Abstract
Aeroponics efficiently conserves water and fertilizer but faces energy sustainability challenges in maintaining high productivity and quality. This study aimed to identify critical growth phases of lettuce affected by management modes and assess resource/energy efficiency (cost per unit yield) to inform the development of sustainability strategies for lettuce production in a lettuce-dominant aeroponics system integrated with radish. Three management modes were tested: M1 (constant nutrient solution concentrations), M2 (variable nutrient solution concentrations), and M3 (combined variable nutrient solution concentrations and preharvest short-duration continuous light for 48 h). Plant parameters were dynamically measured in a 30-day cultivation cycle. The results showed that the intercropped lettuce exhibited peak growth at 15–25 days after transplanting, and nutrient solution adjustment enhanced the shoot weight and quality, with synergistic quality improvements under M3. However, preharvest lighting reduced the net photosynthetic rate via stomatal closure and lowered the effective quantum yield of photosystem II, preventing biomass increase. The preharvest short-duration continuous light elevated the soluble protein, ascorbic acid, and soluble sugar contents. For yield-focused systems, M2 alone achieved comparable shoot weight to M3 with higher energy efficiency. However, when simultaneously considering lettuce quality enhancement and the yield boost of radish in the intercropping system, M3 demonstrated potential for greater marginal benefits.
1. Introduction
Lettuce (Lactuca sativa L.) is widely cultivated in greenhouses and plant factories worldwide; it is healthy to eat because it is low in calories, contains many essential nutrients, and has up to 90% water content [1]. It was reported in 2023 that the global lettuce production reached 28.1 million tons, with China’s output accounting for approximately 53% of the total, and its export volume reaching 69.88 thousand tons [2,3]. Therefore, lettuce yield and quality are key concerns for both producers and consumers.
Producers are constantly seeking ways to balance the yield and quality of leaf vegetables. However, this proves challenging under the traditional open-field cultivation systems characterized by chemical intensification. Primarily, the complexity of soil environments complicates vegetable quality regulation [4]. Additionally, numerous studies have reported that while excessive nitrogen fertilizer was applied for higher yield, it frequently leads to nitrate accumulation in foliage [5,6]. Soilless culture is considered the most promising way to achieve this balance due to its precise controllability over both nutrient supply and growth environments. Soilless culture systems, such as hydroponics and aeroponics, are known for being very water- and fertilizer-efficient. However, the industrial characteristics of soilless culture are also obvious [7]. Maintaining adequate nutrient and oxygen supply through open-flow hydroponic systems and 24h recirculating operations requires significantly increased energy and resource inputs, particularly in electricity consumption [8,9]. As soilless culture technology has progressed, researchers have been trying to develop it in the direction of sustainable agriculture. Various water-saving and fertilizer-saving strategies have been proposed, such as using closed instead of open recycling nutrient solution systems, using rainwater and wastewater instead of drinking water, and improving the substrate [10]. However, these technological improvements still struggle to balance resource conservation with enhancing vegetable yield and quality.
Intercropping is a bioregulation method that leverages the interspecific relationships between different crops without requiring additional energy input. As an eco-friendly farming method, intercropping is widely used to control pests, regulate the root environment, and share space with other plants in open-field agriculture [11], although it is not commonly practiced in soilless culture. Intercropping, categorized as an interspecific diversification strategy, is recognized as a promising approach for sustainable intensification across diverse agricultural systems, spanning from high-input to low-input scenarios [12]. The integration of hydroponics with aquaculture systems (aquaponics) has inspired attempts to apply vegetable–vegetable intercropping in aeroponic systems to enhance overall production efficiency [8]. After several years of trials, cherry radish has been identified as an ideal companion plant for increasing the yield and quality of lettuce in aeroponic systems by utilizing their interspecific relationships [13,14,15].
The nutrient solution is the key to plants’ survival in soilless culture and has been an important component of soilless culture research [16]. The current related research mainly focuses on improving nutrient solution formulations; not much research on nutrient solution management methods has been performed. Previous studies have demonstrated both the direct effects of the nutrient solution concentration on plant yield and quality, and differential nutrient requirements of vegetables across distinct growth stages [17,18]. It can be seen that the management mode of supplying different concentrations of nutrient solution in different stages is essential.
Although our prior two-season study [19] demonstrated enhanced lettuce and cherry radish production in a 2:1 lettuce/radish intercropping system under aeroponics (via a three-factor, three-level orthogonal design), achieving a 55.57% increase in lettuce leaves and a 9.80% increase in radish bulbs compared to a sole cropping system, it focused solely on optimizing the final yield and quality of intercrops through a variable nutrient solution (NS) concentration at different growth stages combined with preharvest short-duration continuous light for 48 h (48 h-PSCL). This presents three limitations:
- As a non-exhaustive experimental framework, the orthogonal design’s exclusion of NS adjustments without 48 h-PSCL obstructs the systematic evaluation of lighting-driven energy expenditures in sustainable aeroponic systems.
- The lack of attention to the formation processes of yield and quality prevents the provision of critical information for studying intercropping regulation mechanisms, such as identifying key regulation periods, which, in turn, determine sampling time points.
- Although NS adjustments and 48 h-PSCL exhibit synergistic effects in enhancing lettuce yield, the coexistence of fertilizer conservation and electricity consumption makes the changes in production costs per unit yield more complex, necessitating a comprehensive evaluation to determine management modes that are more advantageous for sustainability.
Therefore, as an extension of previous research work, this study investigates the established optimal mode (incorporating variable NS concentrations and 48 h-PSCL in a 2:1 lettuce/radish intercropping aeroponic system), aiming to contribute to further exploration of the mechanisms and development of more efficient, resource- and energy-saving bioregulation methods for lettuce production. Specifically, we evaluate two hypotheses in lettuce-dominant intercropping aeroponic systems under controlled environmental conditions: (1) The nutrient solution adjustment strategy enhances lettuce yield and quality while reduces costs per unit yield compared to conventional management; (2) Integration of preharvest short-duration continuous light with nutrient solution adjustment generates significantly higher agroeconomic benefits than nutrient solution adjustment alone. These objectives directly inform sustainability strategy development for aeroponic lettuce production.
2. Materials and Methods
2.1. Experimental Conditions and Plant Materials
The experiment was conducted in a glasshouse located at Jilin University (43°51′05″ N, 125°19′51″ E). The temperature was maintained at 25 ± 3 °C from 8:00 to 19:59, and 15 ± 3 °C from 20:00 to 7:59 using an environmental control system (Simatic S7-200 smart controller, Chengdu Keyida Automation Engineering Co., Ltd, Chengdu, China). The average air relative humidity (RH) was 65 ± 10%, and the photosynthetic photon flux density (PPFD) under natural light ranged from 200 ± 25 μmol/m2·s from 9:00 to 16:00. The shading nets were activated when the light intensity exceeded 200 μmol·m−2·s−1, and supplemental lighting was engaged when the intensity fell below 200 μmol·m−2·s−1. The aeroponic cultivation area occupied approximately 5% of the glasshouse (200 m2), with no other crops present during the trial. Ventilation maintained the CO2 concentrations at 400–420 ppm, consistent with atmospheric levels.
The aeroponic buckets (Figure 1A, Henan Caiju Dongli Agricultural Technology Co., Ltd., Zhengzhou, China) measured 53 cm × 37 cm × 23 cm, each equipped with an independent nutrient solution control system to enable separate treatment and replicate management. Each bucket can hold 12 plants (8 lettuce and 4 radish) arranged in 3 rows of 4 plants each, with all radishes positioned in the middle row and lettuce occupying the remaining two rows. The interplant spacing is approximately 12 cm.

Figure 1.
Aeroponic bucket (A) and preharvest short-duration continuous light (PSCL) arrangement (B) in the experiment.
The buckets were randomly arranged within a 10 m2 area on the northeastern side of the experimental glasshouse to ensure uniformity of the light conditions across all treatments during the non-supplemental lighting period. To enhance energy efficiency through alignment with circadian-driven physiological rhythms, particularly the reduced transpiration-mediated water/nutrient demand during the night, phase-specific intermittent NS delivery schedules have been implemented [19,20]. In this study, the nutrient solution was sprayed for 15 min at 15-minute intervals during the daytime and for 5 min at 45-minute intervals at night.
The nutrient solution formula is detailed in Table 1 (elemental composition of the nutrient solution shown in Table A1), with an elemental mass ratio of N:P:K = 6:1:8, and this ratio remained unchanged even when the solution was diluted to half-strength (1/2× concentration). The pH and electrical conductivity (EC) were measured daily between 7:00 and 8:00 AM. The electrical conductivity was maintained at 2000 ± 200 µS/cm by adding water, and the pH was adjusted to 5.8–6.2 using 0.1 M HCl to counteract observed increases during growth.

Table 1.
Formula of the nutrient solution.
Italian lettuce (Lactuca sativa L. cv. Parris Island Cos) and cherry radish (Raphanus sativus cv. Cherry Belle) were selected as companion crops due to their distinct nutrient requirements, canopy structures, and root morphologies, following Zhang’s method [19]. Seeds were sourced from Jilin Fengke Seed Industry Co., Ltd. (Changchun, China).
2.2. Experimental Treatment
This experiment ran from 20 March to 24 May 2019. Lettuce seeds were sown on the 20 March and cherry radish on the 10 April. Guided by agronomic growth patterns and operational expertise [19,20], uniform seedlings (lettuce: 3–4 true leaves; cherry radish: 2 true leaves) were simultaneously transplanted on the 25 April and jointly harvested 30 days later, and the cultivation period was divided into three 10-day stages. Although cultivation trials confirmed that radish growth in the intercropping system remained unaffected (as shown in Figure A1 and Table A2 in Appendix A), this study ultimately selected lettuce (the dominant crop in this system) as the research subject due to unmanageable workload in data collection and analysis.
This study investigated the effects of three different cultivation management modes on the lettuce fresh weight (FW); dry weight (DW); and nitrate (NO3−), soluble sugar (SS), soluble protein (SP), and ascorbic acid (AsA) contents in a 2:1 lettuce/cherry radish intercropping aeroponic system. Three management modes (shown in Table 2) for the intercropping system were implemented:

Table 2.
Experimental treatments.
- Treatment M1 (Control): A conventional management mode with a constant NS concentration (1× concentration) across all growth stages without performing additional PSCL.
- Treatment M2: A mode with a variable NS concentration (1/2×, 1×, and 1/2× concentrations for Stages 1–3) optimized through prior trials [19].
- Treatment M3: A mode combining a variable NS concentration (identical to M2) with 48 h-PSCL.
Among these treatments, M3 emerged as the optimal mode with the highest final yield, validated by the earlier orthogonal experimental design [19]. Although excluded from the initial design, M2 provides critical data for systematically evaluating energy/resource expenditures across management protocols to advance sustainability objectives.
Each mode consisted of eight aeroponic buckets, allowing three lettuce plants to be randomly collected from different buckets at each sampling time as biological replicates. The nutrient solutions were replaced at each stage for each bucket. A total of 20 L of solution was supplied per aeroponic bucket, with stage-specific volumes of 4 L, 10 L, and 6 L.
At 28 days after transplanting (DAT), all buckets under the M3 treatment were transferred to a light-supplemented frame. The frame had two layers, equipped with nine full-spectrum LEDs (Prosperity Optoelectronics Technology Co., Ltd., Changsha, China) positioned 20 ± 2 cm above the canopy of the plants. The light intensity was maintained at 175–185 μmol/m2·s, and natural light was excluded using barriers. Plants under the M3 treatment received uninterrupted supplemental light for 48 h.
2.3. Sampling and Analytical Methods
In the 2:1 lettuce/radish intercropping system, the total yield advantage of lettuce and radish was discussed in previous work [19]. The current investigation focused exclusively on conducting regular, consecutive sampling analyses of the dominant vegetable crop (lettuce) within this system for subsequent discussion, whereas radish performance was not quantitatively evaluated.
Parameters were measured exclusively in lettuce plants. Sampling commenced at 10 DAT, corresponding to the minimum leaf area required for relative chlorophyll content (SPAD) measurements, and continued at 5-day intervals until harvest (30 DAT). Additional samples were collected at 28 DAT and 29 DAT to assess the effects of 48 h-PSCL exposure. At 10 and 15 DAT, six lettuce plants per treatment were randomly selected from three distinct aeroponic buckets (two plants per bucket). Half of these plants (one plant per bucket) underwent nondestructive SPAD and photosynthesis measurements between 9:00 and 10:00. All the selected plants were then harvested between 13:00 and 14:00 for destructive analysis. At the other sampling timepoints (20–30 DAT), only three lettuce plants per treatment (one plant per bucket) were used for all the aforementioned procedures, as the plants had grown sufficiently large.
To maintain the 2:1 lettuce-to-cherry radish planting ratio in the aeroponic system, after each sampling event, lettuce plants from a non-sampled aeroponic bucket within the same treatment were transplanted into the sampled buckets to ensure experimental continuity. The source bucket (non-sampled) was subsequently removed from the trial. This procedure was repeated until 28 DAT. During the final experimental phase (29–30 DAT), the last three aeroponic buckets per treatment were retained for staggered harvesting. At each sampling interval, two lettuce plants and one cherry radish plant were randomly harvested from each bucket to preserve the intercropping ratio until the terminal harvest at 30 DAT. One lettuce of harvested plants per bucket was analyzed.
2.3.1. Growth and Yield Parameters
Harvested plants were separated into shoots and roots for FW determination. The maximum leaf area (MLA) was measured with a leaf area meter (Yaxin-1241, Beijing Ya Xin Li Yi Technology Co., Ltd., Beijing, China). After reserving sufficient fresh leaf samples for quality analysis, the remaining shoots were weighed to record their FW. All residual tissues (excluding reserved samples) were oven-dried (105 °C for 30 min, followed by 80 °C until constant weight) to determine the root dry weight (DW) and calculate the whole shoot DW per plant.
2.3.2. SPAD, Photosynthesis Gas Exchange, and Chlorophyll a Fluorescence (CF) Parameters
All the parameters were measured on the second youngest, fully expanded leaf from 9:00 to 12:00 before each sampling. The SPAD values were measured using a chlorophyll meter (SPAD-502, Konica-Minolta, Tōkyō, Japan). The gas exchange parameters (net photosynthetic rate Pn, stomatal conductance Gs, intercellular CO2 Ci, and transpiration rate Tr) were detected using a portable photosynthesis system (Li-6400XT, LI-COR Environmental, Lincoln, NE, USA). The measurements were performed under a CO2 concentration of 400 ppm, controlled by a CO2 injector system (Li-6400-01,LI-COR Environmental, USA); photosynthetically active radiation (PAR) of 1500 µmol/m2·s provided by an LED light source (Li-6400-02, LI-COR Environmental, USA); and relative humidity (RH) of 65% [20]. The CF parameters (the minimal fluorescence yield of the dark-adapted state F0, maximal fluorescence yield of the dark-adapted state Fm, maximal fluorescence yield of the light-adapted state , and steady-state fluorescence yield Fs) were recorded with a fluorometer (OS1P, OPTI-sciences, Hudson, NH, USA) after 20-minute dark adaptation. The maximum quantum yield of photosystem II (PSII) (Fv/Fm) and effective quantum yield of PSII (ΦPSII) were calculated as follows:
2.3.3. Quality and Nutrient Parameters
For each reserved harvested lettuce plant, fresh leaves were rinsed with distilled water, blotted dry with filter paper, finely chopped, and homogenized. Aliquots of 2–3 g, 0.5 g, 0.1–0.3 g, and 0.2–0.3 g were weighed as samples for the determination of NO3−, SS, SP, and AsA, respectively. Three replicate samples per plant were analyzed, with results expressed as the mean values of the replicates. The contents of NO3−, SS, and SP were determined using the nitration of salicylic acid method [21], the anthrone–sulfuric acid method [22], and the Coomassie Brilliant Blue G-250 Assay [23], respectively, while AsA content was measured using a Reflectoquant® reflectometer (RQflex 20, Merck KGaA, Darmstadt, Germany) with corresponding AsA test strips.
2.3.4. Cost–Benefit Analysis
The total resource/energy cost per 1 g of lettuce produced (Pc, CNY) was calculated as follows:
where Pw, Pe, and Pf represent the local costs of water, electricity, and fertilizer per liter of nutrient solution; Qw, Qe, and Qf denote the average consumption of water, power, and fertilizer per plant; Yl is the shoot fresh weight (g/plant).
The input increase rates under the M1, M2, and M3 treatments were compared to the sole cropping system (data shown in Table A3).
2.4. Statistical Analysis
The values represent the mean ± SD of three biological replicates per treatment. Statistical analyses, including one-way ANOVA (for overall significance), Pearson correlation, and LSD multiple-comparison tests, were conducted using SPSS 24.0 (Statistical Package for the Social Sciences, 2016, USA), with the significance level set at 0.05. The figures were generated with Origin 2024 (Origin Lab, Northampton, MA, USA).
3. Results
3.1. Growth and Yield
At harvest (30 DAT), lettuce grown under the M2 and M3 treatments exhibited significantly higher FW, DW, and MLA compared to that grown under the M1 treatment, though no significant differences were observed between M2 and M3 treatments (Figure 2A–E and Figure A2). These results suggest that variable NS concentrations significantly influenced FW, DW, and MLA, whereas the 48 h-PSCL showed no pronounced enhancement effect.
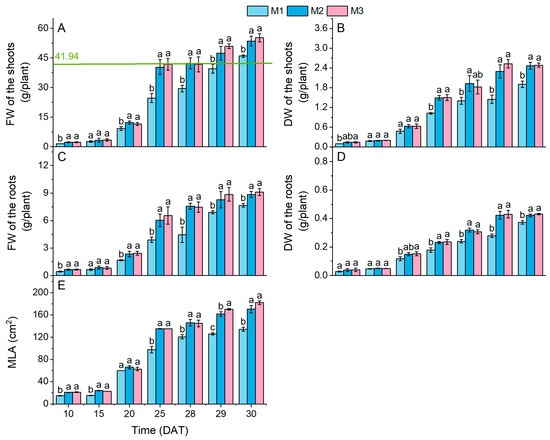
Figure 2.
Fresh weight (FW, (A,C)), dry weight (DW, (B,D)), and maximum leaf area (MLA, (E)) of lettuce plants. Different lowercase letters indicate significant differences (p < 0.05) between treatments at each sampling time. The green line represents the average value of the corresponding index for sole-cropped lettuce grown under identical nutrient solution and light conditions to the M1 treatment (data shown in Table A3). Dashed lines depict temporal trend lines in each indicator.
The growth trends were segmented into three distinct phases—10–15 DAT, 15–25 DAT, and 25–30 DAT—with the 15–25 DAT interval representing the most rapid shoot fresh weight period for lettuce plants under three treatments (M1: 823.90%; M2: 1106.31%; M3: 1114.40%). During the growth period, the FW of lettuce under the M2 and M3 treatments began to exceed that under M1 by 20 DAT, followed by the DW surpassing that under M1 at 25 DAT. The delayed emergence of statistically significant DW differences across treatments, whereas fresh weight (FW) variations manifested earlier, suggests that low-concentration NS application may significantly enhance lettuce FW during the early growth stage (10–15 DAT) through improved water uptake efficiency in intercropped lettuce systems. Notably, 48 h-PSCL did not significantly enhance the FW or DW increase rates between 25 and 30 DAT.
3.2. Photosynthetic Performance
The Pn values were significantly higher under the M2 and M3 treatments than under the M1 treatment throughout the entire growth period (Figure 3A). However, PSCL reduced the Pn value under the M3 treatment, making it significantly lower than that under the M2 treatment, with the highest Pn value recorded under the M2 treatment at harvest (30 DAT). Similar trends were observed for Tr, Gs, and Ci (Figure 3B–D). Specifically, Tr, Gs, and Ci were significantly higher under the M1 treatment at 10 and 15 DAT but shifted to higher values under the M2 and M3 treatment prior to PSCL application. This demonstrated that the lower NS concentration in the M2 and M3 treatments during the early stage (10 DAT) likely contributed to reduced Gs and Ci, as insufficient K+ availability in shoots can induce stomatal closure, and this effect persisted until 15 DAT (Figure 3C).
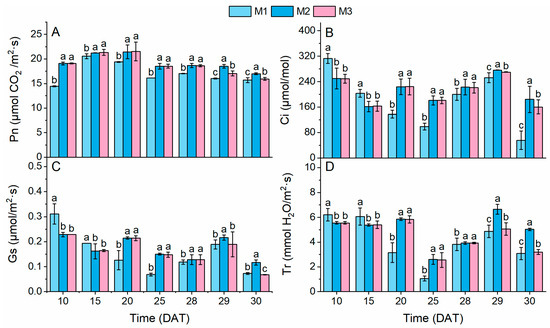
Figure 3.
Gas exchange parameters. Different lowercase letters indicate significant differences (p < 0.05) between treatment modes at each sampling time. Pn, Ci, Gs, and Tr represent the net photosynthetic rate (A), intercellular CO2 concentration (B), stomatal conductance (C), and transpiration rate (D).
3.3. Relative Chlorophyll Content (SPAD)
Regarding the SPAD values (Figure 4), significantly higher SPAD values were observed under the M2 and M3 treatments after 20 DAT, compared to the M1 treatment. The peak SPAD values occurred at 25 DAT: 20.9 ± 1.0 (M1), 22.7 ± 0.4 (M2), and 22.8 ± 0.3 (M3). After 25 DAT, the SPAD declined under both the M1 and M2 treatments, though the values remained higher under M2 than under M1. This demonstrates that variable NS concentrations improved the relative chlorophyll content, with PSCL providing additional enhancement.
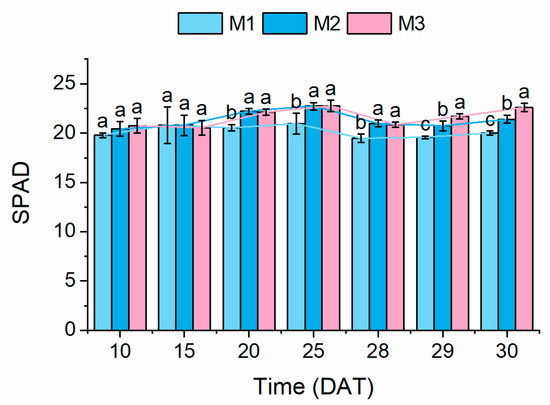
Figure 4.
Relative chlorophyll content (SPAD) of lettuce plants. Different lowercase letters indicate significant differences (p < 0.05) between treatments at each sampling time. Dashed lines depict temporal trend lines in each indicator.
3.4. Chlorophyll a Fluorescence
During the last 48 h before harvest, all three parameters peaked under the M2 treatment without PSCL (Figure 5). The Fv/Fm remained stable across the three treatments, showing no significant differences at any sampling time (Figure 5A). Variable NS concentrations reduced the ΦPSII values of lettuce under the M2 and M3 treatments from 10 to 28 DAT, compared to the M1 treatment, although statistical significance was only achieved at 10 DAT. After 28 DAT, however, the ΦPSII under the M2 treatment rose significantly above the levels under other treatments, indicating that variable NS concentrations enhanced photochemical efficiency during late growth stages. In contrast, the ΦPSII under the M3 treatment remained significantly lower than that under the M2 treatment from 29 DAT onward and even dropped below that under the M1 treatment at 29 DAT.
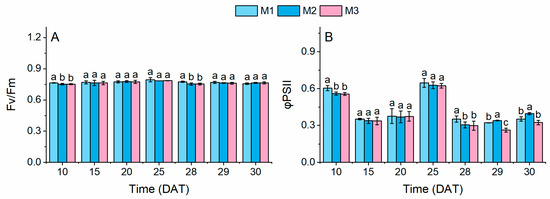
Figure 5.
Chlorophyll a fluorescence parameters. Different lowercase letters indicate significant differences (p < 0.05) between treatment modes at each sampling time. Fv/Fm and ΦPSII represent the maximum quantum yield (A) and effective quantum yield of photosystem II (B), respectively.
3.5. Quality Attributes and Nutrient Content
The trends in lettuce quality and nutrient content between 10 and 28 DAT were similar across all three treatments, with the parameters under the M2 and M3 treatments consistently being higher than those under the M1 treatment at each sampling time (Figure 6A–D). This indicates that variable NS concentrations enhance lettuce quality. However, a significant divergence in trend lines emerged between 29 and 30 DAT. Under the M3 treatment, the NO3− concentrations in leaves decreased significantly, while the SS, SP, and AsA contents increased markedly. These results confirm that 48 h-PSCL further improves lettuce quality when combined with variable NS concentrations.
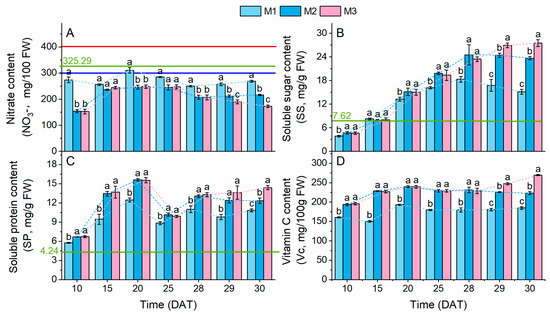
Figure 6.
Nitrate (A) and nutrient contents (B–D) in lettuce plants. Different lowercase letters indicate significant differences (p < 0.05) between treatment modes at each sampling time. The red and blue lines represent the maximum nitrate (NO3−) thresholds established by Commission regulation (EU) No. 1258/2011 [24] and the Chinese National Standards GB 19338-2003 [25], respectively. The green line represents the average values of the corresponding indices for sole-cropped lettuce grown under identical nutrient solution and light conditions to the M1 treatment (data shown in Table A3). Dashed lines depict temporal trend lines in each indicator.
Throughout the growth period, the SS, SP, and AsA contents under the M2 and M3 treatments were significantly higher than under the M1 treatment (excluding SS at 15 DAT). Notably, during early growth (10–20 DAT), the increased rates of SP content under the M2 and M3 treatments (M2: 25.5%; M3: 25.0%) exceeded those of SS content (M2: 14.1%; M3: 13.2%), suggesting prioritized SP synthesis. From 25 DAT onward, the rate of SS content surpassed that of SP content. At harvest (30 DAT), the SS content under the M2 and M3 treatments rose by 56.3% and 81.7%, while the increase rates of SP content were 13.9% and 32.9% (Figure 6B,C), respectively.
Additionally, during the period of 28–30 DAT, PSCL delayed the peak SP and SS levels to 30 DAT, while elevating their maxima. Under the M3 treatment, the SP and SS peaks reached 27.5 ± 0.9 mg/g FW and 14.0 ± 0.3 mg/g FW, respectively, compared to 18.3 ± 0.7 mg/g FW (SP) and 12.4 ± 0.3 mg/g FW (SS) under the M1 treatment. Crucially, 48 h-PSCL under the M3 treatment reversed the decline in SP content observed under the other treatments, highlighting its role in sustaining protein synthesis.
Finally, further Pearson correlation analysis between Pn and SP content in lettuce under the M3 treatment before and after supplementary lighting revealed that the correlation coefficient between the Pn and SP content in intercropped lettuce under the M3 treatment showed a strong positive correlation during the 10–25 DAT period (r = 0.851, p < 0.001). However, the correlation weakened significantly during the 10–30 DAT period (r = 0.074, p > 0.05 for M3).
3.6. Resource and Energy Consumption from Management Modes
Based on Table 3, the increasing rates of the total resource/energy input cost (Pt) required to produce 1 g of lettuce under all three treatments were below zero, demonstrating the dual advantages of aeroponic intercropping in optimizing resource/energy efficiency. The fertilizer inputs in the M2 and M3 treatments decreased by 25% compared to those in the M1 treatment. M3 exhibited the lowest Pt among all treatments, followed by M2. However, it is noteworthy that the absolute resource/energy input (disregarding the extent of yield increase and quality improvement) under the M2 treatment was the lowest. It should also be noted that although the FW values used for Pt calculation were larger in M3, no statistically significant difference in FW per plant was observed between the M3 and M2 treatments.

Table 3.
Rate of increase in shoot fresh weight per plant and resource/energy inputs per 1 g of lettuce produced.
4. Discussion
The nutrient solution serves as the primary source of nutrients and water in aeroponic systems, and variable nutrient solution concentrations can meet the nutrient needs of crops at different growth stages [26,27]. Also, many studies have found that continuous lighting can increase the yield before harvest or during long-term cultivation [28,29,30]. When researchers conducted a 30-day nutrient solution (NS) concentration experiment on glass lettuce varieties in a plant factory, utilizing the collected data to model the precise nutrient requirements at different growth stages (aiming to enhance yield), they also found that during the first growth stage (1–10 DAT), the highest yield increase was achieved with approximately half the concentration of the standard nutrient solution formula [31], which is consistent with the results of the present study. However, PSCL exposure did not amplify the positive effects of variable nutrient solution concentrations on lettuce fresh and dry weight in the intercropping system of this study (Figure 2A,B). This is consistent with these findings that there was no significant difference in fresh weight of lettuce when irradiated for two consecutive days at a light intensity of 150–200 μmol·m−2·s−1 [32,33]. It is evident that continuous supplemental lighting may have varying effects on yield depending on the duration of illumination and the plant species involved.
Variable nutrient solution concentration increased soluble sugar (SS) and soluble (SP) content, with PSCL amplifying the accumulation of SS, which is consistent with the findings of previous studies [32]. SS originates from starch conversion and direct photosynthetic synthesis. However, photosynthates are also allocated to nitrogen assimilation and AsA biosynthesis, explaining the early-stage SP content increase under the M2 and M3 treatments. The enhanced photosynthetic efficiency under the M2 and M3 treatments (Figure 4) during 20–28 DAT facilitated greater SS accumulation. However, after 28 DAT, under the M3 treatment, the simultaneous increase in SS content and decrease in Pn reflect the stimulatory effect of PSCL on the starch-to-sucrose conversion rate. This suggests that, although PSCL reduced the photosynthetic capacity in plants under the M3 treatment, it enhanced carbohydrate partitioning toward sucrose synthesis [34]. In previous studies [35], similar effects of supplemental lighting and varied nutrient solution concentrations on the SP content of sole-cropped lettuce have been reported, which corroborates our present findings.
Intercropped lettuce under the M3 treatment exhibited the lowest NO3− and the highest SP contents. However, its dry weight did not show significant differences from that of intercropped lettuce under the M2 treatment without preharvest supplemental lighting. This result implies that efficient nitrogen assimilation may not have been driven by the carbon skeleton derived from PSCL-enhanced carbon assimilation. Instead, the suppression of carbon assimilation likely reduces competition for assimilatory power (e.g., ATP and NADPH), primarily generated by the light reactions of photosynthesis, between carbon and nitrogen metabolism [36]. Further analysis indicates that the absence of yield enhancement in intercropped lettuce under M3 treatment compared to M2 treatment may be attributed to photosynthetic inhibition by PSCL in intercropped lettuce. Photosynthesis depends not only on chlorophyll content but also on stomatal density, leaf maturity, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity, growth phase, and external factors [37].
Regarding the inhibition of photosynthesis in intercropped lettuce by PSCL in this study, we give explanations based on the data obtained from the experiment as follows. Firstly, the lower Pn, Ci, Gs, and ΦPSII in the M3 treatment relative to the M2 treatment align with prior findings. This suggests that with 48 h-PSCL exposure, the decline in Pn may primarily result from stomatal closure, which limits CO2 supply and subsequently disrupts the Calvin cycle, further suppressing light reaction efficiency and reducing ΦPSII values [38]. It has been reported that excessive light reduces the effective absorption cross-section of light-harvesting pigments in PSII [39]. PSCL significantly increased the SS content in lettuce. However, excessive SS accumulation can trigger the feedback inhibition of photosynthesis by downregulating photosynthetic enzyme genes, such as Rubisco [40]. The correlation results between Pn and SP content also provide indirect evidence for this hypothesis. Given that soluble protein primarily comprises enzymes, including Rubisco, the rate-limiting enzyme in carbon fixation, the weakened correlation aligns with the view that PSCL may inhibit photosynthesis by modulating enzyme activity. Secondly, we also observed that the SPAD values under M3 treatment exceeded those under M2 treatment at 29 and 30 days after transplantation, which contradicted the Pn results. While SPAD is a growth indicator, its correlation with chlorophyll synthesis must be acknowledged [41]. Elevated chlorophyll content under PSCL may not enhance photosynthetic efficiency if the CO2 supply remains inadequate, although chlorophyll content is an important internal factor for photosynthesis [42]. Furthermore, SPAD values alone cannot distinguish chlorophyll a/b ratios. Thus, PSCL-induced shifts in chlorophyll a/b ratios under the M3 treatment might exacerbate light energy surplus, further inhibiting photosynthesis [43]. Additionally, it has been suggested in previous studies that continuous light-induced photodamage in tomato involves phytochrome signaling [44]; however, this hypothesis requires further experimental verification.
A brief discussion follows in response to the benefits analysis. Under the M1, M2, and M3 treatments, the intercropping system demonstrated identical electricity consumption for NS circulation and water usage for nutrient solution preparation. The reduction in production cost under M1 relative to the sole cropping system was solely due to the increased lettuce yield in the intercropping system. In contrast, the lower production costs under M2 and M3 were attributed to both fertilizer savings and yield improvements. Additionally, M3 required extra electricity input for 48 h-PSCL (approximately 0.5 kWh per cultivation bucket), which constituted the sole energy input difference between M2 and M3. From a yield perspective, the average yield per lettuce plant under M3 exceeded that under M2, resulting in the lowest cost per unit fresh weight yield of lettuce under M3. However, statistically, the yield increase per plant in M3 was not significant, leading to a more conservative conclusion: for the yield-focused system, NS adjustment alone (as in M2) represents a viable energy-efficient strategy. However, for M3, the potential of PSCL to enhance lettuce quality should not be overlooked. Higher-quality vegetables may command premium market prices, meaning that the additional energy input in M3 could still justify its economic viability despite the absence of significant yield gains. From an economic perspective, a marginal increase in production costs to attain higher economic returns, which are quantified as marginal benefits (MBs) derived from sales income, is generally justifiable [45]. This cost–benefit equilibrium critically determines the commercial viability of production management models [46].
Additionally, the intercropping system produces not only lettuce but also radish. According to previous research findings [19], under M3 conditions, the total vegetable yield of the intercropping system was significantly higher than that of systems without supplementary lighting or adjusted nutrient solution concentration. This suggests the potential of M3 treatment to reduce the cost per unit yield in the entire intercropping system. Radish, as a companion crop in the intercropping system, was primarily cultivated to simulate authentic intercropping conditions. However, constrained by the predetermined research scope, systematic data collection regarding radish growth parameters was not implemented. Subsequent experiments will incorporate the simultaneous monitoring of both lettuce and radish to investigate the mechanisms underlying intercropping advantages, with the objective of elucidating how interspecific interactions contribute to enhanced vegetable productivity.
5. Conclusions
Based on the experimental results, the research hypotheses have been verified. Variable NS concentrations significantly enhanced the lettuce fresh weight, dry weight, and maximum leaf area compared to constant NS concentrations at different growth stages. The synergistic application of variable NS concentrations and PSCL further improved the SPAD values and lettuce quality. For yield-focused production, variable NS concentrations alone provided statistically comparable shoot fresh weight to plants undergoing 48 h-PSCL without additional energy costs. Conversely, PSCL remains critical for quality-driven markets requiring enhanced phytochemical profiles. Furthermore, if the yield and quality changes of intercropped radish are holistically evaluated, the additional energy inputs required for M3 may achieve greater marginal benefits through system-level productivity improvements that offset energy expenditures. Additionally, we should not overlook the complex interactions between NS concentrations and PSCL. For instance, although the management mode employing a constant NS concentration (l×concentration) throughout all growth stages combined with additional PSCL incurs higher production costs than the M1, M2, and M3 treatments in this study, such combinations might yield unexpectedly superior results due to their synergistic effects. We will further investigate these hypotheses in subsequent studies.
Author Contributions
Conceptualization, L.Z. and F.L.; methodology, L.Z. and F.L.; software, L.W. and Z.P.; validation, L.Z. and F.L.; formal analysis, L.Z. and F.L.; investigation, F.L., Y.S., and Y.Y.; resources, L.Z.; data curation, Z.P., H.F., and Y.X.; writing—original draft preparation, L.Z., F.L., L.W. and Z.P.; writing—review and editing, F.L. and H.Y.; project administration, F.L. and L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Number 32171913), Natural Science Foundation of Sichuan Province (2025ZNSFSC1105), and Sichuan Modern Agricultural Equipment Engineering and Technology Research Center (Grant Number XDNY2024-006).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors would like to thank Chunsheng Yu (Laboratory of Agricultural Engineering, Jilin University) and Haoyu Yang (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences) for their helpful work and advice on assays.
Conflicts of Interest
Author Yaping Yang was employed by the company Sinotest Equipment Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Appendix A
Appendix A.1

Table A1.
Composition of nutrient solution.
Table A1.
Composition of nutrient solution.
| Element | Concentration/(mg/L) | |
|---|---|---|
| 1× Concentration (1 C) | 1/2× Concentration (1/2 C) | |
| N | 242.875 | 121.437 |
| P | 41.193 | 20.596 |
| K | 312.877 | 156.439 |
| Ca | 160.395 | 80.197 |
| Mg | 48.626 | 24.313 |
| S | 64.489 | 32.244 |
| Fe | 3.183 | 1.592 |
| Mn | 0.525 | 0.262 |
| B | 0.500 | 0.250 |
| Zn | 0.050 | 0.025 |
| Cu | 0.020 | 0.010 |
| Mo | 0.011 | 0.005 |
Appendix A.2

Table A2.
Fresh weight of radish bulbs under three treatments (g/plant).
Table A2.
Fresh weight of radish bulbs under three treatments (g/plant).
| Treatment | M1 | M2 | M3 |
|---|---|---|---|
| Radish bulb | 8.39 ± 1.34 b | 11.95 ± 1.11 a | 13.21 ± 1.25 a |
Note: Different lowercase letters indicate significant differences (p < 0.05) between treatments. No significant difference was observed in the fresh weight of radish bulbs between the M1 intercropping system and the sole cropping system [19].
Appendix A.3

Table A3.
Growth and quality of sole-cropped lettuce.
Table A3.
Growth and quality of sole-cropped lettuce.
| Plant | Shoot FW (g/Plant) | NO3− (mg/100g FW) | SS (mg/g FW) | SP (mg/g FW) |
|---|---|---|---|---|
| Sole-cropped lettuce | 41.94 ± 3.72 | 325.29 ± 26.81 | 7.62 ± 1.31 | 4.24 ± 0.37 |
Appendix B
Appendix B.1
Figure A1.
Radish growth in the intercropping system. (A) intercropped vegetable root system. (B) intercropped vegetable canopy.
Appendix B.2

Figure A2.
Maximum leaf area and leaf blade morphology. DAT means days after transplanting.
References
- Oyinlola, L.A.; Obadina, A.O.; Omemu, A.M.; Oyewole, O.B. Prevention of microbial hazard on fresh-cut lettuce through adoption of food safety and hygienic practices by lettuce farmers. Food Sci. Nutr. 2017, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 April 2025).
- General Administration of Customs of the People’s Republic of China. Available online: http://stats.customs.gov.cn/ (accessed on 27 April 2025).
- Zrig, A.; Alsherif, E.A.; Aloufi, A.S.; Korany, S.M.; Selim, S.; Almuhayawi, M.S.; Tarabulsi, M.K.; Nhs, M.; Albasri, H.M.; Bouqellah, N.A. The biomass and health-enhancing qualities of lettuce are amplified through the inoculation of arbuscular mycorrhizal fungi. BMC Plant Biol. 2025, 25, 521. [Google Scholar] [CrossRef]
- Mazahar, S.; Umar, S.; Iqbal, M. Genotypic variability of nitrate-accumulating leafy vegetables as affected by nitrogen doses: Morpho-physiological and biochemical approach. Discov. Plants 2025, 2, 122. [Google Scholar] [CrossRef]
- Tei, F.; Neve, S.D.; Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, G.; Kim, H.; Brown, P.B.; Huang, J. Comparative life cycle assessment of aquaponics and hydroponics in the Midwestern United States. J. Clean. Prod. 2020, 275, 122888. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A review on hydroponics and the technologies associated for medium- and small-scale operations. Agronomy 2022, 12, 646. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Fallovo, C.; Cardarelli, M.; Graifenberg, A. Use of Salsola soda as a companion plant to improve greenhouse pepper (Capsicum annuum) performance under saline conditions. N. Z. J. Crop Hortic. Sci. 2006, 34, 283–290. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Zhang, C.; Li, H.; Zhang, F.; Van der Werf, W. Syndromes of production in intercropping impact yield gains. Nat. Plants 2020, 6, 653–660. [Google Scholar] [CrossRef]
- Wang, L.L.; Yu, H.Y.; Zhang, L.; Tian, D.X.; Zhang, Y.Q.; Zhao, G. Evaluation of intercropping pattern based on Niche-fitness model and TOPSIS model. Trans. CSAM 2017, 4, 291–297. [Google Scholar] [CrossRef]
- Wang, L.L.; Yu, H.Y.; Zhang, L.; Yao, N.N.; Lu, X.L.; Zhao, H.X.; Fu, Q.; Sui, Y.Y. Soilless Cultivation of Lettuce Leaves for Nitrate Reduction. China Patent No. 201510125427.9, 4 January 2017. [Google Scholar]
- Yu, H.; Wang, L.; Zhang, L.; Liu, S.; Zhang, Y. Effects of intercropping on growth and nitrate accumulation of lettuce in aeroponics. Trans. Chin. Soc. Agric. Eng. 2017, 33, 228–234. [Google Scholar] [CrossRef]
- Kang, Z.; Jiang, Z.; Liu, Z.; Wang, P.; Zhang, C.; Yuan, M.; Bai, M.; Hu, X. Optimal combination of substrate supply amount coupled with nutrient solution management program for cucumber planting. Hortic. Plant J. 2024, in press. [CrossRef]
- Papadimitriou, D.M.; Daliakopoulos, I.N.; Lydakis-Simantiris, N.; Cheiladaki, I.; Manios, T.; Savvas, D. Nitrogen source and supply level impact water uptake, yield, and nutrient status of golden thistle in a soilless culture. Sci. Hortic. 2024, 336, 113384. [Google Scholar] [CrossRef]
- An, X.; Liu, S.; He, C.; Yang, R.; Guo, B.; Li, X.; Chen, C.; Wang, H. Nutrient dynamics during the growth period of Epimedium pubescens and its impact on growth and Icar-iin-Flavonoids composition. Ind. Crops Prod. 2025, 225, 120520. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.Q.W.; Yao, L.J.; Xiao, F.; Yu, H.Y. Optimization of management modes for intercropping aeroponics of lettuce and cherry radish. Trans. Chin. Soc. Agric. Mach. 2020, 51, 337–343. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Li, F.; Xiao, F.; Yu, H. Effect of divalent manganese (Mn 2+) concentration on the growth and nitrate nitrogen content of lettuce during aeroponic intercropping with cherry radish. Hortic. Environ. Biotechnol. 2021, 62, 243–251. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Leng, F.F.; Sun, S.; Jing, Y.; Wang, F.; Wei, Q.; Wang, X.; Zhu, X.L. A rapid and sensitive method for determination of trace amounts of glucose by anthrone-sulfuric acid method. Bulg. Chem. Commun 2016, 48, 109–113. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Ministry of Health of the People’s Republic of China. GB 19338-2003; Maximum Levels of Nitrate and Nitrite in Foods. Standardization Administration of China: Beijing, China, 2003.
- Cho, Y.Y.; Cha, M.K.; Ku, Y.G.; Kim, H.C.; Bae, J.H. Effect of different culture nutrient solution EC on carrot top growth and nutritional contents in a closed-type plant factory system. Hortic. Sci. Technol. 2018, 36, 37–45. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Carmona, J.; Martínez, V.; Garcia-Sánchez, F.; Mestre, T.C.; Navarro-Pérez, V.; Cámara-Zapata, J.M. Reducing nitrate accumulation through the management of nutrient solution in a floating system lettuce (Lactuca sativa, L.). Sci. Hortic. 2024, 336, 113377. [Google Scholar] [CrossRef]
- Murakami, K.; Yamamoto, T.; Fujimoto, K.; Okabe, K.; Masuda, M.; Abe, T.; Maeda, K. Low-pungent sweet pepper selected under continuous fluorescent illumination. In Proceedings of the VI International Symposium on Light in Horticulture, Tsukuba, Japan, 15 November 2009; Volume 907, pp. 243–246. [Google Scholar] [CrossRef]
- Zha, L.; Zhang, Y.; Liu, W. Dynamic responses of ascorbate pool and metabolism in lettuce to long-term continuous light provided by red and blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Liu, J.Y.; Zhang, Y.B.; Liu, W.K. Effects of preharvest red and blue continuous light intensity on growth, quality and AsA metabolism of hydroponics lettuce. J. Agric. Sci. Technol. 2022, 24, 76–84. [Google Scholar] [CrossRef]
- Zhang, X.H.; Xia, J.H.; Chen, Z.R.; Zhu, J.X.; Wang, H. A nutrient optimization method for hydroponic lettuce based on multi-strategy improved grey wolf optimizer algorithm. Comput. Electron. Agric. 2024, 224, 109167. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, W.; Li, J.; Huang, Z.; Tao, Y.; Hong, J.; Zhou, Y. Pre-harvest short-term continuous LED lighting improves the nutritional quality and flavor of hydroponic purple-leaf lettuce. Sci. Hortic. 2024, 334, 113304. [Google Scholar] [CrossRef]
- Yang, X.; Hu, J.; Wang, Z.; Huang, T.; Ang, Y.; Zhang, L.; Yang, Q. Pre-harvest nitrogen limitation and continuous lighting improve the quality and flavor of lettuce (Lactuca sativa L.) under hydroponic conditions in greenhouse. J. Agric. Food Chem. 2022, 71, 710–720. [Google Scholar] [CrossRef]
- Zhang, Y.; Zha, L.; Liu, W.; Zhou, C.; Shao, M.; Yang, Q. LED light quality of continuous light before harvest affects growth and AsA metabolism of hydroponic lettuce grown under increasing doses of nitrogen. Plants 2021, 10, 176. [Google Scholar] [CrossRef]
- Fang, S.L.; Hu, X.T.; Wang, W.; Li, X.J.; Yang, X.; Wang, R. Effects of light intensity and nutrient solution concentration on yield and quality of hydroponic lettuce. Northern Hortic. 2017, 214, 97–102. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.; Gao, W.; Chen, Y.; Li, H.; Lawlor, D.W.; Paul, M.J.; Pan, W. Exogenous trehalose improves growth un-der limiting nitrogen through upregulation of nitrogen metabolism. BMC Plant Biol. 2017, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Ye, Z.P.; Hu, W.H.; Yan, X.H. Comparison on light-response models of actual photochemical efficiency in photosystem II. Chin. J. Plant Ecol. 2016, 40, 1208. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Avramova, V.; Baggerman, G.; Van Raemdonck, G.; Valkenborg, D.; Van Ostade, X.; Guisez, Y.; Prinsen, E.; Asard, H.; Ende, W.V.; et al. Starch biosynthesis contributes to the maintenance of photosynthesis and leaf growth under drought stress in maize. Plant Cell Environ. 2020, 43, 2254–2271. [Google Scholar] [CrossRef]
- Ates, F.; Kaya, O. The relationship between iron and nitrogen concentrations based on Kjeldahl method and SPAD-502 readings in grapevine (Vitis vinifera L. cv. ‘Sultana Seedless’). Erwerbs-Obstbau 2021, 63 (Suppl. S1), 53–59. [Google Scholar] [CrossRef]
- Xu, J.P.; Yu, Y.C.; Zhang, T.; Ma, Q.; Yang, H.B. Effects of ozone water irrigation and spraying on physiological characteristics and gene expression of tomato seedlings. Horticult. Res. 2021, 8, 180–190. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Vreugdenhil, D.; Millenaar, F.F.; Van Ieperen, W. Phytochrome A protects tomato plants from injuries induced by continuous light. Front. Plant Sci. 2019, 10, 19. [Google Scholar] [CrossRef]
- Mankiw, N.G. Principles of Economics, 8th ed.; Beijing University: Beijing, China, 2020; pp. 6–7. [Google Scholar]
- Kabir, M.H.; Nur-e-Alam, S.M.; Datta, A.; Tan, M.L.; Rahman, M.S. Understanding vegetable farmers’ adoption, dis-adoption, and non-adoption decisions of pest management by pheromone trapping. PLoS ONE 2023, 18, e0292254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).