Slightly Acidic Electrolyzed Water Improves the Postharvest Quality of Litchi Fruit by Regulating the Phenylpropane Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. SAEW and Samples
2.2. Measurement of the Pericarp Browning Index, Fruit Disease Index, and Commercially Acceptable Fruit Rate
2.3. Determination of Respiration Rate and Weight Loss of Litchi Fruit and Cell Membrane Permeability in Litchi Pericarp
2.4. Measurement of Color Variations
2.5. Measurement of Nutritional Properties
2.6. Appraisal of Pericarp Lignin, Flavonoid, and Total Phenolic Contents
2.7. Appraisal of Litchi Pericarp DRE Activities
2.8. Statistical Analysis
3. Results
3.1. Influences of the SAEW Treatment on Pericarp Browning and Fruit Disease Development and Commercially Acceptable Fruit Rate of Litchi Fruit
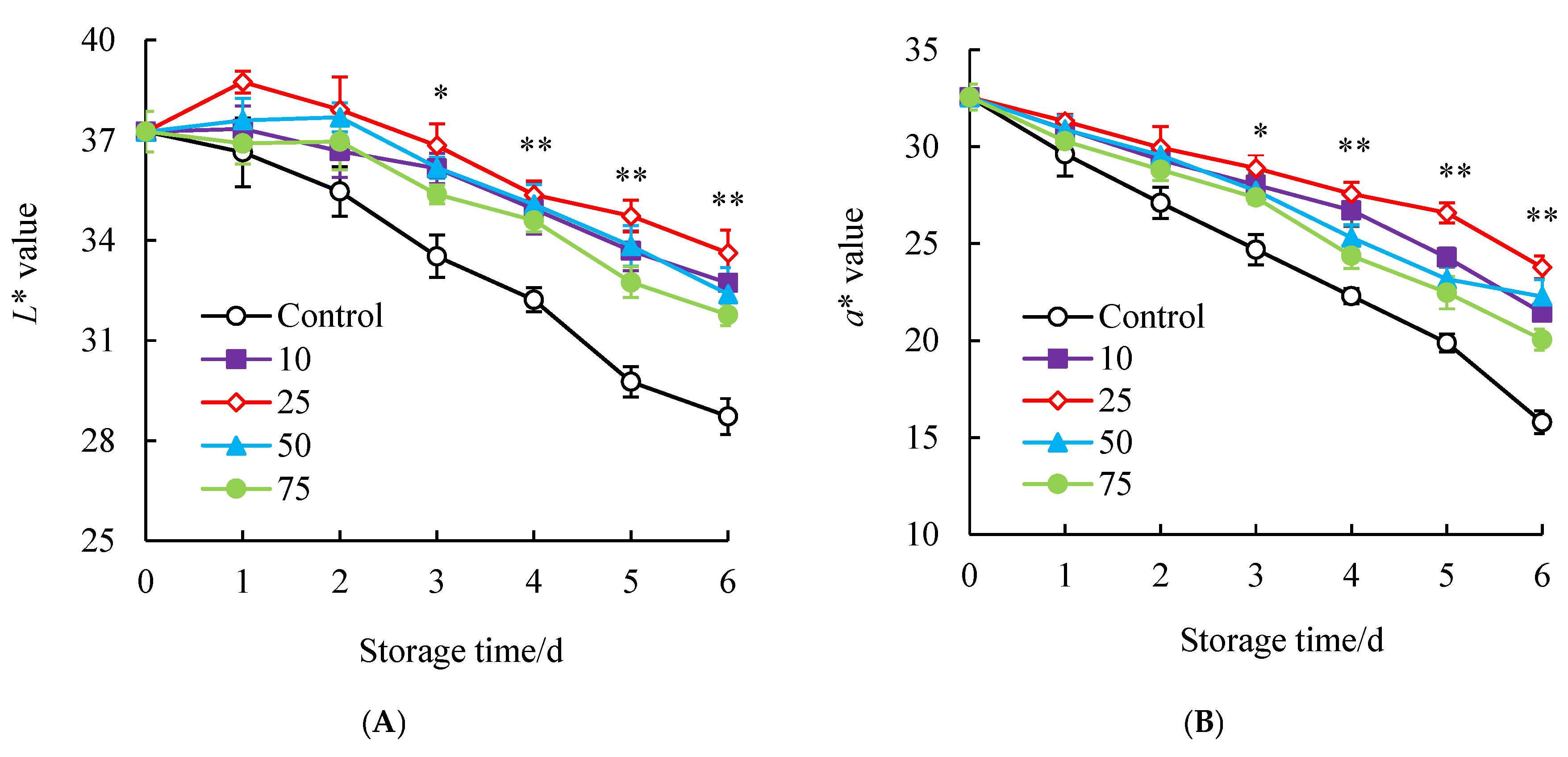
3.2. Influences of SAEW Treatment on the Pericarp Color of Litchi Fruit
3.3. Influences of the SAEW Treatment on the Storage Behavior of Litchi Fruit
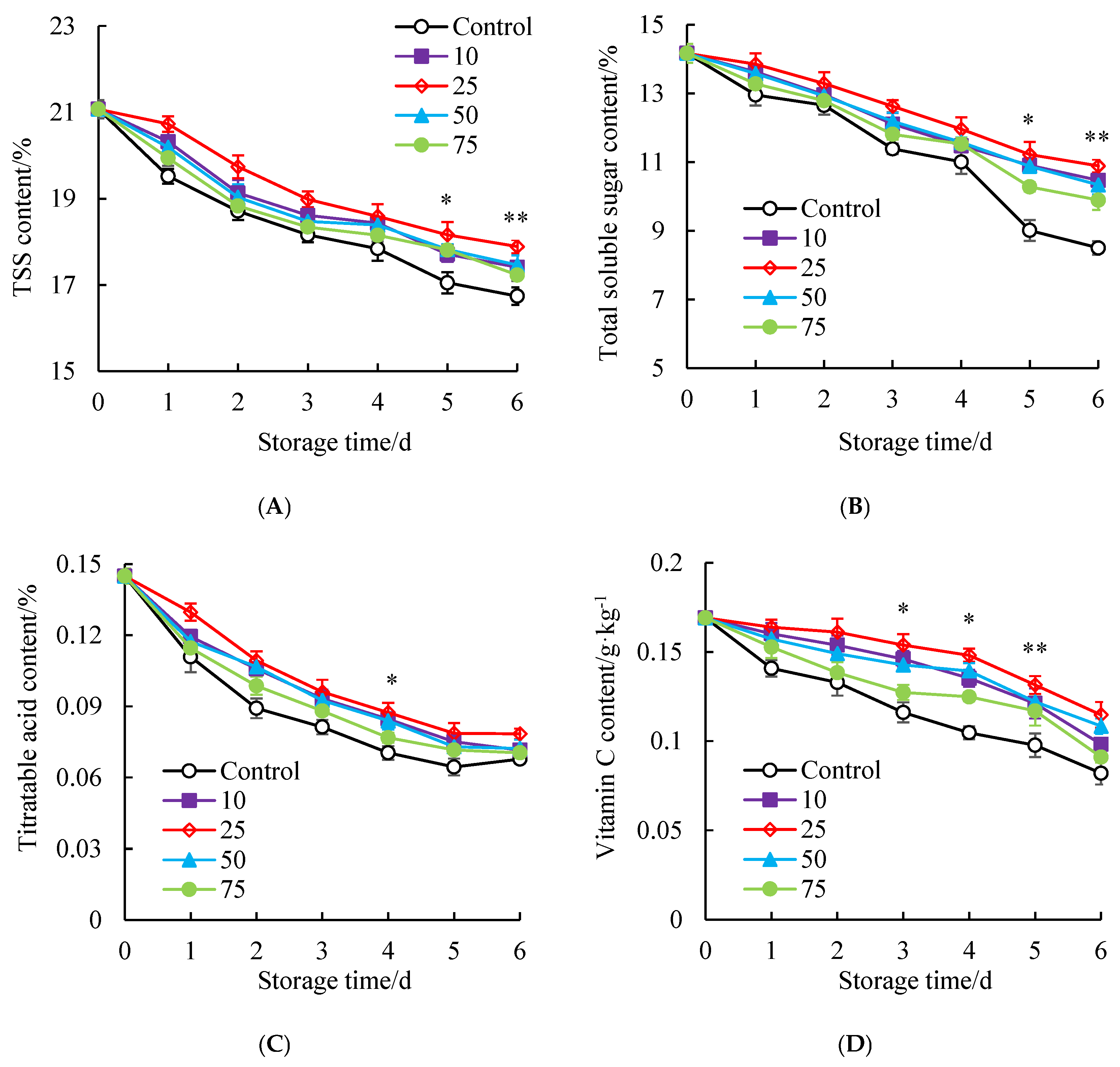
3.4. Influences of SAEW Treatment on the Nutrient Content of Litchi Aril
3.5. Influences of SAEW Treatment on the Disease Resistance-Related Substances of Litchi Pericarp
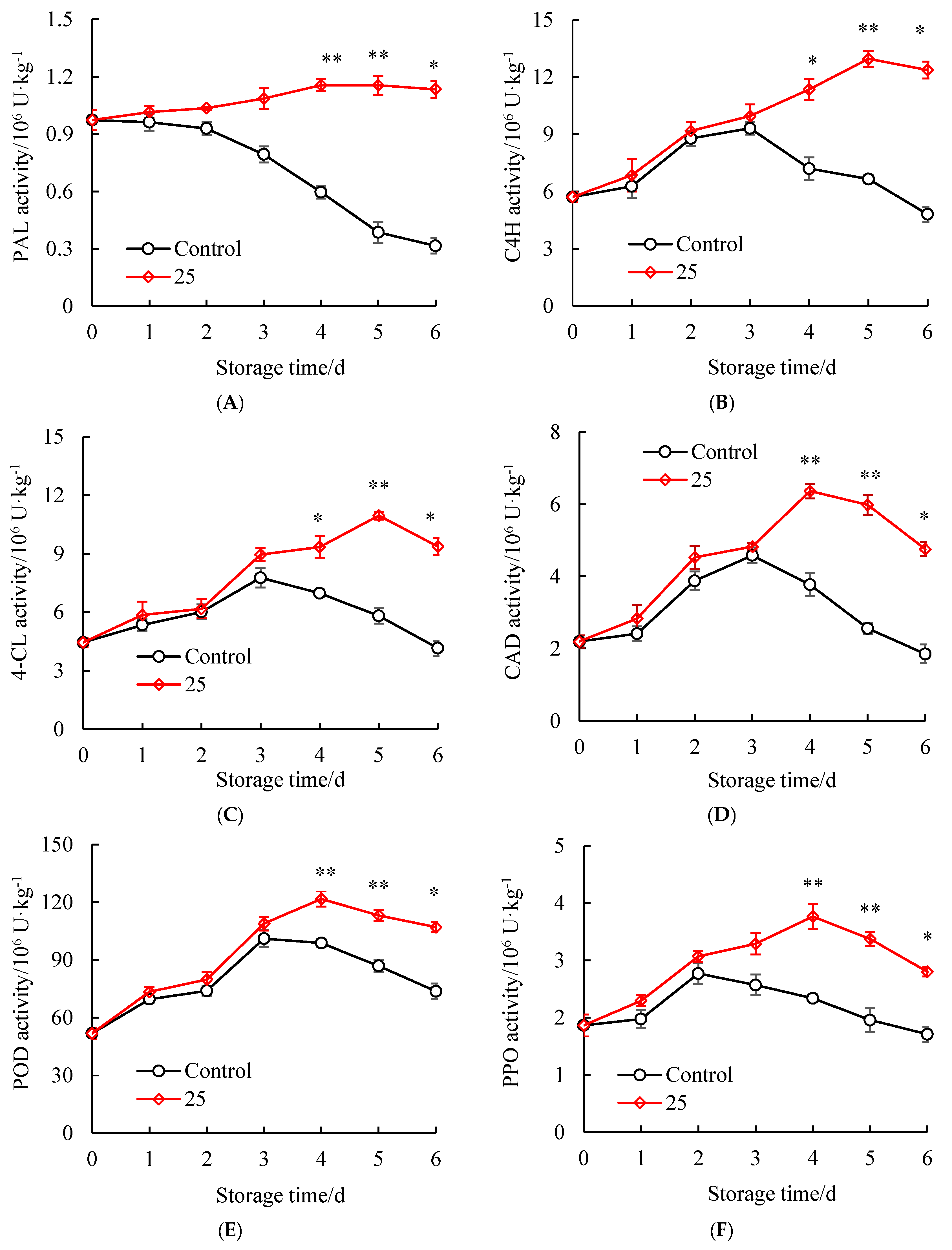
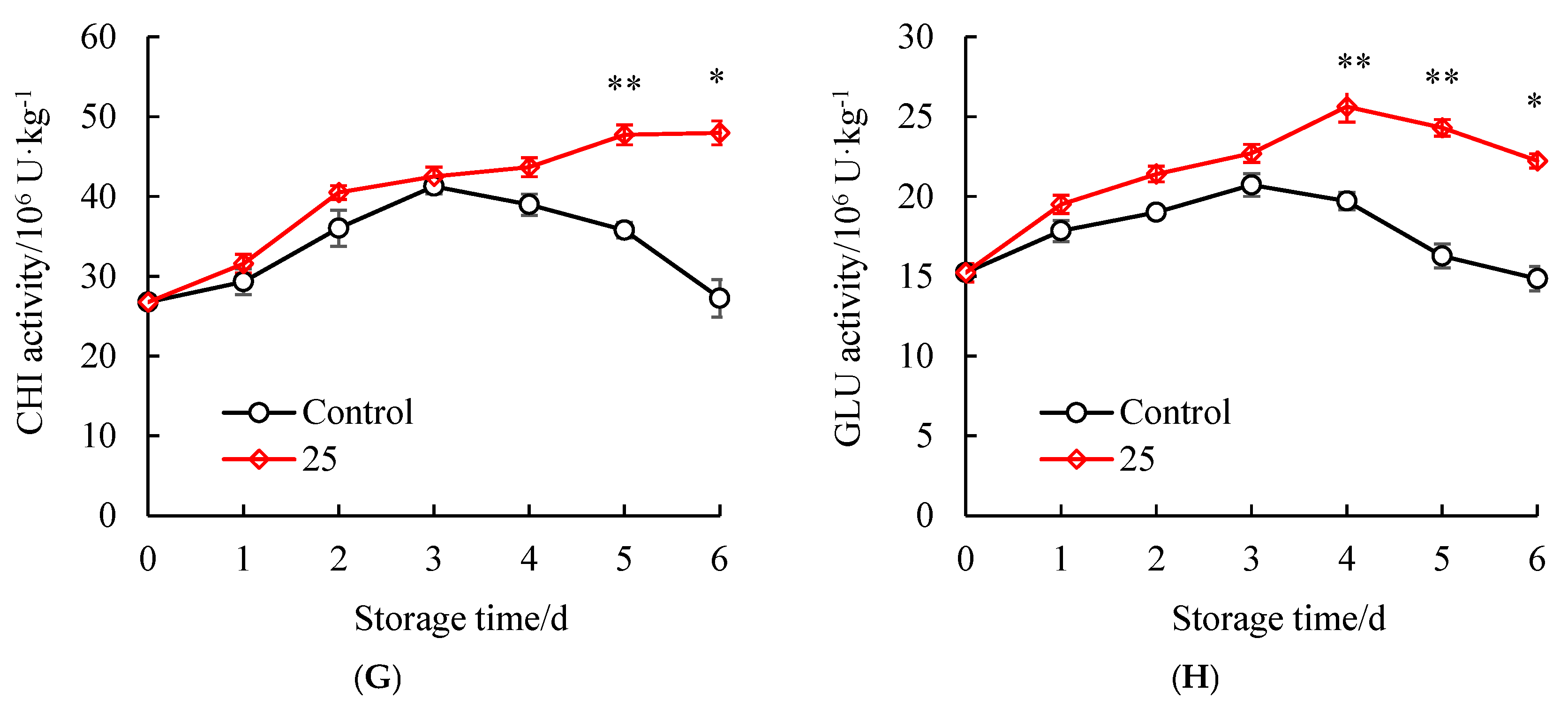
3.6. Influences of SAEW Treatment on the Disease Resistance Enzyme Activities of Litchi Pericarp
4. Discussion
4.1. SAEW Treatment Improved the Postharvest Quality and Storability of Fresh Produce
4.2. Induced Disease Resistance by SAEW Treatment Improved the Storability of Postharvest Litchi Fruit
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-CL | 4-coumarate CoA ligase |
| ACC | Available chlorine concentration |
| C4H | Cinnamate-4-hydroxylase |
| CAD | Cinnamyl alcohol dehydrogenase |
| CAFR | Commercially acceptable fruit rate |
| CHI | Chitinase |
| DI | Disease index |
| DRE | Disease resistance enzyme |
| GLU | β-1,3-glucanase |
| SAEW | Slightly acidic electrolyzed water |
| PAL | Phenylalanine ammonia-lyase |
| PBI | Pericarp browning index |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| ROS | Reactive oxygen species |
| TA | Titratable acidity |
References
- Jiang, X.J.; Lin, H.T.; Shi, J.; Neethirajan, S.; Lin, Y.F.; Chen, Y.H.; Wang, H.; Lin, Y.X. Effects of a novel chitosan formulation treatment on quality attributes and storage behavior of harvested litchi fruit. Food Chem. 2018, 252, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, B.; Fu, L.; Tang, H.; Li, Y.; Pang, X.; Zhang, Z. Water supply via pedicel reduces postharvest pericarp browning of litchi (Litchi chinensis) fruit. Foods 2024, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Shi, D.; Ren, Y.; Yang, C.; Qu, H.; Jiang, Y.; Li, T. Vitexin is a potential postharvest treatment for ameliorating litchi fruit pericarp browning by regulating autophagy. Postharvest Biol. Technol. 2024, 216, 113061. [Google Scholar] [CrossRef]
- Cao, X.; Li, F.; Xu, H.; Li, H.; Wang, S.; Wang, G.; West, J.S.; Wang, J. Characterization of Colletotrichum species infecting litchi in Hainan, China. J. Fungi 2023, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xie, Y.; Kong, X.; Peng, K.; Zhu, H.; Jiang, M.; Qu, H. Involvement of LcmiR482e-LcACA2 mediated energy metabolism in senescence of harvested litchi fruit. Postharvest Biol. Technol. 2024, 208, 112676. [Google Scholar] [CrossRef]
- Situ, J.; Zheng, L.; Xu, D.; Gu, C.; Xi, P.; Deng, Y.; Hsiang, T.; Jiang, Z. Screening of effective biocontrol agents against postharvest litchi downy blight caused by Peronophythora litchii. Postharvest Biol. Technol. 2023, 198, 112249. [Google Scholar] [CrossRef]
- Akther, S.; Islam, M.R.; Alam, M.; Alam, M.J.; Ahmed, S. Impact of slightly acidic electrolyzed water in combination with ultrasound and mild heat on safety and quality of fresh cut cauliflower. Postharvest Biol. Technol. 2023, 197, 112189. [Google Scholar] [CrossRef]
- Du, Y.; Tian, Q.; Li, G.; Yi, J.; Hu, X.; Jiang, Y. Advanced application of slightly acidic electrolyzed water for fresh-cut fruits and vegetables preservation. Food Res. Intl. 2024, 195, 114996. [Google Scholar] [CrossRef]
- Sun, Y.; Qiu, W.; Fang, X.; Zhao, X.; Xu, X.; Li, W. Hydrogen-rich water treatment of fresh-cut kiwifruit with slightly acidic electrolytic water: Influence on antioxidant metabolism and cell wall stability. Foods 2023, 12, 426. [Google Scholar] [CrossRef]
- Tyagi, A.; Chen, X.; Shabbir, U.; Chelliah, R.; Oh, H.D. Effect of slightly acidic electrolyzed water on amino acid and phenolic profiling of germinated brown rice sprouts and their antioxidant potential. LWT Food Sci. Technol. 2022, 157, 113119. [Google Scholar] [CrossRef]
- Suo, K.; Zhang, Y.; Feng, Y.; Yang, Z.; Zhou, C.; Chen, W.; Wang, J. Ultrasonic synergistic slightly acidic electrolyzed water processing to improve postharvest storage quality of Chinese bayberry. Ultrason. Sonochem. 2023, 101, 106668. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoon, S.; Ha, J. Effects of slightly acidic electrolysed water spraying and ultraviolet C light-emitting diode irradiation combination on the inactivation kinetics of Escherichia coli biofilms on food contact surfaces. Food Control 2023, 152, 109879. [Google Scholar] [CrossRef]
- Tango, C.N.; Khan, I.; Ngnitcho Kounkeu, P.-F.; Momna, R.; Hussain, M.S.; Oh, D.H. Slightly acidic electrolyzed water combined with chemical and physical treatments to decontaminate bacteria on fresh fruits. Food Microbiol. 2017, 67, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Ge, Z.; Shi, J.; Xu, Y.T.; Jones, C.L.; Liu, D.H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT Food Sci. Technol. 2015, 60, 1195–1199. [Google Scholar] [CrossRef]
- Guentzel, J.L.; Callan, M.A.; Liang Lam, K.; Emmons, S.A.; Dunham, V.L. Evaluation of electrolyzed oxidizing water for phytotoxic effects and pre-harvest management of gray mold disease on strawberry plants. Crop Prot. 2011, 30, 1274–1279. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Dong, S.; Chen, Y.; Jiang, X.; Chen, Y. Acidic electrolyzed water maintains the storage quality of postharvest wampee fruit by activating the disease resistance. Foods 2024, 13, 1556. [Google Scholar] [CrossRef]
- Tang, J.Y.; Chen, H.B.; Lin, H.T.; Hung, Y.C.; Xie, H.L.; Chen, Y.H. Acidic electrolyzed water treatment delayed fruit disease development of harvested longans through inducing the disease resistance and maintaining the ROS metabolism systems. Postharvest Biol. Technol. 2021, 171, 111349. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Fang, L.; Liu, Q.Q.; Lin, H.T.; Lin, M.S.; Lin, Y.F.; Wang, H.; Hung, Y.C.; Chen, Y.H. Comparative transcriptome and metabolome reveal the role of acidic electrolyzed oxidizing water in improving postharvest disease resistance of longan fruit. Food Chem. 2024, 449, 139235. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Jiang, W. Application of electrolyzed water in postharvest fruits and vegetables storage: A review. Trends Food Sci. Technol. 2021, 114, 599–607. [Google Scholar] [CrossRef]
- Li, P.Q.; Liang, C.Q.; Jiao, J.H.; Ruan, Z.; Sun, M.J.; Zhao, J.C.; Wang, T.; Zhong, S.Y. Exogenous priming of chitosan induces resistance in Chinese prickly ash against stem canker caused by Fusarium zanthoxyli. Int. J. Biol. Macromol. 2024, 259, 129119. [Google Scholar] [CrossRef]
- Li, M.Q.; Zhang, X.H.; Li, J.Q.; Ali, M.; Wang, Y.T.; Liu, X.L.; Li, F.J.; Li, X.A. GABA primes defense responses against Botrytis cinerea in tomato fruit by modulating ethylene and JA signaling pathway. Postharvest Biol. Technol. 2024, 208, 112665. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Zuo, X.; Li, Y.; Li, M.; Wang, K.; Jin, P.; Zheng, Y. PpWRKY45 is involved in methyl jasmonate primed disease resistance by enhancing the expression of jasmonate acid biosynthetic and pathogenesis-related genes of peach fruit. Postharvest Biol. Technol. 2021, 172, 111390. [Google Scholar] [CrossRef]
- Chen, O.; Deng, L.L.; Ruan, C.Q.; Yi, L.H.; Zeng, K.F. Pichia galeiformis induces resistance in postharvest citrus by activating the phenylpropanoid biosynthesis pathway. J. Agric. Food Chem. 2021, 69, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bi, Y.; Zhang, Z.; Xie, D.; Ge, Y.; Li, W.; Wang, J.; Wang, Y. Postharvest oxalic acid treatment induces resistance against pink rot by priming in muskmelon (Cucumis melo L.) fruit. Postharvest Biol. Technol. 2015, 106, 53–61. [Google Scholar] [CrossRef]
- Shen, W.; Li, W.; Shao, Y.; Zeng, J. Proanthocyanidin delays litchi peel browning by inhibiting ethylene biosynthesis, respiratory metabolism, and phenol oxidase activities. Sci. Hort. 2023, 309, 111677. [Google Scholar] [CrossRef]
- Lai, D.; Shao, X.; Xiao, W.; Fan, C.; Liu, C.; He, H.; Tian, S.; Kuang, S. Suppression of fruit decay and maintenance of storage quality of litchi by Photorhabdus luminescens Hb1029 treatment. Sci. Hortic. 2020, 259, 108836. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Han, L.; Shang, H.; Tang, S.; Rui, H.; Duan, Y.; Kong, F.; Kai, X.; Zheng, Y. Methyl jasmonate induces resistance against Penicillium citrinum in Chinese bayberry by priming of defense responses. Postharvest Biol. Technol. 2014, 98, 90–97. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of proteindye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Han, Y.; He, X.; Luo, S.; Hu, H.; Li, P. The regulatory effect of slightly acidic electrolyzed water ice on the postharvest quality decline and glucosinolate metabolism of broccoli. Postharvest Biol. Technol. 2024, 215, 113015. [Google Scholar] [CrossRef]
- Feng, Y.; Suo, K.; Zhang, Y.; Yang, Z.; Zhou, C.; Shi, L.; Chen, W.; Wang, J.; Wang, C.; Zheng, Y. Ultrasound synergistic slightly acidic electrolyzed water treatment of grapes: Impacts on microbial loads, wettability, and postharvest storage quality. Ultrason. Sonochem. 2024, 103, 106751. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Liu, Q.; Li, M.; Feng, S.; Lin, M.; Chen, Y.; Lin, H. Slightly acidic electrolyzed water treatment enhances the quality attributes and the storability of postharvest litchis through regulating the metabolism of reactive oxygen species. Food Chem. X 2024, 23, 101644. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Wang, Z.Y.; Wei, C.H.; Amo, A.; Ahmed, B.; Yang, X.Z. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Zhang, J.; Ru, X.; Xu, F.; Wu, Z.; Jin, P.; Zheng, Y.; Cao, S. CaCl2 promoted phenolics accumulation via the CmCAMTA4-mediated transcriptional activation of phenylpropane pathway and energy metabolism in fresh-cut cantaloupe. Postharvest Biol. Technol. 2024, 207, 112599. [Google Scholar] [CrossRef]
- Zou, Y.Y.; Yang, S.S.; Ren, Q.M.; Chen, J.Y.; Wang, K.T.; Li, C.H.; Zheng, Y.H. Extracellular self-DNA as a DAMP signal for induced systemic resistance to anthracnose rot in postharvest loquat fruit via EjRAV2-EjERF39 module. Postharvest Biol. Technol. 2024, 216, 113023. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; Van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Lin, X.; Lin, Y.; Chen, Y.; Chen, Y.; Chen, H. Slightly Acidic Electrolyzed Water Improves the Postharvest Quality of Litchi Fruit by Regulating the Phenylpropane Pathway. Horticulturae 2025, 11, 751. https://doi.org/10.3390/horticulturae11070751
Jiang X, Lin X, Lin Y, Chen Y, Chen Y, Chen H. Slightly Acidic Electrolyzed Water Improves the Postharvest Quality of Litchi Fruit by Regulating the Phenylpropane Pathway. Horticulturae. 2025; 11(7):751. https://doi.org/10.3390/horticulturae11070751
Chicago/Turabian StyleJiang, Xuanjing, Xiangzhi Lin, Yuzhao Lin, Yazhen Chen, Yihui Chen, and Hongbin Chen. 2025. "Slightly Acidic Electrolyzed Water Improves the Postharvest Quality of Litchi Fruit by Regulating the Phenylpropane Pathway" Horticulturae 11, no. 7: 751. https://doi.org/10.3390/horticulturae11070751
APA StyleJiang, X., Lin, X., Lin, Y., Chen, Y., Chen, Y., & Chen, H. (2025). Slightly Acidic Electrolyzed Water Improves the Postharvest Quality of Litchi Fruit by Regulating the Phenylpropane Pathway. Horticulturae, 11(7), 751. https://doi.org/10.3390/horticulturae11070751





