Transcriptomic and Proteomic Analyses Provide Insight into Sugar Metabolism-Induced Dormancy Release of Flower Buds of Pyrus pyrifolia ‘Cuiguan’
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Enzyme Activities and Sugar and Starch Contents
2.3. RNA-Sequencing and Analysis
2.4. Ultra-Deep Quantitative Proteomics Analysis
2.5. Integrated Transcriptomic and Proteomic Analysis
2.6. Weighted Gene Co-Expression Correlation Network Analysis (WGCNA)
2.7. qRT–PCR Validation
2.8. Data Analysis
3. Results
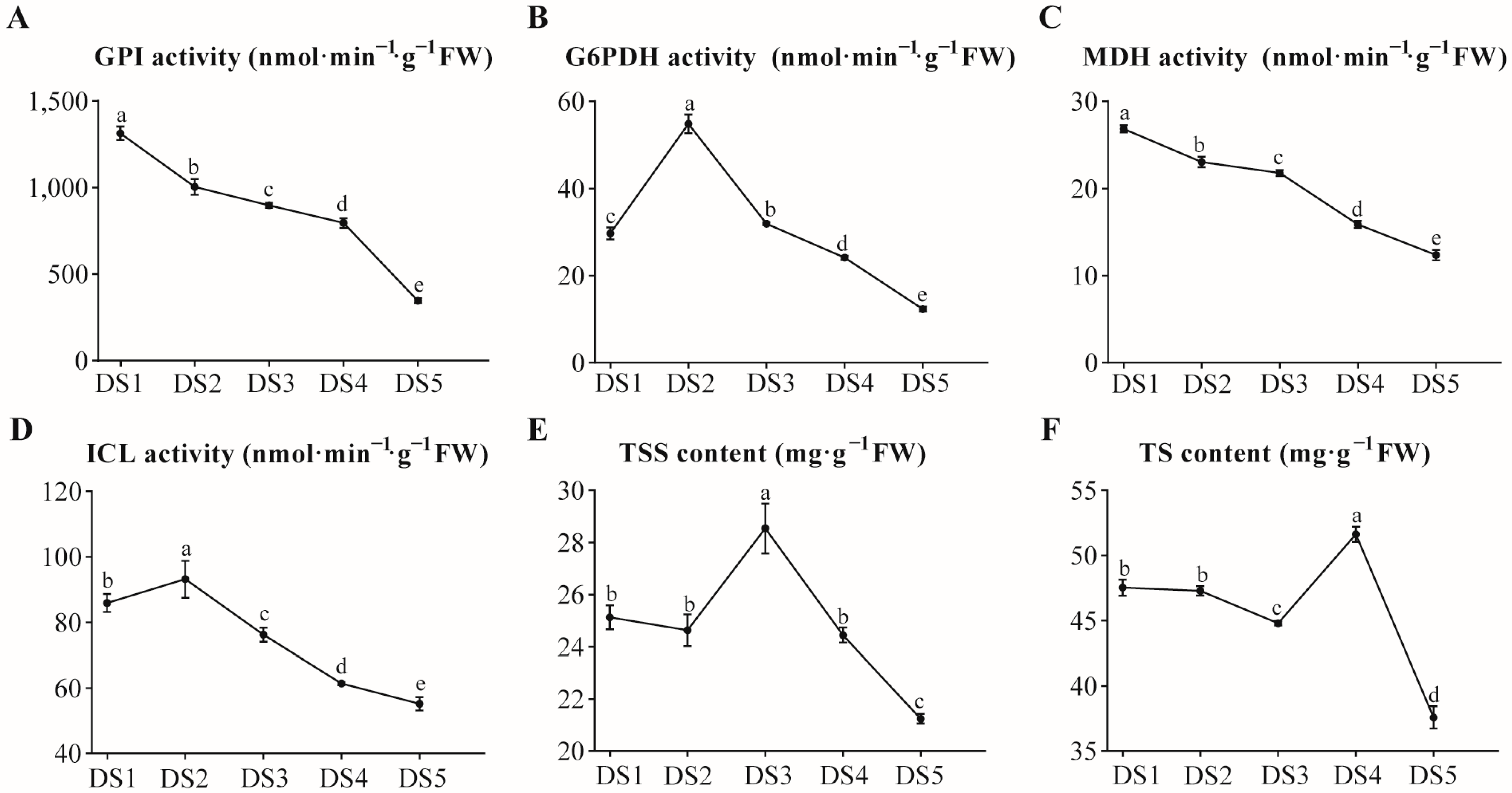
3.1. Physiological Characteristics During Dormancy Process in Flower Buds
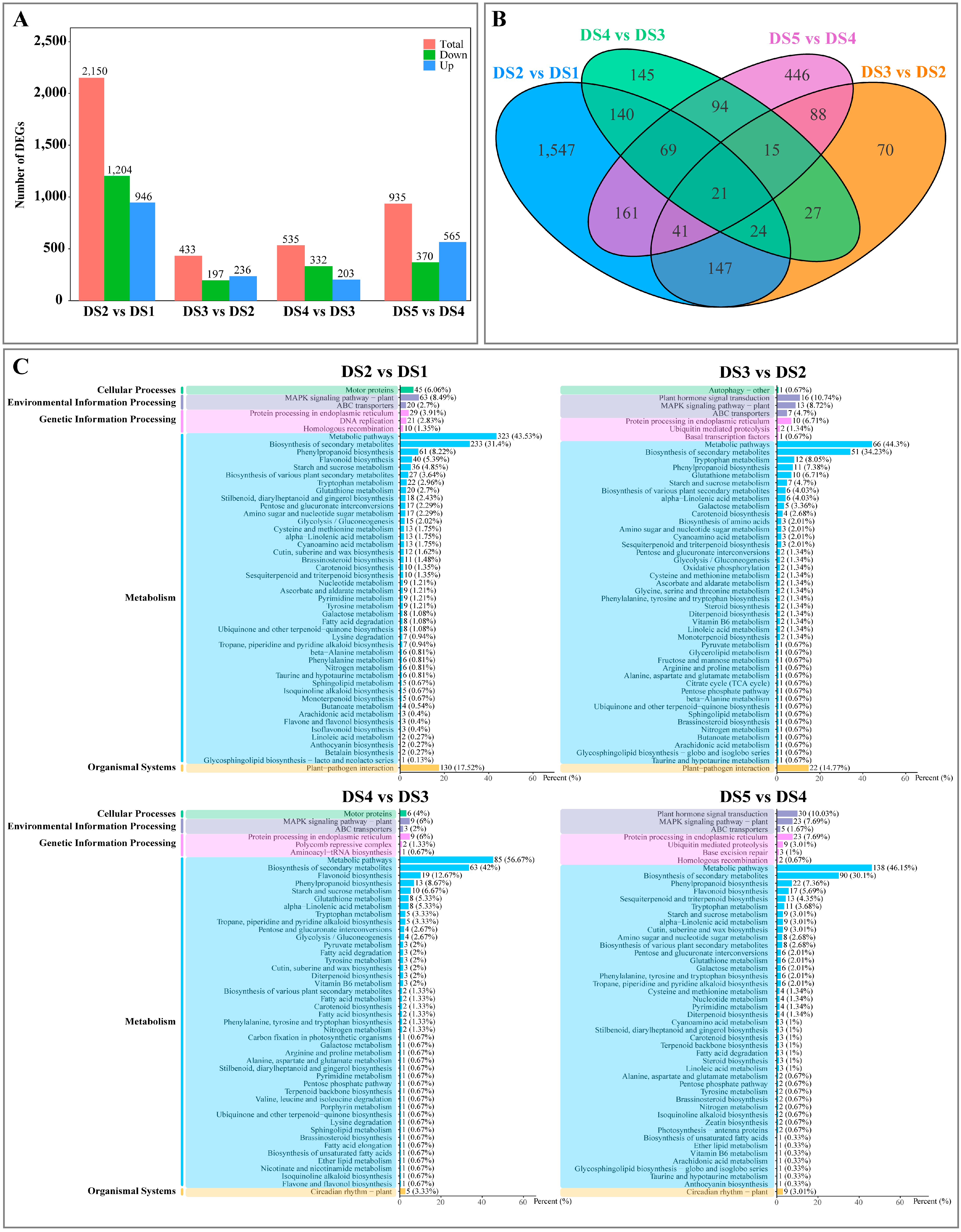
3.2. Transcriptomics Analysis
3.3. Proteomics Analysis
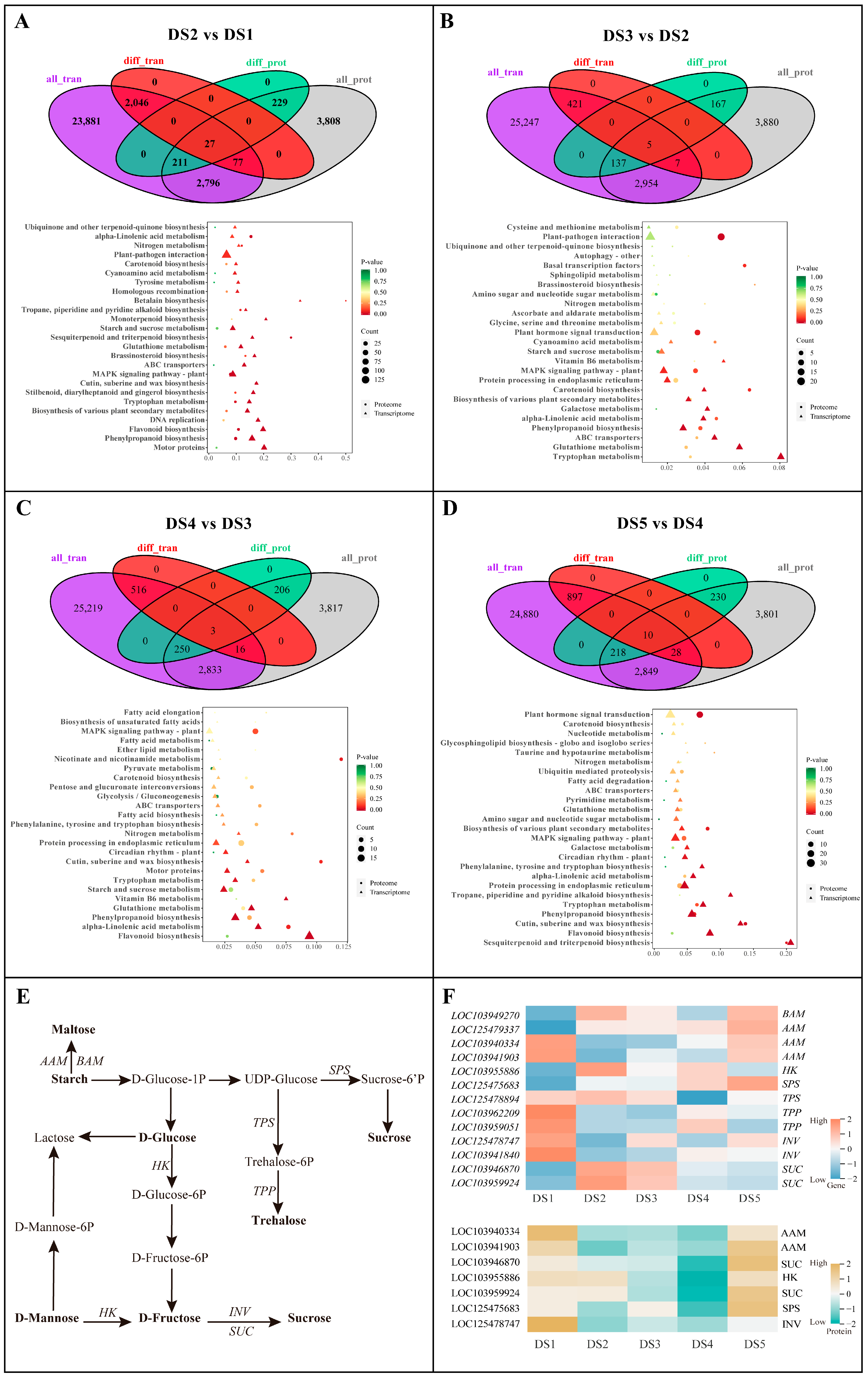
3.4. Multi-Omics Integrative Analysis
3.5. Co-Expression Analysis Identified Key TFs in Dormancy Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chao, W.S.; Doğramacı, M.; Horvath, D.P.; Foley, M.E.; Anderson, J.V. Dormancy Induction and Release in Buds and Seeds; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 235–256. [Google Scholar]
- Zhao, X.H.; Liu, J.T.; Fu, X.L.; Xiao, L.; Wang, Q.J.; Wang, C.R.; Chen, Z.Z.; Li, J.K.; Lu, C.K.; Cao, H.; et al. Whole-genome characterization of CKX genes in Prunus persica and their role in bud dormancy and regrowth. J. Integr. Agric. 2024, 23, 4058–4073. [Google Scholar]
- Yi, X.K.; Xu, Y.L.; Gao, Z.H.; Zhang, J.Y.; Pan, H.F.; Qi, Y.J.; Qin, G.H.; Li, B. Warm winter temperature induced changes in the dormant buds of ‘Dangshansuli’ pear (Pyrus bretschneideri Rehd.). Indian J. Hortic. 2020, 77, 273–278. [Google Scholar]
- Ito, A.; Sakaue, T.; Fujimaru, O.; Iwatani, A.; Ikeda, T.; Sakamoto, D.; Sugiura, T.; Moriguchi, T. Comparative phenology of dormant Japanese pear (Pyrus pyrifolia) flower buds: A possible cause of ‘flowering disorder’. Tree Physiol. 2018, 38, 825–839. [Google Scholar]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar]
- Rohde, A.; Bhalerao, P.R. Plant dormancy in the perennial context. Trends Plant Sci. 2007, 12, 217–223. [Google Scholar]
- Singh, R.K.; Svystun, T.; AlDahmash, B.; Jönsson, A.M.; Bhalerao, R.P. Photoperiod-and temperature-mediated control of phenology in trees–a molecular perspective. New Phytol. 2017, 213, 511–524. [Google Scholar]
- Shu, P.T.; Ding, M.; Chun, L. The Physiological and biochemical changes of lily bulbs during dormancy release with cold treatment. Acta Agric. Univ. Jiangxiensis 2005, 27, 404–407. [Google Scholar]
- Li, X.; Yang, L.Y.; Chen, M.M. Hormone change during dormancy and flower bud differentiation process in Lilium oriental× trumpet hybrid ‘ConcaD Or’. Chin. Agric. Sci. Bull. 2020, 36, 42–47. [Google Scholar]
- Patrick, W.J.; Botha, F.C.; Birch, R.G. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar]
- Salam, B.B.; Barbier, F.; Danieli, R.; Teper-Bamnolker, P.; Ziv, C.; Spíchal, L.; Aruchamy, K.; Shnaider, Y.; Leibman, D.; Shaya, F.; et al. Sucrose promotes stem branching through cytokinin. Plant Physiol. 2021, 185, 1708–1721. [Google Scholar]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [PubMed]
- Jiang, S.; Li, S.G.; Luo, J.; Wang, X.Q.; Shi, C.H. QTL mapping and transcriptome analysis of sugar content during fruit ripening of Pyrus pyrifolia. Front. Plant Sci. 2023, 14, 1137104. [Google Scholar]
- McWhite, C.D.; Papoulas, O.; Drew, K.; Cox, R.M.; June, V.; Dong, O.X.; Kwon, T.; Wan, C.; Salmi, M.L. A pan-plant protein complex map reveals deep conservation and novel assemblies. Cell 2020, 181, 460–474. [Google Scholar] [PubMed]
- Zhang, T.; Yuan, Y.; Zhan, Y.; Cao, X.; Liu, C.; Zhang, Y.; Gai, S. Metabolomics analysis reveals Embden Meyerhof Parnas pathway activation and flavonoids accumulation during dormancy transition in tree peony. BMC Plant Biol. 2020, 20, 484. [Google Scholar]
- Zhang, G.; Tang, R.; Niu, S.; Si, H.; Yang, Q.; Rajora, O.P.; Li, X.Q. Heat-stress-induced sprouting and differential gene expression in growing potato tubers: Comparative transcriptomics with that induced by postharvest sprouting. Hortic. Res. 2021, 8, 226. [Google Scholar]
- Xu, G.G.; Li, L.J.; Zhou, J.; He, M.Q.; Lyu, D.G.; Zhao, D.Y.; Qin, S.J. Integrated transcriptomics and metabolomics analyses reveal key genes and essential metabolic pathways for the acquisition of cold tolerance during dormancy in apple. Environ. Exp. Bot. 2023, 213, 105413. [Google Scholar]
- Li, J.Z.; Yan, X.H.; Ahmad, M.; Yu, W.J.; Song, Z.Z.; Ni, J.B.; Yang, Q.S.; Teng, Y.W.; Zhang, H.G.; Bai, S.L. Alternative splicing of the dormancy-associated MADS-box transcription factor gene PpDAM1 is associated with flower bud dormancy in ‘Dangshansu’ pear (Pyrus pyrifolia white pear group). Plant Physiol. Biochem. 2021, 166, 1096–1108. [Google Scholar]
- Tominaga, A.; Ito, A.; Sugiura, T.; Yamane, H. How is global warming affecting fruit tree blooming? “Flowering (dormancy) disorder” in Japanese pear (Pyrus pyrifolia) as a case study. Front. Plant Sci. 2022, 12, 787638. [Google Scholar]
- Li, J.Z.; Xu, Y.; Niu, Q.F.; He, L.F.; Teng, Y.W.; Bai, S.L. Abscisic acid (ABA) promotes the induction and maintenance of pear (Pyrus pyrifolia White Pear Group) flower bud endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar]
- Liu, G.Q.; Li, W.S.; Zheng, P.H.; Tong, X.; Chen, L.J.; Liu, D.F.; Hussain, S.; Teng, Y.W. Transcriptomic analysis of ‘Suli’pear (Pyrus pyrifolia white pear group) buds during the dormancy by RNA-Seq. BMC Genom. 2012, 13, 700. [Google Scholar]
- Hussain, S.; Niu, Q.; Yang, F.; Hussain, N.; Teng, Y. The possible role of chilling in floral and vegetative bud dormancy release in Pyrus pyrifolia. Biol. Plant. 2015, 59, 726–734. [Google Scholar]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [PubMed]
- Xue, Y.L. Plant Physiology Experimentation Handbook; Shanghai Technology Publishing Company: Shanghai, China, 1985; pp. 183–184. (In Chinese) [Google Scholar]
- Meillon, S.D.; Small, J.G.C.; Venter, H.A.V.D. The respiratory metabolism of Strelitzia juncea Ait. seeds: The effect of dormancy release through oxygen incubation of the seeds on the activity of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. J. Exp. Bot. 1990, 41, 709–714. [Google Scholar]
- Bogatek, R.; Zarska-Maciejewska, B.; Sińska, I.; Lewak, S. The embryonic axis controls lipid catabolism in cotyledons of apple seeds during germination. Physiol. Plant. 1989, 76, 557–562. [Google Scholar]
- Du, Y.L.; Zhao, Q.; Chen, L.R.; Yao, X.D.; Zhang, W.; Zhang, B.; Xie, F.T. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar]
- Zhao, D.L.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods 2001, 4, 402–408. [Google Scholar]
- Li, X.; Li, X.L.; Gao, D.S.; Yang, X.P. Influence of shading on respiratory metabolism of sweet cherry dormant buds. Chin. J. Eco-Agric. 2005, 13, 57–59. [Google Scholar]
- Li, D.M.; Li, L.; Tan, Y.; Chen, X.D.; Zhang, H.S.; Gao, D.S.; Li, J. Effect of photoperiod on key enzyme activities of respiration in nectarine buds during dormancy induction. Agric. Sci. China 2011, 10, 1026–1031. [Google Scholar]
- Tan, Y.; Gao, D.S.; Li, L.; Chen, X.D.; Xu, A.H. Respiratory response of dormant nectarine floral buds on chilling deficiency. Agric. Sci. China 2010, 9, 1448–1454. [Google Scholar]
- Pu, X.C.; Han, J.G.; Li, M.; Du, G.P.; Ni, X.Q. Studies on respiratory pathways of zoysiagrass seed when breaking dormancy. Acta Prataculturae Sin. 1996, 5, 56–60. [Google Scholar]
- Li, X.L.; Yan, T.L.; Ga, D.S.; Yuan, Z.Y. Metabolic changes during low temperature induced dormancy release in ‘NJ72’nectarine (Prunus persica L. Batsch). Chin. J. Eco-Agric. 2002, 10, 27–29. [Google Scholar]
- Borek, S.; Nuc, K. Sucrose controls storage lipid breakdown on gene expression level in germinating yellow lupine (Lupinus luteus L.) seeds. J. Plant Physiol. 2011, 168, 1795–1803. [Google Scholar] [PubMed]
- Ito, A.; Sakamoto, D.; Moriguchi, T. Carbohydrate metabolism and its possible roles in endodormancy transition in Japanese pear. Sci. Hortic. 2012, 144, 187–194. [Google Scholar]
- Horikoshi, H.M.; Sekozawa, Y.; Sugaya, S. Inhibition of carbohydrate metabolism by thermal fluctuations during endodormancy lead to negative impacts on bud burst and incidence of floral necrosis in ‘Housui’ Japanese pear flower buds. Sci. Hortic. 2017, 224, 324–331. [Google Scholar]
- Sauter, J.J. Temperature-induced changes in starch and sugars in the stem of Populus× canadensis «robusta». J. Plant Physiol. 1988, 132, 608–612. [Google Scholar]
- Zhu, H.R.; Chen, Z.Q.; Zhang, K.Z.; Cui, J.T. The key role of sugar metabolism in the dormancy release and bud development of Lilium brownii var. viridulum Baker bulbs. Sci. Hortic. 2024, 336, 113453. [Google Scholar]
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar]
- Guo, Y.Y.; An, L.Z.; Yu, H.Y.; Yang, M.M. Endogenous hormones and biochemical changes during flower development and florescence in the buds and leaves of Lycium ruthenicum Murr. Forests 2022, 13, 763. [Google Scholar] [CrossRef]
- Xuan, L.J.; Wang, Q.Q.; Liu, Z.G.; Xu, B.; Cheng, S.Y.; Zhang, Y.J.; Lu, D.Y.; Dong, B.; Zhang, D.M.; Zhang, L.; et al. Metabolic analysis of the regulatory mechanism of sugars on secondary flowering in Magnolia. BMC Mol. Cell Biol. 2022, 23, 56. [Google Scholar]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyere, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar]
- Brill, E.; Thournout, M.; White, R.G.; Llewellyn, D.; Campbell, P.M.; Engelen, S.; Ruan, Y.L.; Arioli, T.; Furbank, R.T. A novel isoform of sucrose synthase is targeted to the cell wall during secondary cell wall synthesis in cotton fiber. Plant Physiol. 2011, 157, 40–54. [Google Scholar]
- Tian, X.C.; Zhu, L.C.; Yang, N.X.; Song, J.Y.; Zhao, H.Y.; Zhang, J.; Ma, F.W.; Li, M.G. Proteomics and metabolomics reveal the regulatory pathways of ripening and quality in post-harvest kiwifruits. J. Agric. Food Chem. 2021, 69, 824–835. [Google Scholar] [PubMed]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar]
- Wingler, A.; Fritzius, T.; Wiemken, A.; Boller, T.; Aeschbacher, R.A. Trehalose induces the ADP-glucose pyrophosphorylase gene, ApL3, and starch synthesis in Arabidopsis. Plant Physiol. 2000, 124, 105–114. [Google Scholar] [PubMed]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y.H. Trehalose metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [PubMed]
- Zhang, W.; Wang, Y.; Guo, Y. Research progress of trehalose 6- phosphate signal regulation in higher plants. Plant Physiol. 2016, 52, 394–400. [Google Scholar]
- Wu, C.Q.; Jia, L.T.; Fan, J.B.; Zhang, Z.; Ma, M.; Zhang, S.L.; Liu, Z.Q.; Ren, Y.Q.; Wang, L.B.; Zhang, S.L. Research progress of sugar and acid transformation in horticultural fruits. China Fruit Veg. 2020, 40, 39–47. [Google Scholar]
- Lloret, A.; Martínez-Fuentes, A.; Agustí, M.; Badenes, M.L.; Ríos, G. Chromatin-associated regulation of sorbitol synthesis in flower buds of peach. Plant Mol. Biol. 2017, 95, 507–517. [Google Scholar]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Adamakis, I.; Karamanoli, K.; Martens, S.; Molassiotis, A. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018, 155, 1–11. [Google Scholar]
- George, I.S.; Fennell, A.Y.; Haynes, P.A. Shotgun proteomic analysis of photoperiod regulated dormancy induction in grapevine. J. Proteom. 2018, 187, 13–24. [Google Scholar]
- Galimba, K.D.; Denew, M.; Waite, J.M. Application of ABA and ACC in autumn affects dormancy and cold hardiness of pear (Pyrus communis). HortTechnology 2025, 35, 385–391. [Google Scholar]
- Moser, M.; Asquini, E.; Miolli, G.V.; Weigl, K.; Hanke, M.V.; Flachowsky, H.; Si-Ammour, A. The MADS-box gene MdDAM1 controls growth cessation and bud dormancy in apple. Front. Plant Sci. 2020, 11, 1003. [Google Scholar]
- Niu, Q.F.; Li, J.Z.; Cai, D.Y.; Qian, M.J.; Jia, H.M.; Bai, S.L.; Hussain, S.; Liu, G.Q.; Teng, Y.W.; Zheng, X.Y. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J. Exp. Bot. 2016, 67, 239–257. [Google Scholar]
- Vimont, N.; Fouché, M.; Campoy, J.A.; Tong, M.X.Z.; Arkoun, M.; Yvin, J.; Wigge, P.A.; Dirlewanger, E.; Cortijo, S.; Wenden, B. From bud formation to flowering: Transcriptomic state defines the cherry developmental phases of sweet cherry bud dormancy. BMC Genom. 2019, 20, 974. [Google Scholar]
- Zhu, Y.Y.; Liu, X.L.; Gao, Y.D.; Li, K.; Guo, W.D. Transcriptome-based identification of AP2/ERF family genes and their cold-regulated expression during the dormancy phase transition of Chinese cherry flower buds. Sci. Hortic. 2021, 275, 109666. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ding, L.; Ye, Q.; Huang, X.; Xu, L.; Wu, S.; He, D. Transcriptomic and Proteomic Analyses Provide Insight into Sugar Metabolism-Induced Dormancy Release of Flower Buds of Pyrus pyrifolia ‘Cuiguan’. Horticulturae 2025, 11, 813. https://doi.org/10.3390/horticulturae11070813
Wang H, Ding L, Ye Q, Huang X, Xu L, Wu S, He D. Transcriptomic and Proteomic Analyses Provide Insight into Sugar Metabolism-Induced Dormancy Release of Flower Buds of Pyrus pyrifolia ‘Cuiguan’. Horticulturae. 2025; 11(7):813. https://doi.org/10.3390/horticulturae11070813
Chicago/Turabian StyleWang, Huiquan, Ling Ding, Qinghua Ye, Xueying Huang, Lei Xu, Shaohua Wu, and Dongjin He. 2025. "Transcriptomic and Proteomic Analyses Provide Insight into Sugar Metabolism-Induced Dormancy Release of Flower Buds of Pyrus pyrifolia ‘Cuiguan’" Horticulturae 11, no. 7: 813. https://doi.org/10.3390/horticulturae11070813
APA StyleWang, H., Ding, L., Ye, Q., Huang, X., Xu, L., Wu, S., & He, D. (2025). Transcriptomic and Proteomic Analyses Provide Insight into Sugar Metabolism-Induced Dormancy Release of Flower Buds of Pyrus pyrifolia ‘Cuiguan’. Horticulturae, 11(7), 813. https://doi.org/10.3390/horticulturae11070813





