Advancing Light-Mediated Technology in Plant Growth and Development: The Role of Blue Light
Abstract
1. Introduction
2. Blue Light
3. Effects of Blue Light on Plant Morphology and Development
3.1. Growth and Development of Young Plants
| Species | Varieties | Peak Wavelength (nm) | PPFD (µ mol m−2 s−1) | Photoperiod (h d−1) | Morphological Responses | Reference |
|---|---|---|---|---|---|---|
| Arabidopsis (Arabidopsis thaliana) | - | - | 100 | - | Promote stem length | [13] |
| ‘ler’ | - | 120 | 16 | Inhibit hypocotyl length and plant height | [44] | |
| ‘col-0’ | - | 120 | 16 | Inhibit hypocotyl length and plant height | [44] | |
| Arugula (Brassica eruca) | ‘Rocket’ | 450 | 50/100 | 24 | Promote hypocotyl length | [29] |
| ‘Rocket’ | 455 | 100 | 24 | Promote hypocotyl length | [30] | |
| ‘Rocket’ | 440 | 100 | 24 | Promote hypocotyl length | [31] | |
| ‘Rocket’ | 440 | 20–650 | 24 | Promote hypocotyl length | [32] | |
| Artichokes (Cynara cardunculus var. scolymus) | ‘Green Globe’ | 448 | 41 | 16 | Inhibit plant height | [65] |
| ‘Cardoon’ | 448 | 41 | 16 | Inhibit plant height | [65] | |
| ‘Violetto’ | 448 | 41 | 16 | Inhibit plant height | [65] | |
| Bamboo (Phyllostachys edulis) | ‘Moso Bamboo’ | 450 | 30 | - | Inhibit stem length | [66] |
| Barley (Hordeum vulgare) | ‘Luch’ | 415 | 70 | 16 | Inhibit plant height | [38] |
| Bitter gourd (Momordica charantia) | ‘QX001’ | 465 | 50 | 12 | Inhibit plant height | [40] |
| Cabbage (Brassica oleracea var. capitata) | - | 450 | 100 | 24 | Promote hypocotyl length | [29] |
| - | 455 | 100 | 16/24 | Promote hypocotyl length | [30] | |
| - | 440 | 100 | 24 | Promote hypocotyl length | [31] | |
| - | 455 | 100 | 24 | Promote hypocotyl length | [46] | |
| ‘Kinshun’ | 470 | 50 | 16 | Promote stem length | [45] | |
| ‘Red Rookie’ | 470 | 50 | 16 | Inhibit stem length | [45] | |
| Calibrachoa (Calibrachoa × hybrida) | ‘Kabloom Deep Blue’ | 455 | 100 | 24 | Promote hypocotyl length | [33] |
| ‘Kabloom Deep Blue’ | 440 | 100 | 16/24 | Promote stem length | [34] | |
| Cherry tomato (Solanum lycopersicum var cerasiforme) | ‘Cuty’ | 456 | 205 | 12 | Promote plant height | [49] |
| - | 450 | 320 | 12 | Inhibit plant height | [47] | |
| - | - | 320 | 12 | Inhibit plant height | [48] | |
| Coriander (Coriandrum sativum) | ‘Sumai’ | 450 | 200 | 16 | Inhibit plant height | [43] |
| Cucumber (Cucumis sativus) | ‘Cumlaude’ | 455 | 100 | 18 | Promote hypocotyl length | [24] |
| ‘Cumlaude’ | 455 | 100 | 18 | Promote plant height, hypocotyl length, and epicotyl length | [50] | |
| ‘Xiamei No. 2’ | 454 | 100 | 16 | Promote stem length | [51] | |
| ‘Sweet Slice’ | - | 200/500 | 16 | Inhibit stem length | [52] | |
| Eggplant (Solanum melongena) | ‘Kokuyo’ | 470 | 20–150 | 16 | Promote stem length | [54] |
| ‘Jingqiejingang’ | 458 | 300 | 12 | Promote plant height | [67] | |
| Geranium (Pelargonium hortorum) | ‘Pinto Premium Salmon’ | 440 | 100 | 16/24 | Promote stem length | [34] |
| Impatiens (Impatiens walleriana) | ‘SuperElfin XP Red’ | 446 | 160 | 18 | Inhibit plant height | [61] |
| ‘SuperElfin XP Red’ | 446 | 160 | 18 | Inhibit plant height | [62] | |
| Kiwi (Actinidia chinensis) | ‘Hayward’ | 470 | 200 | 16 | Inhibit stem length | [41] |
| Kale (Brassica napus) | ‘Red Russian’ | 450 | 50/100 | 24 | Promote hypocotyl length | [29] |
| ‘Red Russian’ | 455 | 100 | 24 | Promote hypocotyl length | [30] | |
| ‘Red Russian’ | 455 | 100 | 24 | Promote hypocotyl length | [45] | |
| ‘Scarlet’ | 430 | 100 | 16 | Inhibit hypocotyl length | [68] | |
| Lettuce (Lactuca sativa) | ‘Okayamasaradana’ | 470 | 20–150 | 16 | Inhibit stem length | [54] |
| ‘Okayamasaradana’ | 450 | 85/170 | 16 | Inhibit stem length | [55] | |
| ‘Waldmann’s Green’ | - | 200/500 | 16 | Inhibit stem length | [52] | |
| ‘Rouxai’ | 449 | 180 | 20 | Inhibit stem length | [56] | |
| ‘Rouxai’ | 449 | 180 | 20 | Promote leaf length | [56] | |
| ‘Cheong Chi Ma’ | 460 | 200 | 18 | Promote shoot length | [57] | |
| Maize (Zea mays) | ‘Zheng58’ | 450 | 13 | 12 | Inhibit mesocotyl length and coleoptile length | [39] |
| Marigold (Tagetes erecta) | Antigua Orange’ | 440 | 100 | 24 | Promote stem length | [34] |
| ‘Antigua Orange’ | 440 | 100 | 16 | Promote hypocotyl length | [34] | |
| ‘Antigua Orange’ | 455 | 100 | 24 | Promote stem length | [33] | |
| Mulberry (Morus alba) | ‘Longsang No. 1’ | 465 | 100 | 14 | Inhibit stem length | [42] |
| Mustard (Brassica juncea) | ‘Ruby Streaks’ | 440 | 100 | 24 | Promote hypocotyl length | [31] |
| ‘Ruby Streaks’ | 440 | 250–650 | 24 | Promote hypocotyl length | [32] | |
| ‘Ruby Streaks’ | 450 | 50 | 24 | Inhibit hypocotyl length | [29] | |
| ‘Ruby Streaks’ | 450 | 110 | 12 | Inhibit plant height | [58] | |
| Pea (Pisum sativum) | - | - | - | 8 | Promote plant height | [69] |
| Pepper (Capsicum annuum) | ‘Hangjiao No. 12’ | 460 | 180 | 12 | Inhibit plant height | [59] |
| ‘HA-2502’ | 457 | 300 | 12 | Inhibit plant height and hypocotyl length | [60] | |
| ‘California Wonder’ | - | 200/500 | 16 | Promote stem length | [52] | |
| Petunia (Petunia × hydrida) | ‘Duvet Red’ | 455 | 100 | 24 | Promote hypocotyl length | [33] |
| Duvet Red’ | 440 | 100 | 16/24 | Promote stem length | [34] | |
| ‘Dwarf varieties mix’ | - | - | 12 | Promote hypocotyl length | [35] | |
| Radish (Raphanus sativus) | ‘Cherry Belle’ | - | 200 | 16 | Inhibit stem length | [52] |
| Rice (Oryza sativa) | ‘XZX24’ | 450 | 100 | 12 | Inhibit plant height | [37] |
| ‘HZY261’ | 450 | 100 | 12 | Inhibit plant height | [37] | |
| ‘IR1552’ | 460 | 100 | 12 | Inhibit plant height | [36] | |
| ‘TS10’ | 460 | 100 | 12 | Inhibit plant height | [36] | |
| Salvia (Salvia splendens) | ‘Red Vista’ | 446 | 160 | 18 | Inhibit plant height | [61] |
| ‘Red Vista’ | 446 | 160 | 18 | Inhibit plant height | [62] | |
| Sesame (Sesamum indicum) | ‘Gomazou’ | 470 | 80 | 24 | Promote stem length | [70] |
| Soybean (Glycine max) | ‘Pungwon’ | 447 | 50 | 24 | Promote plant height | [27] |
| ‘Hoyt’ | - | 200/500 | 16 | Inhibit stem length | [52] | |
| Tomato (Solanum lycopersicum) | ‘cry1’ | 447 | 150 | 18 | Promote stem length | [64] |
| ‘Komeett’ | 455 | 100 | 18 | Inhibit hypocotyl length | [50] | |
| ‘Early Girl’ | 446 | 160 | 18 | Inhibit plant height | [62] | |
| ‘Early Girl’ | - | 200/500 | 16 | Inhibit stem length | [52] | |
| ‘Early Girl’ | 446 | 160 | 18 | Inhibit plant height | [61] | |
| ‘Piennolo’ | 446 | 190 | 12 | Inhibit plant height and internode length | [63] | |
| ‘Moneymaker’ | 454 | 100 | 16 | Inhibit stem length | [51] | |
| ‘Moneymaker’ | - | 120 | 16 | Inhibit hypocotyl length and plant height | [44] | |
| Zinnia (Zinnia elegans) | ‘Art Deco’ | - | - | 12 | Inhibit hypocotyl length and stem height | [35] |
3.2. Stem Elongation and Leaf Expansion of Mature Plants
| Species | Varieties | Peak Wavelength (nm) | PPFD (µ mol m−2 s−1) | Photoperiod (h d−1) | Morphological Responses | Reference |
|---|---|---|---|---|---|---|
| Arabidopsis (Arabidopsis thaliana) | ‘col-0’ | 455 | 100 | 24 | Promote stem length | [71] |
| ‘col-0’ | 455 | 100 | 24 | Promote stem length Inhibit hypocotyl length | [73] | |
| ‘col-0’ | 455 | 100 | 24 | Promote stem length | [72] | |
| ‘phot1’ | 455 | 100 | 24 | Promote stem length | [71] | |
| ‘cry1’ | 455 | 100 | 24 | Promote stem length Promote hypocotyl length | [73] | |
| ‘cry2’ | 455 | 100 | 24 | Promote stem length Inhibit hypocotyl length | [73] | |
| ‘cry1cry2’ | 455 | 100 | 24 | Promote stem length Promote hypocotyl length | [73] | |
| ‘CRY1OX’ | 455 | 100 | 24 | Promote stem length Inhibit hypocotyl length | [73] | |
| ‘CRY2OX’ | 455 | 100 | 24 | Inhibit hypocotyl length | [73] | |
| ‘phyAphyBphyCphyDphyE’ | 455 | 100 | 24 | Inhibit hypocotyl length | [72] | |
| Calibrachoa (Calibrachoa × hybrida) | ‘Kabloom Deep Blue’ | 450 | 50/100 | 24 | Promote stem length and canopy height | [74] |
| ‘Kabloom Deep Blue’ | 455 | 100 | 24 | Promote stem length | [75] | |
| ‘Kabloom Deep Blue’ | 440 | 100 | 16/24 | Promote stem length | [34] | |
| Cannabis (Cannabis sativa) | ‘Babbas Erkle Cookies’ | 430 | 250–270 | 18 | Inhibit plant height | [80] |
| Chrysanthemum (Dendranthema grandiflorum) | ‘Token’ | 469 | 25 | - | Inhibit shoot height | [81] |
| Geranium (Pelargonium hortorum) | ‘Pinto Premium Salmon’ | 450 | 50/100 | 24 | Promote stem length and canopy height | [74] |
| Pinto Premium Salmon’ | 455 | 100 | 24 | Promote stem length and canopy height | [75] | |
| Pinto Premium Salmon’ | 440 | 100 | 16/24 | Promote stem length | [34] | |
| Lettuce (Lactuca sativa) | ‘Green Oak Leaf’ | 460 | 133 | 14 | Inhibit stem length | [82] |
| Mint (Mentha) | ‘Spear mint’ | 460–475 | 500 | 16 | Inhibit plant height | [82] |
| ‘Pepper mint’ | 460–475 | 500 | 16 | Inhibit plant height | [82] | |
| ‘Horse mint’ | 460–475 | 500 | 16 | Inhibit plant height | [82] | |
| Marigold (Tagetes erecta) | ‘Antigua Orange’ | 450 | 50/100 | 24 | Promote canopy height and stem length | [74] |
| ‘Antigua Orange’ | 455 | 100 | 24 | Promote canopy height | [75] | |
| ‘Antigua Orange’ | 440 | 100 | 24 | Promote stem length | [34] | |
| ‘Orange Boy’ | 440 | 90 | 16 | Promote plant height | [76] | |
| Petunia (Petunia × hydrida) | ‘Duvet Red’ | 450 | 50/100 | 24 | Promote stem length and canopy height | [74] |
| Duvet Red’ | 455 | 100 | 24 | Promote stem length | [75] | |
| Duvet Red’ | 440 | 100 | 16/24 | Promote stem length | [34] | |
| ‘Baccarat blue’ | 470 | 70/150 | 12 | Promote hypocotyl length | [77] | |
| ‘Baccarat blue’ | 450 | 100/150 | 14 | Promote plant height | [78] | |
| ‘Baccarat blue’ | 470 | 100 | 14 | Promote plant height | [79] | |
| ‘Merlin blue Moon’ | 470 | 100 | 14 | Promote plant height | [79] | |
| Salvia (Salvia splendens) | ‘Red Vista’ | 440 | 90 | 16 | Promote plant height | [76] |
| Sunflower (Helianthus annuus) | ‘Pacino Gold’ | 450 | 60 | 22 | Promote stem length | [84] |
| ‘Pacino Cola’ | 460 | 60 | 18 | Promote stem length and internode length | [85] | |
| Tulip (Tulipa × gesneriana) | ‘Lasergame’ | 447 | 200 | 12 | Promote internode length | [83] |
3.3. Root Development and Architecture
3.4. Other Factors Influencing Blue Light Responses
3.4.1. Spectral Interactions and Light Recipes
3.4.2. Environmental and Cultivation Conditions
3.4.3. Night Interruption
4. Photosynthetic Efficiency
5. Flowering and Photoperiodic Responses
6. Plant Secondary Metabolism
6.1. Flavonoids and Anthocyanins
6.2. Phenolic Acids
6.3. Carotenoids
7. Blue Light-Mediated Stress Resilience
7.1. Drought Stress
7.2. Salt Stress
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, A.; Gerlach, C.; Karlsson, M.; Penn, H. Controlled environment agriculture and containerized food production in northern north america. Agric. Food Syst. Community Dev. 2021, 10, 127–142. [Google Scholar] [CrossRef]
- Dohlman, E.; Maguire, K.; Davis, W.; Husby, M.; Bovay, J.; Weber, C.; Lee, Y. Trends, Insights, and Future Prospects for Production in Controlled Environment Agriculture and Agrivoltaics Systems (Report No. Eib-264); U.S. Department of Agriculture, Economic Research Service: Washington, DC, USA, 2023. [Google Scholar]
- Kotilainen, T.; Robson, T.M.; Hernández, R. Light quality characterization under climate screens and shade nets for controlled-environment agriculture. PLoS ONE 2018, 13, e0199628. [Google Scholar] [CrossRef] [PubMed]
- Pocock, T. Advanced lighting technology in controlled environment agriculture. Light. Res. Technol. 2016, 48, 83–94. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Al Murad, M.; Razi, K.; Jeong, B.R.; Samy, P.M.A.; Muneer, S. Light emitting diodes (LEDs) as agricultural lighting: Impact and its potential on improving physiology, flowering, and secondary metabolites of crops. Sustainability 2021, 13, 1985. [Google Scholar] [CrossRef]
- Sumida, S.; Ehara, T.; Osafune, T.; Ohkuro, I.; Hase, E. Effects of blue-light on chloroplast development in dark-grown euglena-gracilis z. J. Electron Microsc. 1984, 33, 304–305. [Google Scholar]
- Zeiger, E. Light perception in guard-cells. Plant Cell Environ. 1990, 13, 739–744. [Google Scholar] [CrossRef]
- Senger, H. The effect of blue-light on plants and microorganisms. Photochem. Photobiol. 1982, 35, 911–920. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Rapid suppression of growth by blue-light—Occurrence, time course, and general-characteristics. Plant Physiol. 1981, 67, 584–590. [Google Scholar] [CrossRef]
- Talbott, L.D.; Hammad, J.W.; Harn, L.C.; Nguyen, V.H.; Patel, J.; Zeiger, E. Reversal by green light of blue light-stimulated stomatal opening in intact, attached leaves of Arabidopsis operates only in the potassium-dependent, morning phase of movement. Plant Cell Physiol. 2006, 47, 332–339. [Google Scholar] [CrossRef]
- Frechilla, S.; Talbott, L.D.; Bogomolni, R.A.; Zeiger, E. Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y.B. Magic blue light: A versatile mediator of plant elongation. Plants 2024, 13, 115. [Google Scholar] [CrossRef]
- Danaila-Guidea, S.M.; Delian, E. An overview on blue light benefits on plants physiological performances and on plant products qualities. Sci. Pap. Ser. B Hortic. 2020, 64, 643–652. [Google Scholar]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Sena, S.; Kumari, S.; Kumar, V.; Husen, A. Light emitting diode (LED) lights for the improvement of plant performance and production: A comprehensive review. Curr. Res. Biotechnol. 2024, 7, 100184. [Google Scholar] [CrossRef]
- McCree, K.J. Action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agr. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Li, J.G.; Terzaghi, W.; Deng, X.W. Genomic basis for light control of plant development. Protein Cell 2012, 3, 106–116. [Google Scholar] [CrossRef]
- Cashmore, A.R.; Jarillo, J.A.; Wu, Y.J.; Liu, D.M. Cryptochromes: Blue light receptors for plants and animals. Science 1999, 284, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Appelgren, M. Effects of light quality on stem elongation of pelargonium invitro. Sci. Hortic. 1991, 45, 345–351. [Google Scholar] [CrossRef]
- Wheeler, R.M.; Mackowiak, C.L.; Sager, J.C. Soybean stem growth under high-pressure sodium with supplemental blue lighting. Agron. J. 1991, 83, 903–906. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting-diodes with supplemental blue or far-red lighting. J. Am. Soc. Hort. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Lee, S.W.; Seo, J.M.; Lee, M.K.; Chun, J.H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al-Dhabi, N.A.; Kim, S.J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crop Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Kulus, D.; Błażejewska, A.; Nadolna, K.; Kulpińska, A.; Pietrzykowski, K. Application of wide-spectrum light-emitting diodes in the indoor production of cucumber and tomato seedlings. Acta Agrobot. 2023, 76, 762. [Google Scholar] [CrossRef]
- Li, C.X.; Xu, Z.G.; Dong, R.Q.; Chang, S.X.; Wang, L.Z.; Khalil-Ur-Rehman, M.; Tao, J.M. An RNA-Seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red LED light, and white fluorescent light. Front. Plant Sci. 2017, 8, 78. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kwon, S.J.; Eom, S.H. Red and blue light-specific metabolic changes in soybean seedlings. Front. Plant Sci. 2023, 14, 1128001. [Google Scholar] [CrossRef] [PubMed]
- Hahn, E.J.; Kozai, T.; Paek, K.Y. Combinations of blue and red LEDs increase the morphophysiological performance and furanocoumarin production of Brosimum gaudichaudii Trécul in vitro. Front. Plant Sci. 2021, 12, 680545. [Google Scholar]
- Kong, Y.; Schiestel, K.; Zheng, Y.B. Pure blue light effects on growth and morphology are slightly changed by adding low-level UVA or far-red light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y.B. Maximum elongation growth promoted as a shade-avoidance response by blue light is related to deactivated phytochrome: A comparison with red light in four microgreen species. Can. J. Plant Sci. 2020, 100, 314–326. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y.B. Blue versus red light can promote elongation growth independent of photoperiod: A study in four brassica microgreens species. HortScience 2019, 54, 1955–1961. [Google Scholar] [CrossRef]
- Johnson, R.E.; Kong, Y.; Zheng, Y.B. Elongation growth mediated by blue light varies with light intensities and plant species: A comparison with red light in arugula and mustard seedlings. Environ. Exp. Bot. 2020, 169, 103898. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Does “blue” light invariably cause plant compactness? Not really: A comparison with red light in four bedding plant species during the transplant stage. Acta Hortic. 2020, 1296, 621–628. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue-light-promoted elongation and flowering are not artifacts from 24-h lighting: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1295, 659–666. [Google Scholar] [CrossRef]
- Akbarian, B.; Matloobi, M.; Mahna, N. Effects of LED light on seed emergence and seedling quality of four bedding flowers. J. Ornam. Hortic. Plants 2016, 6, 115–123. [Google Scholar]
- Chen, C.C.; Huang, M.Y.; Lin, K.H.; Wong, S.L.; Huang, W.D.; Yang, C.M. Effects of light quality on the growth, development and metabolism of rice seedlings (Oryza sativa L.). Res. J. Biotechnol. 2014, 9, 15–24. [Google Scholar]
- Ren, M.F.; Liu, S.Z.; Tang, C.Z.; Mao, G.L.; Gai, P.P.; Guo, X.L.; Zheng, H.B.; Tang, Q.Y. Photomorphogenesis and photosynthetic traits changes in rice seedlings responding to red and blue light. Int. J. Mol. Sci. 2023, 24, 11333. [Google Scholar] [CrossRef]
- Kochetova, G.V.; Avercheva, O.V.; Bassarskaya, E.M.; Kushunina, M.A.; Zhigalova, T.V. Effects of red and blue LED light on the growth and photosynthesis of barley (Hordeum vulgare L.) seedlings. J. Plant Growth Regul. 2023, 42, 1804–1820. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Niu, Y.N.; Hossain, Z.; Zhao, B.Y.; Bai, X.D.; Mao, T.T. New insights into light spectral quality inhibits the plasticity elongation of maize mesocotyl and coleoptile during seed germination. Front. Plant Sci. 2023, 14, 1152399. [Google Scholar] [CrossRef]
- Wan, Y.L.; Wu, Y.; Zhang, M.; Hong, A.Y.; Liu, Y. Effects of photoperiod extension via red-blue light-emitting diodes and high-pressure sodium lamps on the growth and photosynthetic characteristics in Paeonia lactiflora. Acta Physiol. Plant 2020, 42, 174. [Google Scholar] [CrossRef]
- Correia, C.; Magnani, F.; Pastore, C.; Cellini, A.; Donati, I.; Pennisi, G.; Paucek, I.; Orsini, F.; Vandelle, E.; Santos, C.; et al. Red and blue light differently influence Actinidia chinensis performance and its interaction with Pseudomonas syringae pv. Actinidiae. Int. J. Mol. Sci. 2022, 23, 13145. [Google Scholar] [CrossRef]
- Hu, J.W.; Dai, X.; Sun, G.Y. Morphological and physiological responses of Morus alba seedlings under different light qualities. Not. Bot. Horti Agrobot. 2016, 44, 382–392. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.H.; Li, Q.M.; Yang, Q.C.; Wang, F.; Li, J.M. Effects of LED red and blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Spaninks, K.; Lamers, G.; van Lieshout, J.; Offringa, R. Light quality regulates apical and primary radial growth of Arabidopsis thaliana and Solanum lycopersicum. Sci. Hortic. 2023, 317, 112082. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y.B. Growth and morphology responses to narrow-band blue light and its co-action with low-level UVB or green light: A comparison with red light in four microgreen species. Environ. Exp. Bot. 2020, 178, 104189. [Google Scholar] [CrossRef]
- Mizuno, T.; Amaki, W.; Watanabe, H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Hortic. 2011, 907, 179–184. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chang, T.T.; Guo, S.R.; Xu, Z.G.; Li, J. Effect of different light quality of LED on growth and photosynthetic character in cherry tomato seedling. Acta Hortic. 2011, 907, 325–330. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.; Chang, T.; Xu, Z.; Tezuka, T. Regulation of the growth and photosynthesis of cherry tomato seedlings by different light irradiations of light emitting diodes (LED). Afr. J. Biotechnol. 2012, 11, 6169–6177. [Google Scholar]
- Kim, E.Y.; Park, S.A.; Park, B.J.; Lee, Y.; Oh, M.M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Hernández, R.; Eguchi, T.; Kubota, C. Growth and morphology of vegetable seedlings under different blue and red photon flux ratios using light-emitting diodes as sole-source lighting. Acta Hortic. 2016, 1134, 195–200. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.Q.; Kaiser, E.; Kuang, Y.; Yang, Q.C.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, R.; Dabrowa, A.; Kolton, A. How monochromatic and composed light affect the kale ‘scarlet’ in its initial growth stage. Acta Sci. Pol. Hortorum Cultus 2023, 22, 93–100. [Google Scholar] [CrossRef]
- Hirai, T.; Amaki, W.; Watanabe, H. Action of blue or red monochromatic light on stem internodal growth depends on plant species. Sci. Hortic. 2006, 711, 345–350. [Google Scholar] [CrossRef]
- Yanagi, T.; Okamoto, K.; Takita, S. Effects of blue, red, and blue/red lights of two different PPF levels on growth and morphogenesis of lettuce plants. In International Symposium on Plant Production in Closed Ecosystems; International Society for Horticultural Science: Leuven, Belgium, 1997; pp. 117–122. [Google Scholar]
- Meng, Q.W.; Runkle, E.S. Growth responses of red-leaf lettuce to temporal spectral changes. Front. Plant Sci. 2020, 11, 571788. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.S.; Park, S.H.; Jang, Y.J.; Lee, G.W.; Kim, J.S.; Kim, H.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Blue LED (light-emitting diodes)-mediated growth promotion and control of disease in lettuce. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2013, 63, 271–277. [Google Scholar] [CrossRef]
- Kong, Y.; Masabni, J.; Niu, G. Effect of temperature variation and blue and red LEDs on the elongation of arugula and mustard microgreens. Horticulturae 2023, 9, 608. [Google Scholar] [CrossRef]
- Nie, W.F.; Li, Y.; Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.H.; Yang, Y.X. Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B Biol. 2023, 241, 112673. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.F.; Shi, Q.H.; Yang, F.J.; Wei, M. Response of photomorphogenesis and photosynthetic properties of sweet pepper seedlings exposed to mixed red and blue light. Front. Plant Sci. 2023, 13, 984051. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Zhang, X.; Bisbis, M.; Heuvelink, E.; Jiang, W.J.; Marcelis, L.F.M. Green light reduces elongation when partially replacing sole blue light independently from cryptochrome 1a. Physiol. Plant. 2021, 173, 1946–1955. [Google Scholar] [CrossRef]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of spectral quality of monochromatic LED lights on the growth of artichoke seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ji, L.Y.; Xing, Y.Y.; Zuo, Z.C.; Zhang, L. Data-independent acquisition proteomics reveals the effects of red and blue light on the growth and development of moso bamboo (Phyllostachys edulis) seedlings. Int. J. Mol. Sci. 2023, 24, 5103. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.H.; Li, J.; Du, Y.F.; Wei, M.; Shi, Q.H.; Li, Y.; Yang, F.J. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. J. Plant Growth Regul. 2021, 40, 1477–1492. [Google Scholar] [CrossRef]
- Brazaityte, A.; Miliauskiene, J.; Vastakaite-Kairiene, V.; Sutuliene, R.; Lauzike, K.; Duchovskis, P.; Malek, S. Effect of different ratios of blue and red LED light on brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Awasthi, K. Effect of different light on the growth and development of pea plant. Int. J. Res. Eng. Sci. 2023, 11, 94–98. [Google Scholar]
- Hata, N.; Hayashi, Y.; Ono, E.; Satake, H.; Kobayashi, A.; Muranaka, T.; Okazawa, A. Differences in plant growth and leaf sesamin content of the lignan-rich sesame variety ‘gomazou’ under continuous light of different wavelengths. Plant Biotechnol. 2013, 30, 1–8. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y.B. Phototropin is partly involved in blue-light-mediated stem elongation, flower initiation, and leaf expansion: A comparison of phenotypic responses between wild Arabidopsis and its phototropin mutants. Environ. Exp. Bot. 2020, 171, 103967. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y.B. Phytochrome contributes to blue-light-mediated stem elongation and flower initiation in mature Arabidopsis thaliana plants. Can. J. Plant Sci. 2022, 102, 449–458. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y.B. Low-activity cryptochrome 1 plays a role in promoting stem elongation and flower initiation of mature Arabidopsis under blue light associated with low phytochrome activity. Can. J. Plant Sci. 2022, 102, 755–759. [Google Scholar] [CrossRef]
- Kong, Y.; Stasiak, M.; Dixon, M.A.; Zheng, Y.B. Blue light associated with low phytochrome activity can promote elongation growth as shade-avoidance response: A comparison with red light in four bedding plant species. Environ. Exp. Bot. 2018, 155, 345–359. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Blue light associated with low phytochrome activity can promote flowering: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 621–628. [Google Scholar] [CrossRef]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Fukuda, N.; Ishii, Y.; Ezura, H.; Olsen, J.E. Effects of light quality under red and blue light emitting diodes on growth and expression of FBP28 in petunia. Acta Hortic. 2011, 907, 361–366. [Google Scholar] [CrossRef]
- Fukuda, N.; Oba, H.; Mizuta, D.; Yoshida, H.; Olsen, J.E. Timing of blue and red light exposure and CPPU application during the raising of seedlings can control flowering timing of petunia. Acta Hortic. 2016, 1134, 171–178. [Google Scholar] [CrossRef]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.S.; MacPherson, S.; Lefsrud, M. Light quality impacts vertical growth rate, phytochemical yield and cannabinoid production efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef]
- Bergstrand, K.J.; Schüssler, H.K. Recent progresses on the application of LEDs in the horticultural production. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Greenhouse 2010 and Soilless Cultivation, Lisbon, Portugal, 22–27 August 2010; International Society for Horticultural Science: Leuven, Belgium, 2012; Volume 927, pp. 529–534. [Google Scholar] [CrossRef]
- Chen, X.L.; Guo, W.Z.; Xue, X.Z.; Wang, L.C.; Qiao, X.J. Growth and quality responses of ‘green oak leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Roh, Y.S.; Yoo, Y.K. Light quality of light emitting diodes affects growth, chlorophyll fluorescence and phytohormones of tulip ‘lasergame’. Hortic. Environ. Biotechnol. 2023, 64, 245–255. [Google Scholar] [CrossRef]
- Schwend, T.; Prucker, D.; Mempel, H. Red light promotes compact growth of sunflowers. Eur. J. Hortic. Sci. 2015, 80, 56–61. [Google Scholar] [CrossRef]
- Bergstrand, K.; Asp, H.; Schüssler, H.K. Development and acclimatisation of horticultural plants subjected to narrow-band lighting. Eur. J. Hortic. Sci. 2014, 79, 45–51. [Google Scholar] [CrossRef]
- Canamero, R.C.; Bakrim, N.; Bouly, J.P.; Garay, A.; Dudkin, E.E.; Habricot, Y.; Ahmad, M. Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 2006, 224, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrink, J.P.; Herranz, R.; Medina, F.J.; Edelmann, R.E.; Kiss, J.Z. A novel blue-light phototropic response is revealed in roots of Arabidopsis thaliana in microgravity. Planta 2016, 244, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Iacona, C.; Muleo, R. Light quality affects adventitious rooting and performance of cherry rootstock colt. Sci. Hortic. 2010, 125, 630–636. [Google Scholar] [CrossRef]
- Jin, D.Z.; Su, X.F.; Li, Y.F.; Shi, M.M.; Yang, B.B.; Wan, W.C.; Wen, X.; Yang, S.J.; Ding, X.T.; Zou, J. Effect of red and blue light on cucumber seedlings grown in a plant factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Zhai, S.; Cai, W.; Xiang, Z.X.; Chen, C.Y.; Lu, Y.T.; Yuan, T.T. Pin3-mediated auxin transport contributes to blue light-induced adventitious root formation in Arabidopsis. Plant Sci. 2021, 312, 111044. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Heo, J.W.; Lee, C.W.; Paek, K.Y. Influence of mixed LED radiation on the growth of annual plants. J. Plant Biol. 2006, 49, 286–290. [Google Scholar] [CrossRef]
- Mckay, M.; Faust, J.E.; Taylor, M.; Adelberg, J. The effects of blue light and supplemental far-red on an in vitro multiple harvest system for the production of Cannabis sativa. Plants 2025, 14, 966. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Kurata, K. Analysis of the relationship between blue-light photon flux density and the photosynthetic properties of spinach (Spinacia oleracea L.) leaves with regard to the acclimation of photosynthesis to growth irradiance. Soil Sci. Plant Nutr. 2007, 53, 459–465. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; Taylor, C.; Calders, K.; Hogervorst, M.; van Ieperen, W.; Harbinson, J.; de Visser, P.; Nicole, C.C.S.; Marcelis, L.F.M. Unraveling the effects of blue light in an artificial solar background light on growth of tomato plants. Environ. Exp. Bot. 2021, 184, 104377. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Wheeler, R.M.; Sager, J.C.; Goins, G.D. A comparison of growth and photosynthetic characteristics of lettuce grown under red and blue light-emitting diodes (LEDs) with and without supplemental green LEDs. In Proceedings of the 7th International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition, Kissimmee, FL, USA, 23–27 March 2004; Volume 1 and 2, pp. 467–475. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Ma, D.B.; Li, X.; Guo, Y.X.; Chu, J.F.; Fang, S.; Yan, C.Y.; Noel, J.P.; Liu, H.T. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci. USA 2016, 113, 224–229. [Google Scholar] [CrossRef]
- Johansson, H.; Jones, H.J.; Foreman, J.; Hemsted, J.R.; Stewart, K.; Grima, R.; Halliday, K.J. Cell expansion is controlled by a photothermal switch. Nat. Commun. 2014, 5, 4848. [Google Scholar] [CrossRef]
- Innes, S.N.; Jakobsen, S.B.; Niday, A.; Ali, H.; Arve, L.E.; Torre, S. The aerial environment modulates plant responses to blue light. Acta Hortic. 2018, 1227, 525–532. [Google Scholar] [CrossRef]
- Hahn, E.J.; Kozai, T.; Paek, K.Y. Blue and red light-emitting diodes with or without sucrose and ventilation affect in vitro growth of Rehmannia glutinosa plantlets. J. Plant Biol. 2000, 43, 247–250. [Google Scholar] [CrossRef]
- Vince-Prue, D.; Canham, A.E. Horticultural significance of photomorphogenesis. Encycl. Plant Physiol. 1983, 16, 518–544. [Google Scholar]
- Park, Y.G.; Jeong, B.R. Night interruption light quality changes morphogenesis, flowering, and gene expression in Dendranthema grandiflorum. Hortic. Environ. Biotechnol. 2019, 60, 167–173. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. How supplementary or night-interrupting low-intensity blue light affects the flower induction in chrysanthemum, a qualitative short-day plant. Plants 2020, 9, 1694. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Both the quality and positioning of the night interruption light are important for flowering and plant extension growth. J. Plant Growth Regul. 2020, 39, 583–593. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. The Quality and Quality Shifting of the Night Interruption Light Affect the Morphogenesis and Flowering in Floricultural Plants. In Smart Plant Factory: The Next Generation Indoor Vertical Farms, 1st ed.; Kozai, T., Ed.; Springer Nature Singapore Pte Ltd: Singapore, 2018; pp. 223–238. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Shift in the light quality of night interruption affects flowering and morphogenesis of petunia hybrida. Plants 2023, 12, 2049. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Photoreceptors modulate the flowering and morphogenesis responses of Pelargonium × hortorum to night-interruption light quality shifting. Agronomy 2023, 13, 857. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Muneer, S.; Soundararajan, P.; Manivannan, A.; Jeong, B.R. Light quality during night interruption affects morphogenesis and flowering in Petunia hybrida, a qualitative long-day plant. Hortic. Environ. Biotechnol. 2016, 57, 371–377. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Soundararajan, P.; Manivannan, A.; Jeong, B.R. Light quality during night interruption affects morphogenesis and flowering in geranium. Hortic. Environ. Biotechnol. 2017, 58, 212–217. [Google Scholar] [CrossRef]
- Yamada, A.; Tanigawa, T.; Suyama, T.; Matsuno, T.; Kunitake, T. Night break treatment using different light sources promotes or delays growth and flowering of Eustoma grandiflorum (Raf.) shinn. J. Jpn Soc. Hortic. Sci. 2008, 77, 69–74. [Google Scholar] [CrossRef]
- Pérez, C.P.; Ulrichs, C.; Huyskens-Keil, S.; Schreiner, M.; Krumbein, A.; Schwarz, D.; Kläring, H.P. Composition of carotenoids in tomato fruits as affected by moderate UV-B radiation before harvest. Int. Symp. Tomato Trop. 2009, 821, 217–221. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Molecular mechanisms of photodamage in the photosystem ii complex. Biochim. Biophys. Acta BBA Bioenerg. 2012, 1817, 209–217. [Google Scholar] [CrossRef]
- Nilsson, A.K.; Pěnčík, A.; Johansson, O.N.; Bånkestad, D.; Fristedt, R.; Suorsa, M.; Trotta, A.; Novák, O.; Mamedov, F.; Aro, E.-M. PSB33 protein sustains photosystem ii in plant chloroplasts under UV-A light. J. Exp. Bot. 2020, 71, 7210–7223. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem ii damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Chotewutmontri, P.; Barkan, A. Light-induced psbA translation in plants is triggered by photosystem ii damage via an assembly-linked autoregulatory circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 21775–21784. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and physiological responses of cucumber seedlings to supplemental LED light under extremely low irradiance. Agronomy 2020, 10, 1698. [Google Scholar] [CrossRef]
- Haupt, W.; Scheuerlein, R. Chloroplast movement. Plant Cell Environ. 1990, 13, 595–614. [Google Scholar] [CrossRef]
- Yatsuhashi, H. Photoregulation systems for light-oriented chloroplast movement. J. Plant Res. 1996, 109, 139–146. [Google Scholar] [CrossRef]
- DeBlasio, S.L.; Luesse, D.L.; Hangarter, R.P. A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiol. 2005, 139, 101–114. [Google Scholar] [CrossRef]
- Hermanowicz, P.; Banaś, A.K.; Sztatelman, O.; Gabryś, H.; Łabuz, J. UV-B induces chloroplast movements in a phototropin-dependent manner. Front. Plant Sci. 2019, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Eberly, S.L.; Spremulli, G.H.; Spremulli, L.L. Light induction of the euglena chloroplast protein synthesis elongation factors: Relative effectiveness of different wavelength ranges. Arch. Biochem. Biophys. 1986, 245, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zheng, Y.B. Diverse flowering response to blue light manipulation: Application of electric lighting in controlled-environment plant production. Horticulturae 2024, 10, 578. [Google Scholar] [CrossRef]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef]
- Walters, K.J.; Hurt, A.A.; Lopez, R.G. Flowering, stem extension growth, and cutting yield of foliage annuals in response to photoperiod. HortScience 2019, 54, 661–666. [Google Scholar] [CrossRef]
- Chandel, A.; Thakur, M.; Singh, G.; Dogra, R.; Bajad, A.; Soni, V.; Bhargava, B. Flower regulation in floriculture: An agronomic concept and commercial use. J. Plant Growth Regul. 2023, 42, 2136–2161. [Google Scholar] [CrossRef]
- Izawa, T. Daylength measurements by rice plants in photoperiodic short-day flowering. Int. Rev. Cytol. 2007, 256, 191–222. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef]
- Whitman, C.M.; Heins, R.D.; Cameron, A.C.; Carlson, W.H. Lamp type and irradiance level for daylength extensions influence flowering of ‘Blue clips’, ‘Early sunrise’, and ‘Moonbeam’. J. Am. Soc. Hort. Sci. 1998, 123, 802–807. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Low-intensity blue light in night-interruption lighting does not influence flowering of herbaceous ornamentals. Sci. Hortic. 2015, 186, 230–238. [Google Scholar] [CrossRef]
- Lopez, R.G.; Meng, Q.W.; Runkle, E.S. Blue radiation signals and saturates photoperiodic flowering of several long-day plants at crop-specific photon flux densities. Sci. Hortic. 2020, 271, 109470. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants 2022, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.I.; Jeong, H.K.; Park, Y.G.; Jeong, B.R. Flowering and morphogenesis of kalanchoe in response to quality and intensity of night interruption light. Plants 2019, 8, 90. [Google Scholar] [CrossRef]
- Nissim-Levi, A.; Kitron, M.; Nishri, Y.; Ovadia, R.; Forer, I.; Oren-Shamir, M. Effects of blue and red LED lights on growth and flowering of Chrysanthemum morifolium. Sci. Hortic. 2019, 254, 77–83. [Google Scholar] [CrossRef]
- Magar, Y.G.; Ohyama, K.; Noguchi, A.; Amaki, W.; Furufuji, S. Effects of light quality during supplemental lighting on the flowering in an everbearing strawberry. Acta Hortic. 2018, 1206, 279–284. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kadowaki, M.; Che, J.; Takahashi, S.; Horiuchi, N.; Ogiwara, I. Influence of light quality on flowering characteristics, potential for year-round fruit production and fruit quality of blueberry in a plant factory. Fruits 2019, 74, 3–10. [Google Scholar] [CrossRef]
- Javanmardi, J.; Emami, S. Response of tomato and pepper transplants to light spectra provided by light emitting diodes. Int. J. Veg. Sci. 2013, 19, 138–149. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Y.C.; Zhou, L.; Yang, L.Y. Growth and flowering of saffron (Crocus sativus L.) with three corm weights under different LED light qualities. Sci. Hortic. 2022, 303, 111202. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Li, H.S.; Lyu, Y.M.; Chen, X.H.; Wang, C.Q.; Yao, D.H.; Ni, S.S.; Lin, Y.L.; Chen, Y.K.; Zhang, Z.H.; Lai, Z.X. Exploration of the effect of blue light on functional metabolite accumulation in longan embryonic calli via RNA sequencing. Int. J. Mol. Sci. 2019, 20, 441. [Google Scholar] [CrossRef]
- Li, H.S.; Lin, Y.L.; Chen, X.H.; Bai, Y.; Wang, C.Q.; Xu, X.P.; Wang, Y.; Lai, Z.X. Effects of blue light on flavonoid accumulation linked to the expression of miR393, miR394 and miR395 in longan embryogenic calli. PLoS ONE 2018, 13, e0191444. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. J. Am. Soc. Hort. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Samuoliene, G.; Sirtautas, R.; Brazaityte, A.; Duchovskis, P. Led lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Choi, M.; Kim, K.; Bang, G.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y.I. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett. 2013, 587, 1543–1547. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A.R. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome a or phytochrome b in Arabidopsis thaliana. Plant J. 1997, 11, 421–427. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Zhang, T.T.; Folta, K.M. Green light signaling and adaptive response. Plant Signal Behav. 2012, 7, 75–78. [Google Scholar] [CrossRef]
- Larsen, D.H.; Li, H.; Shrestha, S.; Verdonk, J.C.; Nicole, C.C.S.; Marcelis, L.F.M.; Woltering, E.J. Lack of blue light regulation of antioxidants and chilling tolerance in basil. Front. Plant Sci. 2022, 13, 852654. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Kitayama, M.; Lu, N.; Takagaki, M. Improving secondary metabolite accumulation, mineral content, and growth of coriander (Coriandrum sativum L.) by regulating light quality in a plant factory. J. Horticult. Sci. Biotechnol. 2020, 95, 356–363. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Nie, J.W.; Yan, X.Y.; Xue, W.T. Identifying the critical LED light condition for optimum yield and flavonoid of pea sprouts. Sci. Hortic 2024, 327, 112801. [Google Scholar] [CrossRef]
- Zhang, L.C.; Ma, G.; Kato, M.; Yamawaki, K.; Takagi, T.; Kiriiwa, Y.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Nesumi, H. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs. J. Exp. Bot. 2012, 63, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.L.; Yuan, Z.Y.; Xie, J.; Yao, S.X.; Zeng, K.F. Sensitivity to ethephon degreening treatment is altered by blue LED light irradiation in mandarin fruit. J. Agric. Food Chem. 2017, 65, 6158–6168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Deng, L.L.; Yin, B.F.; Yao, S.X.; Zeng, K.F. Effects of blue LED light irradiation on pigment metabolism of ethephon-degreened mandarin fruit. Postharvest Biol. Technol. 2017, 134, 45–54. [Google Scholar] [CrossRef]
- Zhang, L.C.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Effect of blue LED light intensity on carotenoid accumulation in citrus juice sacs. J. Plant Physiol. 2015, 188, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Stanley, L.; Yuan, Y.W. Transcriptional regulation of carotenoid biosynthesis in plants: So many regulators, so little consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef]
- Yamauchi, S.; Takemiya, A.; Sakamoto, T.; Kurata, T.; Tsutsumi, T.; Kinoshita, T.; Shimazaki, K. The plasma membrane H+-ATPase AHA1 plays a major role in stomatal opening in response to blue light. Plant Physiol. 2016, 171, 2731–2743. [Google Scholar] [CrossRef]
- Merlot, S.; Leonhardt, N.; Fenzi, F.; Valon, C.; Costa, M.; Piette, L.; Vavasseur, A.; Genty, B.; Boivin, K.; Müller, A.; et al. Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 2007, 26, 3216–3226. [Google Scholar] [CrossRef]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G.; Lamminmäki, A.; Brosché, M.; Moldau, H.; Desikan, R.; et al. SLAC1 is required for plant guard cell s-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Scuffi, D.; Alvarez, C.; Laspina, N.; Gotor, C.; Lamattina, L.; García-Mata, C. Hydrogen sulfide generated by l-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 2014, 166, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Takemiya, A.; Shimazaki, K. Phototropin signaling and stomatal opening as a model case. Curr. Opin. Plant Biol. 2010, 13, 587–593. [Google Scholar] [CrossRef]
- Sato, A.; Sato, Y.; Fukao, Y.; Fujiwara, M.; Umezawa, T.; Shinozaki, K.; Hibi, T.; Taniguchi, M.; Miyake, H.; Goto, D.B.; et al. Threonine at position 306 of the kat1 potassium channel is essential for channel activity and is a target site for aba-activated snrk2/ost1/snrk2.6 protein kinase. Biochem. J. 2009, 424, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ebisu, Y.; Kinoshita, T.; Doi, M.; Okuma, E.; Murata, Y.; Shimazaki, K. bHLH transcription factors that facilitate K+ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Sci. Signal 2013, 6, ra48. [Google Scholar] [CrossRef] [PubMed]
- Sutter, J.U.; Sieben, C.; Hartel, A.; Eisenach, C.; Thiel, G.; Blatt, M.R. Abscisic acid triggers the endocytosis of the kat1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 2007, 17, 1396–1402. [Google Scholar] [CrossRef]
- Inoue, S.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef]
- Zhou, X.F.; Jin, Y.H.; Yoo, C.Y.; Lin, X.L.; Kim, W.Y.; Yun, D.J.; Bressan, R.A.; Hasegawa, P.M.; Jin, J.B. Cyclin h;1 regulates drought stress responses and blue light-induced stomatal opening by inhibiting reactive oxygen species accumulation in Arabidopsis. Plant Physiol. 2013, 162, 1030–1041. [Google Scholar] [CrossRef]
- Zhang, R.X.; Yang, W.J.; Pan, Q.M.; Zeng, Q.; Yan, C.T.; Bai, X.; Liu, Y.; Zhang, L.G.; Li, B.H. Effects of long-term blue light irradiation on carotenoid biosynthesis and antioxidant activities in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Res. Int. 2023, 174, 113661. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. Improvement in drought tolerance of lemon balm, melissa officinalis l. Under the pre-treatment of LED lighting. Plant Physiol. Biochem. 2019, 139, 548–557. [Google Scholar] [CrossRef]
- Ginzburg, D.N.; Klein, J.D. Led pre-exposure shines a new light on drought tolerance complexity in lettuce (Lactuca sativa) and rocket (Eruca sativa). Environ. Exp. Bot. 2020, 180, 104240. [Google Scholar] [CrossRef]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Fiebig, A.; Noga, G.; Hunsche, M. Influence of light quality on leaf physiology of sweet pepper plants grown under drought. Theor. Exp. Plant Phys. 2018, 30, 287–296. [Google Scholar] [CrossRef]
- Terfa, M.T.; Olsen, J.E.; Torre, S. Blue light improves stomatal function and dark-induced closure of rose leaves (Rosa × hybrida) developed at high air humidity. Front. Plant Sci. 2020, 11, 1036. [Google Scholar] [CrossRef]
- Barillot, R.; De Swaef, T.; Combes, D.; Durand, J.L.; Escobar-Gutiérrez, A.J.; Martre, P.; Perrot, C.; Roy, E.; Frak, E. Leaf elongation response to blue light is mediated by stomatal-induced variations in transpiration in Festuca arundinacea. J. Exp. Bot. 2021, 72, 2642–2656. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Kambale, R.D.; Tzeng, J.-H.; Amy, G.L.; Ladner, D.A.; Karthikeyan, R. The growing trend of saltwater intrusion and its impact on coastal agriculture: Challenges and opportunities. Sci. Total Environ. 2025, 966, 178701. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Peng, Y.X.; Zhu, H.Y.; Wang, Y.T.; Kang, J.; Hu, L.X.; Li, L.; Zhu, K.Y.; Yan, J.R.; Bu, X.; Wang, X.J.; et al. Revisiting the role of light signaling in plant responses to salt stress. Hortic. Res. 2025, 12, uhae262. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, W.; Jia, Y.B.; Wang, M.C.; Xia, G.M. The wheat gene is involved in the blue-light response and salt tolerance. Plant J. 2015, 84, 1219–1230. [Google Scholar] [CrossRef]
- Zha, L.Y.; Liu, W.K.; Yang, Q.C.; Zhang, Y.B.; Zhou, C.B.; Shao, M.J. Regulation of ascorbate accumulation and metabolism in lettuce by the red: Blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.H.; Park, H.S.; Min, C.W.; Woo, J.H.; Choi, B.R.; Rahman, M.M.; Lee, K.W. Light quality plays a crucial role in regulating germination, photosynthetic efficiency, plant development, reactive oxygen species production, antioxidant enzyme activity, and nutrient acquisition in alfalfa. Int. J. Mol. Sci. 2025, 26, 360. [Google Scholar] [CrossRef]

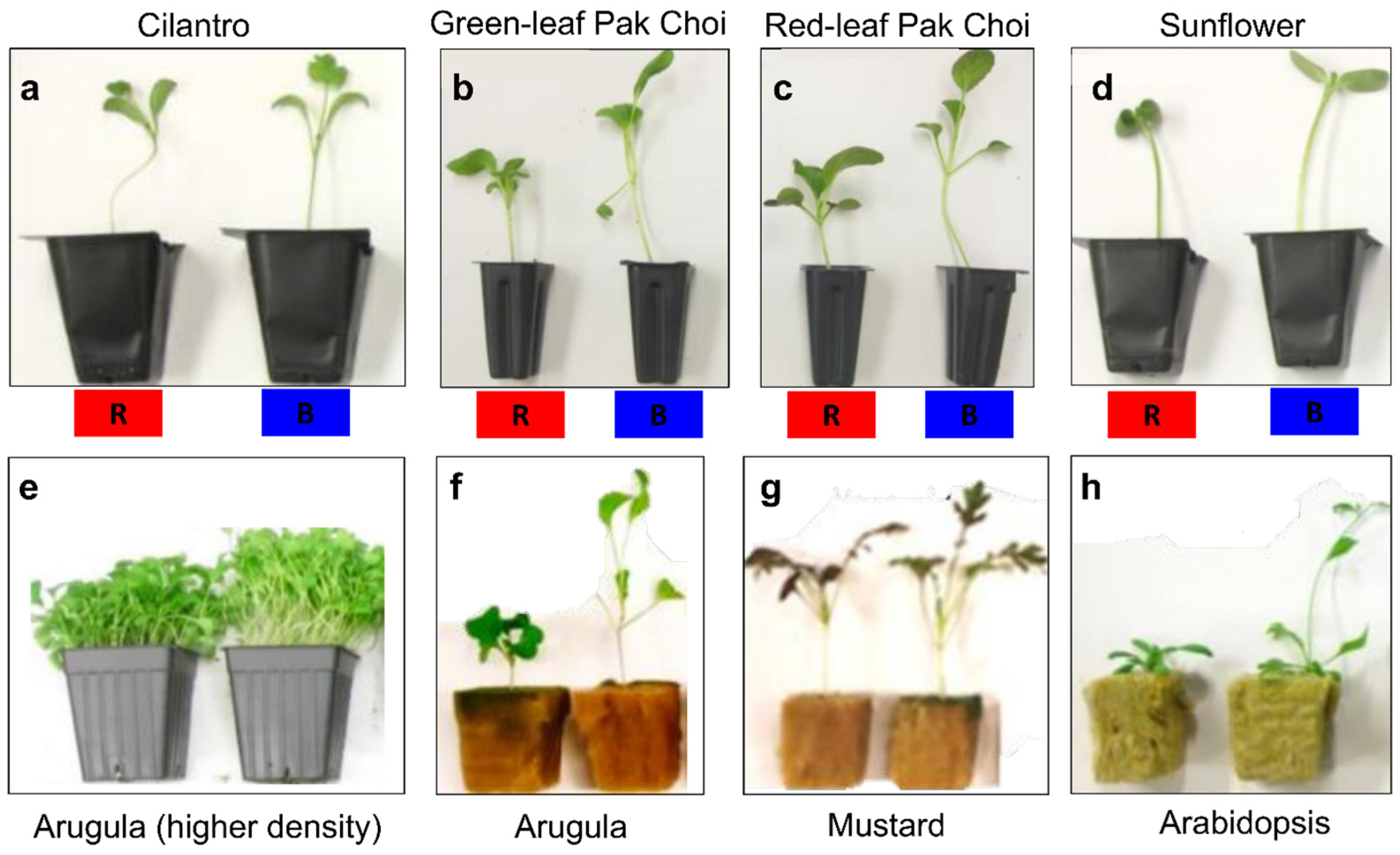
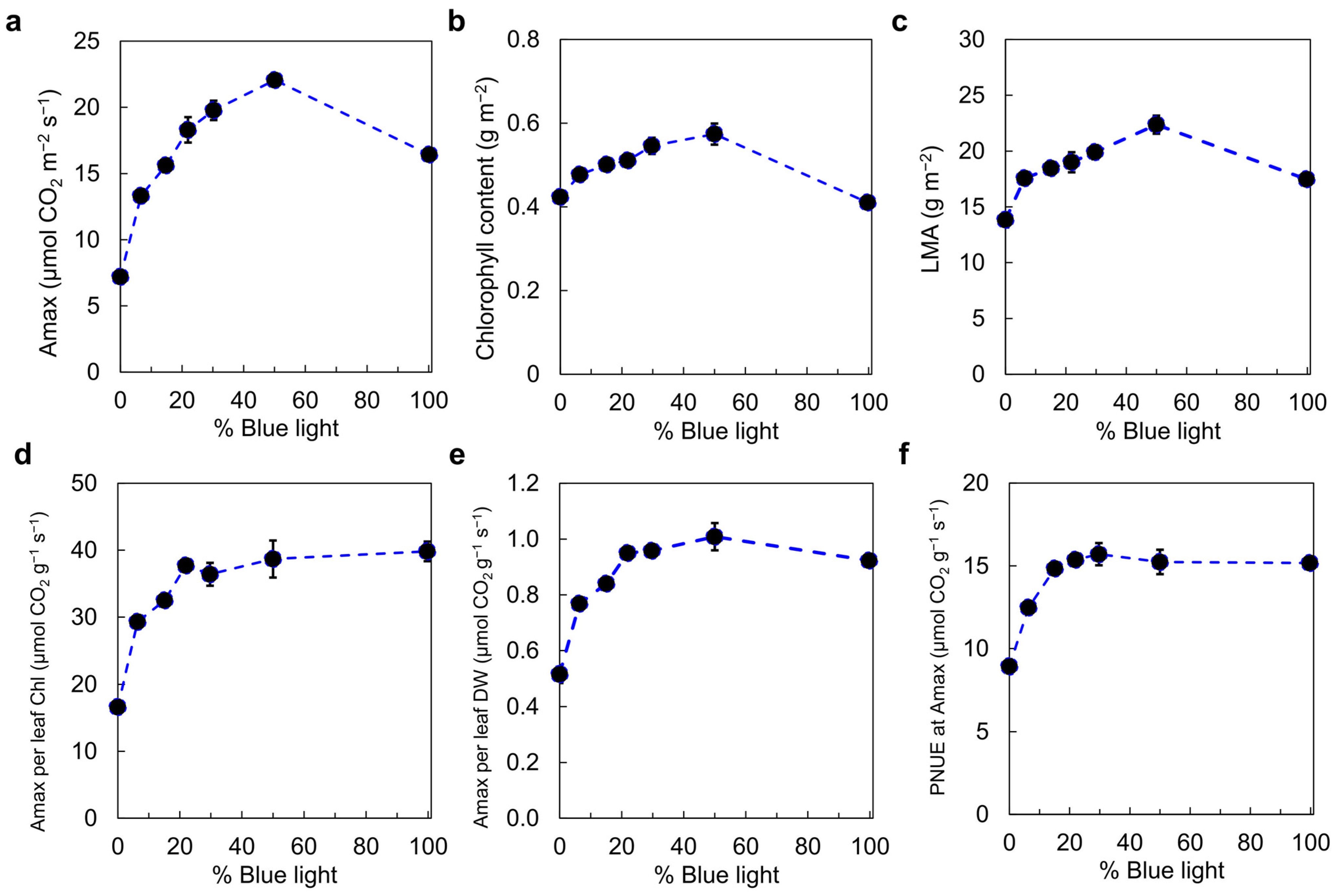
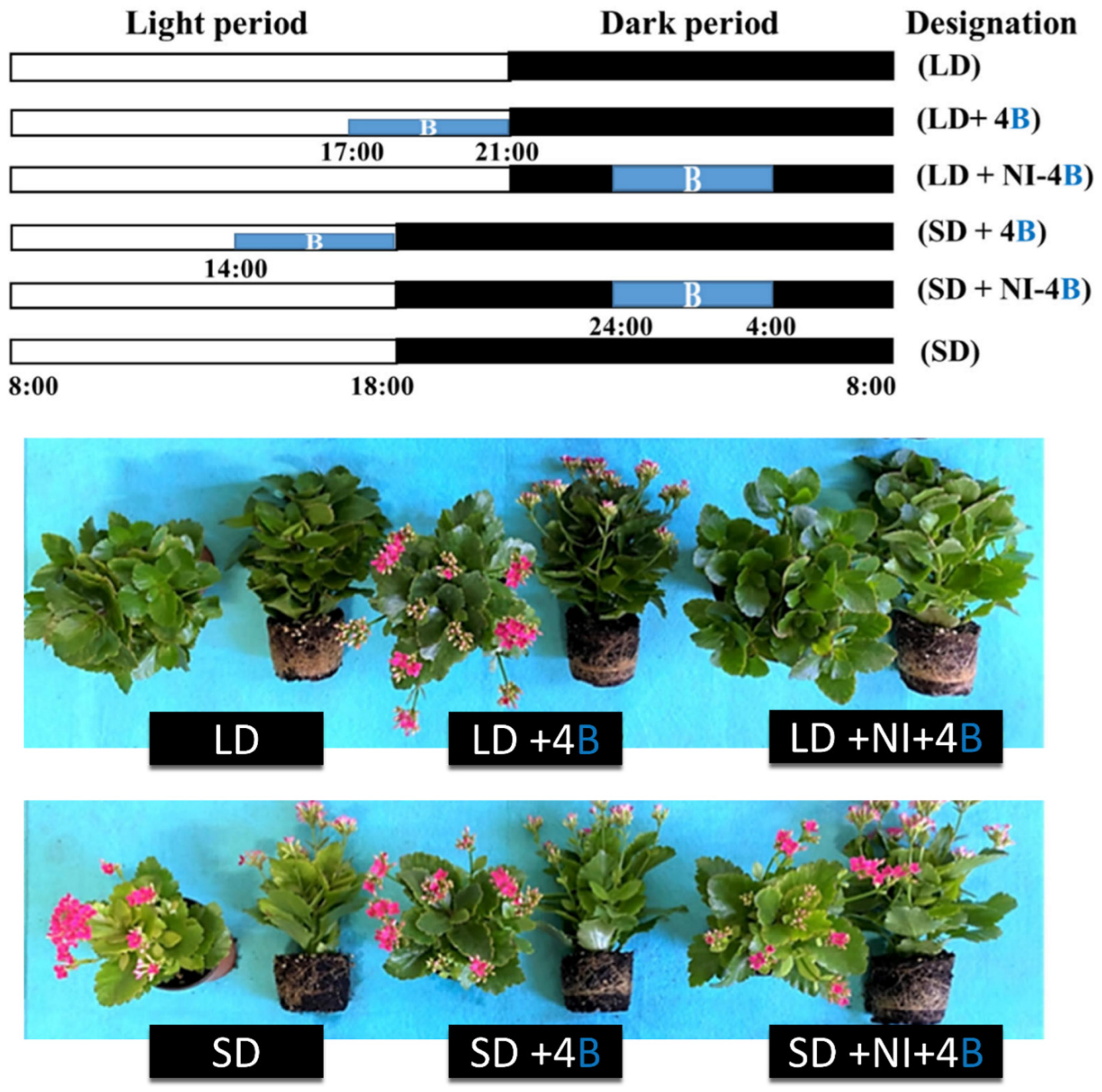
| Species | Photoperiodic Classification | Blue Light Intensity (μmol m−2 s−1) | Observed Responses |
|---|---|---|---|
| Chrysanthemum (Dianthus chinensis) | Qualitative long-day plant | 1–2 | Flowered |
| Rudbeckia (Rudbeckia hirta) | Qualitative long-day plant | 1–2 | Not flowered |
| 15 | Flowered | ||
| Coreopsis (Coreopsis grandiflora) | Qualitative long-day plant | 5 | Flowered |
| Snapdragon (Antirrhinum majus) | Qualitative long-day plant | 5 | Flowered |
| Petunia (Petunia hybrida) | Qualitative long-day plant | 15 | Flowered |
| Chrysanthemum (Dendranthema grandiflorum) | Qualitative short-day plants | 1–2 | Flowered |
| 10 | Flowered | ||
| Kalanchoe (Kalanchoe blossfeldiana ‘Spain’) | Qualitative short-day plants | 10 | Promoted flowering |
| Kalanchoe (Kalanchoe blossfeldiana ‘Lipstick’) | Qualitative short-day plants | 10 | Not flowered |
| Cosmos (Cosmos sulfureus) | Qualitative short-day plants | 1–2 | Flowered |
| Dahlia (Dahlia pinnata) | Qualitative short-day plants | 1–2 | Not flowered |
| Marigold (Tagetes erecta) | Qualitative short-day plants | 1–2 | Flowered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Park, Y.G.; Kambale, R.D.; Adelberg, J.; Karthikeyan, R.; Jeong, B.R. Advancing Light-Mediated Technology in Plant Growth and Development: The Role of Blue Light. Horticulturae 2025, 11, 795. https://doi.org/10.3390/horticulturae11070795
Su Q, Park YG, Kambale RD, Adelberg J, Karthikeyan R, Jeong BR. Advancing Light-Mediated Technology in Plant Growth and Development: The Role of Blue Light. Horticulturae. 2025; 11(7):795. https://doi.org/10.3390/horticulturae11070795
Chicago/Turabian StyleSu, Qiong, Yoo Gyeong Park, Rohit Dilip Kambale, Jeffrey Adelberg, Raghupathy Karthikeyan, and Byoung Ryong Jeong. 2025. "Advancing Light-Mediated Technology in Plant Growth and Development: The Role of Blue Light" Horticulturae 11, no. 7: 795. https://doi.org/10.3390/horticulturae11070795
APA StyleSu, Q., Park, Y. G., Kambale, R. D., Adelberg, J., Karthikeyan, R., & Jeong, B. R. (2025). Advancing Light-Mediated Technology in Plant Growth and Development: The Role of Blue Light. Horticulturae, 11(7), 795. https://doi.org/10.3390/horticulturae11070795







