Salinity Stress and Calcium in Pomegranate: Impacts on Growth, Ion Homeostasis, and Photosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Measurements
2.2. Chlorophyll Content and Photosynthesis Parameters
2.3. Electrolyte Leakage of Cell Membranes
2.4. Inorganic Elements Analysis
2.5. Statistical Data Analysis
3. Results
3.1. Plant Growth Parameters
3.2. Permeability of Leaf Cell Membranes
3.3. Leaf Chlorophyll
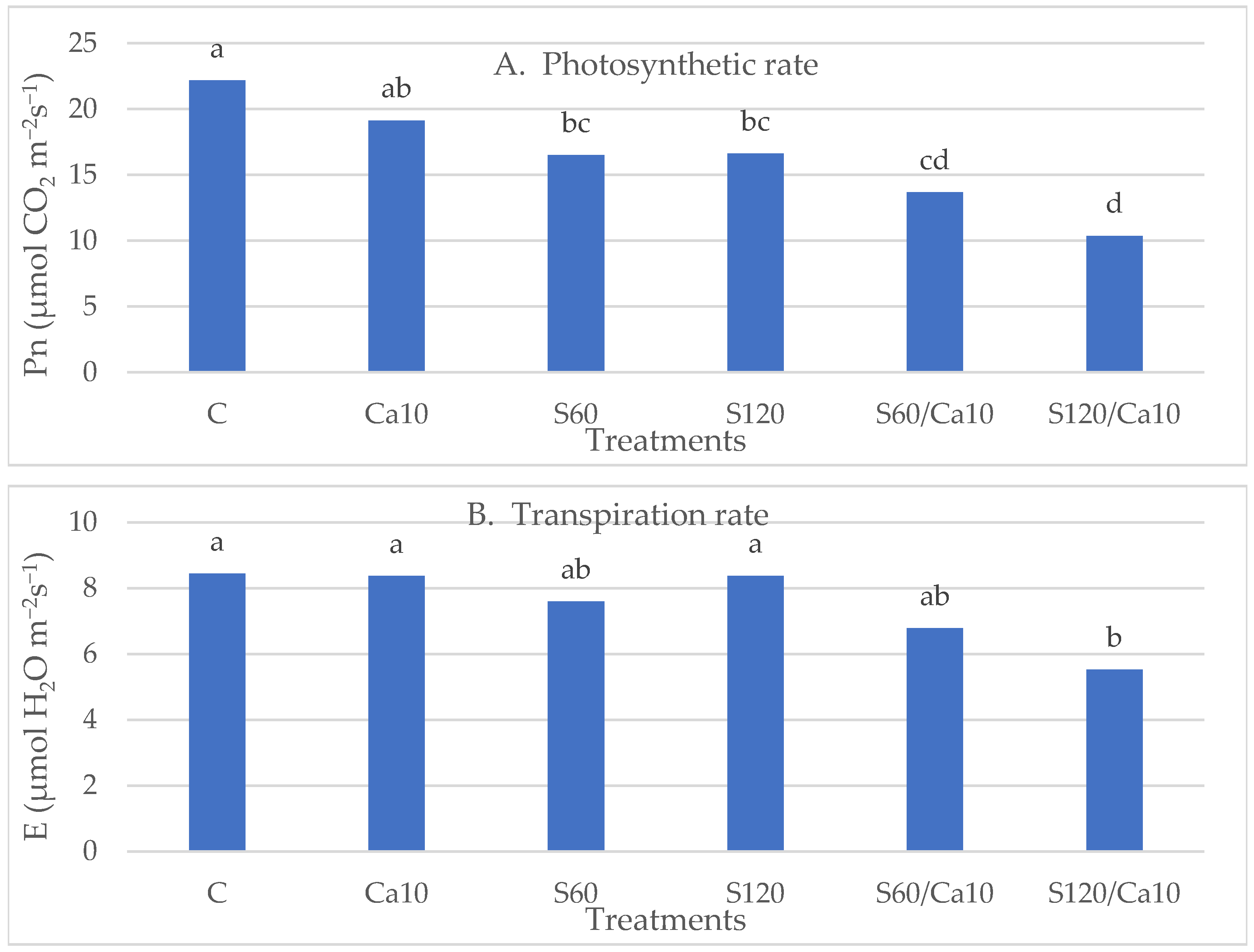
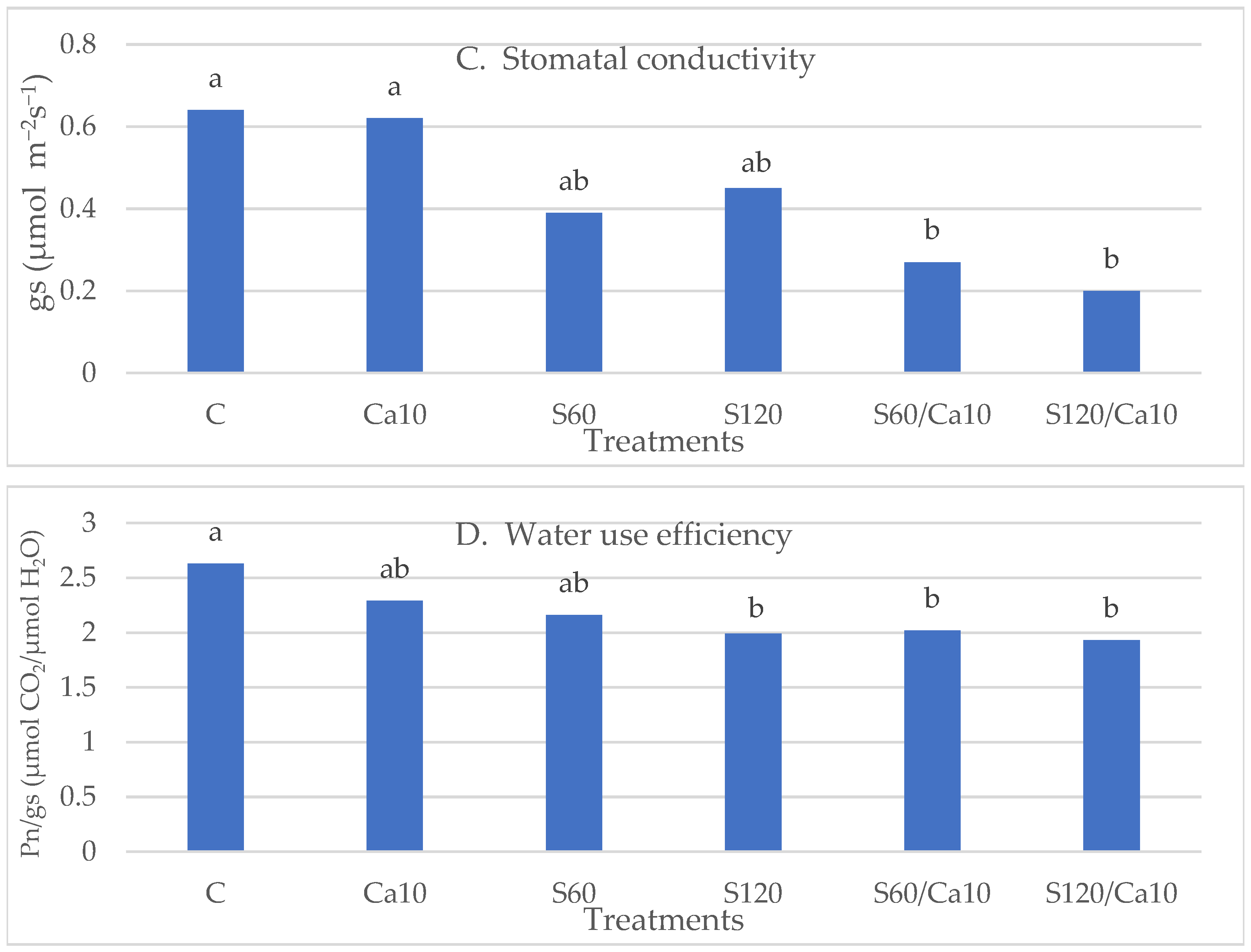
3.4. Photosynthesis
3.5. Chemical Composition of Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Therios, I. Inorganic Nutrition and Fertilizers; Gartaganis Publications: Thessaloniki, Greece, 2021; 873p. (In Greek) [Google Scholar]
- Keren, R. Salt-affected soil reclamation. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 454–461. ISBN 9780123485304. [Google Scholar] [CrossRef]
- Pearson, K.E.; Warrence, N.J.; Bauder, J.W. Basics of Salinity and Sodicity Effects on Soil Physical Properties; Montana State University: Bozeman, MT, USA, 2003; Available online: https://waterquality.montana.edu/energy/cbm/background/soil-prop.html#Growth (accessed on 25 June 2025).
- Flowers, T. Plants and salinity-Preface. J. Exp. Bot. 2006, 57, 3395–3403. [Google Scholar]
- Munns, R.; Husain, S.; Rivelli, A.R.; Ritchard, A.; James, R.A.; Condon, A.G.T.; Lindsay, M.P.; Lagudah, E.S.; Daniel, P.; Schachtman, D.P.; et al. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil 2002, 247, 93–105. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Breeding for salinity resistance in crop plants, where next? Aust. J. Plant Physiol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; 672p. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, N.; Martinez, V.; Carvajal, M. Effect of salinity on growth, mineral composition, and water relations of grafted tomato plants. J. Plant Nutr. Soil Sci. 2004, 167, 616–622. [Google Scholar] [CrossRef]
- Brugnoli, E.; Bjorkman, O. Growth of cotton under continuous salinity stress: Influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 1992, 187, 335–347. [Google Scholar] [CrossRef]
- Pearson, R.W. Soil environment and root development. In Plant Environment and Efficient Water Use; Peirre, W.H., Kirkham, D., Pesek, J., Shaw, R., Eds.; ASA and SSSA: Madison, WI, USA, 1996; pp. 95–126. [Google Scholar]
- Del Amor, F.M.; Martinez, V.; Cerda, A. Salt tolerance of tomato plants as affected by stage of plant development. Hortic. Sci. 2001, 36, 1260–1263. [Google Scholar] [CrossRef]
- Jenks, M.A.; Hasegawa, P.M.; Jain, S.M. Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Debouda, M.; Gouia, H.; Ghorbel, M.H. NaCl effects growth, ions and water status of tomato (Lycopersicon esculentum) seedlings. Acta Bot. Gall. 2006, 153, 297–307. [Google Scholar] [CrossRef]
- Patil, V.K.; Waghmare, P.R. Salinity tolerance of pomegranate. J. Maharashtra Agric. Univ. 1982, 7, 268–269. [Google Scholar]
- Jain, B.L.; Daas, H.C. Effect of saline water on pomegranate of sapling of jujube (Ziziphus mauritiana), Indian cherry (Cordia dichotoma var. Wallichi) and pomegranate (Punica granatum) at nursery stage. Indian J. Agric. Sci. 1988, 58, 420–421. [Google Scholar]
- Mastrogiannidou, E.; Chatzissavvidis, C.; Antonopoulou, C.; Tsabardoukas, V.; Giannakoula, A.; Therios, I. Response of pomegranate cv. Wonderful plants to salinity. J. Soil Sci. Plant Nutr. 2016, 16, 621–636. [Google Scholar]
- Döring, J.; Lüdders, P. Effect of different salt treatment on Punica granatum at different root temperatures. Gartenbauwissenschaft 1986, 52, 92–96. [Google Scholar]
- Doring, J.; Lüdders, P. Influence of sodium salts on Na, Cl and SO4 content in leaves, shoots and roots of Punica granatum. Gartenbauwissenschaft 1987, 52, 26–31. [Google Scholar]
- Rengel, Z. The role of calcium in salt toxicity. Plant Cell Environ. 1992, 15, 625–632. [Google Scholar] [CrossRef]
- Faust, M.; Shear, C.E. The effect of calcium on respiration of apples. J. Am. Soc. Hortic. Sci. 1972, 97, 437–439. [Google Scholar] [CrossRef]
- Gerald, C.J. Influence of osmotic potential, temperature and calcium on growth of plants roots. Agron. J. 1971, 63, 555–558. [Google Scholar]
- Ehret, D.L.; Remann, R.E.; Harvey, B.L.; Cipywnyk, A. Salinity-induced calcium deficiencies in wheat and barley. Plant Soil 1990, 128, 143–151. [Google Scholar] [CrossRef]
- Navarro, J.M.; Martinez, V.; Carvajal, M. Ammonium, bicarbonate and calcium effects on tomato plants grown under saline conditions. Plant Sci. 2000, 157, 89–96. [Google Scholar] [CrossRef]
- Cramer, G.R.; Lauchli, A.; Epstein, E. Effect of NaCl and CaCl2 on ion activities in complex nutrient solutions and root growth of cotton. Plant Physiol. 1986, 81, 792–797. [Google Scholar] [CrossRef]
- Bush, D.S. Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. 1995, 46, 95–122. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Melgarejo-Sánchez, P.; Martínez, J.J.; Hernández, F.; Legua, P.; Martínez, R.; Melgarejo, P. The pomegranate tree in the world: New cultivars and uses. Acta Hortic. 2015, 1089, 327–332. [Google Scholar] [CrossRef]
- Sarafi, E.; Chatzissavvidis, C.; Therios, I. Response of two pomegranate (Punica granatum L.) cultivars to six boron concentrations: Growth performance, nutrient status, gas exchange parameters, chlorophyll fluorescence, and proline and carbohydrate content. J. Plant Nutr. 2017, 40, 983–994. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2018, 235, 214–223. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, G.; Masabni, J.G.; Ganjegunte, G. Relative salt tolerance of 22 pomegranate (Punica granatum) cultivars. HortScience 2018, 53, 1513–1519. [Google Scholar] [CrossRef]
- Wintermans, J.F.; de Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Kjeldahl Method for Nitrogen (N) Determination; Micro-Macro Publishing: Athens, Georgia, 1991. [Google Scholar]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. In Agriculture Handbook; Richards, L.A., Ed.; U.S. Department of Agriculture: Washington, DC, USA, 1954; pp. 157–160. [Google Scholar]
- Dichala, O.; Therios, I.; Papadopoulos, A.; Chatzistathis, T.; Chatzisavvidis, C.; Antonopoulou, C. Effects of varying concentrations of different salts on mineral composition of leaves and roots of three pomegranate (Punica granatum L.) cultivars. Sci. Hortic. 2021, 275, 109718. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Tattini, M.; Panzio, C.; Coradeschi, M.A.; Tafani, R.; Traversi, M.L. Mechanisms of salt tolerance in olive plants. Acta Hortic. 1994, 356, 181–184. [Google Scholar] [CrossRef]
- Shiyab, S.M.; Shibli, R.A.; Mohammad, M.M. Influence of sodium chloride salt stress on growth and nutrient acquisition of sour orange in vitro. J. Plant Nutr. 2003, 26, 985–996. [Google Scholar] [CrossRef]
- Shibli, R.; Shatnawi, M.A.; Swaidat, I.Q. Growth, osmotic adjustment, and nutrient acquisition of bitter almond under induced sodium chloride salinity in vitro. Commun. Soil Sci. Plant Anal. 2003, 34, 1969–1979. [Google Scholar] [CrossRef]
- Amri, E.; Mirzaei, M.; Moradi, M.; Zare, K. The effects of spermidine and putrescine polyamines on growth of pomegranate (Punica granatum L. cv ‘Rabbab’) in salinity circumstance. Int. J. Plant Physiol. Biochem. 2011, 3, 43–49. [Google Scholar]
- Liu, C.; Yan, M.; Huang, X.; Yuan, Z. Effects of NaCl stress on growth and ion homeostasis in pomegranate tissues. Eur. J. Hortic. Sci. 2020, 85, 42–50. [Google Scholar]
- El-Agamy, S.Z.; Mostafa, R.A.; Shaaban, M.M.; El-Mahdy, M.T. In vitro salt and drought tolerance of Manfalouty and Nab El-Gamal pomegranate cultivars. Aust. J. Basic Appl. Sci. 2010, 4, 1076–1082. [Google Scholar]
- Naeini, M.R.; Khoshgoftarmanesh, A.H.; Fallahi, E. Partitioning of chlorine, sodium, and potassium and shoot growth of three pomegranate cultivars under different levels of salinity. J. Plant Nutr. 2006, 29, 1835–1843. [Google Scholar] [CrossRef]
- Zhong, H.; Lauchli, A. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J. Exp. Bot. 1993, 44, 773–778. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous application of calcium ameliorates salinity stress tolerance of tomato (Solanum lycopersicum L.) and enhances fruit quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef]
- Zidan, I.; Azaizeh, H.; Neumann, P.M. Does salinity reduce growth in maize root epidermal cells by inhibiting their capacity for cell wall acidification? Plant Physiol. 1990, 93, 7–11. [Google Scholar] [CrossRef]
- Yeo, A.R.; Lee, K.S.; Izard, P.; Boursier, P.J.; Flowers, T.J. Short-and long-term effects of salinity on leaf growth in rice (Oryza sativa). J. Exp. Bot. 1991, 42, 881–889. [Google Scholar] [CrossRef]
- Rashedy, A.A.; Abd-El Nafea, M.H.; Khedr, E.H. Co-application of proline or calcium and humic acid enhances productivity of salt stressed pomegranate by improving nutritional status and osmoregulation mechanisms. Sci. Rep. 2022, 12, 14285. [Google Scholar] [CrossRef]
- Sharma, D.; Jamra, G.; Singh, U.M.; Sood, S.; Kumar, A. Calcium biofortification: Three pronged molecular approaches for dissecting complex traits of calcium nutrition in finger millet (Eleusine coracana) for devising strategies of enrichment of food crops. Front. Plant Sci. 2017, 7, 2028. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, M.; Tehranifar, A.; Davarynejad, G.H.; Sayyari-Zahan, M.H. Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-Saveh’ and ‘Shishe-Kab’ pomegranates in response to salinity stress. Photosynthetica 2014, 52, 301–312. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.; Jifon, J.L.; Carvajal, M.; Syvertsen, J.P. Gas exchange, chlorophyll and nutrient contents in relation to Na+ and Cl- accumulation in “Sunburst” mandarin grafted on different rootstock. Plant Sci. 2002, 162, 705–712. [Google Scholar] [CrossRef]
- Khan, A.M.; Ungar, I.A.; Showalter, A.M. Effects of sodium chloride treatments on growth and ion accumulation of the halophyte Haloxylon recurvum. Commun. Soil Sci. Plant Anal. 2000, 31, 2763–2774. [Google Scholar] [CrossRef]
- Montesano, F.; van Iersel, M.W. Calcium can prevent toxic effects of Na+ on tomato leaf photosynthesis but does not restore growth. J. Am. Soc. Hortic. Sci. 2007, 132, 310–318. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhang, Y.; Liu, J.; Gul, Z.; Guo, X.-R.; Abozeid, A.; Tang, Z.-H. Effects of exogenous calcium on adaptive growth, photosynthesis, ion homeostasis and phenolics of Gleditsia sinensis Lam. plants under salt stress. Agriculture 2021, 11, 978. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High temperature stress tolerance in wheat genotypes: Role of antioxidant defense enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity–mineral nutrient relations in horticultural crops. Sci. Hortic. 1999, 78, 127–157. [Google Scholar] [CrossRef]
- Dubey, R.S. Photosynthesis in plants under stressful conditions. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; CRC Press, Taylor and Francis Group: New York, NY, USA, 2005; pp. 717–737. [Google Scholar]
- Paranychianakis, N.V.; Chartzoulakis, K.S.; Angelakis, A.N. Influence of rootstock, irrigation level and recycled water on water relations and gas exchange of Soultanina grapevines. Environ. Exp. Bot. 2004, 52, 185–198. [Google Scholar] [CrossRef]
- Moya, J.L.; Tadeo, F.R.; Gomez-Cadenas, A.; Primo-Millo, E.; Talon, M. Transmissible salt tolerance traits identified through reciprocal grafts between sensitive Carrizo and tolerance Cleopatra citrus genotypes. J. Plant Physiol. 2002, 159, 991–998. [Google Scholar] [CrossRef]
- Calzone, A.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E. Hyperspectral detection and monitoring salt stress in pomegranate cultivars. Agronomy 2021, 11, 1038. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Effects of salinity on growth, ion content, and osmotic relations in Halopyrum mocoronatum (L.) Stapf. J. Plant Nutr. 1999, 22, 191–204. [Google Scholar] [CrossRef]
- Maas, E.V. Salinity and citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [CrossRef]
- Zekri, M.; Parsons, L.R. Salinity tolerance of Citrus rootstocks: Effect of salt on root and leaf mineral concentrations. Plant Soil 1992, 147, 171–181. [Google Scholar] [CrossRef]
- Banuls, J.; Serna, M.D.; Legaz, F.; Talon, M.; Primo-Millo, E. Growth and gas exchange parameters of citrus plants stressed with different salts. J. Plant Physiol. 1997, 150, 194–199. [Google Scholar] [CrossRef]
- Kulkarni, T.S.; Desai, U.T.; Kshirsagar, D.B.; Kamble, A.B. Effects of salt regimes on growth and mineral uptake of pomegranate (Punica granatum L.) cv. Mrudula. Ann. Arid Zone 2007, 46, 77. [Google Scholar]
- Cerezo, M.; Garcia-Agustin, P.; Serna, M.D.; Primo-Millo, E. Kinetics of nitrate uptake by citrus seedlings and inhibitory effects of salinity. Plant Sci. 1997, 126, 105–112. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef]
- Wakeel, A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, K.; Ohta, E.; Sakata, M. Protective effects of external Ca2+ on elongation and intracellular concentration of K+ in intact mung bean roots under high NaCl stress. Plant Cell Physiol. 1990, 31, 815–821. [Google Scholar]
- Subbarrao, G.V.; Johansen, C.; Jana, M.K.; Kumar Rao, J.V.D.K. Effects of the sodium/calcium ratio in modifying salinity response of pigeonpea (Cajanus cajan). J. Plant Physiol. 1990, 136, 439–443. [Google Scholar] [CrossRef]
- Izzo, R.; Scagnozzi, A.; Belligno, A.; Navari-Izzo, F. Influence of NaCl treatment on Ca, K and Na interrelations in maize shoots. In Optimization of Plant Nutrition; Fragoso, M.A.C., van Beusichem, M.L., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 577–582. [Google Scholar]
- Lopez, M.V.; Satti, S.M.E. Calcium and potassium-enhanced growth and yield of tomato under sodium chloride stress. Plant Sci. 1996, 144, 19–27. [Google Scholar] [CrossRef]
- Humphery, A.E.; Rodriguez, V. Ion compartmentation in salinity-stressed alfalfa seedling growing under different temperature regimes. Commun. Soil Sci. Plant Anal. 1998, 29, 2607–2618. [Google Scholar]
- Tozlu, I.; Moore, G.A.; Guy, C.L. Effects of increasing NaCl concentration on stem elongation, dry mass production, and macro-and-micro-nutrient accumulation in Poncirus trifoliata. Aust. J. Plant Physiol. 2000, 27, 35–42. [Google Scholar]
- Banuls, J.; Legaz, F.; Primo-Millo, E. Effect of salinity on uptake and distribution of chloride and sodium in some Citrus scion-rootstock combinations. J. Hortic. Sci. 1990, 65, 715–724. [Google Scholar] [CrossRef]
- Ruiz, D.; Martinez, V.; Cerda, A. Demarcating specific ion (NaCl, Cl-, Na+) and osmotic effects in the response of two Citrus rootstocks to salinity. Sci. Hortic. 1999, 80, 213–224. [Google Scholar] [CrossRef]
- López-Lefebre, L.R.; Rivero, R.M.; García, P.C.; Sánchez, E.; Ruiz, J.M.; Romero, L. Effect of calcium on mineral nutrient uptake and growth of tobacco. J. Sci. Food Agric. 2001, 81, 1334–1338. [Google Scholar] [CrossRef]
- Naeini, M.R.; Khoshgoftarmanesh, A.H.; Lessani, H.; Fallahi, E. Effect of sodium chloride-induced salinity on mineral nutrients and soluble sugars in three commercial cultivars of pomegranate. J. Plant Nutr. 2004, 27, 1319–1326. [Google Scholar] [CrossRef]
- Okhovatian-Ardakani, A.R.; Mehrabanian, M.; Dehghani, E.; Akbarzadeh, A. Salt tolerance evaluation and relative comparison in cuttings of different pomegranate cultivars. Plant Soil Environ. 2010, 56, 176–185. [Google Scholar] [CrossRef]
- Karimi, H.R.; Hassanpour, Z. Effects of salinity and water stress on growth and macro nutrients concentration of pomegranate (Punica granatum L.). J. Plant Nutr. 2014, 37, 1937–1951. [Google Scholar] [CrossRef]
- Khayyat, M.; Tehranifar, A.; Davarynejad, G.H.; Sayyari-Zahan, M.H. Effects of NaCl salinity on some leaf nutrient concentrations, non-photochemical quenching and the efficiency of the PSII photochemistry of two Iranian pomegranate varieties under greenhouse and field conditions: Preliminary results. J. Plant Nutr. 2016, 39, 1752–1765. [Google Scholar] [CrossRef]
- Karimi, H.R.; Hassanpour, H. Effects of salinity, rootstock, and position of sampling on macro nutrient concentration of pomegranate cv. Gabri. J. Plant Nutr. 2017, 40, 2269–2278. [Google Scholar] [CrossRef]
- Marathe, R.A.; Chandra, R.; Jadhav, V.T.; Singh, R. Soil and nutritional aspects in pomegranate (Punica granatum L.). Environ. Ecol. 2009, 27, 630–637. [Google Scholar]
- Calzone, A.; Cotrozzi, L.; Pellegrini, E.; Guidi, L.; Lorenzini, G.; Nali, C. Differential response strategies of pomegranate cultivars lead to similar tolerance to increasing salt concentrations. Sci. Hortic. 2020, 271, 109441. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.; Papadakis, I.; Therios, I. Effect of calcium on the ion status and growth performance of a citrus rootstock grown under NaCl stress. Soil Sci. Plant Nutr. 2008, 54, 910–915. [Google Scholar] [CrossRef]
- Hasegawa, P.H.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Al-Harbi, A.R. Growth and nutrient composition of tomato and cucumber seedlings as affected by sodium chloride salinity and supplemental calcium. J. Plant Nutr. 1995, 18, 1403–1416. [Google Scholar] [CrossRef]
- Niazi, B.H.; Ahmed, T. Effect of sodium chloride and zinc on the growth of tomato. II. Uptake of ions. Geobios 1984, 11, 155–160. [Google Scholar]
- Ruiz, D.; Martinez, V.; Cerda, A. Citrus response to salinity: Growth and nutrient uptake. Tree Physiol. 1997, 17, 141–150. [Google Scholar] [CrossRef]

| Treatments | NaCl (mM) | CaCl2·2H2O (mM) | |
|---|---|---|---|
| 1 | C | 0 | 0 |
| 2 | Ca10 | 0 | 10 |
| 3 | S60 | 60 | 0 |
| 4 | S120 | 120 | 0 |
| 5 | S60/Ca10 | 60 | 10 |
| 6 | S120/Ca10 | 120 | 10 |
| Treatments | Leaf Fresh Weight (g) | Stem Fresh Weight (g) | Root Fresh Weight (g) | Plant Fresh Weight (g) | Shoot/Root |
|---|---|---|---|---|---|
| C | 17.76 a | 9.99 a | 15.32 a | 43.07 a | 2.23 a |
| Ca10 | 11.80 b | 6.71 b | 11.07 a | 29.58 b | 1.69 a |
| S60 | 12.10 b | 6.22 b | 13.78 a | 32.10 b | 1.35 a |
| S60/Ca10 | 9.38 bc | 4.46 b | 10.25 a | 24.09 b | 1.46 a |
| S120 | 9.79 bc | 5.19 b | 10.65 a | 25.64 b | 1.41 a |
| S120/Ca10 | 7.95 c | 4.38 b | 9.06 a | 21.39 b | 1.34 a |
| Treatments | Plant Height (cm) | Leaf Number per Plant | Leaf Dry Weight (g) | Stem Dry Weight (g) | Root Dry Weight (g) | Plant Dry Weight (g) | Shoot/Root |
|---|---|---|---|---|---|---|---|
| C | 50.42 a | 88.67 a | 6.45 a | 4.14 a | 2.77 a | 13.36 a | 3.97 b |
| Ca10 | 46.82 a | 61.92 bc | 4.50 bc | 2.86 b | 1.95 b | 9.31 b | 3.77 bc |
| S60 | 42.13 a | 73.17 ab | 4.17 bc | 2.52 b | 1.88 b | 8.57 b | 3.59 bc |
| S60/Ca10 | 40.38 a | 52.83 bc | 6.75 a | 1.80 b | 1.71 b | 10.26 ab | 5.30 a |
| S120 | 42.83 a | 60.42 bc | 3.28 c | 2.24 b | 1.84 b | 7.37 b | 2.97 c |
| S120/Ca10 | 39.38 a | 40.58 c | 5.74 ab | 1.90 b | 1.70 b | 9.34 b | 4.54 ab |
| C1/C2 | C1/C1+C2 | K1/K2 | K1/K1+K2 | |
|---|---|---|---|---|
| C | 0.57 b | 0.36 b | 0.94 a | 0.48 a |
| Ca10 | 0.66 ab | 0.39 ab | 0.98 a | 0.49 a |
| S60 | 0.65 ab | 0.39 ab | 0.92 a | 0.48 a |
| S60/Ca10 | 0.67 ab | 0.40 ab | 1.01 a | 0.50 a |
| S120 | 1.01 a | 0.49 a | 0.95 a | 0.49 a |
| S120/Ca10 | 1.57 a | 0.52 a | 0.99 a | 0.50 a |
| Treatments | Chlorophyll a + b (mg/g Fresh Weight) | Chlorophyll a + b (mg/cm2) | CCI Units |
|---|---|---|---|
| C | 3.84 a | 0.040 a | 40.21 a |
| Ca10 | 3.80 a | 0.041 a | 32.50 a |
| S60 | 4.19 a | 0.037 a | 30.26 a |
| S60/Ca10 | 2.56 ab | 0.031 ab | 30.34 a |
| S120 | 3.17 ab | 0.034 ab | 37.36 a |
| S120/Ca10 | 1.79 b | 0.025 b | 41.75 a |
| Treatments | N | K | Ca | Mg | Na | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|---|
| (% Dry Weight) | (mg kg−1 Dry Weight) | |||||||
| Basal leaves | ||||||||
| C | 1.91 c | 1.31 ab | 1.88 a | 0.19 a | 0.19 c | 91.50 a | 19.33 c | 12.17 c |
| Ca10 | 1.82 c | 1.38 a | 1.92 a | 0.20 a | 0.14 c | 118.17 a | 26.50 bc | 15.17 bc |
| S60 | 3.14 bc | 1.22 bc | 1.29 c | 0.16 b | 0.94 b | 145.17 a | 26.17 bc | 13.67 c |
| S60/Ca10 | 5.21 a | 1.16 c | 1.52 b | 0.16 b | 0.94 b | 90.00 a | 25.00 bc | 19.67 ab |
| S120 | 2.79 bc | 1.09 cd | 1.17 c | 0.15 b | 1.22 ab | 129.83 a | 30.83 ab | 14.83 bc |
| S120/Ca10 | 4.85 a | 0.97 d | 1.22 c | 0.15 b | 1.40 a | 106.77 a | 34.51 a | 22.87 a |
| Top leaves | ||||||||
| C | 2.26 ab | 1.31 a | 1.20 a | 0.16 a | 0.16 c | 50.50 a | 13.33 c | 11.17 b |
| Ca10 | 2.40 a | 1.29 ab | 1.08 a | 0.13 b | 0.09 c | 51.00 a | 17.83 ab | 15.33 a |
| S60 | 2.42 a | 1.21 b | 0.59 c | 0.13 b | 0.78 b | 46.50 a | 17.83 ab | 10.83 b |
| S60/Ca10 | 1.99 b | 1.06 c | 0.83 b | 0.10 c | 0.78 b | 53.33 a | 19.50 a | 13.33 ab |
| S120 | 2.21 ab | 1.02 c | 0.48 c | 0.09 cd | 1.14 a | 50.00 a | 16.33 abc | 10.33 b |
| S120/Ca10 | 2.19 ab | 1.00 c | 0.50 c | 0.08 d | 1.09 a | 44.67 a | 15.67 bc | 11.83 b |
| Treatments | K | Ca | Mg | Na | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|
| (% Dry Weight) | (mg kg−1 Dry Weight) | ||||||
| C | 0.97 ab | 1.08 a | 0.097 bc | 0.28 c | 67.67 a | 11.33 b | 31.83 a |
| Ca10 | 1.09 a | 1.27 a | 0.085 d | 0.22 c | 59.17 a | 11.17 b | 31.83 a |
| S60 | 0.85 bc | 1.22 a | 0.111 a | 0.78 ab | 44.83 a | 17.67 b | 32.67 a |
| S60/Ca10 | 0.79 c | 1.32 a | 0.084 d | 0.70 b | 89.29 a | 17.35 b | 57.93 a |
| S120 | 0.74 c | 1.11 a | 0.108 ab | 0.90 a | 220.67 a | 24.17 a | 97.33 a |
| S120/Ca10 | 0.76 c | 1.36 a | 0.093 cd | 0.87 a | 43.35 a | 17.61 b | 39.33 a |
| Treatments | K | Ca | Mg | Na | Fe | Mn | Zn |
|---|---|---|---|---|---|---|---|
| (% Dry Weight) | (mg kg−1 Dry Weight) | ||||||
| C | 0.96 a | 1.52 b | 0.35 a | 0.51 c | 326.17 a | 40.17 c | 79.83 c |
| Ca10 | 1.22 a | 1.75 a | 0.37 a | 0.39 c | 274.00 a | 38.17 c | 95.83 bc |
| S60 | 0.93 a | 1.33 cd | 0.38 a | 1.41 a | 343.17 a | 61.17 a | 86.83 c |
| S60/Ca10 | 1.14 a | 1.45 bc | 0.32 a | 1.02 b | 307.50 a | 45.17 bc | 113.50 ab |
| S120 | 0.94 a | 1.13 e | 0.31 a | 1.38 a | 306.33 a | 53.50 ab | 82.17 c |
| S120/Ca10 | 1.11 a | 1.24 de | 0.34 a | 1.56 a | 329.67 a | 57.83 a | 119.50 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzissavvidis, C.; Devetzi, N.; Antonopoulou, C.; Papadakis, I.E.; Therios, I.; Koundouras, S. Salinity Stress and Calcium in Pomegranate: Impacts on Growth, Ion Homeostasis, and Photosynthesis. Horticulturae 2025, 11, 786. https://doi.org/10.3390/horticulturae11070786
Chatzissavvidis C, Devetzi N, Antonopoulou C, Papadakis IE, Therios I, Koundouras S. Salinity Stress and Calcium in Pomegranate: Impacts on Growth, Ion Homeostasis, and Photosynthesis. Horticulturae. 2025; 11(7):786. https://doi.org/10.3390/horticulturae11070786
Chicago/Turabian StyleChatzissavvidis, Christos, Nina Devetzi, Chrysovalantou Antonopoulou, Ioannis E. Papadakis, Ioannis Therios, and Stefanos Koundouras. 2025. "Salinity Stress and Calcium in Pomegranate: Impacts on Growth, Ion Homeostasis, and Photosynthesis" Horticulturae 11, no. 7: 786. https://doi.org/10.3390/horticulturae11070786
APA StyleChatzissavvidis, C., Devetzi, N., Antonopoulou, C., Papadakis, I. E., Therios, I., & Koundouras, S. (2025). Salinity Stress and Calcium in Pomegranate: Impacts on Growth, Ion Homeostasis, and Photosynthesis. Horticulturae, 11(7), 786. https://doi.org/10.3390/horticulturae11070786







