Optimized In Vitro Method for Conservation and Exchange of Zygotic Embryos of Makapuno Coconut (Cocos nucifera)
Abstract
1. Introduction
2. Materials and Methods
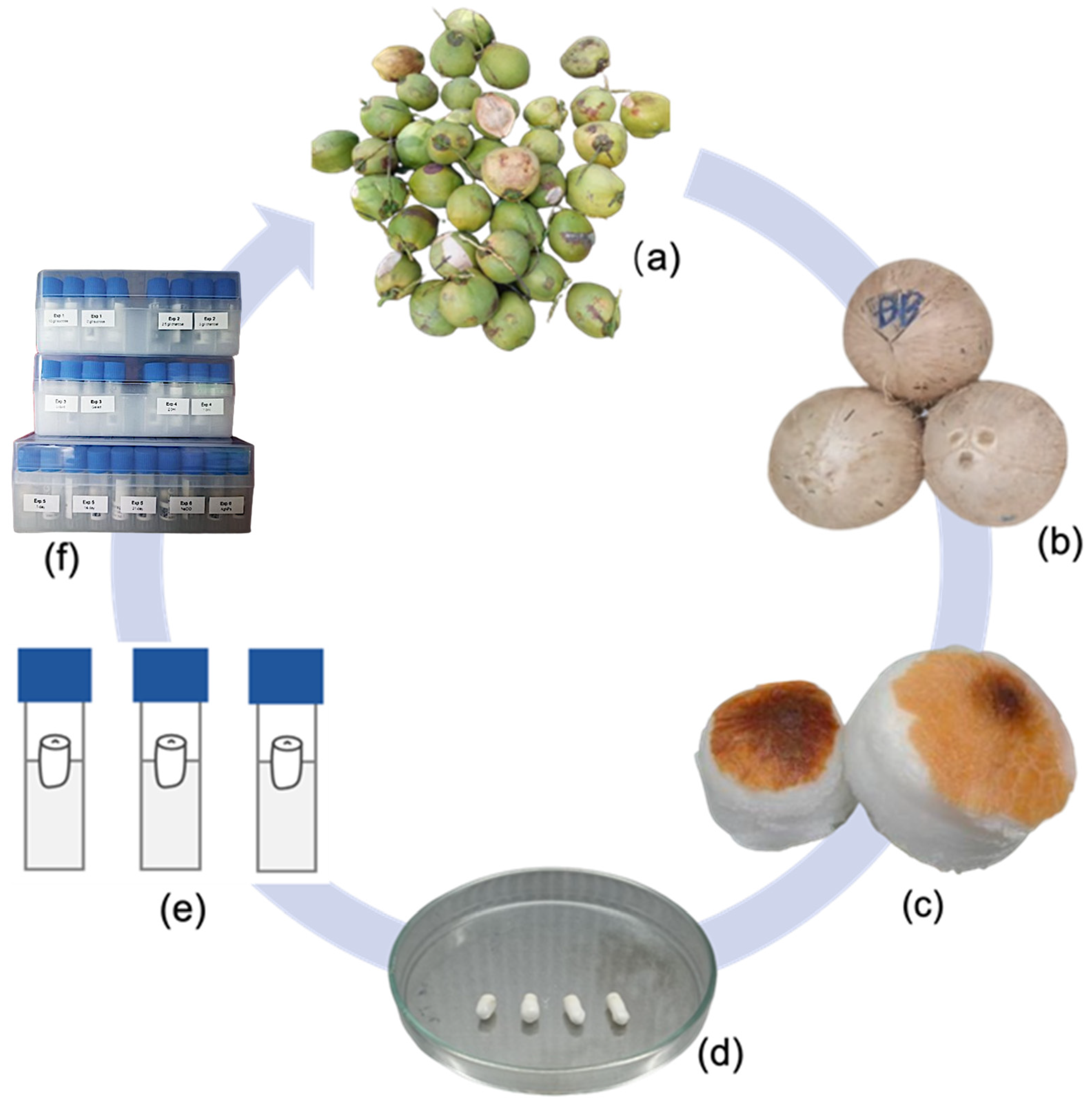
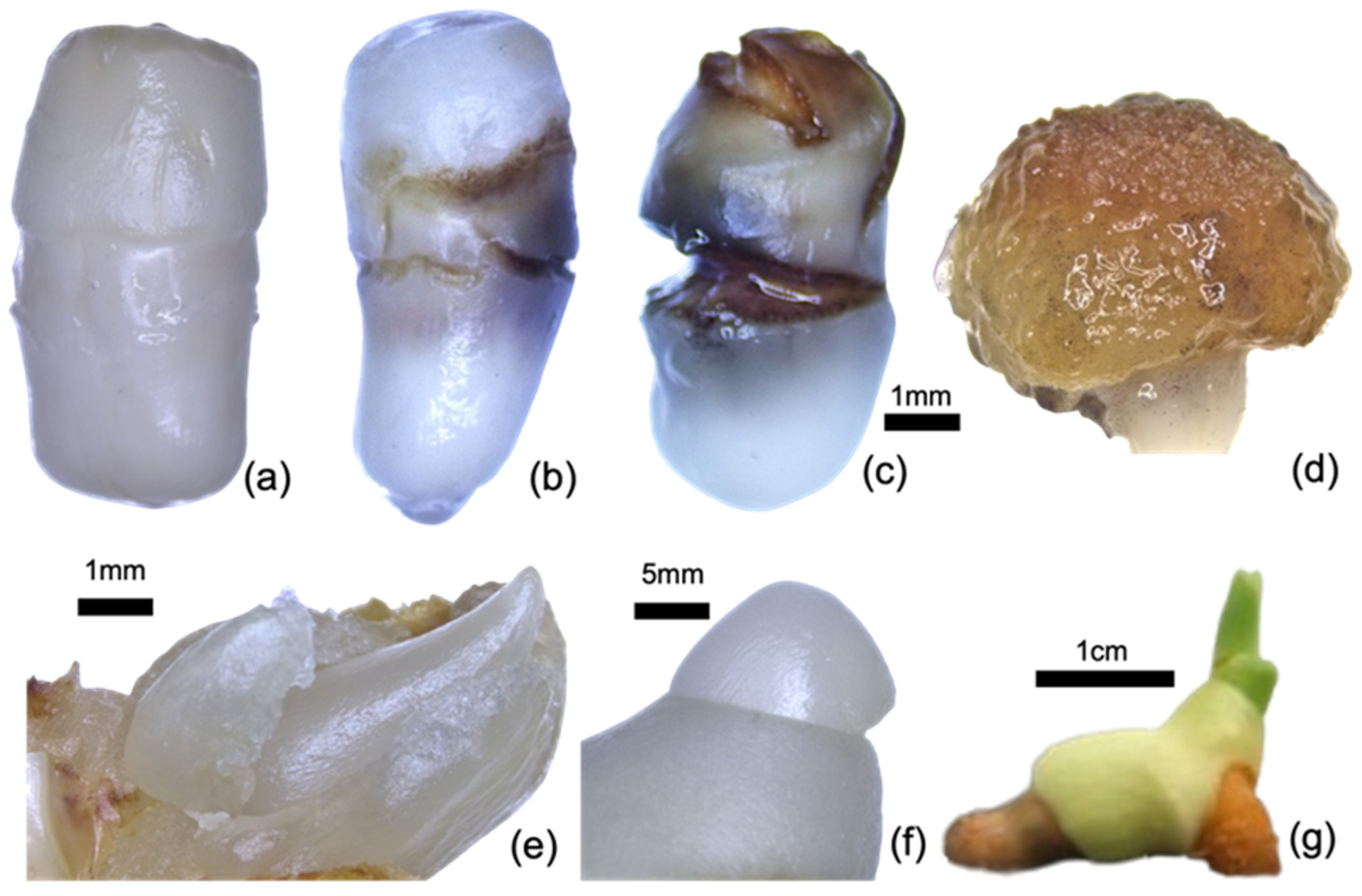
2.1. Plant Materials and Basal Medium Preparations
2.2. Experiment Set-Up
2.3. Assessment and Data Analysis
3. Results
3.1. Experiment 1: Effects of Sucrose Concentration on Germplasm Exchange
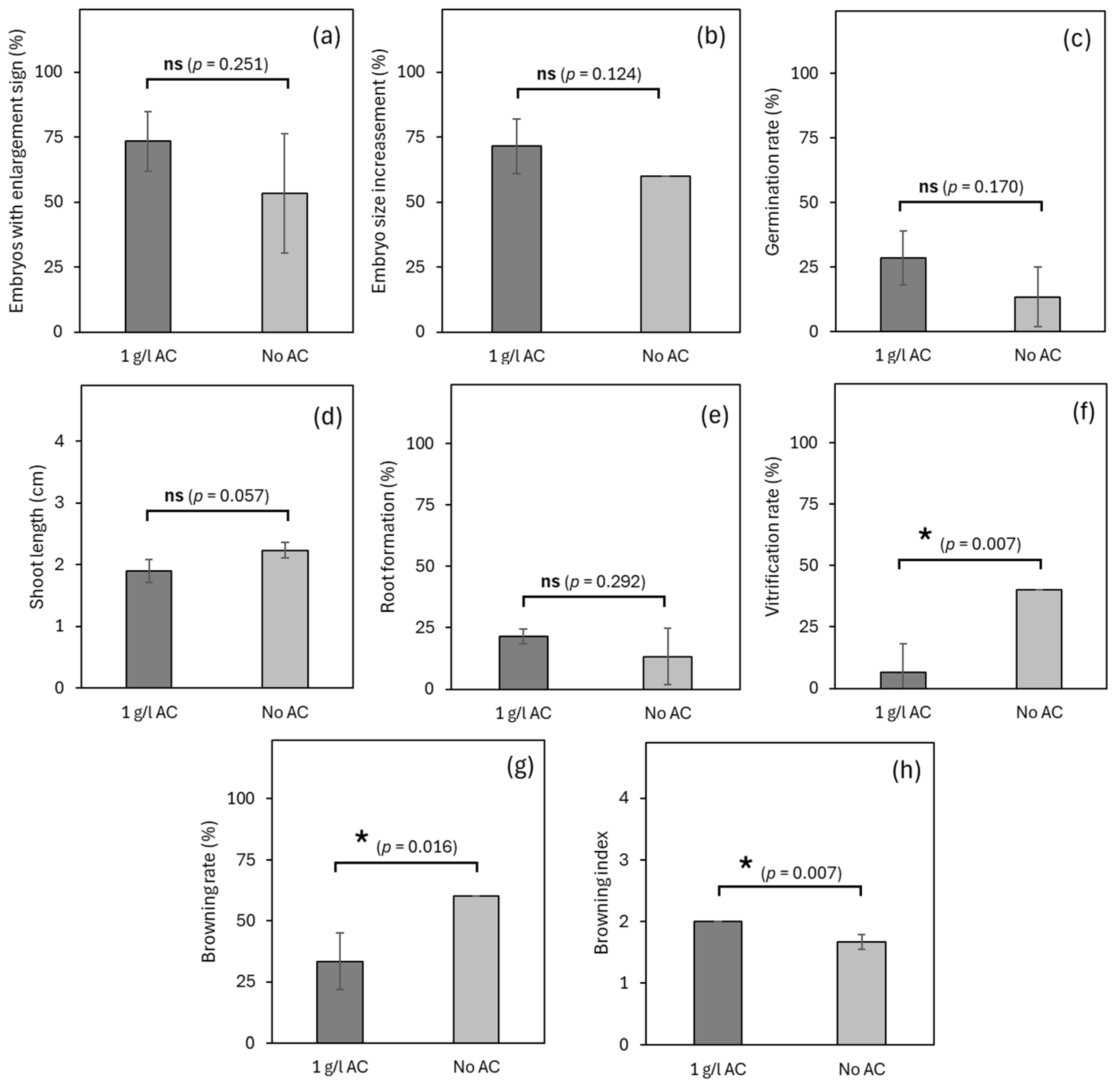
3.2. Experiment 2: Effects of Activated Charcoal on Germplasm Exchange
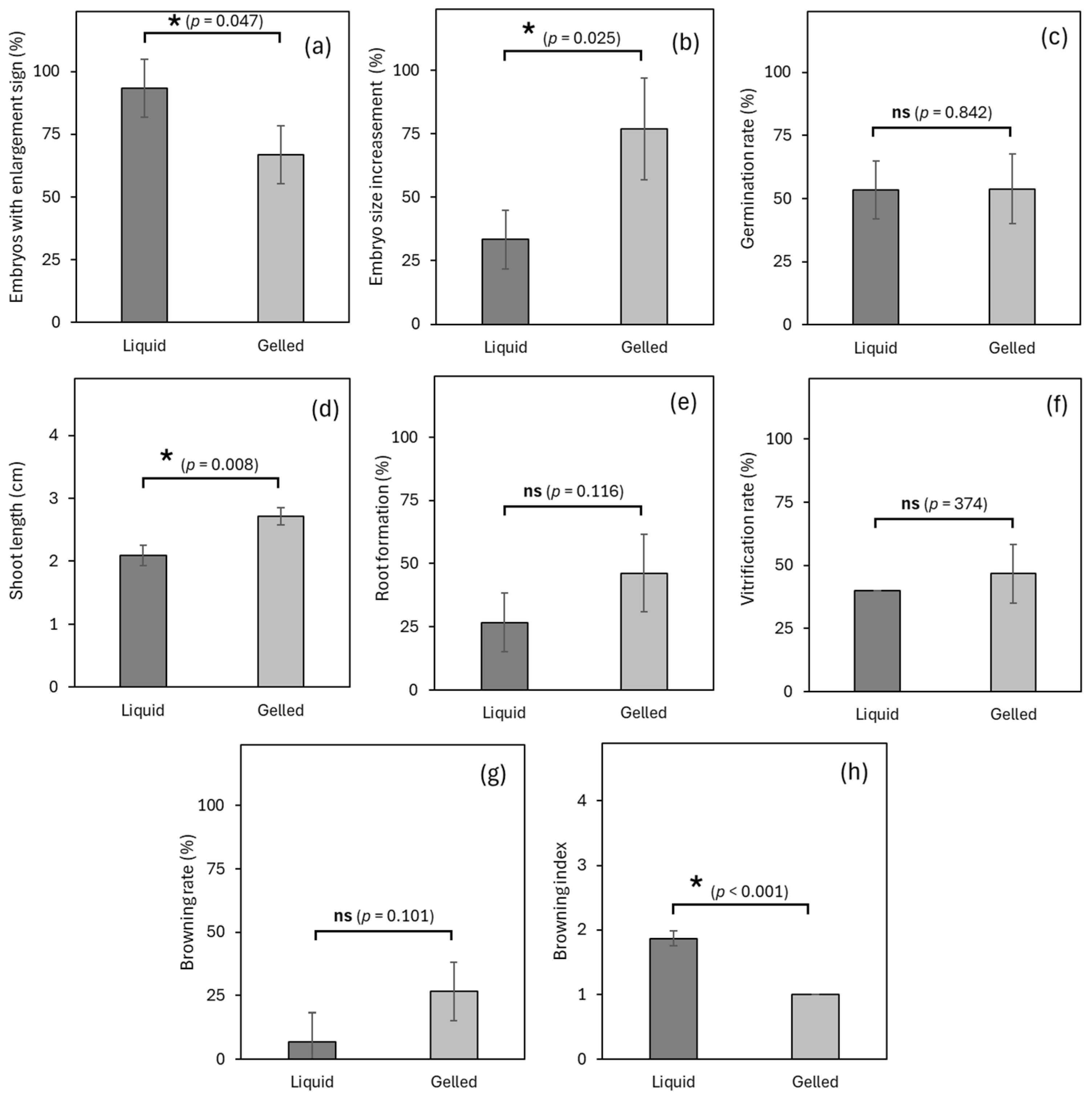
3.3. Experiment 3: Effects of Liquid Medium/Solid Medium on Germplasm Exchange
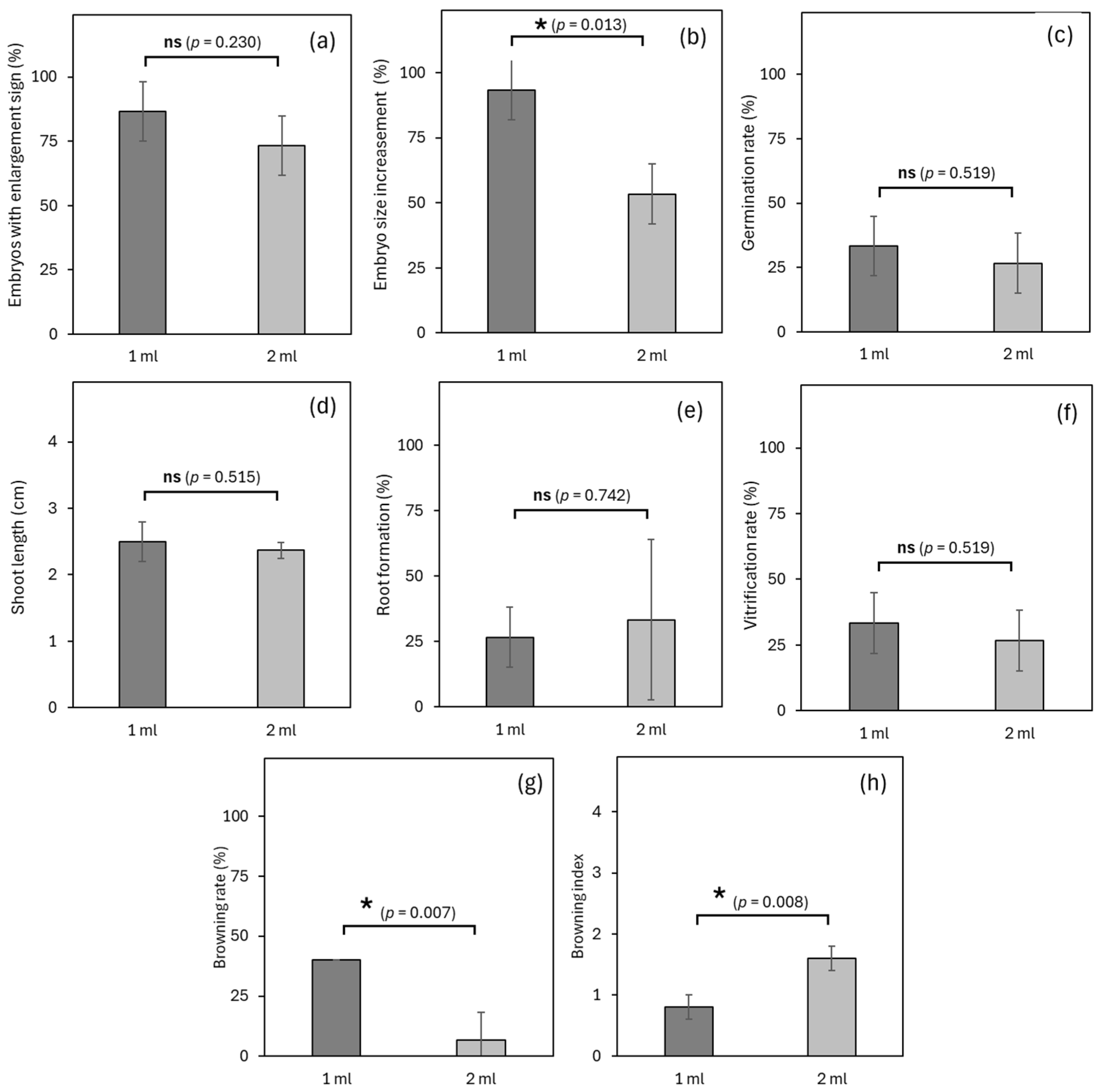
3.4. Experiment 4: Effects of Medium Volume on Germplasm Exchange
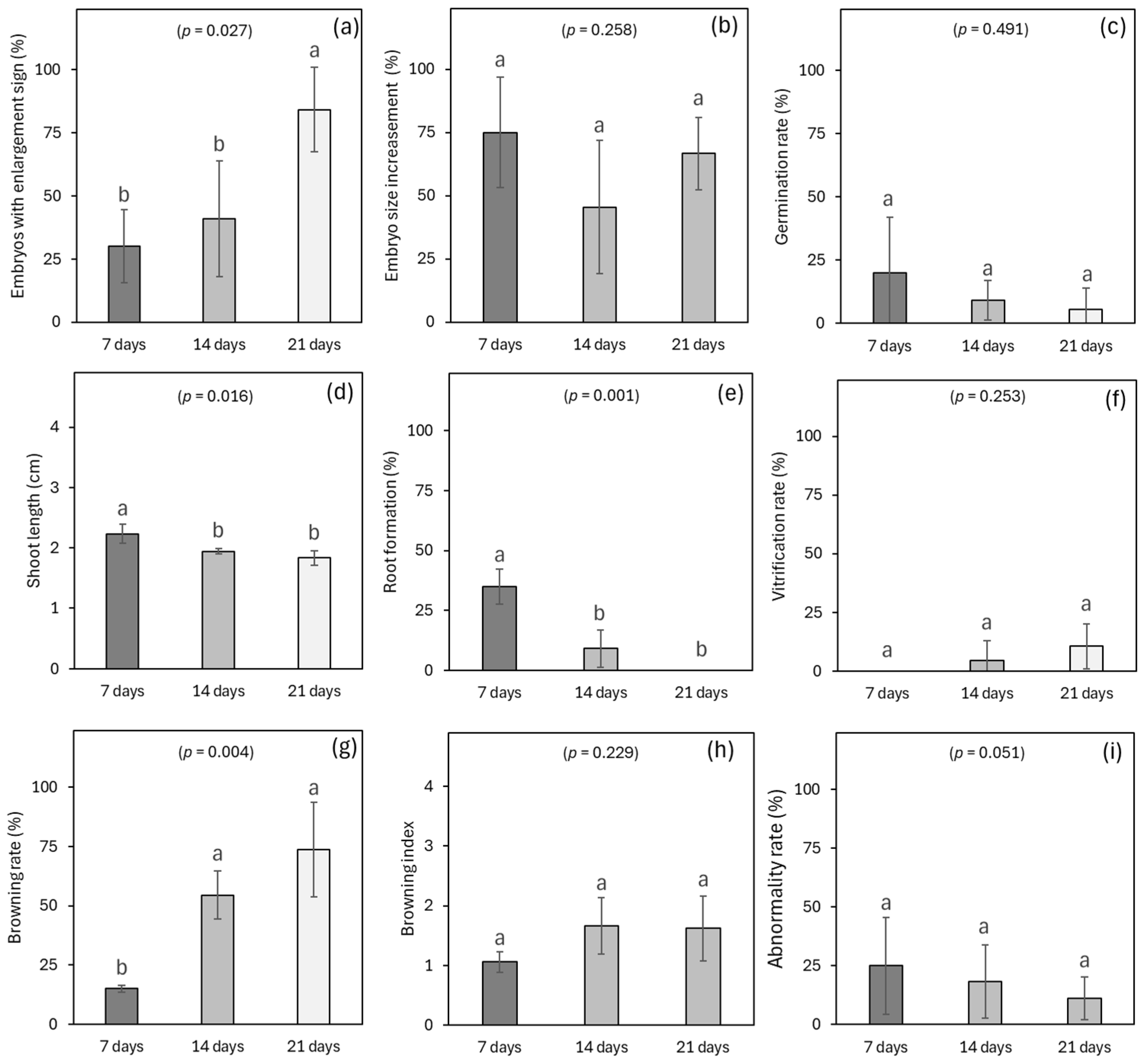
3.5. Experiment 5: Effects of Simulated Transportation Time on Germplasm Exchange
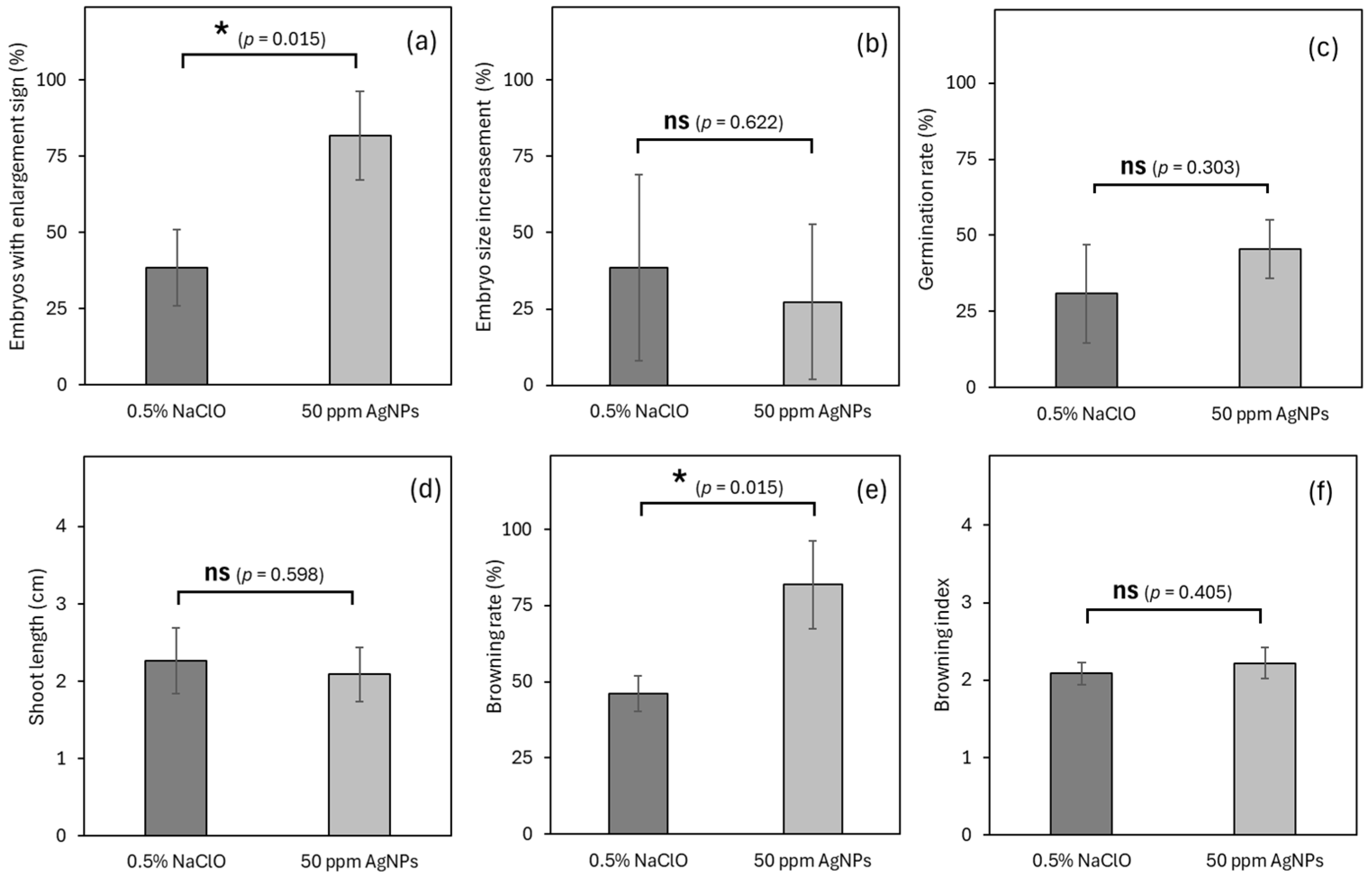
3.6. Experiment 6: Effects of Sterilant on Germplasm Exchange
4. Discussion and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| NaOCl | Sodium Hypochlorite |
| COGENT | International Coconut Genetic Resources Network |
| KCl | Potassium Chloride |
| HCMC | Ho Chi Minh City |
| GL | Growlab Company Limited |
| SIBM | Sanya Institute of Breeding and Multiplication |
| AC | Activated Charcoal |
| ANOVA | Analysis of Variance |
| ST | Sucrose Treatment |
| NST | No Sucrose treatment |
| NA | Non-Activated Charcoal |
| LM | Liquid Medium |
| GM | Gelled Medium |
References
- Yang, Z.; Liu, Z.; Xu, H.; Li, Y.; Huang, S.; Cao, G.; Shi, M.; Zhu, J.; Zhou, J.; Li, R.; et al. ArecaceaeMDB: A comprehensive multi-omics database for Arecaceae breeding and functional genomics studies. Plant Biotechnol. J. 2023, 21, 11. [Google Scholar]
- Mu, Z.; Tran, B.-M.; Xu, H.; Yang, Z.; Qamar, U.Z.; Wang, X.; Xiao, Y.; Luo, J. Exploring the potential application of coconut water in healthcare and biotechnology: A review. Beverage Plant Res. 2024, 4, 1–9. [Google Scholar]
- Sun, X.; Kaleri, G.A.; Mu, Z.; Feng, Y.; Yang, Z.; Zhong, Y.; Dou, Y.; Xu, H.; Zhou, J.; Luo, J.; et al. Comparative Transcriptome Analysis Provides Insights into the Effect of Epicuticular Wax Accumulation on Salt Stress in Coconuts. Plants 2024, 13, 141. [Google Scholar] [CrossRef]
- Mu, Z.; Yang, S.; Xu, H.; Yang, Z.; Haque, M.M.; Tran, B.-M.; Chen, J.; Wang, X.; Peng, H.; Luo, J. The Influence of Maturity, Storage, and Embryo Size on Coconut Callus Induction Success. Forests 2024, 15, 764. [Google Scholar] [CrossRef]
- Welewanni, I.; Bandupriya, D. Coconut cryopreservation: Present status and future prospects. Cord 2017, 33, 21. [Google Scholar]
- Salum, U.; Foale, M.; Biddle, J.; Bazrafshan, A.; Adkins, S. Towards the Sustainability of the “Tree of Life”: An Introduction. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar]
- Karun, A.; Ramesh, S.V.; Rajesh, M.K.; Niral, V.; Sudha, R.; Muralikrishna, K.S. Conservation and Utilization of Genetic Diversity in Coconut (Cocos nucifera L.). In Cash Crops: Genetic Diversity, Erosion, Conservation and Utilization; Springer: Berlin/Heidelberg, Germany, 2022; pp. 197–250. [Google Scholar]
- Bazrafshan, A. Ex Situ Conservation of Coconut (Cocos nucifera L.) Germplasm Using Cryopreservation. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Austrilia, 2022. [Google Scholar]
- Sthapit, B.; Rao, V.R.; Jarvis, D. In situ conservation of coconut diversity. In Coconut Genetic Resources; IPGRI-APO: Serdang, Malaysia, 2005; p. 149. [Google Scholar]
- Arumugam, T.; Hatta, M.A.M. Improving coconut using modern breeding technologies: Challenges and opportunities. Plants 2022, 11, 3414. [Google Scholar] [CrossRef] [PubMed]
- Lédo, A.d.S.; Oliveira, L.A.; Machado, C.D.; Silva, A.V.; Ramos, S.R. Strategies for exchange of coconut germplasm in Brazil. Ciência Rural. 2017, 47, e20160391. [Google Scholar]
- Malaurie, B. Medium-and long-term conservation and safe international exchange of germplasm from food and cash tropical crops. In Proceedings of the IV International Symposium on In Vitro Culture and Horticultural Breeding, Tampere, Finland, 2–7 July 2000; p. 560. [Google Scholar]
- Yang, C.; Nguyen, V.A.; Nulu, N.P.C.; Kalaipandian, S.; Beveridge, F.C.; Biddle, J.; Young, A.; Adkins, S.W. Towards pathogen-free coconut germplasm exchange. Plants 2024, 13, 1809. [Google Scholar] [CrossRef]
- Sisunandar; Rival, A.; Turquay, P.; Samosir, Y.; Adkins, S.W. Cryopreservation of coconut (Cocos nucifera L.) zygotic embryos does not induce morphological, cytological or molecular changes in recovered seedlings. Planta 2010, 232, 435–447. [Google Scholar]
- Kong, E.Y.; Mu, Z.; Vidhanaarachchi, V.R.M.; Nguyen, Q.T.; Sisunandar, S.; Kalaipandian, S.; Panis, B. Chapter 10 Coconut Biotechnology. In The Coconut: Botany, Production and Uses; CABI Publishing: Wallingford, UK, 2024; p. 143. [Google Scholar]
- Samosir, Y.M.; Adkins, S. Improving acclimatization through the photoautotrophic culture of coconut (Cocos nucifera) seedlings: An in vitro system for the efficient exchange of germplasm. Vitr. Cell. Dev. Biol. Plant 2014, 50, 493–501. [Google Scholar]
- Sajini, K.; Karun, A.; Amamath, C.H.; Engelmann, F. Cryopreservation of coconut (Cocos nucifera L.) zygotic embryos by vitrification. CryoLetters 2011, 32, 317–328. [Google Scholar]
- McGregor, A. The export of horticultural and high-value agricultural products from the Pacific islands. Pac. Econ. Bull. 2007, 22, 81–99. [Google Scholar]
- Foale, M.; Julianne, B.; Amirhossein, B.; Steve, A. Biology, ecology, and evolution of coconut. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 17–27. [Google Scholar]
- Chan, E.; Elevitch, C.R. Cocos nucifera (coconut). Species Profiles Pac. Isl. Agrofor. 2006, 2, 1–27. [Google Scholar]
- Sharma, K.M.; Kumar, M.; Muralidharan, C.; Salomón-Torres, R. Date palm pollination management. Crop Prod. Sci. Hortic. 2023, 209–240. [Google Scholar]
- Karun, A.; Sajini, K.K.; Niral, V.; Amarnath, C.H.; Remya, P.; Rajesh, M.K.; Samsudeen, K.; Jerard, B.A.; Engelmann, F. Coconut (Cocos nucifera L.) pollen cryopreservation. CryoLetters 2014, 35, 407–417. [Google Scholar]
- Engelmann, F.; Malaurie, B.; N’Nan, O. In vitro culture of coconut (Cocos nucifera L.) zygotic embryos. In Plant Embryo Culture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 63–72. [Google Scholar]
- Adkins, S.; Foale, M.; Bourdeix, R.; Nguyen, Q.; Biddle, J. Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Mu, Z. Overcoming Bottlenecks in the Pathway of Clonal Propagation of Coconut (Cocos nucifera L.). Ph.D. Thesis, University of Queensland, St Lucia, QLD, Austrilia, 2022. [Google Scholar]
- Nguyen, T.Q. Clonal Propagation of Coconut (Cocos nucifera L.) for Elite Seedling Production and Germplasm Exchange. Ph.D. Thesis, University of Queensland, St Lucia, QLD, Austrilia, 2018. [Google Scholar]
- Eeuwens, C. Mineral requirements for growth and callus initiation of tissue explants excised from mature coconut palms (Cocos nucifera) and cultured in vitro. Physiol. Plant. 1976, 36, 23–28. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar]
- Biddle, J.; Nguyen, Q.; Mu, Z.H.; Foale, M.; Adkins, S. Germplasm Reestablishment and Seedling Production: Embryo Culture. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–225. [Google Scholar]
- Ashburner, G.; Faure, M.G.; Tomlinson, D.R.; Thompson, W.K. A Guide to the Zygotic Embryo Culture of Coconut Palms (Cocos nucifera L.); Australian Centre for International Agricultural Research (ACIAR): Bruce, ACT, Australia, 1995.
- Nguyen, Q.T.; Bandupriya, H.D.; López-Villalobos, A.; Sisunandar, S.; Foale, M.; Adkins, S.W. Tissue culture and associated biotechnological interventions for the improvement of coconut (Cocos nucifera L.): A review. Planta 2015, 242, 1059–1076. [Google Scholar] [PubMed]
- Sáenz, L.; Herrera-Herrera, G.; Uicab-Ballote, F.; Chan, J.L.; Oropeza, C. Influence of form of activated charcoal on embryogenic callus formation in coconut (Cocos nucifera). Plant Cell Tissue Organ Cult. (PCTOC) 2010, 100, 301–308. [Google Scholar]
- Perera, P.; Hocher, V.; Verdeil, J.; Doulbeau, S.; Yakandawala, D.; Weerakoon, L. Unfertilized ovary: A novel explant for coconut (Cocos nucifera L.) somatic embryogenesis. Plant Cell Rep. 2007, 26, 21–28. [Google Scholar]
- Ake, A.P.; Souza, R.; Maust, B.; Santamaría, J.M.; Oropeza, C. Enhanced aerobic respiration improves in vitro coconut embryo germination and culture. Vitr. Cell. Dev. Biol. Plant 2004, 1, 90–94. [Google Scholar]
- Assy-Bah, B.; Engelmann, F. Cryopreservation of mature embryos of coconut (Cocos nucifera L.) and subsequent regeneration of plantlets. Cryoletters 1992, 13, 117–126. [Google Scholar]
- Lédo, A.d.S.; Ramos, S.; Diniz, L.; Konan, J.; George, M. Performance of coconut embryo culture accessions introduced at the International Coconut Genebank for Latin America and the Caribbean (ICG-LAC). In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): III International Symposium on 918, Lisbon, Portugal, 22–27 August 2010. [Google Scholar]
- EL-Gioushy, S.; RLiu; Fan, H. A complete protocol to reduce browning during coconut (Cocos nucifera L.) tissue culture through shoot tips and inflorescence explants. Plant Arch. 2020, 20, 2196–2204. [Google Scholar]
- Bangkok Post PCL. Southeast Asian Coconuts Gain Popularity in China. 2022. Available online: https://www.bangkokpost.com/business/general/2424071/southeast-asian-coconuts-gain-popularity-in-china (accessed on 29 January 2024).
- Pratama, B.R.; Tooy, D.; Kim, J. Price Competition and Shifting Demand: The Relation between Palm and Coconut Oil Exports. Sustainability 2023, 16, 101. [Google Scholar] [CrossRef]
- Vietnam News Agency. Opportunity for Vietnam’s Coconut Exports to US, China. 2023. Available online: https://en.vietnamplus.vn/opportunity-for-vietnams-coconut-exports-to-us-china/271437.vnp (accessed on 29 January 2024).
- Mu, Z.; Peng, H.; Jayarathna, S.; Indrachapa, M.; Yang, C.; Yang, S.; Zhou, J.; Xu, W.; Jiang, Y.; Vidhanaarachchi, V.; et al. The coconut palm in China: Distribution, germplasm, and sustainable development through genomic and biotechnological approaches. Technol. Hortic. 2025, 5, e005. [Google Scholar]
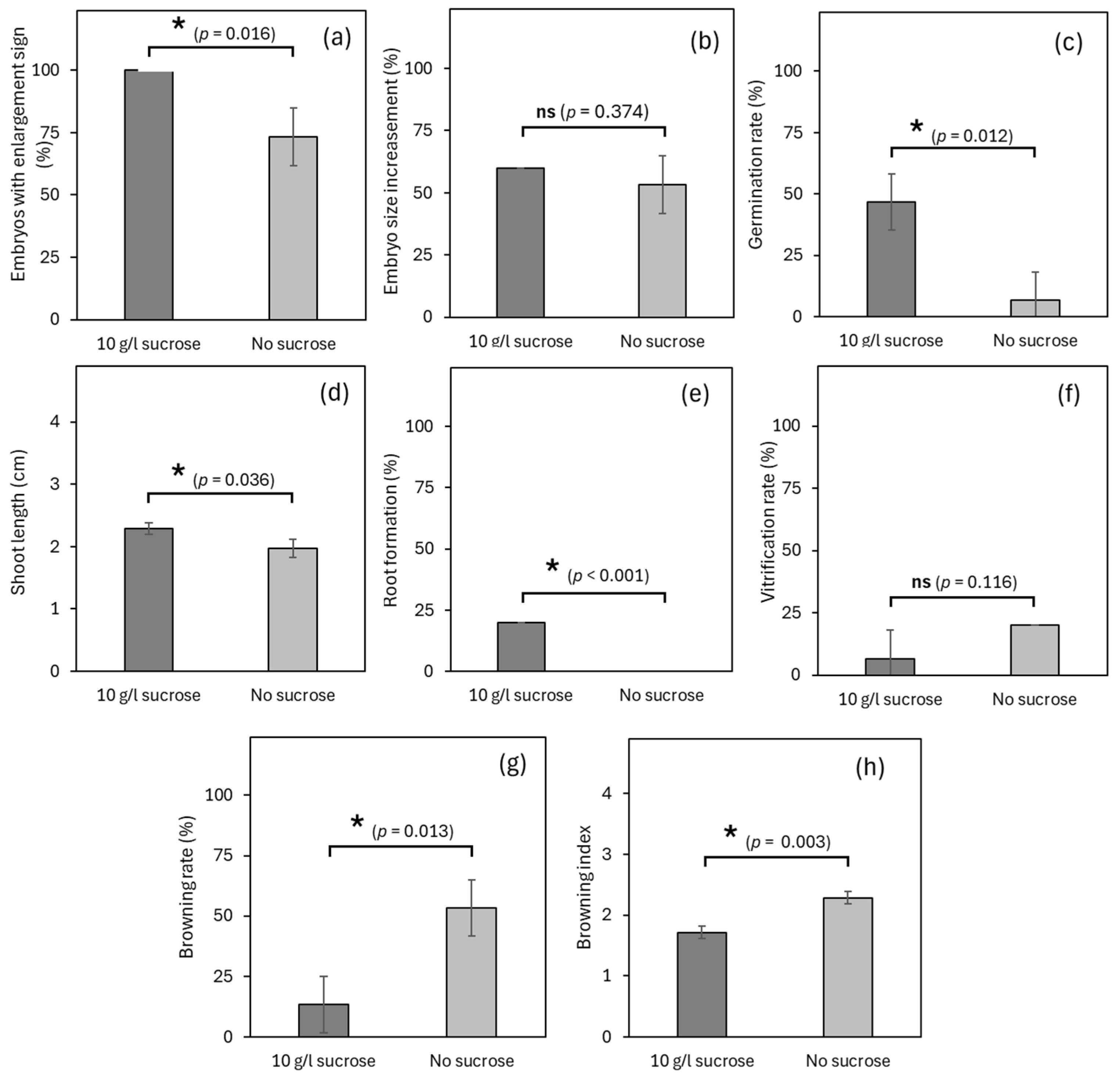
| Experiment 1: Effects of Sucrose Concentration on Germplasm Exchange | ||||
| Culture medium | Y3 + vitamins + sucrose * + no AC + 2 g/L Gelrite | |||
| Treatments | Without sucrose (0 g/L) | With sucrose (10 g/L) | ||
| Replicates | 15 embryos | 15 embryos | ||
| Experiment 2: Effects of Activated Charcoal on Germplasm Exchange | ||||
| Culture medium | Y3 + vitamins + no sucrose + AC * + 2 g/L Gelrite | |||
| Treatments | Without AC (0 g/L) | With AC (2.5 g/L) | ||
| Replicates | 15 embryos | 15 embryos | ||
| Experiment 3: Effects of Liquid Medium/Solid Medium on Germplasm Exchange | ||||
| Culture medium | Y3 + vitamins + no sucrose + no AC + Gelrite * | |||
| Treatments | Gelled medium (2.0 g/L Gelrite) | Liquid medium (0 g/L Gelrite) | ||
| Replicates | 15 embryos | 15 embryos | ||
| Experiment 4: Effects of Medium Volume on Germplasm Exchange | ||||
| Culture medium | Y3 + vitamins + no sucrose + no AC + 2 g/L Gelrite + medium volume * | |||
| Treatments | 1 mL medium | 2 mL medium | ||
| Replicates | 15 embryos | 15 embryos | ||
| Experiment 5: Effects of Transportation Time on Germplasm Exchange | ||||
| Culture medium | Y3 + vitamins + no sucrose + no AC + 2 g/L Gelrite + transportation period * | |||
| Treatments | 7 days | 14 days | 21 days | |
| Replicates | 15 embryos | 15 embryos | 15 embryos | |
| Experiment 6: Effects of Sterilant on Germplasm Exchange | ||||
| Culture medium | Y3 + vitamins + no sucrose + no AC + 2 g/L Gelrite + sterilizing agent * | |||
| Treatments | NaOCl (0.5% w/v) | AgNPs (50 ppm) | ||
| Replicates | 15 embryos | 15 embryos | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Z.; Tran, B.-M.; Wang, X.; Yang, S.; Pham, T.T.-T.; Le, M.-A.; Indrachapa, M.T.N.; Nguyen, P.T.; Luo, J. Optimized In Vitro Method for Conservation and Exchange of Zygotic Embryos of Makapuno Coconut (Cocos nucifera). Horticulturae 2025, 11, 816. https://doi.org/10.3390/horticulturae11070816
Mu Z, Tran B-M, Wang X, Yang S, Pham TT-T, Le M-A, Indrachapa MTN, Nguyen PT, Luo J. Optimized In Vitro Method for Conservation and Exchange of Zygotic Embryos of Makapuno Coconut (Cocos nucifera). Horticulturae. 2025; 11(7):816. https://doi.org/10.3390/horticulturae11070816
Chicago/Turabian StyleMu, Zhihua, Binh-Minh Tran, Xingwei Wang, Shuya Yang, Thi Thanh-Thuy Pham, Minh-An Le, M. T. N. Indrachapa, Phuong Thao Nguyen, and Jie Luo. 2025. "Optimized In Vitro Method for Conservation and Exchange of Zygotic Embryos of Makapuno Coconut (Cocos nucifera)" Horticulturae 11, no. 7: 816. https://doi.org/10.3390/horticulturae11070816
APA StyleMu, Z., Tran, B.-M., Wang, X., Yang, S., Pham, T. T.-T., Le, M.-A., Indrachapa, M. T. N., Nguyen, P. T., & Luo, J. (2025). Optimized In Vitro Method for Conservation and Exchange of Zygotic Embryos of Makapuno Coconut (Cocos nucifera). Horticulturae, 11(7), 816. https://doi.org/10.3390/horticulturae11070816







