Biochemical Characterization of Ornithine Decarboxylases from Solanaceae Plants Producing Tropane Alkaloids
Abstract
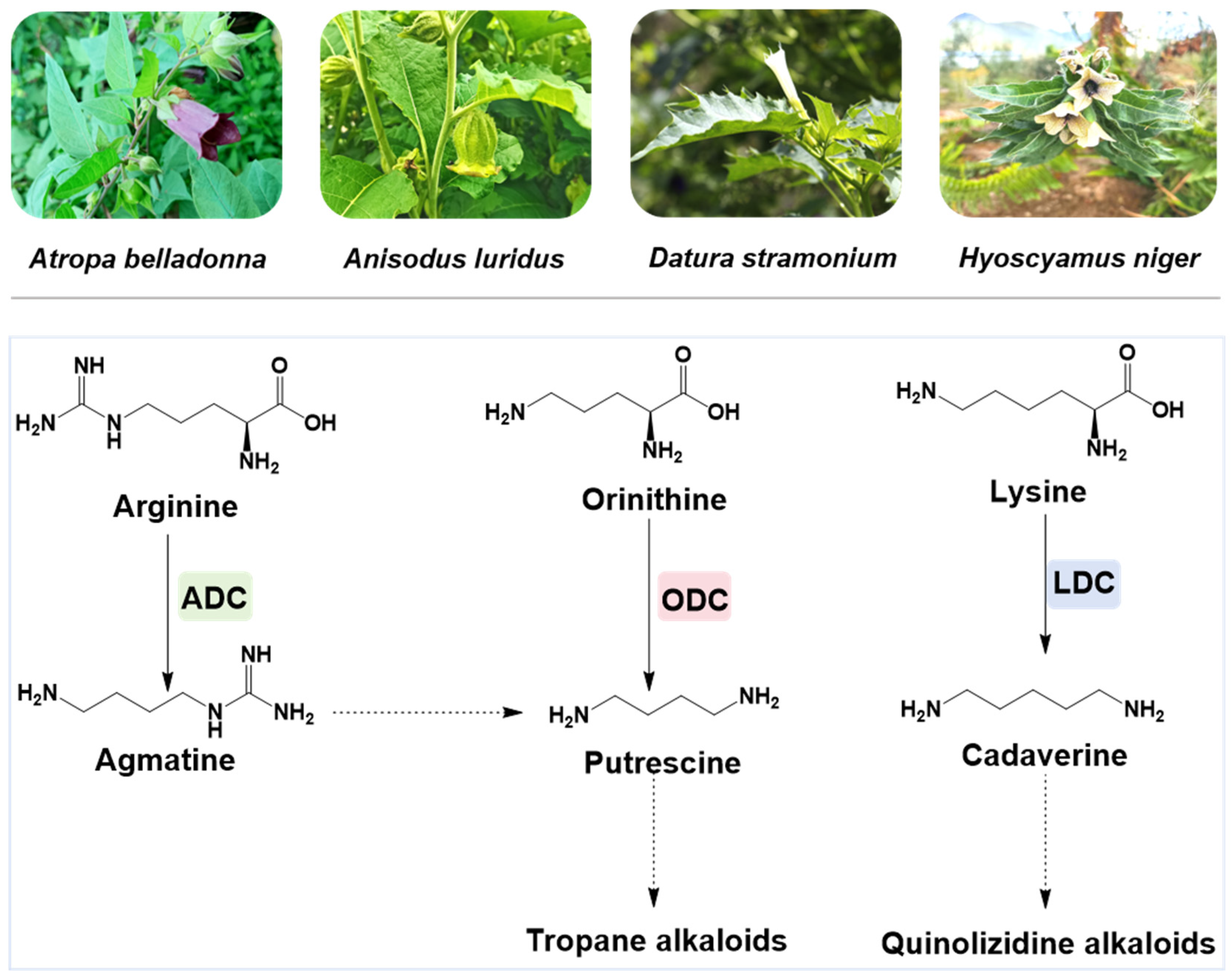
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Gene Cloning and Bioinformatics Analysis
2.3. Gene Expression Analysis
2.4. Protein Purification and ODC Activity Assays
2.5. LDC Activity Analysis
3. Results
3.1. Gene Cloning and Protein Sequence Alignment
3.2. Evolutionary Relationship Analysis
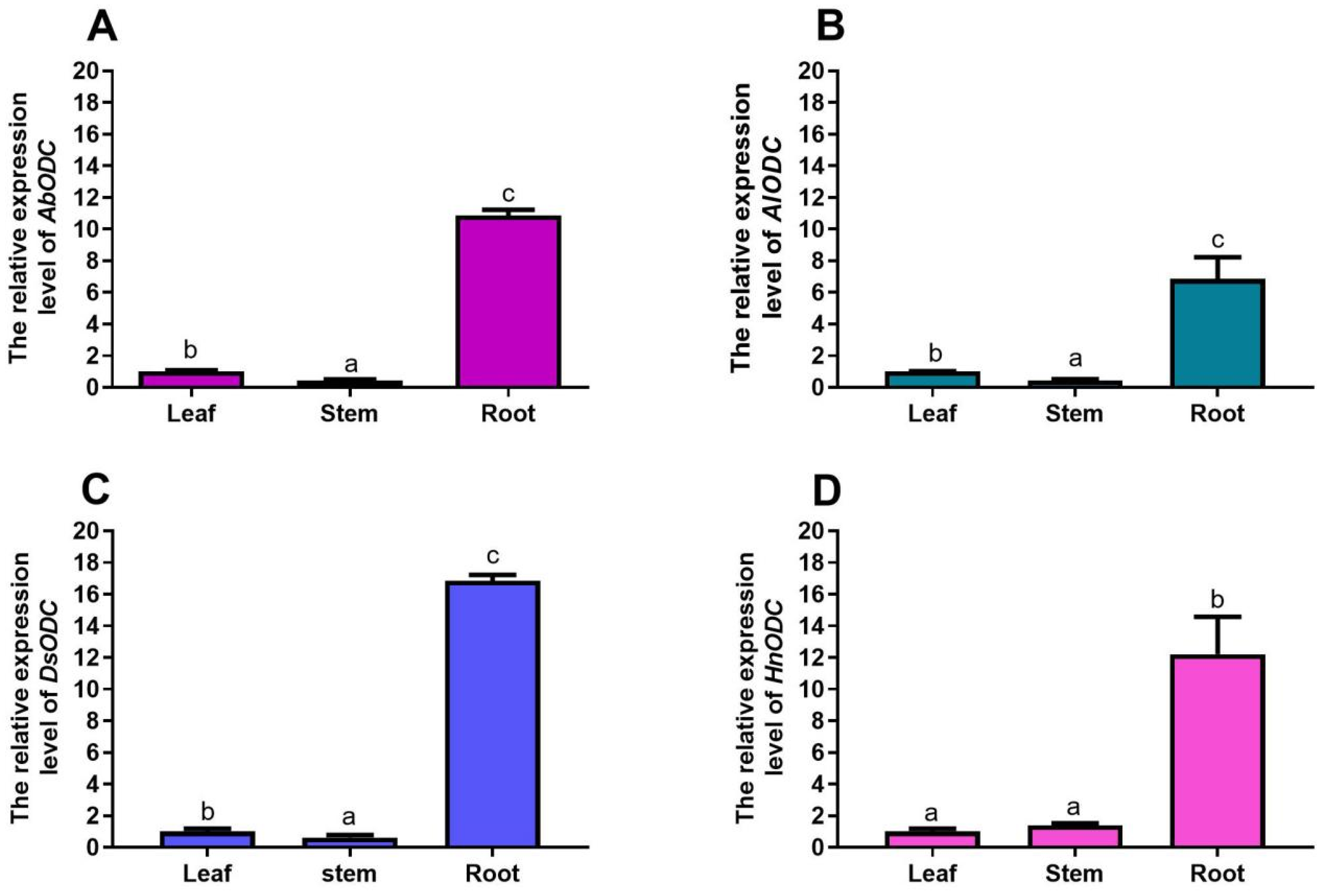
3.3. Gene Expression Analysis
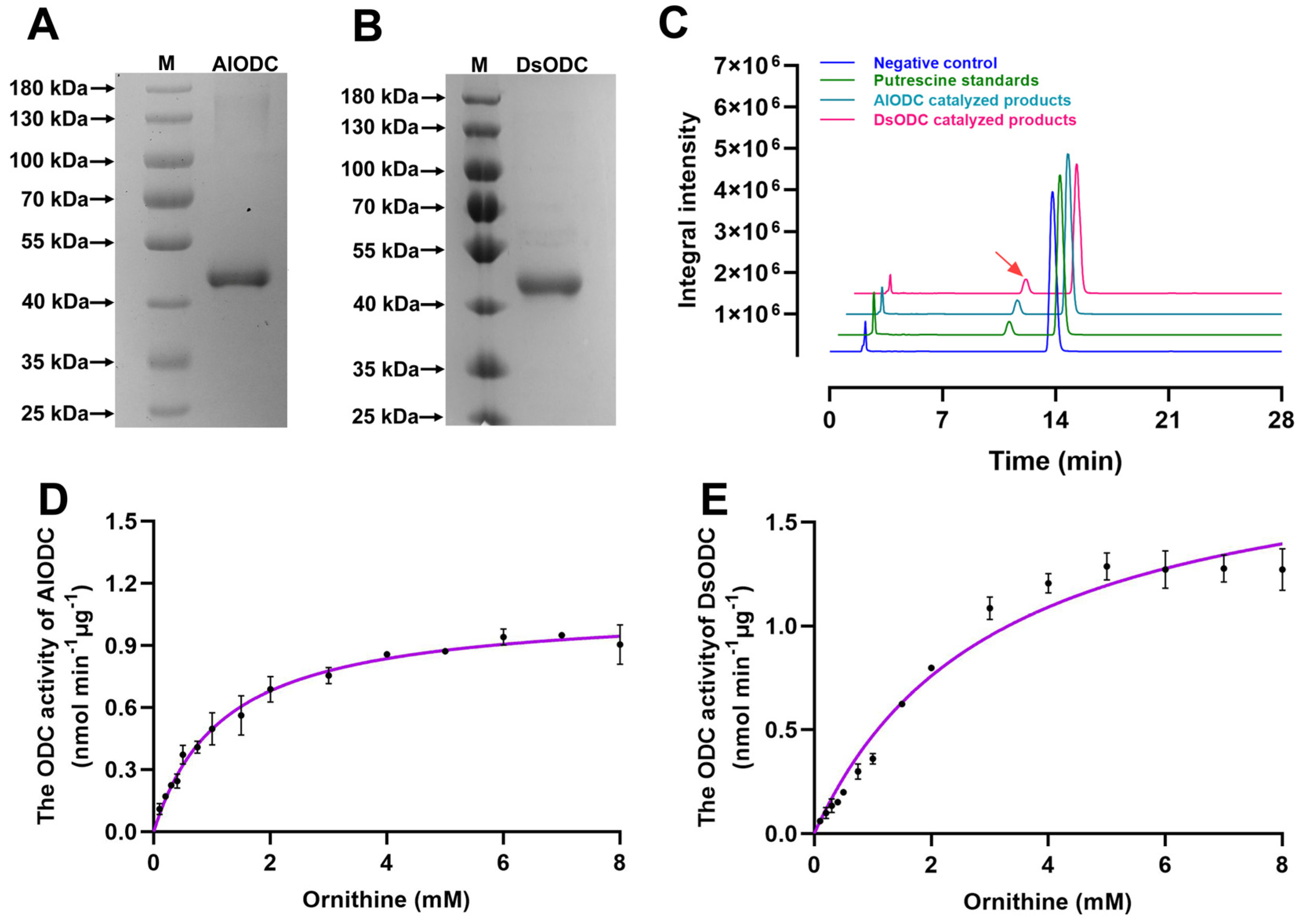
3.4. Enzyme Kinetics Analysis of AlODC and DsODC for Their ODC Activities
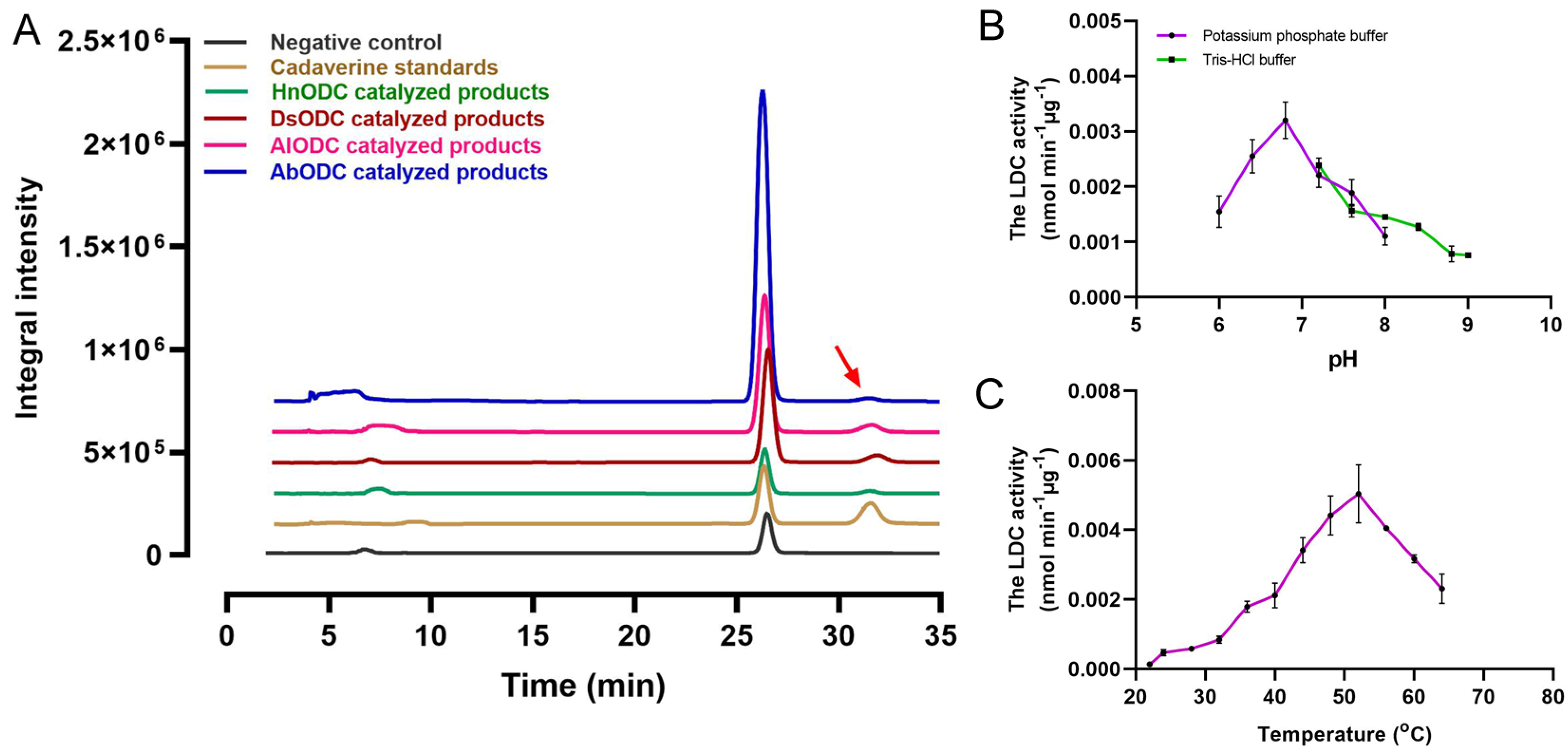
3.5. LDC Activity Identification and Optimal Condition Selection
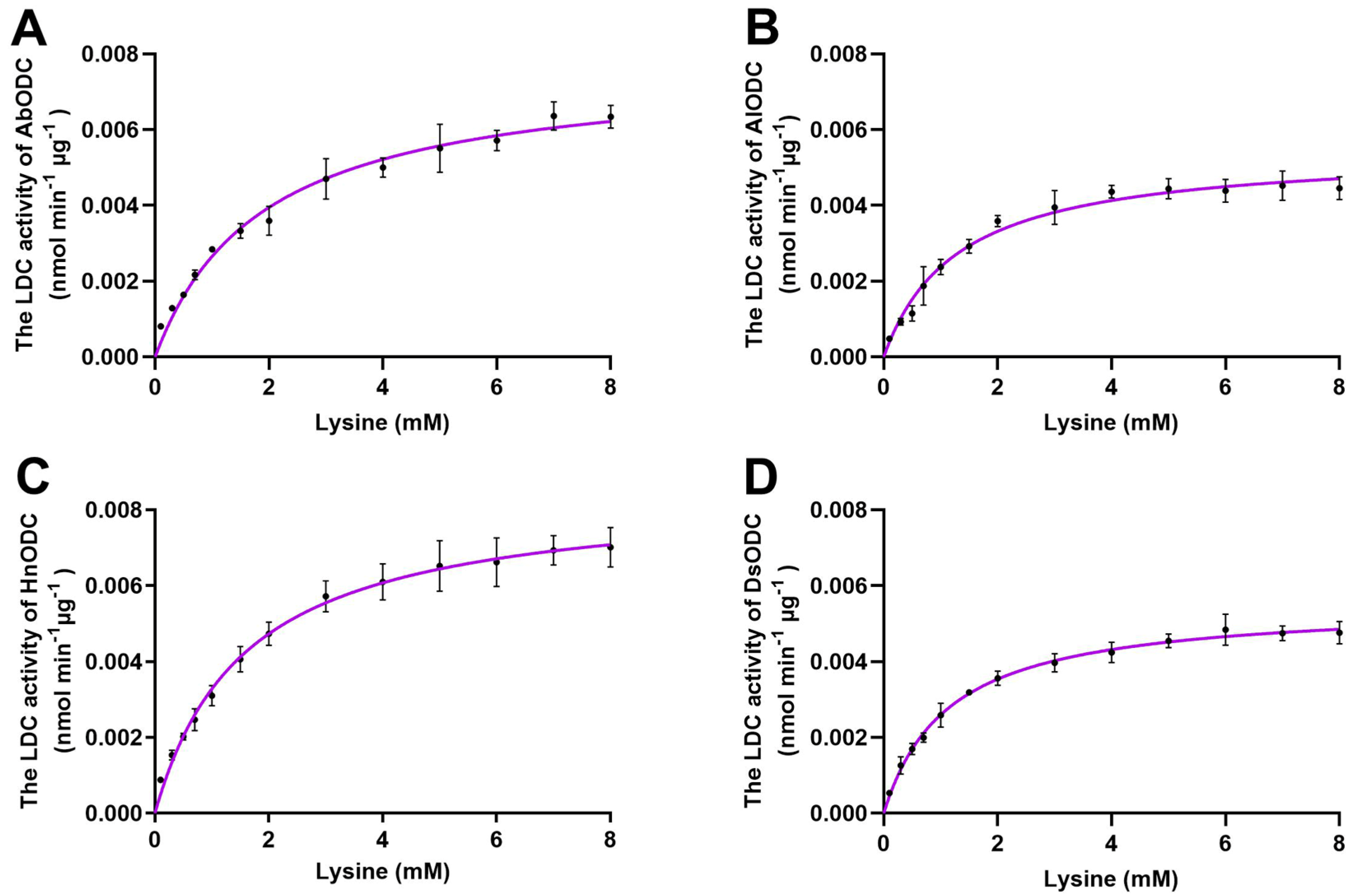
3.6. Enzyme Kinetic Analysis of ODCs for Their LDC Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine Catabolism: Target for Antiproliferative Therapies in Animals and Stress Tolerance Strategies in Plants. Amino Acids 2012, 42, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Wohlleben, W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Jancewicz, A.L.; Gibbs, N.M.; Masson, P.H. Cadaverine’s Functional Role in Plant Development and Environmental Response. Front. Plant Sci. 2016, 7, 870. [Google Scholar] [CrossRef]
- Bunsupa, S.; Katayama, K.; Ikeura, E.; Oikawa, A.; Toyooka, K.; Saito, K.; Yamazakia, M. Lysine Decarboxylase Catalyzes the First Step of Quinolizidine Alkaloid Biosynthesis and Coevolved with Alkaloid Production in Leguminosae. Plant Cell 2012, 24, 1202–1216. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kaur, Y.; Das, N. Roles of Polyamines in Growth and Development of the Solanaceous Crops Under Normal and Stressful Conditions. J. Plant Growth Regul. 2023, 42, 4989–5010. [Google Scholar] [CrossRef]
- Bunsupa, S.; Yamazaki, M.; Saito, K. Quinolizidine Alkaloid Biosynthesis: Recent Advances and Future Prospects. Front. Plant Sci. 2012, 3, 239. [Google Scholar] [CrossRef]
- Bunsupa, S.; Yamazaki, M.; Saito, K. Lysine-Derived Alkaloids: Overview and Update on Biosynthesis and Medicinal Applications with Emphasis on Quinolizidine Alkaloids. Mini-Rev. Med. Chem. 2017, 17, 1002–1012. [Google Scholar] [CrossRef]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Gadzikowska, M. Tropane Alkaloids as Medicinally Useful Natural Products and Their Synthetic Derivatives as New Drugs. Pharmacol. Rep. 2008, 60, 439–463. [Google Scholar]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef] [PubMed]
- Chavez, B.G.; Srinivasan, P.; Glockzin, K.; Kim, N.; Montero Estrada, O.; Jirschitzka, J.; Rowden, G.; Shao, J.; Meinhardt, L.; Smolke, C.D.; et al. Elucidation of Tropane Alkaloid Biosynthesis in Erythroxylum coca Using a Microbial Pathway Discovery Platform. Proc. Natl. Acad. Sci. USA 2022, 119, e2215372119. [Google Scholar] [CrossRef]
- Dunn, M.F.; Becerra-Rivera, V.A. The Biosynthesis and Functions of Polyamines in the Interaction of Plant Growth-Promoting Rhizobacteria with Plants. Plants 2023, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Hanfrey, C.; Sommer, S.; Mayer, M.J.; Burtin, D.; Michael, A.J. Arabidopsis Polyamine Biosynthesis: Absence of Ornithine Decarboxylase and the Mechanism of Arginine Decarboxylase Activity. Plant J. 2001, 27, 551–560. [Google Scholar] [CrossRef]
- Shimizu, Y.; Rai, A.; Okawa, Y.; Tomatsu, H.; Sato, M.; Kera, K.; Suzuki, H.; Saito, K.; Yamazaki, M. Metabolic diversification of nitrogen-containing metabolites by the expression of a heterologous lysine decarboxylase gene in Arabidopsis. Plant J. 2019, 100, 505–521. [Google Scholar] [CrossRef]
- Kumria, R.; Rajam, M.V. Ornithine Decarboxylase Transgene in Tobacco Affects Polyamines, in Vitro-Morphogenesis and Response to Salt Stress. J. Plant Physiol. 2002, 159, 983–990. [Google Scholar] [CrossRef]
- Choubey, A.; Rajam, M.V. Transcriptome response and developmental implications of RNAi-mediated ODC knockdown in tobacco. Funct. Integr. Genom. 2017, 17, 399–412. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, M.; Htun, Z.L.L.; Zhou, J.; Zeng, J.; Qiu, F.; Zhang, H.; Lan, X.; Chen, M.; Liao, Z. Engineering the Production of Scopolamine and Cold Tolerance through Overexpressing ODC and H6H in Atropa belladonna. Ind. Crops Prod. 2024, 208, 117886. [Google Scholar] [CrossRef]
- Zhao, T.; Li, S.; Wang, J.; Zhou, Q.; Yang, C.; Bai, F.; Lan, X.; Chen, M.; Liao, Z. Engineering Tropane Alkaloid Production Based on Metabolic Characterization of Ornithine Decarboxylase in Atropa belladonna. ACS Synth. Biol. 2020, 9, 437–448. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, Y.D. Identification of Essential Active-Site Residues in Ornithine Decarboxylase of Nicotiana glutinosa Decarboxylating Both L-Ornithine and L-Lysine. Biochem. J. 2001, 360, 657–665. [Google Scholar] [CrossRef]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The First Step in the Biosynthesis of Cocaine in Erythroxylum coca: The Characterization of Arginine and Ornithine Decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Zeng, J.; Qiu, F.; Zhang, F.; Liao, Z. De Novo Synthesis of Anticholinergic Hyoscyamine and Scopolamine in Nicotiana benthamiana Based on Elucidating Tropane Alkaloid Biosynthetic Pathway of Anisodus luridus. Agronomy 2024, 14, 2460. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Qiu, F.; Qin, B.; Liu, W.; Wu, N.; Lan, X.; Wang, Q.; Liao, Z.; Tang, K. Reference Gene Selection for Gene Expression Studies Using Quantitative Real-Time PCR Normalization in Atropa belladonna. Plant Mol. Biol. Rep. 2014, 32, 1002–1014. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Lu, C.; Ma, W.; Guan, J.; Gong, C.; Yang, J.; Zhang, H.; Zhang, K.; ByteDance AML AI4Science Team; et al. Protenix—Advancing Structure Prediction Through a Comprehensive AlphaFold3 Reproduction. bioRxiv 2025. [Google Scholar] [CrossRef]
- Bedewitz, M.A.; Góngora-Castillo, E.; Uebler, J.B.; Gonzales-Vigil, E.; Wiegert-Rininger, K.E.; Childs, K.L.; Hamilton, J.P.; Vaillancourt, B.; Yeo, Y.S.; Chappell, J.; et al. A root-expressed L-phenylalanine:4-hydroxyphenylpyruvate aminotransferase is required for tropane alkaloid biosynthesis in Atropa belladonna. Plant Cell 2014, 26, 3745–3762. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J.; Eisenthal, R.; Lee, C.K.; Peterson, M.E. The Effect of Temperature on Enzyme Activity: New Insights and Their Implications. Extremophiles 2008, 12, 51–59. [Google Scholar] [CrossRef]
- Pegg, A.E. Regulation of Ornithine Decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef]
- Brewer, M.; Janakiram, N.B.; Biddick, L.; Lightfoot, S.; Ritchie, R.L.; Patlolla, J.M.R.; Mohammed, A.; Madka, V.; Steele, V.E.; Rao, C.V.; et al. Eflornithine (DFMO) Prevents Progression of Pancreatic Cancer by Modulating Ornithine Decarboxylase Signaling. Cancer Prev. Res. 2014, 7, 1198–1209. [Google Scholar]
- Illingworth, C.; Michael, A.J. Interactions of the Human, Plant and Yeast Ornithine Decarboxylase Subunits and Human Antizyme. Biochem. Soc. Trans. 1998, 26, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine Metabolism and Cancer: Treatments, Challenges and Opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Love, R.R.; Astrow, S.H.; Cheeks, A.M.; Havighurst, T.C. Ornithine Decarboxylase (ODC) as a Prognostic Factor in Operable Breast Cancer. Breast Cancer Res. Treat. 2003, 79, 329–334. [Google Scholar] [CrossRef]
- Kusano, T.; Kim, D.W.; Liu, T.; Berberich, T. Polyamine catabolism in plants. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Springer: Tokyo, Japan, 2015; pp. 77–88. [Google Scholar]
- Michael, A.J.; Furze, J.M.; Rhodes, M.J.C.; Burtin, D. Molecular Cloning and Functional Identification of a Plant Ornithine Decarboxylase CDNA. Biochem. J. 1996, 314, 241–248. [Google Scholar] [CrossRef]
- Dalton, H.L.; Blomstedt, C.K.; Neale, A.D.; Gleadow, R.; Deboer, K.D.; Hamill, J.D. Effects of Down-Regulating Ornithine Decarboxylase upon Putrescine-Associated Metabolism and Growth in Nicotiana tabacum L. J. Exp. Bot. 2016, 67, 3367–3381. [Google Scholar] [CrossRef] [PubMed]
- Tashi, T.; Hu, X.; Zhao, T.; Lan, X.; Liao, Z. Effects of Ornithine Decarboxylase Inhibitor on the Growth of Hyoscyamus niger and the Biosynthesis of Tropine Alkaloids. Zhiwu Shengli Xuebao/Plant Physiol. J. 2020, 56, 1818–1824. [Google Scholar]
- Carrizo, C.N.; Pitta-Alvarez, S.I.; Kogan, M.J.; Giulietti, A.M.; Tomaro, M.L. Occurrence of cadaverine in hairy roots of Brugmansia candida. Phytochemistry 2001, 57, 759–763. [Google Scholar] [CrossRef]

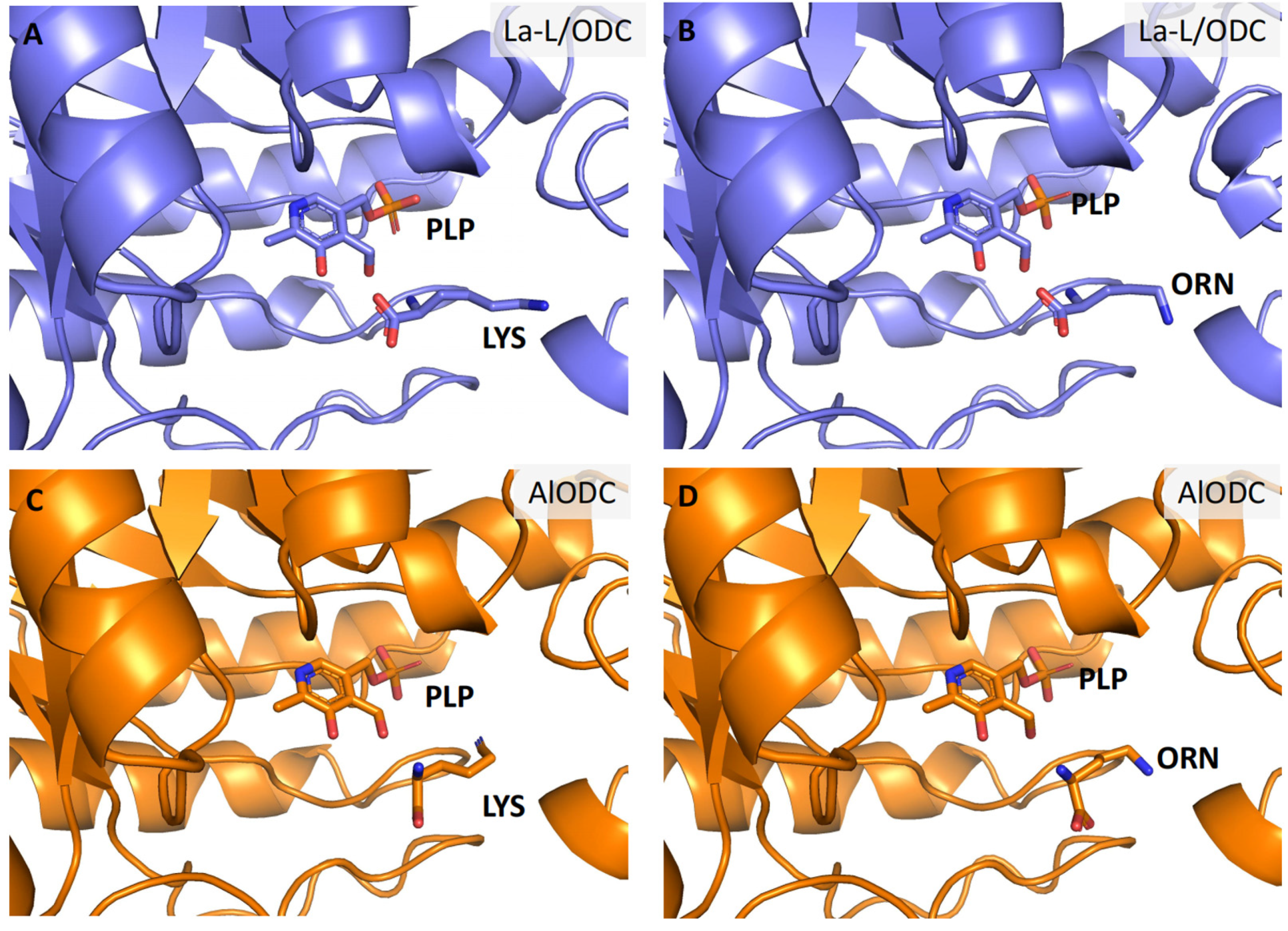
 represents those reported ODCs.
represents those reported ODCs.  represents those reported L/ODCs.
represents those reported L/ODCs.
 represents those reported ODCs.
represents those reported ODCs.  represents those reported L/ODCs.
represents those reported L/ODCs.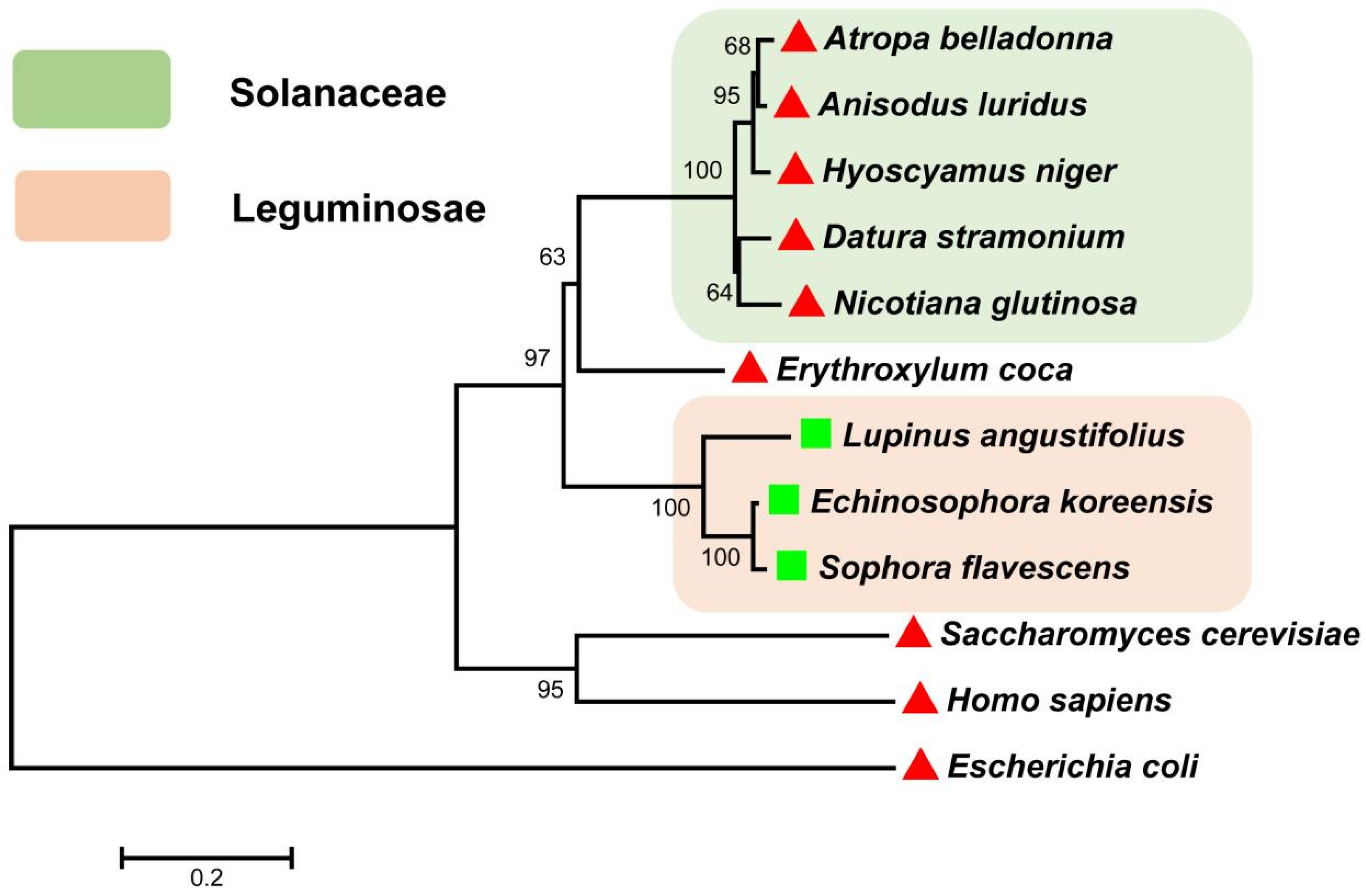
| Km (mM) | Vmax (nmol min−1 μg−1) | Kcat (s−1) | Kcat/Km (M−1s−1) | Ratio of Kcat/Km | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | ODC | LDC | ODC | LDC | ODC | LDC | ODC | LDC | ODC/LDC | |
| AlODC | 1.19 | 1.30 | 1.09 | 5.47 × 10−3 | 0.91 | 4.57 × 10−3 | 761 | 3.52 | 216 | this study |
| DsODC | 3.11 | 1.13 | 1.94 | 5.54 × 10−3 | 1.62 | 4.64 × 10−3 | 523 | 4.11 | 127 | this study |
| AbODC | 1.03 | 1.90 | 0.93 | 7.69 × 10−3 | 0.67 | 6.42 × 10−3 | 649 | 3.38 | 192 | this study and [19] |
| HnODC | 2.62 | 1.59 | 1.87 | 8.49 × 10−3 | 1.57 | 7.10 × 10−3 | 599 | 4.47 | 134 | this study and [19] |
| EcODC | 0.40 | ND | 0.25 | ND | 0.18 | ND | 465 | ND | - | [21] |
| NgODC | 0.56 | 1.59 | 1.25 × 10−2 | 1.52 × 10−4 | 9.3 × 10−3 | 1.13 × 10−4 | 16.53 | 0.07 | 233 | [20] |
| La-L/ODC | 1.05 | 2.37 | 1.14 | 1.49 | 0.91 | 1.18 | 859 | 433 | 1.90 | [4] |
| Sf-L/ODC | 1.53 | 2.10 | 1.46 | 2.94 | 1.16 | 2.23 | 755 | 1108 | 0.68 | |
| Ek-L/ODC | 1.32 | 3.85 | 0.76 | 2.42 | 0.73 | 1.91 | 454 | 469 | 0.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Zhao, T.; Wang, M.; Sun, Y.; Liu, C.; Lan, X.; Song, P.; Liao, Z. Biochemical Characterization of Ornithine Decarboxylases from Solanaceae Plants Producing Tropane Alkaloids. Horticulturae 2025, 11, 748. https://doi.org/10.3390/horticulturae11070748
Zeng L, Zhao T, Wang M, Sun Y, Liu C, Lan X, Song P, Liao Z. Biochemical Characterization of Ornithine Decarboxylases from Solanaceae Plants Producing Tropane Alkaloids. Horticulturae. 2025; 11(7):748. https://doi.org/10.3390/horticulturae11070748
Chicago/Turabian StyleZeng, Lingjiang, Tengfei Zhao, Mengxue Wang, Yifan Sun, Chengcun Liu, Xiaozhong Lan, Peng Song, and Zhihua Liao. 2025. "Biochemical Characterization of Ornithine Decarboxylases from Solanaceae Plants Producing Tropane Alkaloids" Horticulturae 11, no. 7: 748. https://doi.org/10.3390/horticulturae11070748
APA StyleZeng, L., Zhao, T., Wang, M., Sun, Y., Liu, C., Lan, X., Song, P., & Liao, Z. (2025). Biochemical Characterization of Ornithine Decarboxylases from Solanaceae Plants Producing Tropane Alkaloids. Horticulturae, 11(7), 748. https://doi.org/10.3390/horticulturae11070748







