Integrated Transcriptomic and Functional Analyses Reveal the Role of the Plant–Pathogen Interaction Pathway in Fusarium solani Infection of Zingiber officinale
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Fungal Pathogen
2.2. Ginger Rhizome Treatment
2.3. Determination of Disease Spot Diameter, Decay Rate, Water Loss, Hardness, and F. solani Biomass
2.4. RNA-Seq Analysis
2.5. qRT-PCR Validation
2.6. Transient Agroinfiltration Assays
2.6.1. Transient Transformation and Inoculation Treatment of N. benthamiana Leaves
2.6.2. DAB Histochemical Staining
2.7. Determination of H2O2, O2− Content, Enzyme Activities, NO Content, and NOS Activity
2.8. Statistical Analysis
3. Results
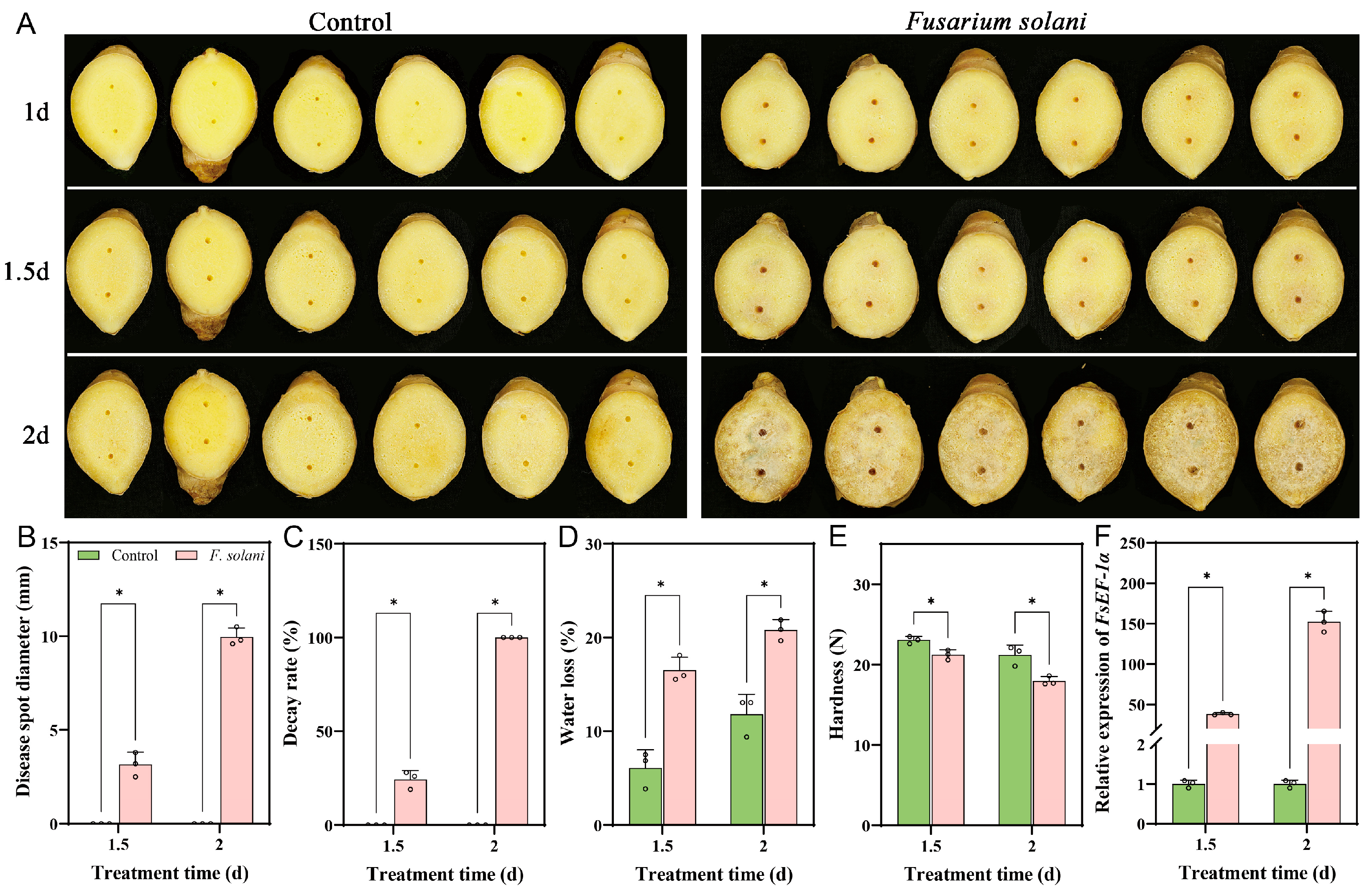
3.1. Symptoms of Ginger Rhizomes Inoculated with F. solani
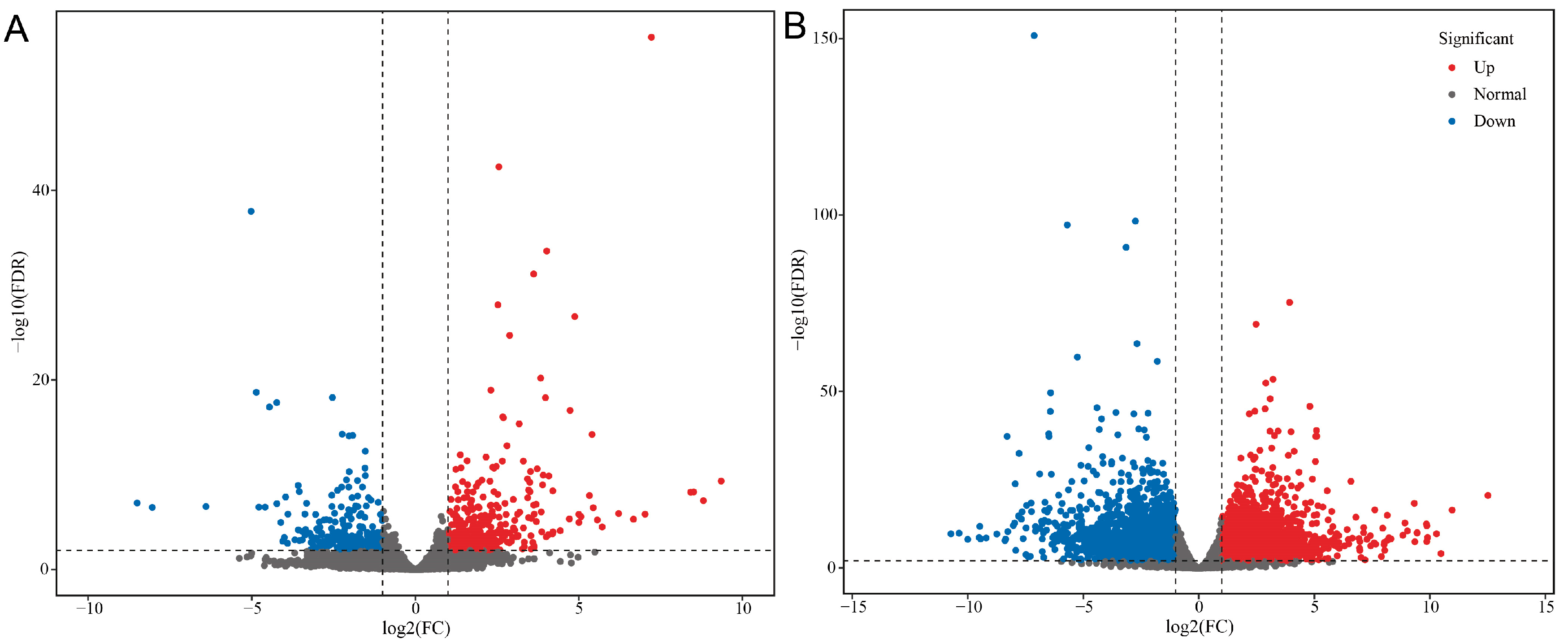
3.2. Sequencing Data Quality Evaluation
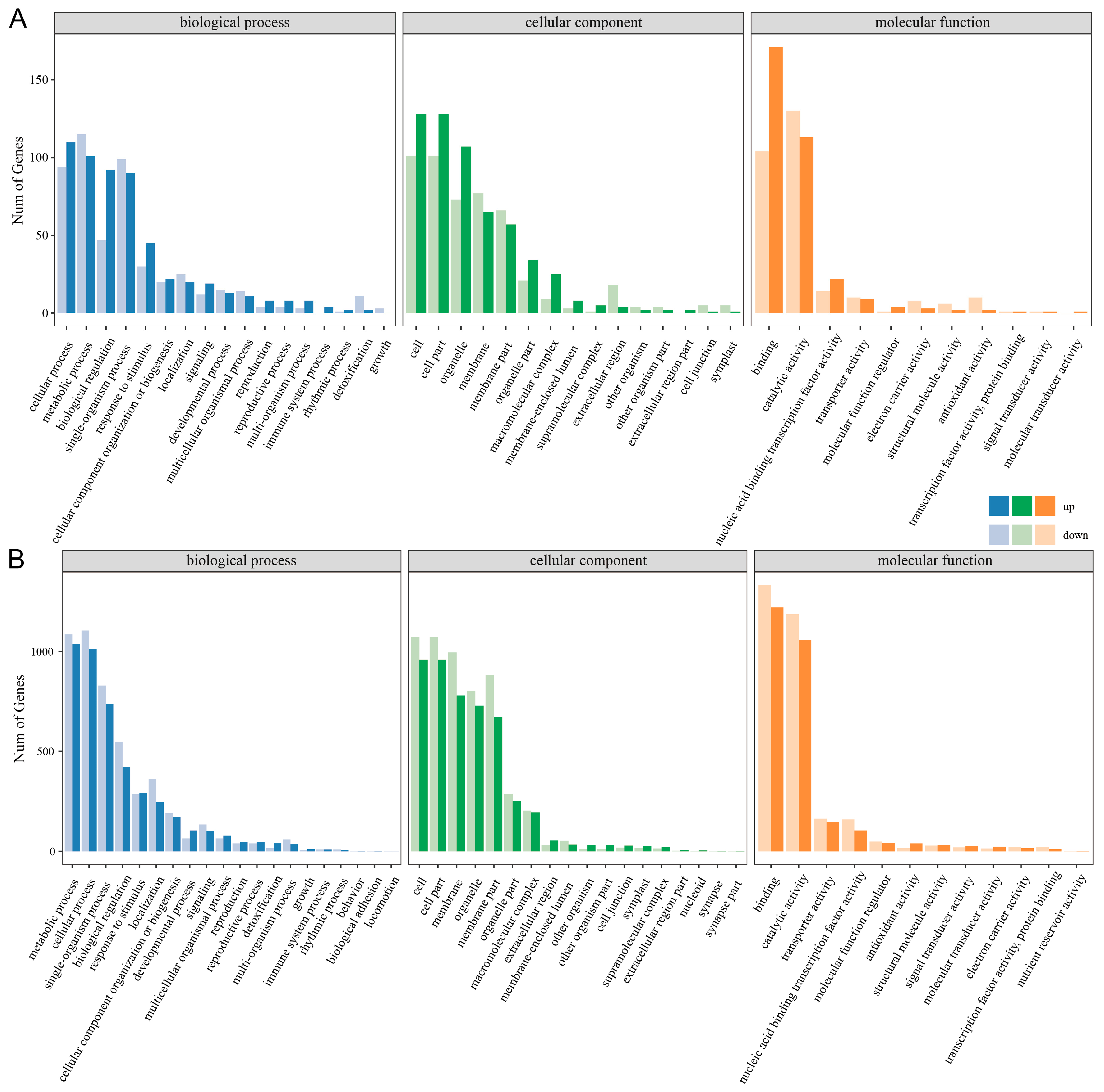
3.3. GO Function Annotation
3.4. KEGG Metabolic Pathway
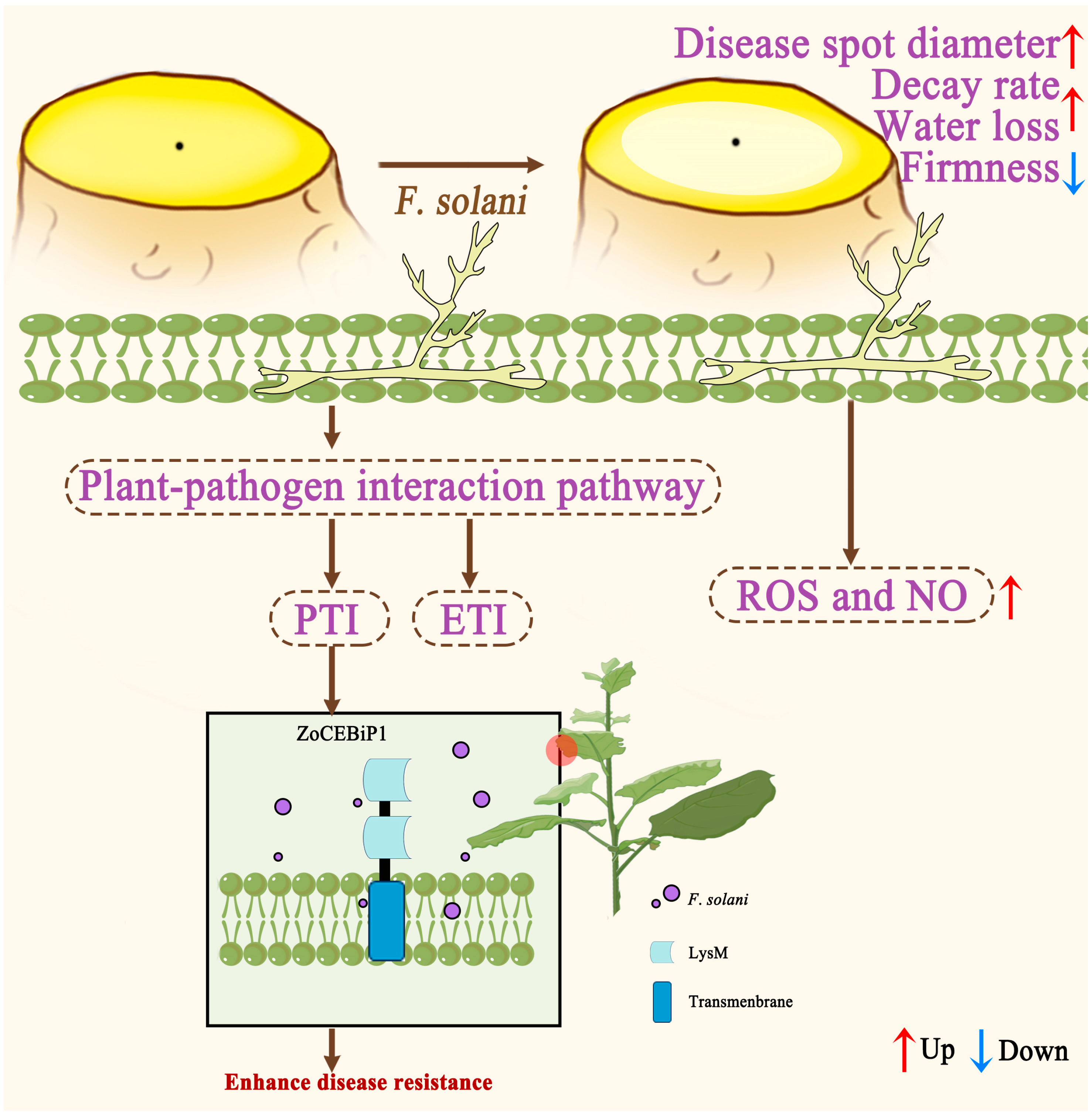
3.5. DEGs Related to Plant–Pathogen Interaction Pathway in Ginger Rhizome Against F. solani
3.6. Functional Analysis of ZoCEBiP1
3.7. Effects of ROS Accumulation, Antioxidant Enzyme Activities, NOS Activity, and NO Content of Ginger Rhizome After Infection with F. solani
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, H.H.; Xiong, S.J.; Zhang, L.L.; Zhu, X.D.; Zhu, Y.X.; Zhou, L.R. Evaluation of the inhibitory efficacy of eugenol against the pathogen of Fusarium wilt in ginger seedlings. Horticulturae 2023, 9, 1024. [Google Scholar] [CrossRef]
- Choi, J.H.; Nah, J.Y.; Lee, M.J.; Yim, S.B.; Jang, J.Y.; Lee, T.; Kim, J. Fungal diversity in ginger and effect of storage conditions on occurrence of Fusarium and its mycotoxins. Food Control 2024, 165, 110631. [Google Scholar] [CrossRef]
- Meng, X.L.; Wu, W.J.; Niu, B.; Liu, R.L.; Chen, H.Z.; Gao, H.Y.; Chen, H.J. Inhibitory effect and action mechanism of perillaldehyde on the Fusarium graminearum in postharvest fresh ginger. Postharvest Biol. Technol. 2024, 209, 112674. [Google Scholar] [CrossRef]
- Zhang, L.L.; Fang, S.Y.; Sun, C.; Liang, H.R.; Ma, J.W.; Jia, Q.; Yin, J.L.; Zhu, Y.X.; Liu, Y.Q. Chitosan boosts ginger disease resistance: Insights from transcriptomic and metabolomic analyses. LWT 2024, 205, 116478. [Google Scholar] [CrossRef]
- Ferreira da Cunha Neto, J.; da Silva Rocha, W.P.; Makris, G.; Sandoval-Denis, M.; Hagen, F.; Crous, P.W.; Chaves, G.M. Fusarioid keratitis and other superficial infections: A 10-years prospective study from Northeastern Brazil. PLOS Neglected Trop. Dis. 2024, 18, e0012247. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wu, X.Q.; Zhong, Y.; Yang, Y.; Wei, S.W.; Sun, C.; Wei, L.J.; Liu, Y.Q. Hydrogen sulfide enhances the disease resistance of ginger to rhizome rot during postharvest storage through modulation of antioxidant response and nitric oxide-mediated S-nitrosylaion. Postharvest Biol. Technol. 2025, 220, 113321. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host-multi-pathogen warfare: Pathogen interactions in co-infected plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef]
- Zhao, L.N.; Zhou, Y.L.; Quan, S.H.; Qiu, J.E.; Dhanasekaran, S.; Li, B.; Gu, X.Y.; Zhang, H.Y. Transcriptome analysis reveals mechanisms of the disease resistance in postharvest kiwifruit induced by Meyerozyma caribbica. Sci. Hortic. 2023, 322, 112452. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Li, R.; Tang, Y.; Cheng, Z.Y.; Qian, M.J.; Li, W.; Shao, Y.Z. Transcriptome analysis reveals the inducing effect of Bacillus siamensis on disease resistance in postharvest mango fruit. Foods 2022, 11, 107. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wang, X.Y.; Liu, X.Q.; Zhu, X.L.; Zhang, J. Integration of metabolome and transcriptome profiling reveals the effect of modified atmosphere packaging (MAP) on the browning of fresh-cut Lanzhou lily (Lilium davidii var. unicolor) bulbs during storage. Foods 2023, 12, 1335. [Google Scholar] [CrossRef]
- Yang, X.M.; Yang, K.X.; Wang, X.H.; Wang, Y.T.; Zhao, Z.Y.; Meng, D.M. Transcriptomic analysis reveals the mechanism of bacterial disease resistance of postharvest button mushroom (Agaricus bisporus). Physiol. Mol. Plant Pathol. 2022, 122, 101903. [Google Scholar] [CrossRef]
- Zhang, L.L.; Yang, Y.; Zhu, Y.X.; Hu, H.J.; Jia, Q.; Sun, C.; Zhu, X.D.; Liu, Y. Antifungal activity and mechanism of chitosan against Fusarium solani caused ginger soft rot during postharvest storage. Postharvest Biol. Technol. 2024, 208, 112680. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, L.; Dong, Z.M.; Jiang, Y.S.; Jiang, S.J.; Xing, H.T.; Li, Q.; Liu, G.C.; Tian, S.M.; Wu, Z.Y.; et al. Haplotype-resolved genome of diploid ginger (Zingiber officinale) and its unique gingerol biosynthetic pathway. Hortic. Res. 2021, 8, 189. [Google Scholar] [CrossRef]
- Li, G.; Ma, J.W.; Yin, J.L.; Guo, F.L.; Xi, K.Y.; Yang, P.H.; Cai, X.D.; Jia, Q.; Li, L.; Liu, Y.Q.; et al. Identification of reference genes for reverse transcription-quantitative PCR analysis of ginger under abiotic stress and for postharvest biology studies. Front. Plant Sci. 2022, 13, 893495. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Liu, X.; Xiao, Y.; Wen, Y.; Li, K.K.; Ma, Z.L.; Yin, J.L.; Yang, L.J.; Zhu, Y.X. Genome-wide characterization and function analysis uncovered roles of wheat LIMs in responding to adverse stresses and TaLIM8-4D function as a susceptible gene. Plant Genome 2022, 15, e20246. [Google Scholar] [CrossRef] [PubMed]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- Sun, Y.I.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Kırgeç, Y.; Batı-Ay, E.; Açıkgöz, M.A. The effects of foliar salicylic acid and zinc treatments on proline, carotenoid, and chlorophyll content and anti-oxidant enzyme activity in Galanthus elwesii Hook. Horticulturae 2023, 9, 1041. [Google Scholar] [CrossRef]
- Wei, L.J.; Zhang, J.; Wei, S.H.; Hu, D.L.; Liu, Y.Y.; Feng, L.; Li, C.X.; Wang, C.L.; Liao, W.B. Nitric oxide enhanced salt stress tolerance in tomato seedlings, involving phytohormone equilibrium and photosynthesis. Int. J. Mol. Sci. 2022, 23, 4539. [Google Scholar] [CrossRef]
- Lei, S.; Rossi, S.; Huang, B. Metabolic and physiological regulation of aspartic acid-mediated enhancement of heat stress tolerance in Perennial Ryegrass. Plants 2022, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Li, Y.J.; Wang, X.Y.; Yao, L.; Sheng, J.P.; Shen, L. Exogenous ferulic acid treatment increases resistance against Botrytis cinerea in tomato fruit by regulating nitric oxide signaling pathway. Postharvest Bio. Technol. 2021, 182, 111678. [Google Scholar] [CrossRef]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2020, 168, 318–344. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.L.; Sun, C.; Li, G.; Yang, P.M.; Jia, Q.; Cai, X.D.; Zhu, Y.X.; Yin, J.L.; Liu, Y.Q. Silica nanoparticles enhance the disease resistance of ginger to rhizome rot during postharvest storage. Nanomaterials 2022, 12, 1418. [Google Scholar] [CrossRef]
- Xu, M.Q.; Zhang, X.Y.; Dhanasekaran, S.; Godana, E.A.; Yang, Q.Y.; Zhao, L.N.; Zhang, H.Y. Transcriptome analysis of postharvest pear (Pyrus pyrifolia Nakai) in response to Penicillium expansum infection. Sci. Hortic. 2021, 288, 110361. [Google Scholar] [CrossRef]
- Ortiz-Morea, F.A.; Liu, J.; Shan, L.B.; He, P. Malectin-like receptor kinases as protector deities in plant immunity. Nat. Plants 2022, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C. Early molecular events in PAMP-triggered immunity. Curr. Opin. Plant Biol. 2009, 12, 414–420. [Google Scholar] [CrossRef]
- Yuan, M.H.; Jiang, Z.Y.; Bi, G.Z.; Nomura, K.; Liu, M.H.; Wang, Y.P.; Cai, B.Y.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Xiong, J.S.; Zhu, H.Y.; Bai, Y.B.; Liu, H.; Cheng, Z.M. RNA sequencing-based transcriptome analysis of mature strawberry fruit infected by necrotrophic fungal pathogen Botrytis cinerea. Physiol. Mol. Plant Pathol. 2018, 104, 77–85. [Google Scholar] [CrossRef]
- Akamatsu, A.; Wong, H.L.; Fujiwara, M.; Okuda, J.; Nishide, K.; Uno, K.; Imai, K.; Umemura, K.; Kawasaki, T.; Kawano, Y.; et al. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe 2013, 13, 465–476. [Google Scholar] [CrossRef]
- Kaku, H.; Nishizawa, Y.; Ishii-Minami, N.; Akimoto-Tomiyama, C.; Dohmae, N.; Takio, K.; Minami, E.; Shibuya, N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 11086–11091. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Kurjogi, M.M.; Khalil-Ur-Rehman, M.; Fiaz, M.; Pervaiz, T.; Jiu, S.T.; Jia, H.F.; Chen, W.; Fang, J.G. Grapevine immune signaling network in response to drought stress as revealed by transcriptomic analysis. Plant Physiol. Biochem. 2017, 121, 187–195. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Moeder, W.; Yoshioka, K. Opening the gates: Insights into cyclic nucleotide-gated channel-mediated signaling. Trends Plant Sci. 2016, 21, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.Y.; Li, J.; Zhang, Q.C.; Jia, S.X.; Zhang, Q.Q.; Wang, R.T.; Ju, M.X.; Gu, P.W. Transcriptional response of wolfberry to infestation with the endophytic Fusarium nematophilum strain NQ8GII4. Plant Dis. 2024, 108, 1514–1525. [Google Scholar] [CrossRef]
- Gao, Q.M.; Venugopal, S.; Navarre, D.; Kachroo, A. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol. 2011, 155, 464–476. [Google Scholar] [CrossRef]
- Liu, X.F.; Song, Y.Z.; Xing, F.Y.; Wang, N.; Wen, F.J.; Zhu, C.X. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma 2016, 253, 1265–1281. [Google Scholar] [CrossRef]
- Peng, H.M.; Hu, H.J.; Xi, K.Y.; Zhu, X.M.; Zhou, J.; Yin, J.L.; Guo, F.L.; Liu, Y.Q.; Zhu, Y.X. Silicon nanoparticles enhance ginger rhizomes tolerance to postharvest deterioration and resistance to Fusarium solani. Front. Plant Sci. 2022, 13, 816143. [Google Scholar] [CrossRef]
- Ding, L.N.; Li, Y.T.; Wu, Y.Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.L. Plant disease resistance-related signaling pathways: Recent progress and future prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Pan, L.Y.; Zhao, X.Y.; Chen, M.; Fu, Y.Q.; Xiang, M.L.; Chen, J.Y. Effect of exogenous methyl jasmonate treatment on disease resistance of postharvest kiwifruit. Food Chem. 2020, 305, 125483. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Cai, Z.P.; Shen, Y.G.; Gan, Z.Y.; Duan, B.; Yuan, J.; Huang, T.H.; Zhang, W.; Du, H.Y.; et al. Transcriptome profiling to elucidate mechanisms of the enhancement of the resistance to Botryosphaeria dothidea by nitric oxide in postharvest kiwifruit during storage. LWT 2022, 159, 113187. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, W.L.; Hu, M.J.; Pan, Y.G.; Jiang, Y.M.; Zhang, Z.K.; Jiang, G.X. Nitric oxide is involved in melatonin-induced cold tolerance in postharvest litchi fruit. Postharvest Bio. Technol. 2023, 196, 112157. [Google Scholar] [CrossRef]
- Capone, R.; Tiwari, B.S.; Levine, A. Rapid transmission of oxidative and nitrosative stress signals from roots to shoots in Arabidopsis. Plant. Physiol. Biochem. 2004, 42, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Weralupitiya, C.; Eccersall, S.; Meisrimler, C.N. Shared signals, different fates: Calcium and ROS in plant PRR and NLR immunity. Cell Rep. 2024, 43, 114910. [Google Scholar] [CrossRef]
- He, Q.; McLellan, H.; Hughes, R.K.; Boevink, P.C.; Armstrong, M.; Lu, Y.; Banfield, M.J.; Tian, Z.; Birch, P.R.J. Phytophthora infestans effector SFI3 targets potato UBK to suppress early immune transcriptional responses. New Phytol. 2019, 222, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Ma, X.Y.; Zhang, C.; Kim, S.I.; Li, B.; Xie, Y.P.; Yeo, I.C.; Thapa, H.; Chen, S.; Devarenne, T.P.; et al. Dual phosphorylation of DGK5-mediated PA burst regulates ROS in plant immunity. Cell 2024, 187, 609–623. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Zhao, M.; Li, T.T.; Wang, L.Z.; Liao, C.L.; Liu, D.X.; Zhang, H.M.; Zhao, Y.P.; Liu, L.S.; Ge, X.Y.; et al. Interactions between Verticillium dahliae and cotton: Pathogenic mechanism and cotton resistance mechanism to Verticillium wilt. Front Plant Sci. 2023, 14, 1174281. [Google Scholar] [CrossRef]
- Chakraborty, N.; Basak, J. Comparative transcriptome profiling of a resistant vs. susceptible Vigna mungo cultivar in response to Mungbean yellow mosaic India virus infection reveals new insight into MYMIV resistance. Curr. Plant Biol. 2018, 15, 8–24. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Jia, Q.; Liu, L.; Liu, Y. Integrated Transcriptomic and Functional Analyses Reveal the Role of the Plant–Pathogen Interaction Pathway in Fusarium solani Infection of Zingiber officinale. Horticulturae 2025, 11, 791. https://doi.org/10.3390/horticulturae11070791
Zhang L, Jia Q, Liu L, Liu Y. Integrated Transcriptomic and Functional Analyses Reveal the Role of the Plant–Pathogen Interaction Pathway in Fusarium solani Infection of Zingiber officinale. Horticulturae. 2025; 11(7):791. https://doi.org/10.3390/horticulturae11070791
Chicago/Turabian StyleZhang, Lingling, Qie Jia, Lei Liu, and Yiqing Liu. 2025. "Integrated Transcriptomic and Functional Analyses Reveal the Role of the Plant–Pathogen Interaction Pathway in Fusarium solani Infection of Zingiber officinale" Horticulturae 11, no. 7: 791. https://doi.org/10.3390/horticulturae11070791
APA StyleZhang, L., Jia, Q., Liu, L., & Liu, Y. (2025). Integrated Transcriptomic and Functional Analyses Reveal the Role of the Plant–Pathogen Interaction Pathway in Fusarium solani Infection of Zingiber officinale. Horticulturae, 11(7), 791. https://doi.org/10.3390/horticulturae11070791





