Bioengineered Indoor Farming Approaches: LED Light Spectra and Biostimulants for Enhancing Vindoline and Catharanthine Production in Catharanthus roseus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Condition
2.2. Experimental Treatments
2.3. Samplings
2.4. Evaluation of Mycorrhizal Colonization
2.5. Quantitative Biochemical Analyses of Alkaloids
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Mycorrhizal Colonization
3.2. ANOVA Results
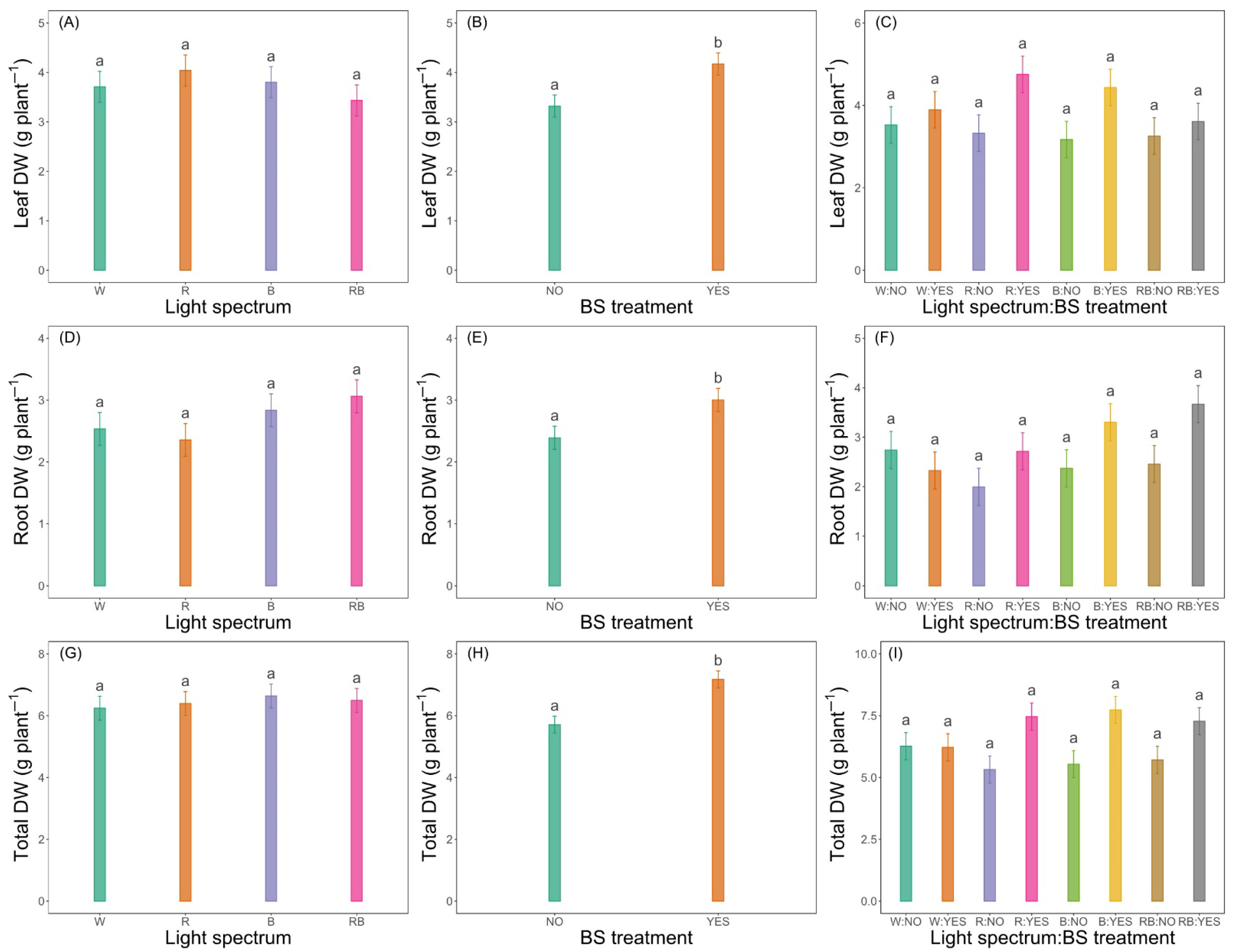
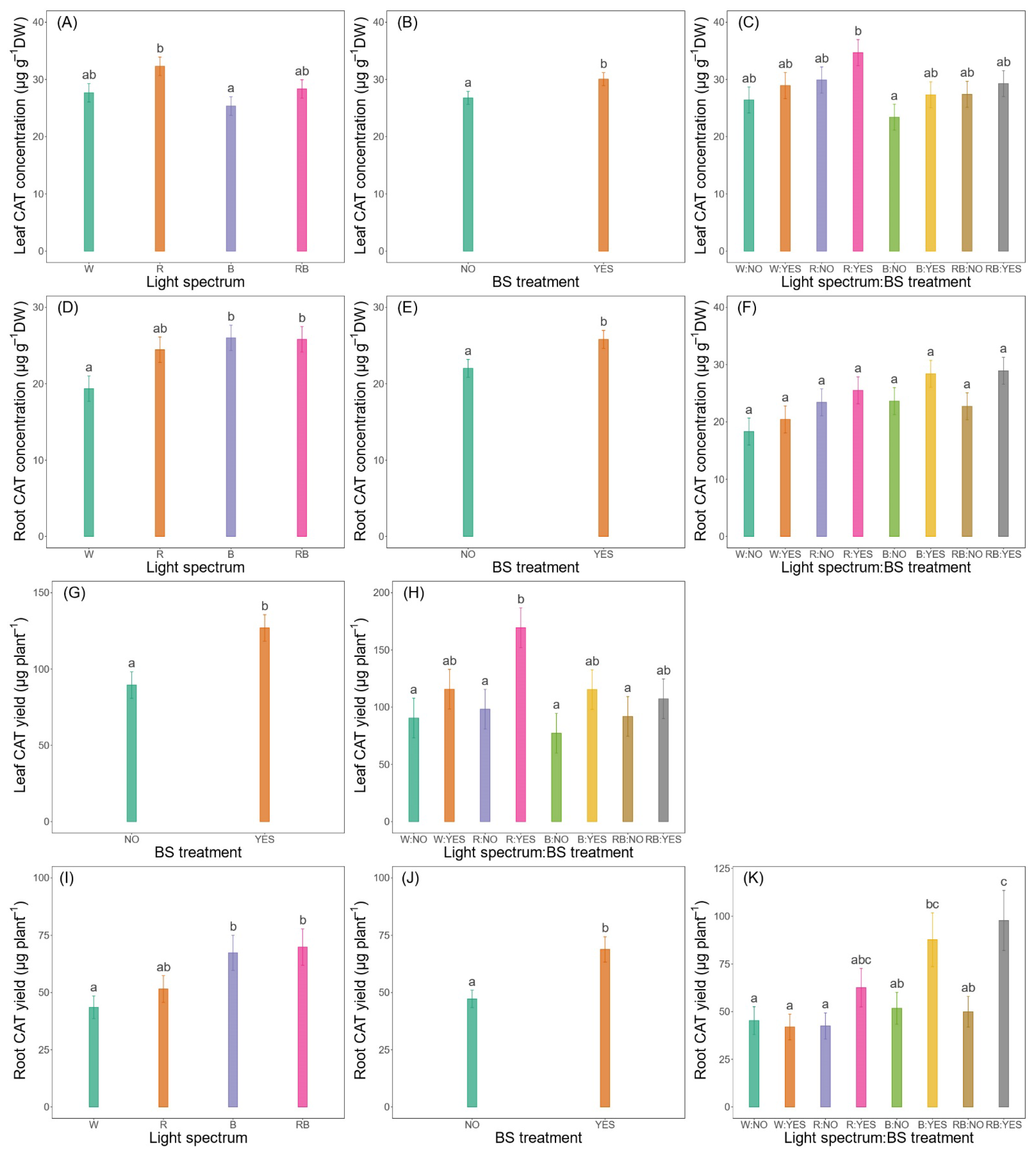
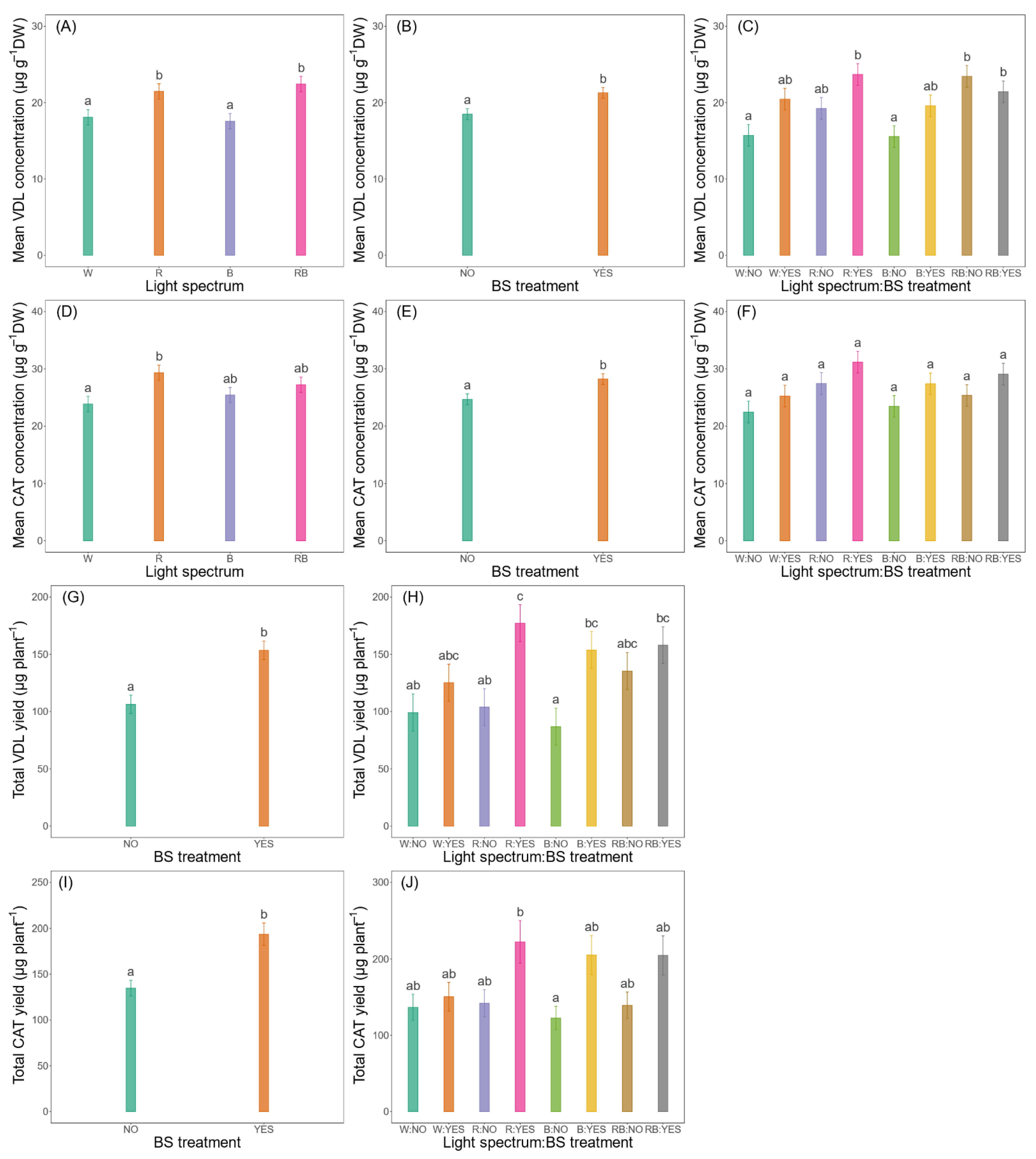
3.3. Effect of LED Light and BS on DW, Concentrations, and Yields
3.3.1. Leaf, Root and Total DW
3.3.2. Leaf and Root Concentrations and Yields
3.3.3. Mean Concentrations and Total Yields
3.4. Effects of Sampling Time on DW, Concentrations, and Yields
4. Conclusions
- (i)
- Investigate the expression of vindoline-related biosynthetic genes in root tissues;
- (ii)
- Examine potential transport processes responsible for alkaloid movement between organs;
- (iii)
- Combine biochemical and molecular approaches to distinguish in situ synthesis from phloem-mediated translocation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, S.K.; Livingston, R.B. Plant products in cancer chemotherapy. Cancer Treat. Rep. 1976, 60, 1141–1156. [Google Scholar] [PubMed]
- Roepke, J.; Salim, V.; Wu, M.; Thamm, A.M.K.; Murata, J.; Ploss, K.; Boland, W.; De Luca, V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc. Natl. Acad. Sci. USA 2010, 107, 15287–15292. [Google Scholar] [CrossRef] [PubMed]
- Guéritte, F.; Fahy, J. The vinca alkaloids. In Anticancer Agents from Natural Products, 2nd ed.; Kingston, D., Cragg, G., Newman, D., Eds.; CRC Press: New York, NY, USA, 2005; pp. 177–188. [Google Scholar]
- Hirata, K.; Akagi, T.; Duangteraprecha, S.-E.; Honda, M.; Sakamoto, Y.; Nagase, H.; Miyamoto, K. Catharanthine Oxidation in Flavin Mononucleotide-Mediated Catharanthine-Vindoline Coupling Reaction for Synthesis of Dimeric Indole Alkaloids under Near-Ultraviolet Light. J. Biosci. Bioeng. 1999, 87, 781–786. [Google Scholar] [CrossRef]
- Blazich, F.A.; Henry, P.H.; Wise, F.C. Seed germination of annual vinca responds to irradiation and temperature. Hortscience 1995, 30, 357–359. [Google Scholar] [CrossRef]
- Nagy, K.; Darkó, É.; Szalai, G.; Janda, T.; Jókai, Z.; Ladányi, M.; Rady, M.R.; Dernovics, M. UPLC-ESI-QTOF-MS assisted targeted metabolomics to study the enrichment of vinca alkaloids and related metabolites in Catharanthus roseus plants grown under controlled LED environment. J. Pharm. Biomed. Anal. 2023, 235, 115611. [Google Scholar] [CrossRef]
- Vatistas, C.; Avgoustaki, D.D.; Bartzanas, T. A Systematic Literature Review on Controlled-Environment Agriculture: How Vertical Farms and Greenhouses Can Influence the Sustainability and Footprint of Urban Microclimate with Local Food Production. Atmosphere 2022, 13, 1258. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Olle, M.; Viršilė, A.; Olle, M.; Viršilė, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Quadri, A.; Barbaresi, A.; Tassinari, P.; Bertaccini, A.; Contaldo, N.; Mercolini, L.; Protti, M.; Montalbetti, R.; Laurita, R.; Torreggiani, D. Enhancement of vindoline and catharanthine production in Catharanthus roseus by LED light and plasma activated water. PLoS ONE 2024, 19, e0315542. [Google Scholar] [CrossRef]
- Eftekhari, M.; Javid, M.G.; Aliniaeifard, S.; Nicola, S. Alteration of Flower Yield and Phytochemical Compounds of Saffron (Crocus sativus L.) by Application of Different Light Qualities and Growth Regulators. Horticulture 2023, 9, 169. [Google Scholar] [CrossRef]
- Quadri, A.; Barbaresi, A.; Tassinari, P.; Bertaccini, A.; Contaldo, N.; Mercolini, L.; Protti, M.; Montalbetti, R.; Laurita, R.; Torreggian, D. Use of LED light and plasma activated water (PAW) to stimulate pharmaceutical compound production in Catharanthus roseus plants. Acta Hortic. 2025, 1423, 255–268. [Google Scholar] [CrossRef]
- Trenta, M.; Quadri, A.; Sambuco, B.; Perez Garcia, C.A.; Barbaresi, A.; Tassinari, P.; Torreggiani, D. Green Roof Management in Mediterranean Climates: Evaluating the Performance of Native Herbaceous Plant Species and Green Manure to Increase Sustainability. Buildings 2025, 15, 640. [Google Scholar] [CrossRef]
- Chiocchio, I.; Barbaresi, A.; Barbanti, L.; Mandrone, M.; Poli, F.; Torreggiani, D.; Trenta, M.; Tassinari, P. Effects of LED supplemental lighting on the growth and metabolomic profile of Taxus baccata cultivated in a smart greenhouse. PLoS ONE 2022, 17, e0266777. [Google Scholar] [CrossRef]
- Fukuyama, T.; Ohashi-Kaneko, K.; Ono, E.; Watanabe, H. Growth and alkaloid yields of Catharanthus roseus (L.) G. Don cultured under red and blue LEDs. Shokubutsu Kankyo Kogaku 2013, 25, 175–182. [Google Scholar] [CrossRef]
- Fukuyama, T.; Ohashi-Kaneko, K.; Watanabe, H. Estimation of optimal red light intensity for production of the pharmaceutical drug components, vindoline and catharanthine, contained in Catharanthus roseus (L.) G. Don. Environ. Control Biol. 2015, 53, 217–220. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, N.; Ebrahimi, M. Red LED light promotes biomass, flowering and secondary metabolites accumulation in hydroponically grown Hypericum perforatum L. (cv. Topas). Ind. Crops Prod. 2022, 175, 114239. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Shi, Y.; Wu, L.; Zhang, T.; Xiang, L. Red and blue light promote the accumulation of artemisinin in Artemisia Annua L. Molecules 2018, 23, 1329. Molecules 2018, 23, 1329. [Google Scholar] [CrossRef]
- Sambuco, B.; Quadri, A.; Trenta, M.; Tassinari, P.; Torreggiani, D.; Barbaresi, A.; Mercolini, L.; Protti, M. Enhancing secondary metabolites accumulation in Coleus blumei through LED light application. Acta Hortic. 2025, 1, 243–254. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Huqail, A.A.; El-Gamal, S.M.A. Improvement of Phytopharmaceutical and Alkaloid Production in Periwinkle Plants by Endophyte and Abiotic Elicitors. Horticulturae 2022, 8, 237. [Google Scholar] [CrossRef]
- Machado VPde, O.; Pacheco, A.C.; Carvalho, M.E.A. Effect of biostimulant application on production and flavonoid content of marigold (Calendula officinalis L.). Rev. Ceres 2014, 61, 983–988. [Google Scholar] [CrossRef]
- Andrade, S.A.L.; Malik, S.; Sawaya, A.C.H.F.; Bottcher, A.; Mazzafera, P. Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol. Plant 2013, 35, 867–880. [Google Scholar] [CrossRef]
- Raei, Y.; Weisany, W. Arbuscular mycorrhizal fungi associated with some aromatic and medicinal plants. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 129–138. [Google Scholar]
- Cartmill, A.D.; Valdez-Aguilar, L.A.; Bryan, D.L.; Alarcón, A. Arbuscular mycorrhizal fungi enhance tolerance of vinca to high alkalinity in irrigation water. Sci. Hortic. 2008, 115, 275–284. [Google Scholar] [CrossRef]
- Ratti, N.; Verma, H.N.; Gautam, S.P. Effect of Glomus species on physiology and biochemistry of Catharanthus roseus. Indian. J. Microbiol. 2010, 50, 355–360. [Google Scholar] [CrossRef][Green Version]
- Rafiee, H.; Mehrafarin, A.; Qaderi, A.; Kalate Jari, S.; Naghdi Badi, H. Phytochemical, agronomical and morphological responses of pot marigold (Calendula officinalis L.) to foliar application of bio-stimulators (bioactive amino acid compounds). J. Med. Plants 2013, 12, 48–61. [Google Scholar]
- Darzi, M.T.; Hazi, M.S. Effects of the application of organic manure and biofertilizer on the fruit yield and yield components in Dill (Anethum graveolens). J. Med. Plants Res. 2012, 6, 3345–3350. [Google Scholar] [CrossRef]
- El-Sharabasy, S.; Farag, M.A.; El-Emerym, G. Effect of amino acids on the growth and production of steroids in date palm using tissue culture technique. Researcher 2012, 4, 75–84. [Google Scholar]
- Ardabili, Z.; Moghadam, A.; Ardebili, N.; Omics, A.P.-P. 2012 undefined The induced physiological changes by foliar application of amino acids in Aloe vera, L. plants. Plant Omics 2012, 5, 279–284. [Google Scholar]
- Omer, E.; Said-Al-Ahl, H.; El-Gendy, A.; Shaban, K. Effect of amino acids application on production, volatile oil and chemical composition of chamomile cultivated in saline soil at sinai. J. Appl. Sci. Res. 2013, 9, 3006–3021. [Google Scholar]
- Haj, M.R.; Hadi, S.; Taghi Darz, M.; Ghandehari, Z.; Riazi, G. Effects of vermicompost and amino acids on the flower yield and essential oil production from Matricaria chamomile L. J. Med. Plants Res. 2011, 5, 5611–5617. [Google Scholar]
- El-Din, K.M.G.; El-Wahed, M.S.A.A. Effect of some amino acids on growth and essential oil content of chamomile plant. Int. J. Agric. Biol. 2005, 7, 376–380. [Google Scholar]
- Rahimi, S.; Mr, D.-M.; Naghdi, B. Changes in essential oil composition and leaf traits of basil affected by bio-stimulators/fertilizers application. J. Med. Plants 2013, 12, 83–92. [Google Scholar]
- Befrozfar, M.R.; Habibi, D.; Asgharzadeh, A.; Sadeghi-Shoae, M.; Tookalloo, M.R. Vermicompost, plant growth promoting bacteria and humic acid can affect the growth and essence of basil (Ocimum basilicum L.). Ann Biol Res 2013, 4, 8–12. [Google Scholar] [CrossRef]
- Mehrafarin, A.; Badi, H.N.; Khalighi, F. Effects of bio-stimulators and bio-fertilizers on morphological traits of basil (Ocimum bacilicum L.). Ann Biol Res 2013, 4, 146–151. [Google Scholar]
- Rafiee, H.; Mehrafarin, A.; Labbafi, M.; Qaderi, A.; Naghdi Badi, H. Mineral elements and biochemical analysis of Calendula officinalis L. affected by bio-stimulators. Trakia J. Sci. 2015, 13, 27–35. [Google Scholar] [CrossRef]
- Vierheilig, H.; Schweiger, P.; Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant 2005, 125, 393–404. [Google Scholar] [CrossRef]
- Lenth, R. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8.4-1. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 8 July 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression +16,000 Citations; Sage: Thousand Oaks, CA, USA, 2019; Volume 2016. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 8 July 2025).
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piaggesi, A. Use of biostimulants for reducing nutrient solution concentration in floating system. Acta Hortic. 2006, 718, 477–484. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Nofal, E.M.S.; Menesy Fardous, A.; Abd El-Hady, W.M.; Shehab El-Deen Eman, G. Effect of bio-stimulants (Humic acid, salicylic acid and Chitosan) on rose periwinkle (Catharanthus roseus L.). Appl Ecol Env. Res 2021, 19, 971–980. [Google Scholar] [CrossRef]
- Endo, T.; Goodbody, A.; Misawa, M. Alkaloid production in root and shoot cultures of Catharanthus roseus. Planta Med. 1987, 53, 479–482. [Google Scholar] [CrossRef]
- Mall, M.; Singh, P.; Kumar, R.; Shanker, K.; Gupta, A.K.; Khare, P.; Ajit, K.; Shasany, A.K.; Khatoon, S.; Sundaresan, V.; et al. Phenotypic, genetic and expression profiling of a vindoline-rich genotype of Catharanthus roseus. South. Afr. J. Bot. 2021, 139, 50–57. [Google Scholar] [CrossRef]
- Shukla, A.K.; Shasany, A.K.; Verma, R.K.; Gupta, M.M.; Mathur, A.K.; Khanuja, S.P.S. Influence of cellular differentiation and elicitation on intermediate and late steps of terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Protoplasma 2010, 242, 35–47. [Google Scholar] [CrossRef]
- Liu, Y.; Patra, B.; Pattanaik, S.; Wang, Y.; Yuan, L. GATA and phytochrome interacting factor transcription factors regulate light-induced vindoline biosynthesis in Catharanthus roseus. Plant Physiol. 2019, 180, 1336–1350. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fragoso, V.; Paranhos, J.T.; Rau, M.R.; Fett-Neto, A.G. The bioactive monoterpene indole alkaloid N.;β-d-glucopyranosyl vincosamide is regulated by irradiance quality and development in Psychotria leiocarpa. Ind. Crops Prod. 2016, 86, 210–218. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Yu, W.; Hu, Y.; Ma, X.; Wu, J.; Ying, J. Light quality modifies camptothecin production and gene expression of biosynthesis in Camptotheca acuminata Decne seedlings. Ind. Crops Prod. 2015, 66, 137–143. [Google Scholar] [CrossRef]
- Kirfel, J.; Magin, T.M.; Reichelt, J. Keratins: A structural scaffold with emerging functions. Cell. Mol. Life Sci. 2003, 60, 56–71. [Google Scholar] [CrossRef]
- Bryndina, L.; Ilyina, N.; Baklanova, O.; Moiseyeva, E. Comparative evaluation of biostimulator efficiency on corn seeds germination: Keratin protein and preparation Ribav Extra. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012068. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216–243. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Skopinska-Wiłniewska, J.; Kozłowska, J.; Płanecka, A.; Kurzawa, M. Photochemical behaviour of hydrolysed keratin. Int. J. Cosmet. Sci. 2011, 33, 503–508. [Google Scholar] [CrossRef]
- Naeem, M.; Aftab, T.; Idrees, M.; Alam, M.M.; Masroor, M.A.K.; Uddin, M. Plant efficacy and alkaloids production in sadabahar (Catharanthus roseus L.): Role of potent pgrs and mineral nutrients. In Catharanthus roseus: Current Research and Future Prospects; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–57. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A.M. Hairy roots: An untapped potential for production of plant products. Front. Plant Sci. 2022, 13, 937095. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Patra, B.; Singh, S.K.; Paul, P.; Zhou, Y.; Li, Y.; Wang, Y.; Pattanaik, S.; Yuan, L. Terpenoid indole alkaloid biosynthesis in Catharanthus roseus: Effects and prospects of environmental factors in metabolic engineering. Biotechnol. Lett. 2021, 43, 2085–2103. [Google Scholar] [CrossRef]
- Moreno, P.R.H.; Van Der Heijden, R.; Verpoorte, R. Elicitor-mediated induction of isochorismate synthase and accumulation of 2,3-dihydroxy benzoic acid in Catharanthus roseus cell suspension and shoot cultures. Plant Cell Rep. 1994, 14, 188–191. [Google Scholar] [CrossRef]
- Shanks, J.V.; Rijhwani, S.K.; Morgan, J.; Vani, S.; Bhadra, R.; Ho, C.-H. Quantification of Metabolic Fluxes for Metabolic Engineering of Plant Products. In Plant Cell and Tissue Culture for the Production of Food Ingredients; Fu, T.J., Singh, G., Curtis, W.R., Eds.; Springer: Boston, MA, USA, 1999; pp. 45–60. [Google Scholar] [CrossRef]
- Hamza, B.; Suggars, A. Biostimulants: Myths and Realities. TurfGrass Trends 2001, 8, 6–10. [Google Scholar]
- Nardi, S.; Ertani, A.; Francioso, O. Soil–root cross-talking: The role of humic substances. J. Plant Nutr. Soil. Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Scientia Agricola Plant biostimulants: Physiological responses induced by protein hydrolyzed-based. Sci. Agric. V 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Aerts, R.J.; Alarco, A.-M.; De Luca, V. Auxins Induce Tryptophan Decarboxylase Activity in Radicles of Catharanthus Seedlings. Plant Physiol. 1992, 100, 1014–1019. [Google Scholar] [CrossRef]
- Papon, N.; Bremer, J.; Vansiri, A.; Andreu, F.; Rideau, M.; Crèche, J. Cytokinin and ethylene control indole alkaloid production at the level of the MEP/terpenoid pathway in Catharanthus roseus suspension cells. Planta Med. 2005, 71, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.-W.; Cong, W.-W.; Chen, Q.; Zu, Y.-G.; Tang, Z.-H. The influence of different forms and concentrations of potassium nutrition on growth and alkaloid metabolism in Catharanthus roseus seedlings. J. Plant Interact. 2014, 9, 370–377. [Google Scholar] [CrossRef]
- Boldyreva, Y.; Velichko, N. Effect of the compounds of mineral nutrition on the Catharanthus roseus callus tissue growth and synthesis of alkaloids. Biotekhnologiya 2003, 19, 53–62. [Google Scholar]
- Ohashi-Kaneko, K.; Fukuyama, T.; Nakai, A.; Usami, H.; Ono, E.; Watanabe, H. Growth and alkaloids production in Madagascar periwinkle plants grown under red LED. IFAC Proc. Vol. 2013, 46, 274–277. [Google Scholar] [CrossRef]
- Pan, Q.; Saiman, M.Z.; Rianika Mustafa, N.; Verpoorte, R.; Tang, K. A simple and rapid HPLC-DAD method for simultaneously monitoring the accumulation of alkaloids and precursors in different parts and different developmental stages of Catharanthus roseus plants. J. Chromatogr. B 2016, 1014, 10–16. [Google Scholar] [CrossRef]
- Guo, X.R.; Chang, B.W.; Zu, Y.G.; Tang, Z.H. The impacts of increased nitrate supply on Catharanthus roseus growth and alkaloid accumulations under ultraviolet-B stress. J. Plant Interact. 2014, 9, 640–646. [Google Scholar] [CrossRef]
- Yu, F.; De Luca, V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2013, 110, 15830–15835. [Google Scholar] [CrossRef]
- González, M. Cloning and Characterization of Transporter Genes Potentially Involved in the Transmembrane Transport of the Alkaloids from the Medicinal Plant. Master’s Thesis, Universidade do Porto, Porto, Portugal, 2013. [Google Scholar]


| Light Spectrum | BS a | Treatment | TC b | Room Conditions |
|---|---|---|---|---|
| White light (W; R = 49.6%, G = 35.5%, B = 13.2%) | NO | W (control) | W:NO | PPFD c = 150 Phot d: 16/8 T e = 23 RH f = 70 |
| YES | W + BS | W:YES | ||
| Red light (R, 658 nm) | NO | R | R:NO | |
| YES | R + BS | R:YES | ||
| Blue light (B, 446 nm) | NO | B | B:NO | |
| YES | B + BS | B:YES | ||
| Mixture of R and B (6:1) | NO | RB | RB:NO | |
| YES | RB + BS | RB:YES |
| Component Category | Description | Details |
|---|---|---|
| Bioactive particles (BPs) | Fragments of colonized roots, spores, and mycelium from five arbuscular mycorrhizal fungi (AMF) species naturally occurring in European soils | Fungal species: - Clariodeoglomus etunicatum - Clariodeoglomus claroideum - Rhizophagus irregularis - Funneliformis geosporus - Funneliformis mosseae |
| BP concentration | Minimum and typical number of infective propagules per kg | Minimum: 200,000 propagules/kg Typical: 325,000 propagules/kg (evaluated by the Most Probable Number test) |
| Inert carrier Components | Materials used for the physical support of bioactive particles and to facilitate their dispersion | - Expanded clay: 500 g/kg (brown particles, fraction: 1–2.5 mm) - Clinoptilolite clay (zeolite): 390 g/kg (green particles, fraction: 0.5–2.5 mm) |
| Bioadditive components | Natural minerals, seaweed extracts, natural keratin, humates, and powdered biodegradable water-storing polymer granules, supporting the development of mycorrhizal symbiosis | Key ingredients (52 g/kg of product): - Keratin - Milled phosphates - Alginates (seaweed) - Humates - Patentkali - Dolomite - Water-storing granules |
| Average product mass | Estimated bulk density | 700–800 kg/m3 |
| Compound | MW (g/mol) | Parent Ion (m/z) | Product Ions (m/z) a | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|
| Catharanthine | 336.4 | 337.5 | 173.4, 144.3 | 25 | 30 |
| Vindoline | 456.6 | 457.5 | 427.5, 397.3 | 35 | 40 |
| IS (vindoline-d3) | 459.6 | 460.5 | 430.4, 400.2 | 35 | 40 |
| Light | BS | Light:BS | |
|---|---|---|---|
| Leaf DW (g plant−1) | ns | ** | ns |
| Root DW (g plant−1) | ns | * | ns |
| Total DW (g plant−1) | ns | *** | ns |
| Leaf VDL concentration (µg g−1 DW) | *** | ns | *** |
| Leaf VDL yield (µg plant−1) | ns | * | ** |
| Root VDL concentration (µg g−1 DW) | *** | *** | ns |
| Root VDL yield (µg plant−1) | ns | *** | ns |
| Mean VDL concentration (µg g−1 DW) | ** | ** | . |
| Total VDL yield (µg plant−1) | ns | *** | ns |
| Leaf CAT concentration (µg g−1 DW) | * | * | ns |
| Leaf CAT yield (µg plant−1) | ns | ** | ns |
| Root CAT concentration (µg g−1 DW) | * | * | ns |
| Root CAT yield (µg plant−1) | * | ** | ns |
| Mean CAT concentration (µg g−1 DW) | * | * | ns |
| Total CAT yield (µg plant−1) | ns | *** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quadri, A.; Sambuco, B.; Trenta, M.; Tassinari, P.; Torreggiani, D.; Mercolini, L.; Protti, M.; Zambonelli, A.; Puliga, F.; Barbaresi, A. Bioengineered Indoor Farming Approaches: LED Light Spectra and Biostimulants for Enhancing Vindoline and Catharanthine Production in Catharanthus roseus. Horticulturae 2025, 11, 828. https://doi.org/10.3390/horticulturae11070828
Quadri A, Sambuco B, Trenta M, Tassinari P, Torreggiani D, Mercolini L, Protti M, Zambonelli A, Puliga F, Barbaresi A. Bioengineered Indoor Farming Approaches: LED Light Spectra and Biostimulants for Enhancing Vindoline and Catharanthine Production in Catharanthus roseus. Horticulturae. 2025; 11(7):828. https://doi.org/10.3390/horticulturae11070828
Chicago/Turabian StyleQuadri, Alessandro, Bianca Sambuco, Mattia Trenta, Patrizia Tassinari, Daniele Torreggiani, Laura Mercolini, Michele Protti, Alessandra Zambonelli, Federico Puliga, and Alberto Barbaresi. 2025. "Bioengineered Indoor Farming Approaches: LED Light Spectra and Biostimulants for Enhancing Vindoline and Catharanthine Production in Catharanthus roseus" Horticulturae 11, no. 7: 828. https://doi.org/10.3390/horticulturae11070828
APA StyleQuadri, A., Sambuco, B., Trenta, M., Tassinari, P., Torreggiani, D., Mercolini, L., Protti, M., Zambonelli, A., Puliga, F., & Barbaresi, A. (2025). Bioengineered Indoor Farming Approaches: LED Light Spectra and Biostimulants for Enhancing Vindoline and Catharanthine Production in Catharanthus roseus. Horticulturae, 11(7), 828. https://doi.org/10.3390/horticulturae11070828











