Morphological and Biochemical Characteristics of a Novel Albino Tea Cultivar (Camellia sinensis ‘Geumda’)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Investigation of Growth Characteristics and Leaf Size of Tea Plants
2.3. Measurement of Chlorophyll Content
2.4. Chloroplast Ultrastructure Observation by Transmission Electron Microscopy (TEM)
2.5. Determination of Free Amino Acids Content
2.6. Determination of Catechin Content
2.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Characteristics of ‘Geumda’
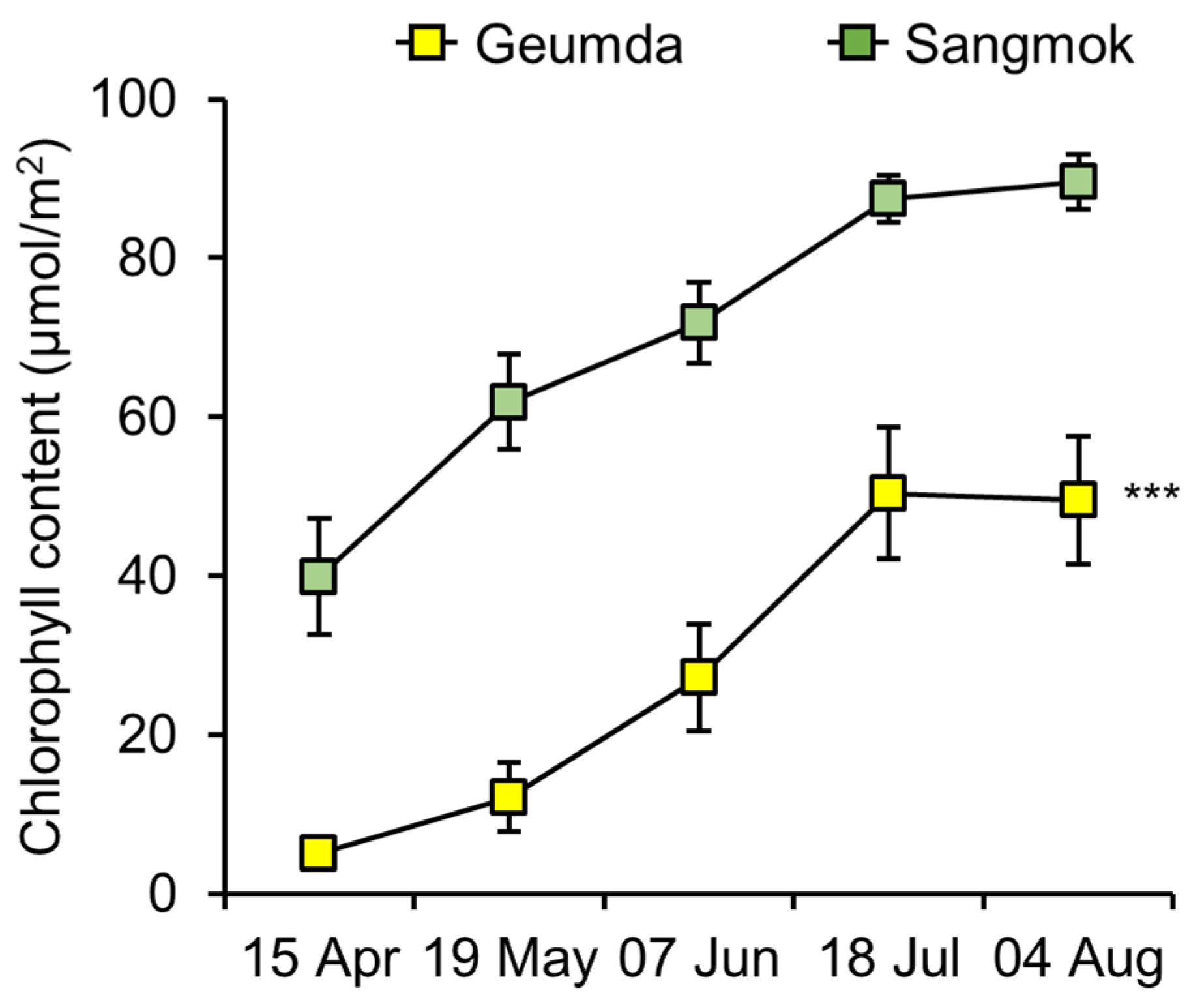
3.2. Chlorophyll Content of ‘Geumda’
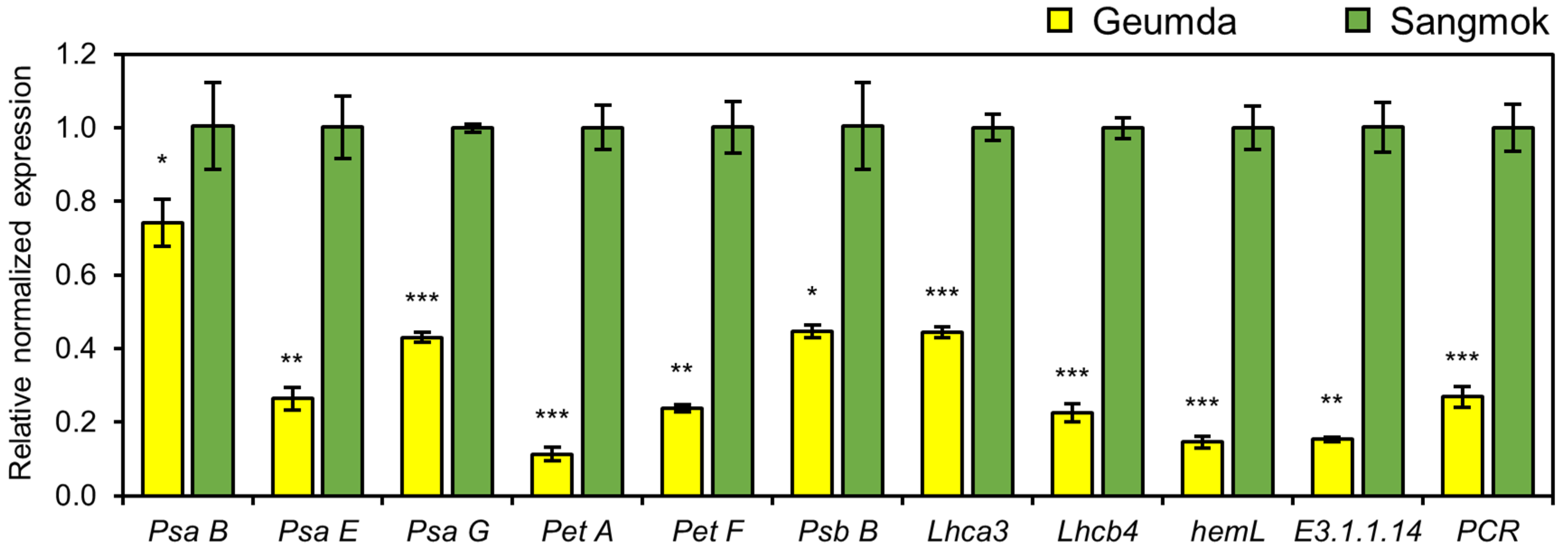
3.3. Abnormal Chloroplast Development of ‘Geumda’
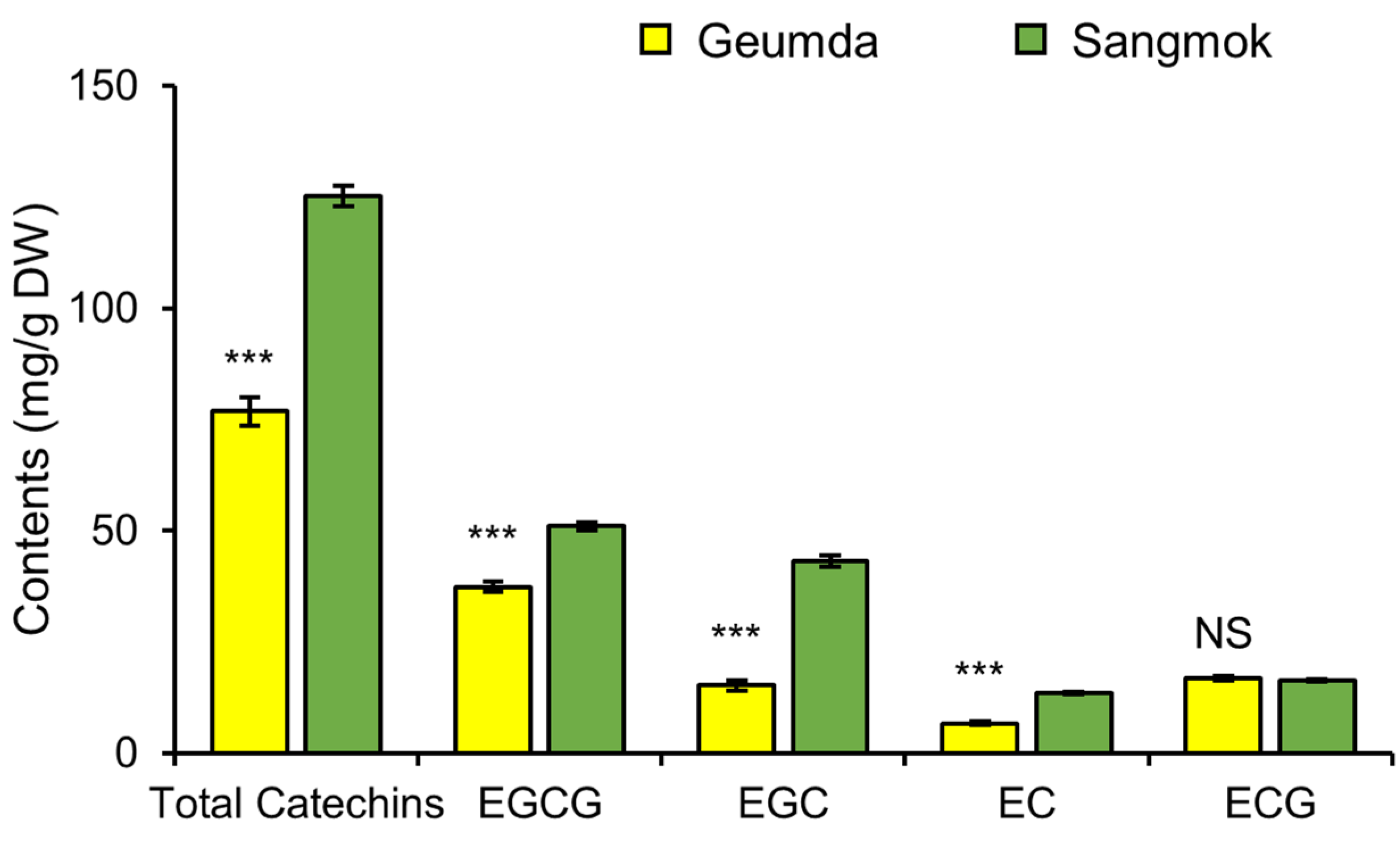
3.4. Contents of Quality-Related Compounds: Amino Acids and Catechins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Apostolides, Z.; Chen, Z.-M. Global Tea Breeding: Achievements, Challenges and Perspectives; Zhejiang University Press: Hangzhou, China; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–11. [Google Scholar]
- Li, J.; Xiao, Y.; Zhou, X.; Liao, Y.; Wu, S.; Chen, J.; Qian, J.; Yan, Y.; Tang, J.; Zeng, L. Characterizing the cultivar-specific mechanisms underlying the accumulation of quality-related metabolites in specific Chinese tea (Camellia sinensis) germplasm to diversify tea products. Food Res. Int. 2022, 161, 111824. [Google Scholar] [CrossRef] [PubMed]
- Kottawa-Arachchi, J.D.; Ranatunga, M.A.B.; Amarakoon, A.M.T.; Gunasekare, M.T.K.; Attanayake, R.N.; Sharma, R.K.; Chaudhary, H.K.; Sood, V.K.; Katoch, R.; Banyal, D.K.; et al. Variation of catechin and caffeine content in exotic collection of tea [Camellia sinensis (L.) O. Kuntze] in Sri Lanka and potential implication in breeding cultivars with enhanced quality and medicinal properties. Food Chem. Adv. 2022, 1, 100108. [Google Scholar] [CrossRef]
- Yan, M.; Huang, X.; Xie, N.; Zhao, T.; Zhu, M.; Li, J.; Wang, K. Advances in purple tea research: Chemical compositions, anthocyanin synthesis and regulation, processing, and health benefits. Horticulturae 2024, 10, 50. [Google Scholar] [CrossRef]
- Jiang, C.; Moon, D.-G.; Ma, J.; Chen, L. Characteristics of non-volatile metabolites in fresh shoots from tea plant (Camellia sinensis) and its closely related species and varieties. Beverage Plant Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Guo, J.; Li, K.; Lin, Y.; Liu, Y. Protective effects and molecular mechanisms of tea polyphenols on cardiovascular diseases. Front. Nutr. 2023, 10, 1202378. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Zhang, C.; Ni, L. Comparative study of the anti-obesity and gut microbiota modulation effects of green tea phenolics and their oxidation products in high-fat-induced obese mice. Food Chem. 2022, 367, 130735. [Google Scholar] [CrossRef]
- Unno, K.; Nakamura, Y. Green tea suppresses brain aging. Molecules 2021, 26, 4897. [Google Scholar] [CrossRef]
- Unno, K.; Hara, A.; Nakagawa, A.; Iguchi, K.; Ohshio, M.; Morita, A.; Nakamura, Y. Anti-stress effects of drinking green tea with lowered caffeine and enriched theanine, epigallocatechin and arginine on psychosocial stress induced adrenal hypertrophy in mice. Phytomedicine 2016, 23, 1365–1374. [Google Scholar] [CrossRef]
- Zheng, G.; Sayama, K.; Okubo, T.; Juneja, L.R.; Oguni, I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004, 18, 55–62. [Google Scholar]
- Ding, Y.; Wang, X.; Cui, H.; Zhao, Y. Biochemical and proteome analysis reveal different nutritional compound compositions and chloroplast development situations between purple-red and white-yellow tea plant cultivars. Horticulturae 2022, 8, 685. [Google Scholar] [CrossRef]
- Wang, L.; Yue, C.; Cao, H.; Zhou, Y.; Zeng, J.; Yang, Y.; Wang, X. Biochemical and transcriptome analyses of a novel chlorophyll-deficient chlorina tea plant cultivar. BMC Plant Biol. 2014, 14, 352. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, Z.; Wen, W.; Yao, M.; Liu, H.; Li, F.; Zhang, S.; Ni, D.; Chen, L. Comprehensive dissection of variation and accumulation of free amino acids in tea accessions. Hortic. Res. 2024, 11, uhad263. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Brennan, M.; Li, S.; Zhao, H.; Lange, K.W.; Brennan, C. How does the tea L-theanine buffer stress and anxiety. Food Sci. Hum. Wellness 2022, 11, 467–475. [Google Scholar] [CrossRef]
- Felice, M.D.; Renard, J.; Hudson, R.; Szkudlarek, H.J.; Pereira, B.J.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. L-Theanine prevents long-term affective and cognitive side effects of adolescent Δ-9-tetrahydrocannabinol exposure and blocks associated molecular and neuronal abnormalities in the mesocorticolimbic circuitry. J. Neurosci. 2021, 41, 739–750. [Google Scholar] [CrossRef]
- Deb, S.; Borah, A. L-theanine, the unique constituent of tea, improves neuronal survivability by curtailing inflammatory responses in MPTP model of Parkinson’s disease. Neurochem. Int. 2024, 179, 105830. [Google Scholar] [CrossRef]
- Unno, K.; Taguchi, K.; Matsuda, T.; Nakamura, Y. Stress-relieving effects of green tea depend on the ratio of its special ingredients and the infusion conditions. Molecules 2024, 29, 4553. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Gao, X.; Zhou, F.; Shen, C.; Liu, Z. Multi-omics research in albino tea plants: Past, present, and future. Sci. Hortic. 2020, 261, 108943. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, H.; Liu, Y.; Wang, H.; Xu, Y.; Huang, J.; Lei, P. Chemical constituents of green teas processed from albino tea cultivars with white and yellow shoots. Food Chem.-Mol. Sci. 2022, 5, 100143. [Google Scholar]
- Du, Y.; Chen, H.-Y.; Zhong, W.l.; Wu, L.; Ye, J.-H.; Lin, C.; Zheng, X.; Lu, J.-L.; Liang, Y. Effect of temperature on accumulation of chlorophylls and leaf ultrastructure of low temperature induced albino tea plant. Afr. J. Biotechnol. 2008, 7, 1881–1885. [Google Scholar] [CrossRef]
- Chen, X.; Yu, H.; Zhu, J.; Chen, Y.; Fu, Z.; Zhao, Y.; Yu, Y.; Chen, X.; Li, X.; Ma, Q. Widely targeted metabolomic analyses of albino tea germplasm ‘Huabai 1’ and ‘Baiye 1’. All Life 2021, 14, 530–540. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, M.; Wu, Q.; Zeng, W.; Chen, Z.; Sun, W. Combined analysis of lipidomics and transcriptomics revealed the key pathways and genes of lipids in light-sensitive albino tea plant (Camellia sinensis cv. Baijiguan). Front. Plant Sci. 2022, 13, 1035119. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-G.; Zhao, T.-T.; Xiang, Q.-Z.; Han, X.-Y.; Yang, S.-S.; Zhang, L.-X.; Ren, L.-J. Multi-omics research accelerates the clarification of the formation mechanism and the influence of leaf color variation in tea (Camellia sinensis) plants. Plants 2024, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-H.; Zheng, X.-Q.; Liang, Y.-R. High-light-induced degradation of photosystem II subunits’ involvement in the albino phenotype in tea plants. Int. J. Mol. Sci. 2022, 23, 8522. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, C.; Zhou, P.; Gao, X.; Wang, M.; Tian, S.; Lu, C.; Wang, K.; Shen, C. Transcriptomic analyses reveal variegation-induced metabolic changes leading to high L-theanine levels in albino sectors of variegated tea (Camellia sinensis). Plant Physiol. Biochem. 2021, 169, 29–39. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Chen, C.; Yue, C.; Hao, X.; Yang, Y.; Wang, X. Complementary transcriptomic and proteomic analyses of a chlorophyll-deficient tea plant cultivar reveal multiple metabolic pathway changes. J. Proteom. 2016, 130, 160–169. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, B.; Zhang, Y.; Li, Y.; Yu, C.; Peng, Z.; Wang, K.; Liu, Z.; Huang, J.-A.; Xiong, L.; et al. Transcriptomic and biochemical analysis reveal differential regulatory mechanism of photosynthetic pigment and characteristic secondary metabolites between high amino acids green-leaf and albino tea cultivars. Sci. Hortic. 2022, 295, 110823. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Liao, Y.; Xu, X.; Zeng, L.; Tang, J.; Li, J.; Lai, J.; Yang, Z. Differential accumulation of specialized metabolite L-theanine in green and albino-induced yellow tea (Camellia sinensis) leaves. Food Chem. 2019, 15, 93–100. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Palta, J.P. Leaf chlorophyll content. Remote Sens. Rev. 1990, 5, 207–213. [Google Scholar] [CrossRef]
- Korea Seed & Variety Service. Available online: http://seed.go.kr/sites/seed_eng/index.do (accessed on 1 May 2025).
- Lee, D.-J.; Kim, C.-K.; Lee, T.-H.; Lee, S.-J.; Moon, D.-G.; Kwon, Y.-H.; Cho, J.-Y. The complete chloroplast genome sequence of economical standard tea plant, Camellia sinensis L. cultivar Sangmok, in Korea. Mitochondrial DNA B Resour. 2020, 5, 2835–2836. [Google Scholar] [CrossRef]
- Lee, D.-J.; Kim, J.-H.; Lee, T.-H.; Park, M.-E.; Ahn, B.-O.; Lee, S.-J.; Cho, J.-Y.; Kim, C.-K. Selection of catechin biosynthesis-related genes and functional analysis from chromosome-level genome assembly in C. sinensis L. variety ‘Sangmok’. Int. J. Mol. Sci. 2024, 25, 3634. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, J.; Liu, S.; Li, J.; Yang, X.; Liu, Y.; Liu, Z. Proteomic analysis of young leaves at three developmental stages in an albino tea cultivar. Proteome Sci. 2011, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, C.; Ma, C.; Chen, L.; Yao, M. Transcriptome and biochemical analyses of a chlorophyll-deficient bud mutant of tea plant (Camellia sinensis). Int. J. Mol. Sci. 2023, 24, 15070. [Google Scholar] [CrossRef]
- Xu, P.; Yu, J.; Ma, R.; Ji, Y.; Hu, Q.; Mao, Y.; Ding, C.; Li, Z.; Ge, S.; Deng, W.-W.; et al. Chlorophyll and carotenoid metabolism varies with growth temperatures among tea genotypes with different leaf colors in Camellia sinensis. Int. J. Mol. Sci. 2024, 25, 10772. [Google Scholar] [CrossRef]
- Li, N.-N.; Lu, J.-L.; Li, Q.-S.; Zheng, X.-Q.; Wang, X.-C.; Wang, L.; Wang, Y.-C.; Ding, C.-Q.; Liang, Y.-R.; Yang, Y.-J. Dissection of chemical composition and associated gene expression in the pigment-deficient tea cultivar ‘Xiaoxueya’ reveals an albino phenotype and metabolite formation. Front. Plant Sci. 2019, 10, 1543. [Google Scholar] [CrossRef]
- Feng, L.; Gao, M.-J.; Hou, R.-Y.; Hu, X.-Y.; Zhang, L.; Wan, X.-C.; Wei, S. Determination of quality constituents in the young leaves of albino tea cultivars. Food Chem. 2014, 155, 98–104. [Google Scholar] [CrossRef]
- Pang, D.; Liu, Y.; Sun, Y.; Tian, Y.; Chen, L. Menghai Huangye, a novel albino tea germplasm with high theanine content and a high catechin index. Plant Sci. 2021, 311, 110997. [Google Scholar] [CrossRef]
- Xiong, L.; Li, J.; Li, Y.; Yuan, L.; Liu, S.; Huang, J.A.; Liu, Z. Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.). Plant Physiol. Biochem. 2013, 71, 132–143. [Google Scholar] [CrossRef]
- Li, M.-Y.; Liu, H.-Y.; Wu, D.-T.; Kenaan, A.; Geng, F.; Li, H.-B.; Gunaratne, A.; Li, H.; Gan, R.-Y. L-Theanine: A unique functional amino acid in tea (Camellia sinensis L.) with multiple health benefits and food applications. Front. Nutr. 2022, 9, 853846. [Google Scholar] [CrossRef]
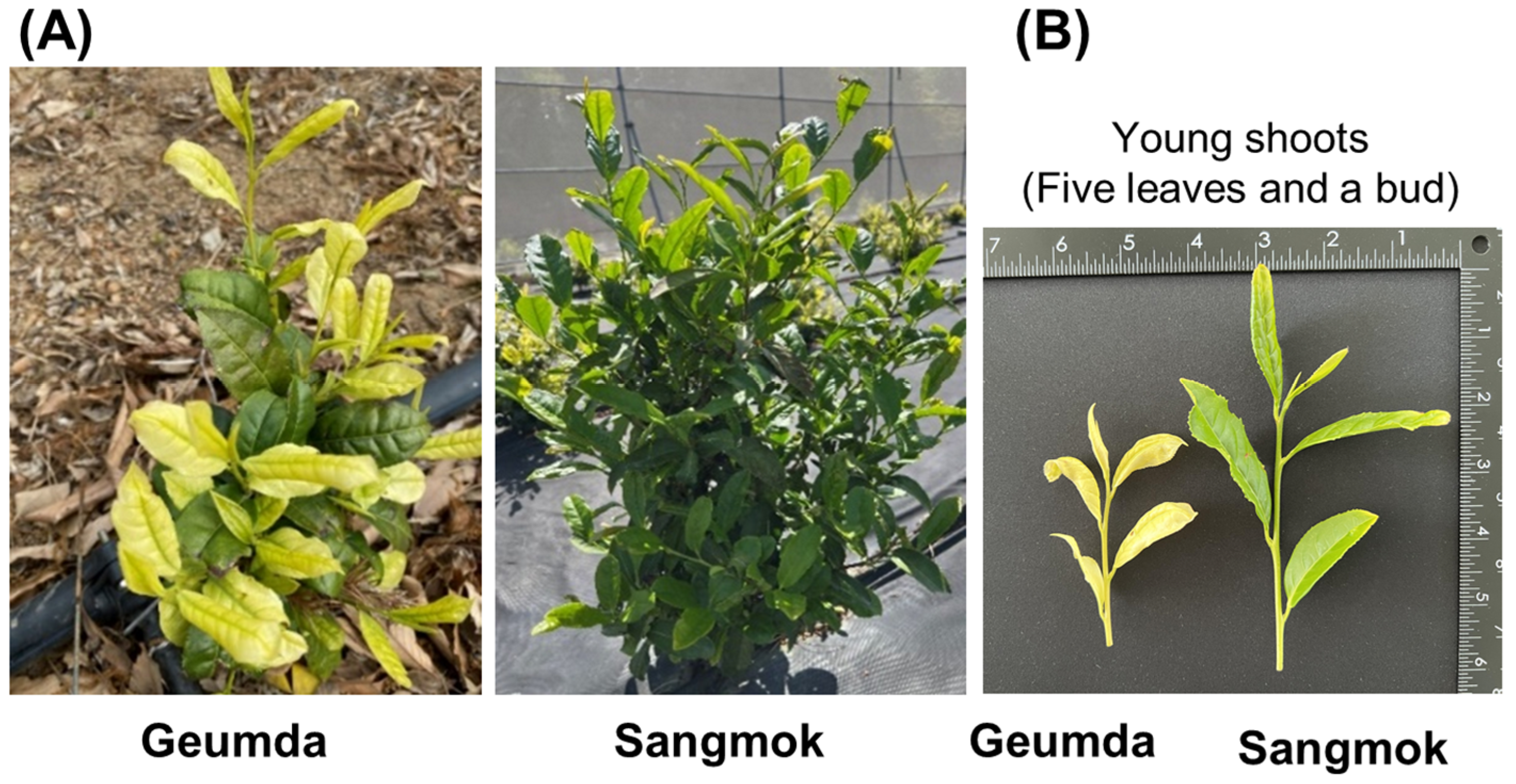

| Characteristics | Phenotypes | Score | Geumda | Sangmok | |
|---|---|---|---|---|---|
| 1 1 (*) 2 QN | Plant: vigor | Weak Middle Strong | 3 5 7 | 3 | 5 |
| 2 (*) 3 PQ | Plant: type | Shrub Semi-arbor Arbor | 1 2 3 | 1 | 1 |
| 3 (*) QN | Plant: growth habit | Upright Semi-upright Spreading | 1 3 5 | 3 | 3 |
| 4 QN | Plant: density of branches | Sparse Medium Dense | 3 5 7 | 3 | 3 |
| 5 4 QL | Branch: zigzagging | Absent Present | 1 9 | 1 | 1 |
| 6 (*) 5 (+) QN | Young shoot: time of beginning of ‘one and a bud’ stage | Early Medium Late | 3 5 7 | 5 | 3 |
| 7 PQ | Young shoot: color of second leaf at ‘two and a bud’ stage | Whitish Yellow Yellow-green Light green Middle green Purple-green | 1 2 3 4 5 6 | 2 | 4 |
| 8 (*) QL | Young shoot: pubescence of a bud | Absent Present | 1 9 | 9 | 9 |
| 9 QN | Young shoot: density of pubescence of a bud | Sparse Medium Dense | 3 5 7 | 3 | 3 |
| 10 QL | Young shoot: anthocyanin coloration at base of the petioles | Absent Present | 1 9 | 1 | 1 |
| 11 (*) QN | Young shoot: length of ‘three and a bud’ | Short Medium Long | 3 5 7 | 3 | 5 |
| 12 (*) QN | Leaf blade: attitude | Upwards Outwards Downwards | 1 3 5 | 3 | 3 |
| 13 (*) QN | Leaf blade: length | Short Medium Long | 3 5 7 | 5 | 5 |
| 14 (*) QN | Leaf blade: width | Narrow Medium Broad | 3 5 7 | 5 | 5 |
| 15 QN | Leaf blade: shape | Very narrow elliptic Narrow elliptic Medium elliptic Wide elliptic | 1 2 3 4 | 3 | 3 |
| 16 QN | Leaf blade: intensity of green color | Light Medium Dark | 3 5 7 | 3 | 7 |
| 17 QN | Leaf blade: shape in cross section | Folded upwards Flat Recurved | 1 2 3 | 2 | 2 |
| 18 QN | Leaf blade: texture of upper surface | Smooth or weakly rugose Moderately rugose Strongly rugose | 3 5 7 | 5 | 3 |
| 19 PQ | Leaf blade: shape of apex | Obtuse Acute Acuminate | 1 2 3 | 2 | 2 |
| 20 QN | Leaf blade: undulation of margin | Absent or weak Medium Strong | 3 5 7 | 3 | 3 |
| 21 QN | Leaf blade: serrations on the margin | Weak Medium Strong | 3 5 7 | 3 | 5 |
| 22 PQ | Leaf blade: shape of base | Acute Obtuse Truncate | 1 2 3 | 2 | 1 |
| 23 QN | Flower: time of full flowering | Early Medium Late | 3 5 7 | 7 | 3 |
| 24 QN | Flower: length of pedicel | Short Medium Long | 3 5 7 | 3 | 5 |
| 25 (*) QL | Flower: pubescence on outer side of sepal | Absent Present | 1 9 | 9 | 9 |
| 26 (*) QL | Flower: anthocyanin coloration on outer side of sepal | Absent Present | 1 9 | 1 | 1 |
| 27 (*) QN | Flower: diameter | Small Medium Large | 3 5 7 | 3 | 5 |
| 28 (+) PQ | Flower: color of inner petals | Greenish White Pink | 1 2 3 | 2 | 2 |
| 29 (*) QL | Flower: pubescence of ovary | Absent Present | 1 9 | 9 | 9 |
| 30 QN | Flower: density of pubescence of ovary | Sparse Medium Dense | 3 5 7 | 7 | 7 |
| 31 QN | Flower: length of style | Short Medium Long | 3 5 7 | 3 | 5 |
| 32 (+) QN | Flower: position of style splitting | Low Medium High | 3 5 7 | 7 | 5 |
| 33 (*) (+) QN | Flower: position of stigma relative to stamens | Below Same level Above | 3 5 7 | 3 | 5 |
| Geumda | Sangmok | |
|---|---|---|
| Length (cm) | 6.64 ± 0.72 *** | 8.22 ± 0.48 |
| Width (cm) | 2.96 ± 0.46 * | 3.41 ± 0.31 |
| Thickness (mm) | 0.41 ± 0.06 | 0.47 ± 0.06 |
| Leaf area (cm2) | 13.85 ± 3.04 *** | 19.68 ± 2.62 |
| Geumda | Sangmok | |
|---|---|---|
| Total free amino acids | 62.48 ± 3.38 ** | 30.00 ± 6.52 |
| Theanine | 54.47 ± 3.29 ** | 27.31 ± 6.00 |
| Aspartic acid | 1.24 ± 0.03 *** | 0.45 ± 0.01 |
| Glutamic acid | 1.78 ± 0.18 ** | 0.95 ± 0.19 |
| Serine | 0.64 ± 0.05 ** | 0.28 ± 0.02 |
| Threonine | 0.14 ± 0.02 * | 0.07 ± 0.00 |
| Alanine | 0.24 ± 0.02 ** | 0.09 ± 0.02 |
| Valine | 0.07 ± 0.00 ** | 0.04 ± 0.00 |
| Leucine | 0.07 ± 0.00 *** | 0.02 ± 0.00 |
| Lysine | 0.16 ± 0.02 | ND |
| Histidine | 0.13 ± 0.01 | ND |
| Arginine | 2.86 ± 0.11 *** | 0.29 ± 0.00 |
| Cysteine | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Methionine | 0.02 ± 0.00 | ND |
| Isoleucine | 0.02 ± 0.01 | ND |
| Phenylalanine | 0.04 ± 0.01 | ND |
| Taurine | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Citrulline | 0.05 ± 0.03 | 0.05 ± 0.01 |
| Ornithine | 0.42 ± 0.03 | 0.39 ± 0.06 |
| Gamma-aminobutyric acid | 0.06 ± 0.01 | 0.04 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-S.; Kim, S.J.; Hong, H.R.; Kim, B.-H.; Song, E.Y.; Kim, C.H.; Chen, L.; Moon, D.-G. Morphological and Biochemical Characteristics of a Novel Albino Tea Cultivar (Camellia sinensis ‘Geumda’). Horticulturae 2025, 11, 747. https://doi.org/10.3390/horticulturae11070747
Kwon Y-S, Kim SJ, Hong HR, Kim B-H, Song EY, Kim CH, Chen L, Moon D-G. Morphological and Biochemical Characteristics of a Novel Albino Tea Cultivar (Camellia sinensis ‘Geumda’). Horticulturae. 2025; 11(7):747. https://doi.org/10.3390/horticulturae11070747
Chicago/Turabian StyleKwon, Yun-Suk, Su Jin Kim, Ha Rim Hong, Byung-Hyuk Kim, Eun Young Song, Chun Hwan Kim, Liang Chen, and Doo-Gyung Moon. 2025. "Morphological and Biochemical Characteristics of a Novel Albino Tea Cultivar (Camellia sinensis ‘Geumda’)" Horticulturae 11, no. 7: 747. https://doi.org/10.3390/horticulturae11070747
APA StyleKwon, Y.-S., Kim, S. J., Hong, H. R., Kim, B.-H., Song, E. Y., Kim, C. H., Chen, L., & Moon, D.-G. (2025). Morphological and Biochemical Characteristics of a Novel Albino Tea Cultivar (Camellia sinensis ‘Geumda’). Horticulturae, 11(7), 747. https://doi.org/10.3390/horticulturae11070747








