/50 | Modified MS + BA | In vitro floral induction and flowering | 90% of success/floral buds withered with TDZ | [27] |
| Dendrobium huoshanase | Seedlings | 25 ± 2/12/27 | Modified MS + PGR (2,4-D and Zeatin) | In vitro floral induction and flowering | Not reported | [31] |
| Geodorum densiflorum (Lam.) Schltr. | Seedlings | 25 ± 2/16/13.5 | Modified MS + PGR (BA and IAA) + AC | In vitro flowering | Not reported | [32] |
| Psygmorchis pusilla Dodson and Dressler | Seedlings | 22–32/dark 6, 8, 12, 16, 20 and 22/30 | Modified VW + additives (ripe banana pulp, AC, and sucrose) | Floral spike development and flower formation | Low rate of success/flower buds did not open | [33] |
| Phalaenopsis cygnus var Silky Moon | Plantlets | 25 ± 2/16/35–40 | VW medium + BA | Flower bud formation | 38–43% of success/no flower abnormality reported | [34] |
| Dendrobium moniliforme | Seedlings | 25 ± 2/12/50–60 | Modified MS + PGR (TDZ or PBZ and ABA) | In vitro floral induction and normal flowering | 45–80% of success/many of the flowers showed abnormalities | [35] |
| Dendrobium var Second Love | Shoots | 26 ± 1/16/50–60 | Modified VW and MS + PGR (TDZ, BA, and [9R]iP) | In vitro floral induction, transition and flowering | 65% of success/no flower abnormality reported | [36] |
| Dendrobium var Madame Thong-In | Protocorms | 26 ± 2/16/35 | Modified KC + BA + additives (CW and AC) | In vitro floral induction, transition and abnormal flowering | 100% of success/75% of the flowers showed abnormalities | [37] |
| Dendrobium var Chao Praya | Protocorms | 25 ± 2/16/40 | Modified KC + BA + CW | In vitro inflorescence induction and flowering, but some incomplete flowers were reported | 18–83% of success/many of the flowers were incomplete | [38] |
| Dendrobium var Sonia 17 | Three-leaf stage plantlets | 25 ± 2/16/25 | Modified MS + BA | In vitro inflorescence induction and flowering, but incomplete flower structures were reported | 20–52% of success/many of the flowers were incomplete | [39] |
| Psygmorchis pusilla | Seedlings | 25 ± 2/16/30 | Modified VW + additives (ripe banana pulp, AC, and sucrose) | Floral spike development and flower formation | Not reported | [40] |
| Dendrobium primulinum | Protocorm-like bodies (PLBs) | 25 ± 2/12/40 | Modified MS + PGR (BA and NAA) + fresh apple juice | Induction of floral buds | Low rate of success/flower buds did not open and deformed flower developed | [41] |
| Dendrobium denndanum | Seedlings | 22–24/12/27 | Modified MS + PGR (BA, NAA, ABA, and 2,4-D) | Induction and formation of floral buds | 10% of success/flower buds did not open | [42] |
| Bulbophyllum auricomum Lindl. | Seedlings | 24/16/80 | Modified MS and KC + PGR (BA, KIN, NAA, and IBA) + CW | In vitro flowering but some flowers with asymmetrical sepals were reported | 50% of success/many of the flowers were incomplete | [43] |
| Friederick’s Dendrobium | Shoots | 26 ± 2/14/22 | Modified MS + PBZ | In vitro induction of floral buds and normal flowering | 7–29% of success/normal morphology | [44] |
| Dendrobium nobile Lindl. | Seedlings | 25/12/60 | Modified MS + PGR (PBZ and TDZ) | In vitro induction of floral buds and flowering, but deformed flowers were reported at high temperature | 33–97% of success/deformed flowers at 25 °C | [45] |
| Dendrobium officinale | Non-rooted plantlets | 26 ± 1/16/13.5–24 | Modified MS + single-factor and multi-factor PGR treatments | In vitro induction of floral buds and normal flowering | 80–90% of success/no deformed flowers reported | [46] |
| Oncidium var Gower Ramsey | Petal-bearing embryos from root callus | 26/16/36 | Modified MS + PGR (2,4-D, dicamba, NAA, IBA, 2iP, BA, kinetin, TDZ, and GA3) | Normal in vitro flowering | 50% of success/abnormal petal-bearing embryos | [47] |
| Dendrobium aphyllum | PLBs | 25 ± 2/14/60 | Culture medium (MS, PM and KC) + PGR (BA and NAA) | Normal in vitro flowering | 10% of success/no deformed flowers reported | [48] |
| Dendrobium wangliangii | Protocorms | 22 ± 2/16/36 | Modified MS + PGR (BA, NAA, and PBZ) | Induction of floral buds | 5–20% of success/no deformed flowers reported | [49] |
| Dendrobium var Chao Praya Smile, Pinky and Kiyomi Beauty | Protocorms | 24/16/35 | Modified KC + BA + additives (sucrose and CW) | Normal in vitro flowering | Not reported/abnormal floral buds on the apex | [50] |
| Dendrobium nobile types | Seedlings | 14/12/80 for 30 days, then at 25 ± 2/12/50 | Modified MS + PGR (PBZ and TDZ) + CW | Flower bud formation | 4–39% of success/no deformed flowers reported | [51] |
| Dendrobium huoshanense | PLBs | 25 ± 2/16/42–55 | Modified MS + PGR (2,4-D, TDZ, BA, NAA, and IBA) | In vitro flowering | Not reported/flowers showed a lack of the gynandrium | [52] |
| Dendrobium Chao Praya Smile | Protocorms | 24/16/-- | Modified KC + BA + CW | Inflorescence stalk formation | Not reported/no deformed flowers reported | [53] |
| Erycina pusilla | Seedlings | 25 ± 2/12/6–55 | Modified MS + additives (sucrose, AC, peptone, potato powder, and CW) | Normal in vitro flowering | Not reported/no deformed flowers reported | [54] |
| Dendrobium ovatum | Protocorms and PLBs | Prechilling at −20, 0, 4, and 15 for 2 h/dark 6, 12, 18, and 24/50 | Modified MS + Zeatin + CW | Normal in vitro flowering | 10–25% of success/presence of lateral petals | [55] |
| Cymbidium ensifolium, Cymbidium sinense, and Cymbidium goeringii | Protocorms | 26 ± 2/12/40 | Culture medium (not reported) + PGR (BA and NAA) + additives (sucrose and AC) | Abnormal in vitro flowering | Not reported/leafless flowers | [56] |
| Dendrobium nobile | Seedlings | 26/12/40–56 | Modified KC + TDZ + additives (sucrose, AC, and CW) | Normal in vitro flowering | 88% of success/no deformed flowers reported | [57] |
| Vanilla planifolia Jacks. ex Andrews | Shoots | 26 ± 2/--/55 | Modified KC + PGR (BA, TDZ, PBZ, and GA3) + additives (sucrose and CW) | Floral differentiation | 0% of success/no abnormalities reported | [58] |
| Cymbidium faberi Rolfe | PLBs | Not measured | Modified MS + PGR (2,4-D, BA, IBA and NAA) + additives (sucrose, glucose, AC, CW, and peptone) | In vitro flowering but changes in leaf and flower morphology were reported | Low rate of success/deformed flower developed and small or curled petals | [59] |
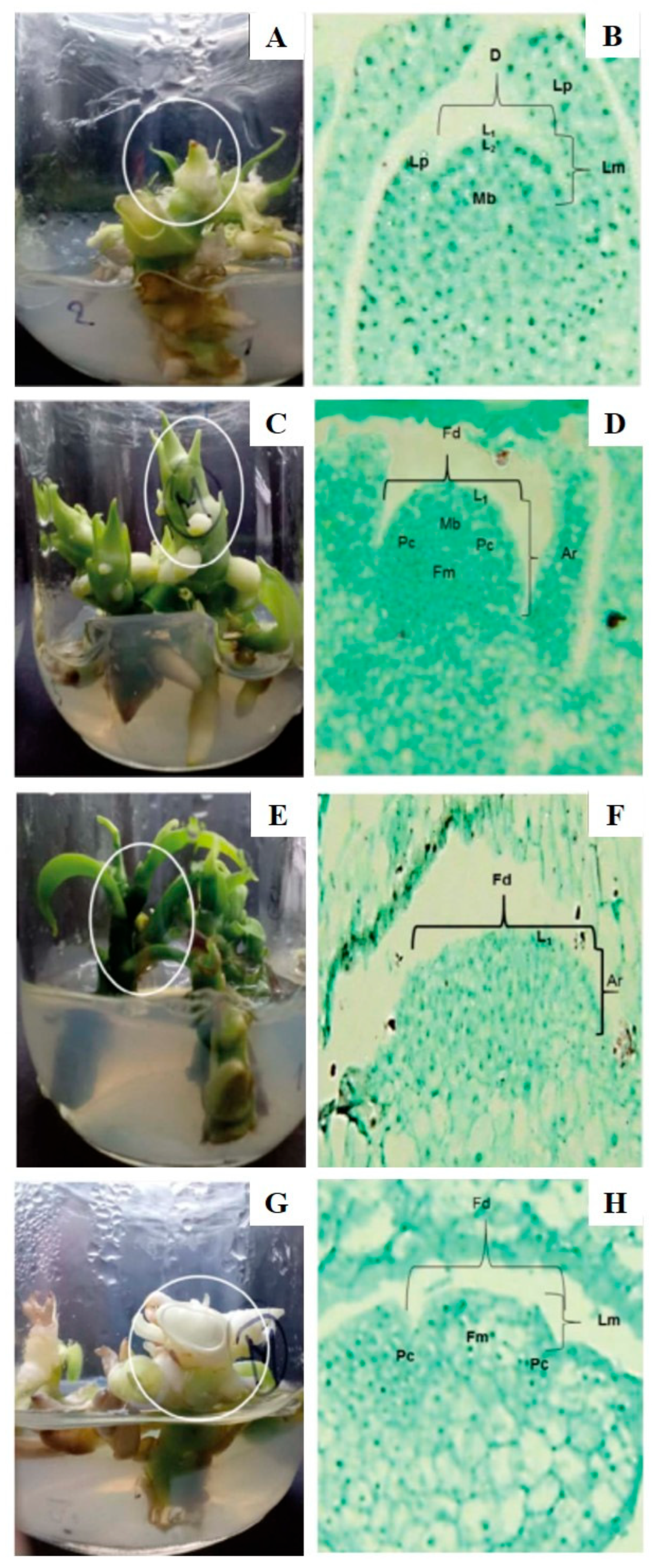
In Vitro Floral Induction: Application to and Importance for Vanilla
4. Factors Controlling In Vitro Flowering of Tropical Orchids Focusing on Vanilla
4.1. Effect of Physical Factors on In Vitro Floral Induction
4.2. Effect of Plant Growth Regulators on In Vitro Floral Induction
4.3. Effect of Nutrition on In Vitro Floral Induction
4.4. Effect of Additives for In Vitro Floral Induction
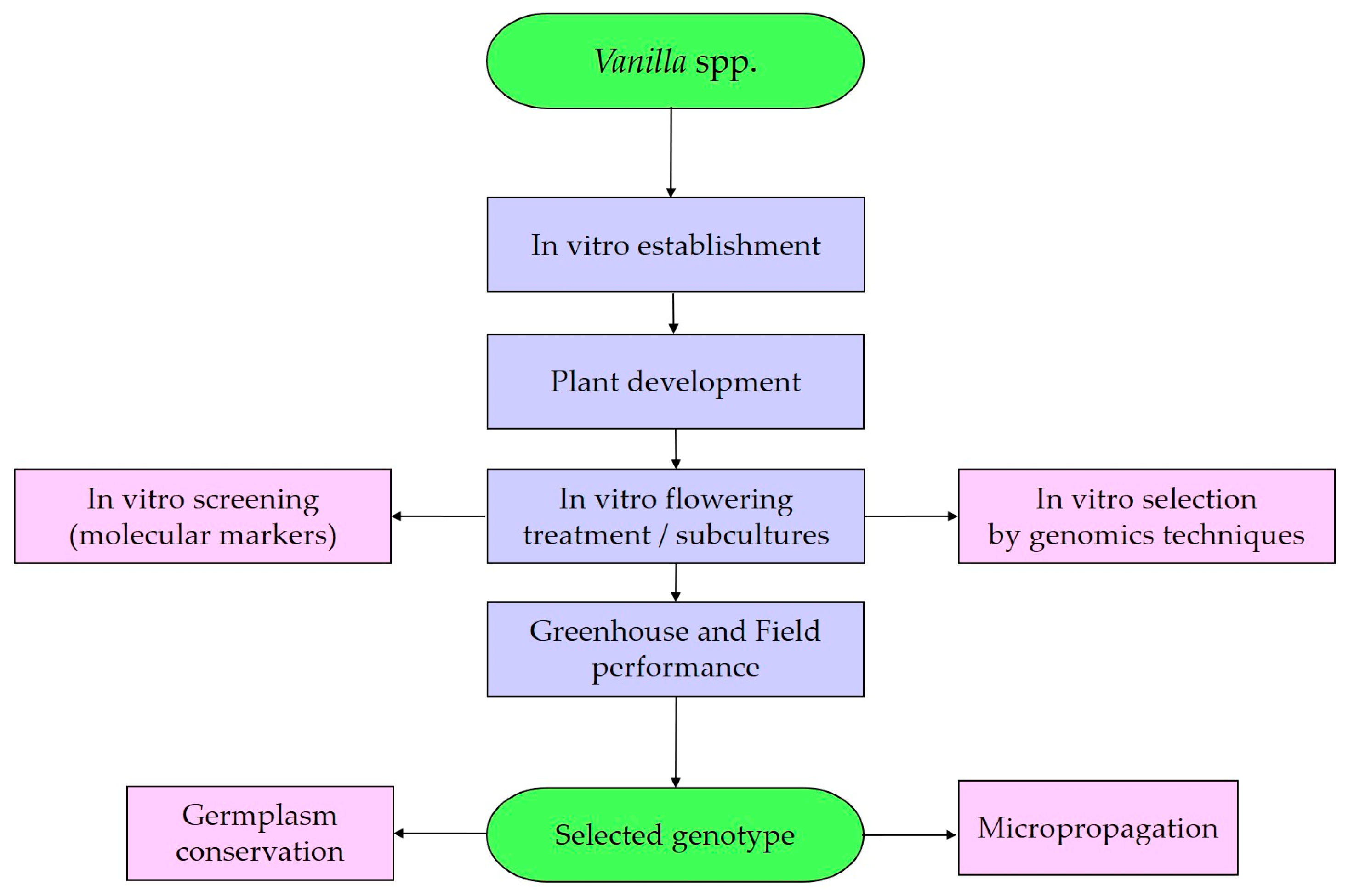
5. Orchid Biotechnology Approach
6. Issues to Consider in a Protocol for the In Vitro Flowering of Vanilla
7. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kean-Galeno, T.; Lopez-Arredondo, D.; Herrera-Estrella, L. The shoot apical meristem: An evolutionary molding of higher plants. Int. J. Mol. Sci. 2024, 25, 1519. [Google Scholar] [CrossRef]
- Rehman, S.; Bahadur, S.; Xia, W. An overview of floral regulatory genes in annual and perennial plants. Gene 2023, 885, 147699. [Google Scholar] [CrossRef]
- Shen, C.; Liu, H.; Guan, Z.; Yan, J.; Zheng, T.; Yan, W.; Wu, C.; Zhang, Q.; Yin, P.; Xing, Y. Structural Insight into DNA Recognition by CCT/NF-YB/YC Complexes in Plant Photoperiodic Flowering. Plant Cell 2020, 32, 3469–3484. [Google Scholar] [CrossRef]
- Phillips, G.C. In vitro morphogenesis in plants-recent advances. In Vitro Cell Dev. Biol-Plant 2004, 40, 342–345. [Google Scholar] [CrossRef]
- Peer, L.A.; Bhat, M.Y.; Ahmad, N.; Mir, B.A. Floral induction pathways: Decision making and determination in plants to flower-A comprehensive review. J. Appl. Biol. Biotech. 2021, 9, 7–17. [Google Scholar] [CrossRef]
- Kaur, S. In vitro florigenesis with special reference to orchids—A Review. Recent. Pat. Biotechnol. 2022, 16, 311–318. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Kerbauy, G.; Zeng, S.; Chen, Z.; Duan, J. In vitro flowering of orchids. Crit. Rev. Biotechnol. 2013, 34, 56–76. [Google Scholar] [CrossRef]
- Wang, S.L.; Viswanath, K.K.; Tong, C.G.; An, H.R.; Jang, S.; Chen, F.C. Floral induction and flower development of orchids. Front. Plant Sci. 2019, 10, 1258. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Bogarín, D.; Przelomska, N.A.S.; Ackerman, J.D.; Balbuena, J.A.; Bellot, S.; Bühlmann, R.P.; Cabrera, B.; Cano, J.A.; Charitonidou, M.; et al. The origin and speciation of orchids. New Phytol. 2024, 242, 700–716. [Google Scholar] [CrossRef]
- Peakall, R. Speciation in the Orchidaceae: Confronting the challenges. Mol. Ecol. 2007, 16, 2834–2837. [Google Scholar] [CrossRef]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley and Sons: Hoboken, NJ, USA, 1992. [Google Scholar]
- Sailo, N.; Rai, D.; De, L. Physiology of Temperate and Tropical Orchids—An Overview. Int. J. Sci. Res. 2019, 3, 3–8. [Google Scholar]
- Silvera, K.; Santiago, L.S.; Cushman, J.C.; Winter, K. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiol. 2009, 149, 1838–1847. [Google Scholar] [CrossRef]
- Hew, C.S.; Yong, J.W.H. The Physiology of Tropical Orchids in Relation to the Industry, 2nd ed.; World Scientific: Singapore, 2004. [Google Scholar]
- Koopowitz, H. Tropical Slipper Orchids: Paphiopedilum and Phragmipedium Species and Hybrids; Timber Press: Portland, OR, USA, 2008; p. 41. [Google Scholar] [CrossRef]
- Dirks-Mulder, A.; Butôt, R.; van Schaik, P.; Wijnands, J.W.P.M.; Berg, R.v.D.; Krol, L.; Doebar, S.; van Kooperen, K.; de Boer, H.; Kramer, E.M.; et al. Exploring the evolutionary origin of floral organs of Erycina pusilla, an emerging orchid model system. BMC Evol. Biol. 2017, 17, 89. [Google Scholar] [CrossRef]
- Freudenstein, J.V. Orchid phylogenetics and evolution: History, current status and prospects. Ann. Bot. 2025, 135, 805–822. [Google Scholar] [CrossRef]
- de Oliveira, R.T.; da Silva Oliveira, J.P.; Macedo, A.F. Vanilla beyond Vanilla planifolia and Vanilla × tahitensis: Taxonomy and historical notes, reproductive biology, and metabolites. Plants 2022, 11, 3311. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tiss. Org. Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Erel, R.; Dag, A.; Ben-Gal, A.; Schwartz, A.; Yermiyahu, U. Flowering and fruit set of olive trees in response to nitrogen, phosphorus, and potassium. J. Am. Soc. Hortic. Sci. 2008, 133, 639–647. [Google Scholar] [CrossRef]
- Anuradha, K.; Shyamala, B.N.; Naidu, M.M. Vanilla—Its Science of Cultivation, Curing, Chemistry, and Nutraceutical Properties. Crit. Rev. Food Sci. Nutr. 2013, 53, 1250–1276. [Google Scholar] [CrossRef]
- Gantait, S.; Sinniah, U.R. Rapid micropropagation of monopodial orchid hybrid (Aranda Wan Chark Kuan ‘Blue’ × Vanda coerulea Grifft. ex. Lindl.) through direct induction of protocorm-like bodies from leaf segments. Plant Growth Regul. 2012, 68, 129–140. [Google Scholar] [CrossRef]
- Yam, T.W.; Arditti, J.; Cameron, K.M. The orchids have been a splendid sport—An alternative look at Charles Darwin’s contribution to orchid biology. Am. J. Bot. 2009, 96, 2128–2154. [Google Scholar] [CrossRef]
- Duan, J.X.; Yazawa, S. Floral induction and development in Phalaenopsis in vitro. Plant Cell Tiss. Org. Cult. 1995, 43, 71–74. [Google Scholar] [CrossRef]
- Kerbauy, G. In vitro flowering of Oncidium varicosum mericlones (Orchidaceae). Plant Sci. Lett. 1984, 35, 73–75. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Zeng, S.; Cardoso, J.C.; Dobránszki, J.; Kerbauy, G.B. In vitro flowering of Dendrobium. Plant Cell Tiss. Org. Cult. 2014, 119, 447–456. [Google Scholar] [CrossRef]
- Kostenyuk, I.; Oh, B.J.; So, I.S. Induction of early flowering in Cymbidium niveo-marginatum Mak in vitro. Plant Cell Rep. 1999, 19, 1–5. [Google Scholar] [CrossRef]
- Chang, C.; Chang, W.C. Cytokinins promotion of flowering in Cymbidium ensifolium var. misericors in vitro. Plant Growth Regul. 2003, 39, 217–221. [Google Scholar] [CrossRef]
- Wang, G.Y.; Liu, P.; Xu, Z.H.; Chua, N.H. Effect of ABA on the in vitro production of flower buds of Dendrobium candidum Wall. ex Lindl. Acta Bot. Sin. 1995, 37, 374–378. [Google Scholar]
- Wang, G.Y.; Xu, Z.H.; Chia, T.F.; Chua, N.H. In vitro flowering of Dendrobium candidum. Sci. China (Ser. C) 1997, 40, 35–42. [Google Scholar] [CrossRef]
- Wen, Y.F.; Lu, R.L.; Xie, Z.L. Rapid propagation and induction of floral buds of Dendrobium huoshanase. Plant Physiol. Commun. 1999, 35, 296–297. [Google Scholar]
- Bhadra, S.K.; Hossain, M.M. In vitro germination and micropropagation of Geodorum densiflorum (Lam.) Schltr., an endangered orchid species. Plant Cell Tiss. Org. Cult. 2003, 13, 165–171. [Google Scholar]
- Vaz, A.P.A.; Rita de Cássia, L.; Kerbauy, G.B. Photoperiod and temperature effects on in vitro growth and flowering of P. pusilla, an epiphytic orchid. Plant Physiol. Biochem. 2004, 42, 411–415. [Google Scholar] [CrossRef]
- Rojanawong, T.; Thepsithar, C.; Thongpukdee, A. Micropropagation of Phalaenopsis cygnus ‘Silky Moon’ from leaf segments. In Proceedings of the 32nd Congress on Science and Technology of Thailand, Bangkok, Thailand, 10–12 October 2006; pp. 10–12. [Google Scholar]
- Wang, Z.H.; Tu, H.Y.; Ye, Q.S. Rapid propagation and in vitro flowering of Dendrobium moniliforme (L.) Sw. Plant Physiol. Commun. 2006, 42, 1143–1144. [Google Scholar]
- Ferreira, W.M.; Kerbauy, G.B.; Kraus, J.E.; Pescador, R.; Suzuki, R.M. Thidiazuron influences the endogenous levels of cytokinins and IAA during flowering of isolated shoots of Dendrobium. J. Plant Physiol. 2006, 163, 1126–1134. [Google Scholar] [CrossRef]
- Sim, G.E.; Loh, C.S.; Goh, C.J. High frequency early in vitro flowering of Dendrobium Madame Thong-In (Orchidaceae). Plant Cell Rep. 2007, 26, 383–393. [Google Scholar] [CrossRef]
- Hee, K.H.; Loh, C.S.; Yeoh, H.H. Early in vitro flowering and seed production in culture in Dendrobium Chao Praya Smile (Orchidaceae). Plant Cell Rep. 2007, 26, 2055–2062. [Google Scholar] [CrossRef]
- Tee, C.S.; Maziah, M.; Tan, C.S. Induction of in vitro flowering in the orchid Dendrobium Sonia 17. Biol. Plant 2008, 52, 723–726. [Google Scholar] [CrossRef]
- Vaz, A.P.A.; Kerbauy, G.B. In vitro precocious orchid flowering: A strategy for basic research and commercial approaches. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books Ltd.: Isleworth, UK, 2008; Volume V, Chapter 45; pp. 421–426. [Google Scholar]
- Deb, C.R.; Sungkumlong, T. Rapid multiplication and induction of early in vitro flowering in Dendrobium primulinum Lindl. J. Plant Biochem. Biotechnol. 2009, 18, 241–244. [Google Scholar] [CrossRef]
- Guan, P.; Shi, J.M. Tissue culture of stem segment and induction of floral buds of Dendrobium denndanum. Lishizhen Med. Mater. Med. Res. 2009, 20, 205–206. [Google Scholar]
- Than, M.M.M.; Pal, A.; Jha, S. In vitro flowering and propagation of Bulbophyllum auricomum Lindl., the royal flower of myanmar. Acta Hortic. 2009, 829, 105–111. [Google Scholar] [CrossRef]
- Te-chato, S.; Nujeen, P.; Muangsorn, S. Paclobutrazol enhance budbreak and flowering of Friederick’s Dendrobium orchid in vitro. J. Agric. Technol. 2009, 5, 157–165. [Google Scholar]
- Wang, Z.H.; Wang, L.; Ye, Q.S. High frequency early flowering from in vitro seedlings of Dendrobium nobile. Sci. Hortic. 2009, 122, 328–331. [Google Scholar] [CrossRef]
- Cen, X.F.; Huang, C.H.; Wei, P.X. Effects of hormone factors on the in vitro culture flowering induction of Dendrobium officinate Kimura et Migo. Agric. Sci. Technol. 2010, 11, 75–79. [Google Scholar]
- Chen, J.T. Induction of petal-bearing embryos from root-derived callus of Oncidium ‘Gower Ramsey’. Acta Physiol. Plant 2012, 34, 1337–1343. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sharma, M.; Pathak, P. In vitro propagation of Dendrobium aphyllum (Orchidaceae)—Seed germination to flowering. J. Plant Biochem. Biotechnol. 2013, 22, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.H. Study of tube flowering of Dendrobium candidum. Seed 2013, 32, 16–23. [Google Scholar]
- Ding, L.; Wang, Y.; Yu, H. Overexpression of DOSOC1, an ortholog of Arabidopsis SOC1, promotes flowering in the orchid Dendrobium Chao Parya Smile. Plant Cell Physiol. 2013, 54, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.J.; Lai, Z.X. Optimization of in vitro culture conditions and in vitro flowering in Dendrobium nobile. Chin. Trop. Crops 2013, 34, 451–458. [Google Scholar]
- Lee, P.L.; Chen, J.T. Plant regeneration via callus culture and subsequent in vitro flowering of Dendrobium huoshanense. Acta Physiol. Plant 2014, 36, 2619–2625. [Google Scholar] [CrossRef]
- Sawettalake, N.; Bunnag, S.; Wang, Y.; Shen, L.; Yu, H. DOAP1 Promotes Flowering in the Orchid Dendrobium Chao Praya Smile. Front. Plant Sci. 2017, 8, 400. [Google Scholar] [CrossRef]
- Chiu, Y.T.; Chang, C. In Vitro Flowering and Breeding of Erycina pusilla. In Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols. Springer Protocols Handbooks; Lee, Y.I., Yeung, E.T., Eds.; Humana Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Pujari, I.; Babu, S.V. Precocious in vitro flowering in threatened ornamental orchid, Dendrobium ovatum—Decoding the causal factors. Curr. Plant Biol. 2022, 31, 100257. [Google Scholar] [CrossRef]
- Ahmad, S.; Chen, J.; Chen, G.; Huang, J.; Hao, Y.; Shi, X.; Liu, Y.; Tu, S.; Zhou, Y.; Zhao, K.; et al. Transcriptional Proposition for Uniquely Developed Protocorm Flowering in Three Orchid Species: Resources for Innovative Breeding. Front. Plant Sci. 2022, 13, 942591. [Google Scholar] [CrossRef]
- Nadal, M.C.; Andrade, G.V.S.; Flores, J.H.N.; Reis, M.V.D.; Rodrigues, V.A.; Pasqual, M. Dendrobium nobile in vitro flowering induction. Ornam. Hortic. 2023, 29, 135–142. [Google Scholar] [CrossRef]
- Ríos-Barreto, Y.; Arellano-Ostoa, G.; Fernández-Pavía, Y.L.; García-Villanueva, E.; Tejeda-Sartorius, O. Plant growth and early in vitro floral differentiation of vanilla (Vanilla planifolia Jacks. ex Andrews). Agro Product. 2023, 17, 127–135. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; An, B.; Zhang, H.; Wu, J.; Li, C.; Long, Y. Mutagenesis and flowering promotion through sodium azide in vitro culture of Cymbidium faberi Rolfe. Horticulturae 2024, 10, 889. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; García-García, G.G.; Iglesias-Andreu, L. Conservación de vainilla (Vanilla planifolia Jacks.) bajo condiciones de lento crecimiento in vitro. Rev. Fitotec. Mex. 2015, 38, 165–171. [Google Scholar] [CrossRef]
- Halim, R.; Akyol, B.; Güner, A. In vitro Regeneration of vanilla (Vanilla planifolia L.). J. Appl. Biol. Sci. 2017, 11, 5–10. [Google Scholar]
- Lee-Espinosa, H.E.; Murguía-González, J.; García-Rosas, B.; Córdova-Contreras, A.L.; Laguna-Cerda, A.; Mijangos-Cortés, J.O.; Santana-Buzzy, N. In vitro clonal propagation of vanilla (Vanilla planifolia ‘Andrews’). HortScience 2008, 43, 454–458. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tiss. Org. Cult. 2015, 123, 657–664. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A. Investigation on in vitro bouquets and flower longevity of micropropagated Dianthus chinensis L. Sci. Hortic. 2021, 275, 109708. [Google Scholar] [CrossRef]
- Divakaran, M.; Babu, K.N. Micropropagation and In Vitro Conservation of Vanilla (Vanilla planifolia Andrews). In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants. Methods in Molecular Biology; Jain, S.M., Saxena, P.K., Eds.; Humana Press: Totowa, NJ, USA, 2009; Volume 547. [Google Scholar] [CrossRef]
- Ramos-Castellá, A.; Iglesias-Andreu, L.G.; Bello-Bello, J.; Lee-Espinosa, H. Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. In Vitro Cell Dev. Biol.-Plant 2014, 50, 576–581. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.; Iglesias Andreu, L.; Luna-Sánchez, I.J. Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. S. Afr. J. Bot. 2017, 109, 288–293. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Prasongsom, S.; Thammasiri, K.; Pritchard, H.W. Seed dormancy concepts in orchids: Dendrobium cruentum as a model species. Seed Sci. Res. 2022, 32, 175–186. [Google Scholar] [CrossRef]
- Goh, C.J.; Arditti, J. Handbook of Flowering; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Arditti, J.; Ernst, R. Micropropagation of Orchids; John Willey and Sons Inc.: Hoboken, NJ, USA, 1993. [Google Scholar]
- Knudson, L. A new nutrient solution for the germination of orchid seed. Am. Orchid. Soc. Bull. 1946, 15, 214–217. [Google Scholar]
- Vacin, E.F.; Went, F.W. Some pH changes in nutrient solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Malmgren, S. Orchid propagation: Theory and practice. In North American Native Terrestrial Orchids: Propagation and Production, Proceedings of the North American Native Terrestrial Orchid Conference, Germantown, MD, USA, 16–17 March 1996; Allen, C., Ed.; National Arboretum: Washington, DC, USA, 1996; pp. 63–71. [Google Scholar]
- Gayatri, M.C.; Kavyashree, R. Influence of carbon sources on in vitro seed germination, protocorm and shoot formation in Vanilla planifolia. J. Curr. Sci. 2005, 7, 43–48. [Google Scholar]
- Sidek, N.; Anuar, N.S.M.; Naher, L.; Rahman, K.A.M.A. The effect of different nutrient media on in vitro shoot and root proliferation of Vanilla planifolia Jacks. ex Andrews. Afr. J. Biotechnol. 2018, 17, 1241–1246. [Google Scholar] [CrossRef]
- Davidonis, G.; Knorr, D. Callus formation and shoot regeneration in Vanilla planifolia. Food Biotechnol. 1991, 5, 59–66. [Google Scholar] [CrossRef]
- Gu, Z.; Arditti, J.; Nyman, L.P. Vanilla planifolia: Callus induction and plantlet production in vitro. Lindleyana 1987, 2, 48–52. [Google Scholar]
- Janarthanam, B.; Seshadri, S. Plantlet regeneration from leaf derived callus of Vanilla planifolia Andr. In Vitro Cell Dev. Biol. 2008, 44, 84–89. [Google Scholar] [CrossRef]
- Romagnoli, L.G.; Knorr, D. Effects of ferulic acid treatment on growth and flavor development of cultured Vanilla planifolia cells. Food Biotechnol. 1988, 2, 93–104. [Google Scholar] [CrossRef]
- Velankar, M.H.; Heble, M.R. Biotransformation of externally added vanillin related compounds by multiple shoot cultures of Vanilla planifolia L. J. Plant Biochem. Biotechnol. 2004, 13, 153–156. [Google Scholar] [CrossRef]
- Goh, C.J.; Yang, A.L. Effects of growth regulators and decapitation on flowering of Dendrobium orchid hybrids. Plant Sci. Lett. 1978, 12, 278–292. [Google Scholar] [CrossRef]
- Ziv, M.; Naor, V. Flowering of geophytes in vitro. Propag. Ornam. Plants 2006, 6, 3–16. [Google Scholar]
- Teixeira da Silva, J.A.; Yam, T.; Fukai, S.; Nayak, N.; Tanaka, M. Establishment of optimum nutrient media for in vitro propagation of Cymbidium Sw. (Orchidaceae) using protocorm-like body segments. Propag. Ornam. Plants 2005, 5, 129–136. [Google Scholar]
- Paiva-Neto, V.B.; Otoni, W.C. Carbon sources and their osmotic potential in plant tissue culture: Does it matter? Sci. Hortic. 2003, 97, 193–202. [Google Scholar] [CrossRef]
- Kane, M.E. Propagation by shoot culture. In Plant Tissue Culture, Development and Biotechnology; Trigiano, R.N., Gray, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 181–192. [Google Scholar]
- Liu, T.H.A.; Lin, J.J.; Wu, R.Y. The effects of using trehalose as a carbon source on the proliferation of Phalaenopsis and Doritaenopsis protocorm-like bodies. Plant Cell Tiss. Org. Cult. 2006, 86, 125–129. [Google Scholar] [CrossRef]
- Zha, X.Q.; Luo, J.; Jiang, P.S.T.; Wan, J.H. Enhancement of polysaccharide production in suspension cultures of protocorm-like bodies from Dendrobium huoshanense by optimization of medium compositions and feeding sucrose. Process Biochem. 2007, 42, 344–351. [Google Scholar] [CrossRef]
- Baker, F.S.; Miller, C.E.; Repik, A.J.; Tolles, E.D. (Eds.) Activated Carbon. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Fridborg, G.; Pedersen, M.; Landstrom, L.E.; Eriksson, T. The effect of activated charcoal on tissue cultures: Adsorption of metabolites inhibiting morphogenesis. Physiol. Plant 1978, 43, 104–106. [Google Scholar] [CrossRef]
- Theander, O.; Nelson, D.A. Aqueous, high temperature transformation of carbohydrates relative to utilization of biomass. Adv. Carbohydr. Chem. Biochem. 1988, 46, 273–326. [Google Scholar]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Liu, M.S.C. Plant regeneration in cell suspension culture of sugarcane as affected by activated charcoal, medium composition and tissue culture. Taiwan. Sugar 1993, 40, 18–25. [Google Scholar]
- Teixeria, J.B.; Sondahl, M.R.; Kirby, E.G. Somatic embryogenesis from immature inflorescences of oil palm. Plant Cell Rep. 1994, 13, 247–250. [Google Scholar] [CrossRef]
- Owen, H.R.; Wengerd, D.; Miller, A.R. Culture medium pH is influenced by basal medium, carbohydrate source, gelling agent, activated charcoal, and medium storage method. Plant Cell Rep. 1991, 10, 583–586. [Google Scholar] [CrossRef]
- Dumas, E.; Monteuuis, O. In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants—Influence of activated charcoal. Plant Cell Tiss. Org. Cult. 1995, 40, 231–235. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Edmond, V.; Moon, P.A.; Bremgartner, M.; Wu, X.; Bassil, E. Agrobacterium-mediated transformation, selection and regeneration of Vanilla pompona. Plant Cell Tiss. Org. Cult. 2024, 158, 41. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based Methods for Genome Engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Hu, Y.; Resende, M.F.R.; Bombarely, A.; Brym, M.; Bassil, E.; Chambers, A.H. Genomics-based diversity analysis of Vanilla species using a Vanilla planifolia draft genome and Genotyping-By-Sequencing. Sci. Rep. 2019, 9, 3416. [Google Scholar] [CrossRef]
- Hasing, T.; Tang, H.; Brym, M.; Khazi, F.; Huang, T.; Chambers, A.H. A phased Vanilla planifolia genome enables genetic improvement of flavour and production. Nat. Food 2020, 1, 811–819. [Google Scholar] [CrossRef]
- Ovcharenko, O.O.; Rudas, V.A. Modern approaches to Genetic Engineering in the Orchidaceae Family. Cytol. Genet. 2023, 57, 142–156. [Google Scholar] [CrossRef]
- Chia, T.F.; Chan, Y.S.; Chua, N.H. Genetic engineering of tolerance to Cymbidium Mosaic Virus. In Proceedings of the 13th World Orchid Conference; Kernohan, J., Bonham, N., Bonham, D., Cobb, L., Eds.; World Orchid Conference Trust: Auckland, New Zealand, 1990; p. 284. [Google Scholar]
- Nan, G.L.; Kuehnle, A.R. Factors affecting gene delivery by particle bombardment of Dendrobium orchids. In Vitro Cell Dev. Biol–Plant 1995, 31, 131–136. [Google Scholar] [CrossRef]
- Retheesh, S.T.; Bhat, A.I. Genetic transformation and regeneration of transgenic plants from protocorm—Like bodies of vanilla (Vanilla planifolia Andrews) using Agrobacterium tumefaciens. J. Plant Biochem. Biotechnol. 2011, 20, 262–269. [Google Scholar] [CrossRef]
- Felix, L.P.; Guerra, M. Chromosome Analysis in Psygmorchis pusilla (L.) Dodson & Dressier: The Smallest Chromosome Number Known in Orchidaceae. Caryologia 1999, 52, 165–168. [Google Scholar]
- Song, C.; Wang, Y.; Manzoor, M.A.; Mao, D.; Wei, P.; Cao, Y.; Zhu, F. Indepth analysis of genomes and functional genomics of orchid using cutting-edge high-throughput sequencing. Front. Plant Sci. 2022, 13, 1018029. [Google Scholar] [CrossRef]
- Bory, S.; Lubinsky, P.; Risterucci, A.M.; Noyer, J.L.; Grisoni, M.; Duval, M.F.; Besse, P. Patterns of introduction and diversification of Vanilla planifolia (Orchidaceae) in Reunion Island (Indian Ocean). Am. J. Bot. 2008, 95, 805–815. [Google Scholar] [CrossRef]
- Lepers-Andrzejewski, S.; Siljak-Yakovlev, S.; Brown, S.C.; Wong, M.; Dron, M. Diversity and dynamics of plant genome size: An example of polysomaty from a cytogenetic study of Tahitian vanilla (Vanilla × tahitensis, Orchidaceae). Am. J. Bot. 2011, 98, 986–997. [Google Scholar] [CrossRef]
- Piet, Q.; Droc, G.; Marande, W.; Sarah, G.; Bocs, S.; Klopp, C.; Bourge, M.; Siljak-Yakovlev, S.; Bouchez, O.; Lopez-Roques, C.; et al. A chromosome-level, haplotype-phased Vanilla planifolia genome highlights the challenge of partial endoreplication for accurate whole-genome assembly. Plant Comm. 2022, 3, 100330. [Google Scholar] [CrossRef]
- Himani; Sharma, A.; Ramkumar, T.R.; Sembi, J.K. Regulatory mechanisms underlying florigenesis in Vanilla planifolia Andrews: A study of MADS-box gene family. J. Hortic. Sci. Biotechnol. 2021, 96, 428–443. [Google Scholar] [CrossRef]
- Brym, M.; Brewer, S.; Wu, X.; Chambers, A.H. CRISPR/Cas9-mediated editing of the phytoene desaturase gene in Vanilla planifolia enabling targeted domestication. J. Hortic. Sci. Biotechnol. 2024, 99, 421–430. [Google Scholar] [CrossRef]
| Factors | Recommended Ranges |
|---|---|
| Photoperiod | 12 to 16 h light |
| Temperature | 18 to 24 °C |
| Culture medium | Knudson C and MS |
| Nitrogen | 1/2 full-strength MS salts |
| Potassium | 1.25 to 1.50 times full-strength MS salts |
| Sucrose | 1.5 to 3% (w/v) |
| Benzyladenine | 0.5 to 5 mg/L |
| Thidiazuron | 0.1 to 3 mg/L |
| Naphthaleneacetic acid | 0.1 to 0.5 mg/L |
| Paclobutrazol | 0.1 to 1 mg/L |
| Gibberellic acid | 0.1 to 2 mg/L |
| Activated charcoal | 0.5 to 2.0 g/L |
| Coconut water | 50 to 200 mL/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltazar-Bernal, O.; Spinoso-Castillo, J.L. Recent Advances in In Vitro Floral Induction in Tropical Orchids: Progress and Prospects in Vanilla Species. Horticulturae 2025, 11, 829. https://doi.org/10.3390/horticulturae11070829
Baltazar-Bernal O, Spinoso-Castillo JL. Recent Advances in In Vitro Floral Induction in Tropical Orchids: Progress and Prospects in Vanilla Species. Horticulturae. 2025; 11(7):829. https://doi.org/10.3390/horticulturae11070829
Chicago/Turabian StyleBaltazar-Bernal, Obdulia, and José Luis Spinoso-Castillo. 2025. "Recent Advances in In Vitro Floral Induction in Tropical Orchids: Progress and Prospects in Vanilla Species" Horticulturae 11, no. 7: 829. https://doi.org/10.3390/horticulturae11070829
APA StyleBaltazar-Bernal, O., & Spinoso-Castillo, J. L. (2025). Recent Advances in In Vitro Floral Induction in Tropical Orchids: Progress and Prospects in Vanilla Species. Horticulturae, 11(7), 829. https://doi.org/10.3390/horticulturae11070829








