Identification of Glucose-6-Phosphate Dehydrogenase Family Members Associated with Cold Stress in Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Identification of G6PDH Genes in Pepper
2.3. Sequence Analysis and Evolutionary Tree Construction of CaG6PDH Members
2.4. Conservative Motif and Gene Structure Analysis of G6PDH in Solanaceae
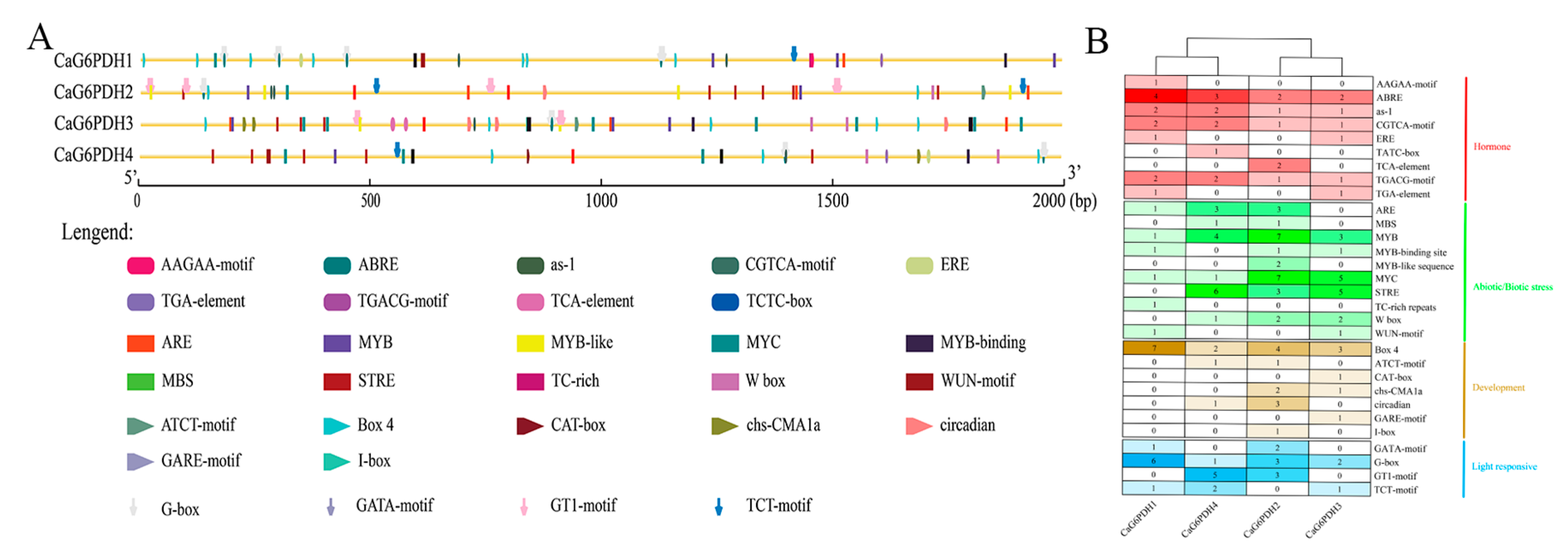
2.5. Analysis of Cis-Acting Elements of CaG6PDH Gene Family in Pepper
2.6. Analysis of Gene Collinearity and Selective Pressure
2.7. G6PDH Gene Expression Analysis of RNA-Seq Data Under Different Phytohormone and Stresses Treatments
2.8. RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
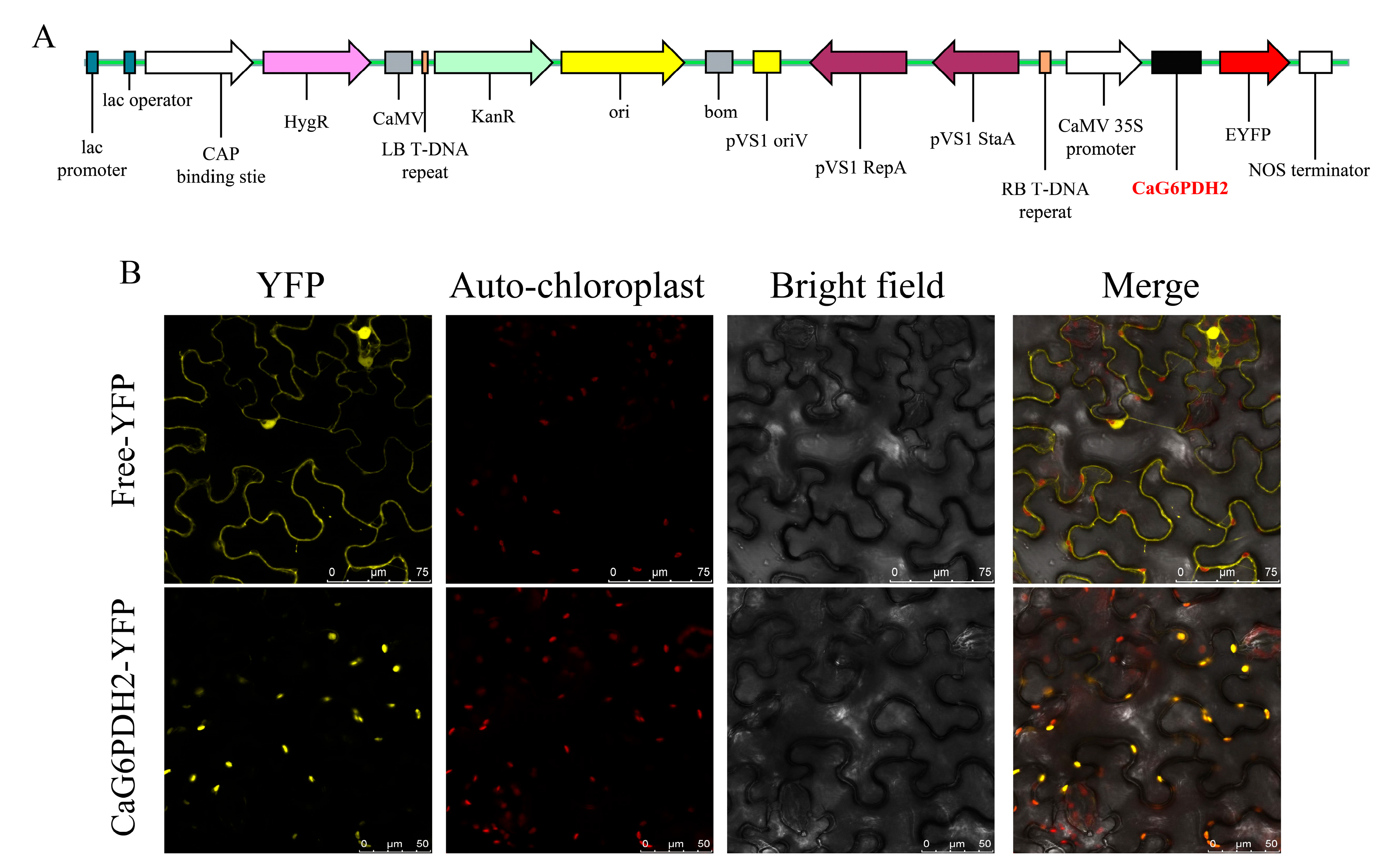
2.9. Subcellular Localization of CaG6PDH2
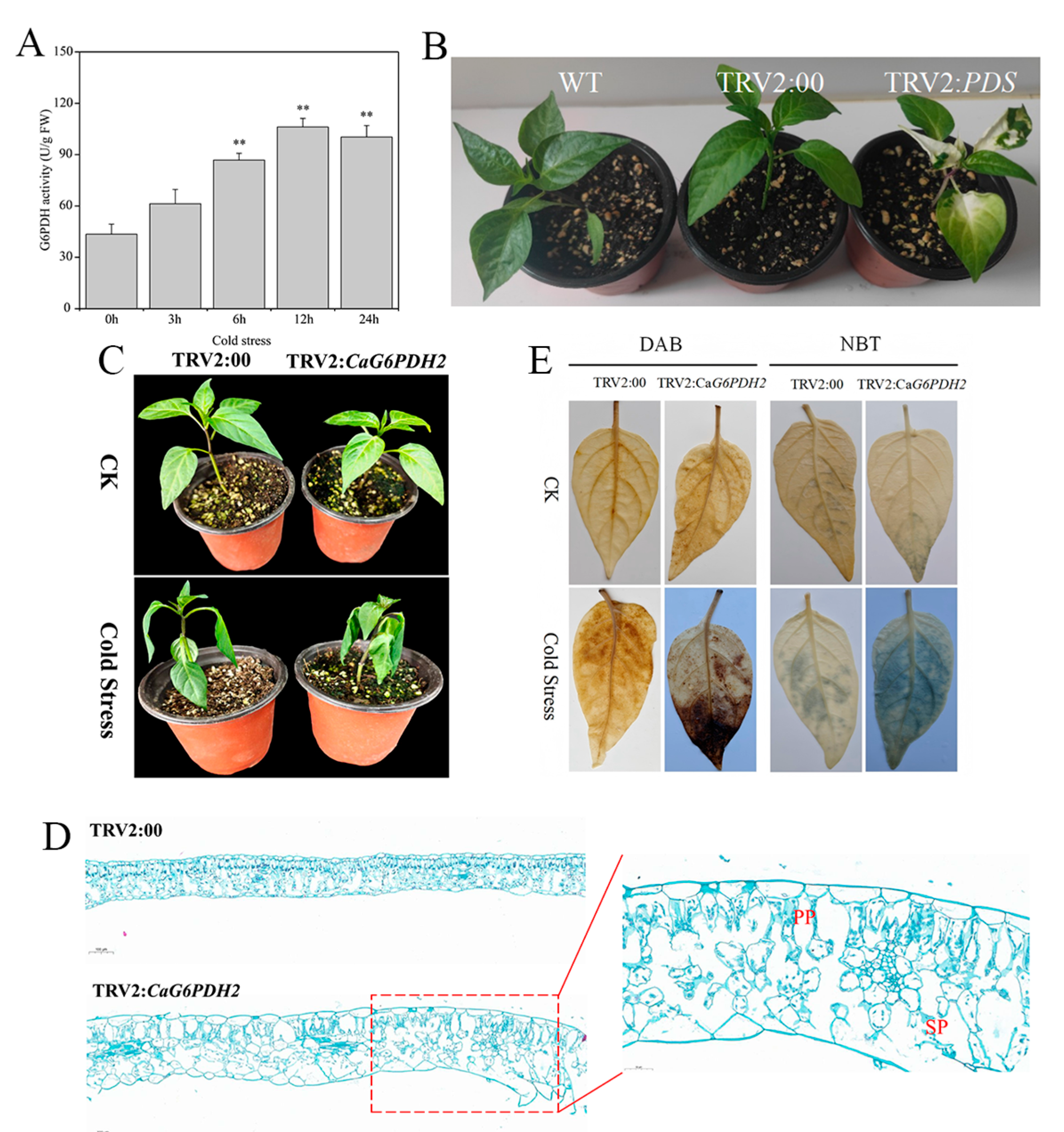
2.10. VIGS of CaG6PDH2 Genes in Pepper
2.11. Pepper Materials and Stress Treatment
2.12. Measurement of Biochemical Indicators
2.13. Statistical Analysis
3. Results
3.1. Identification and Characterization of CaG6PDHs in Pepper Genome
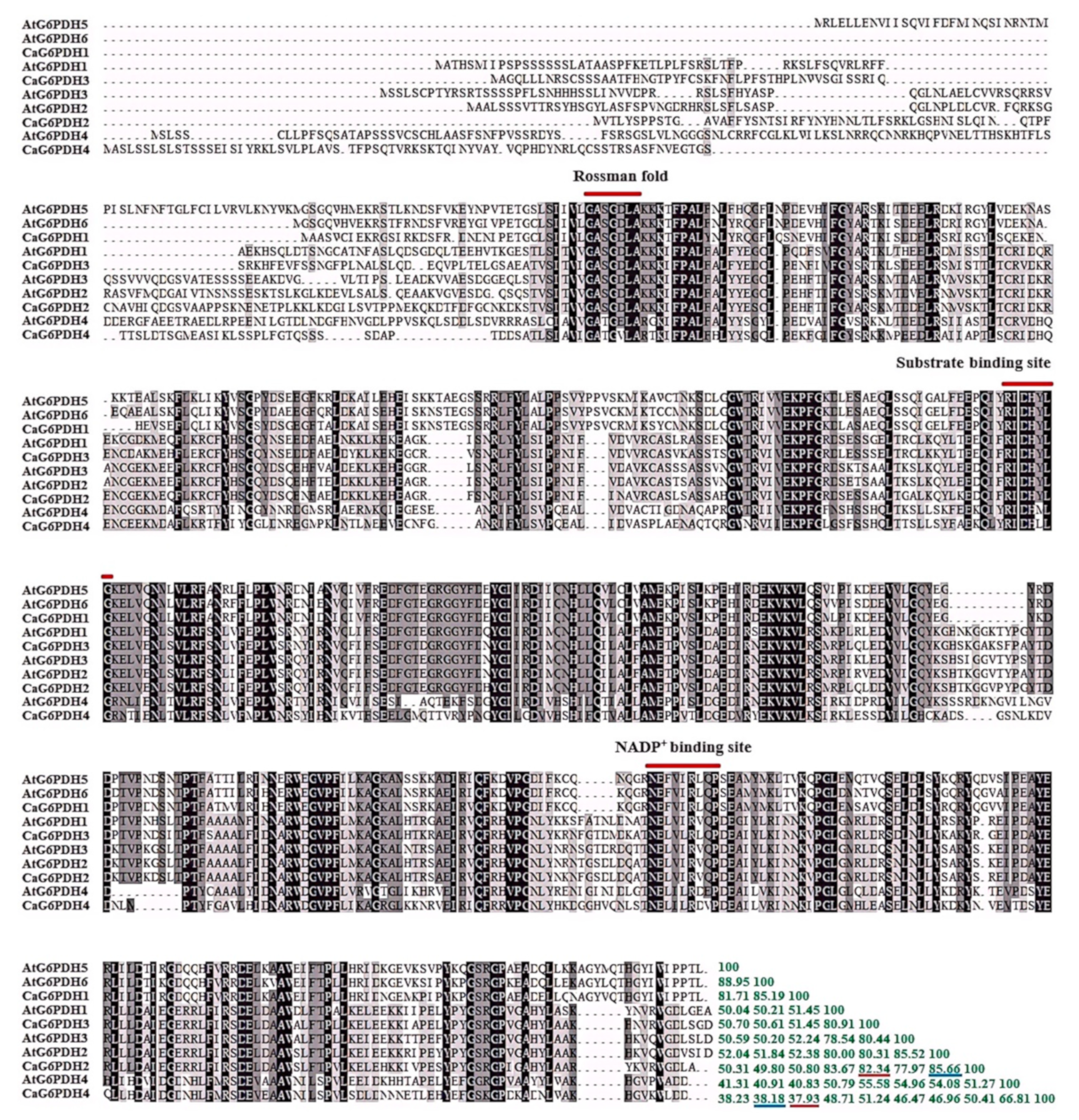
3.2. Sequence Alignment and Conserved Domain Analysis
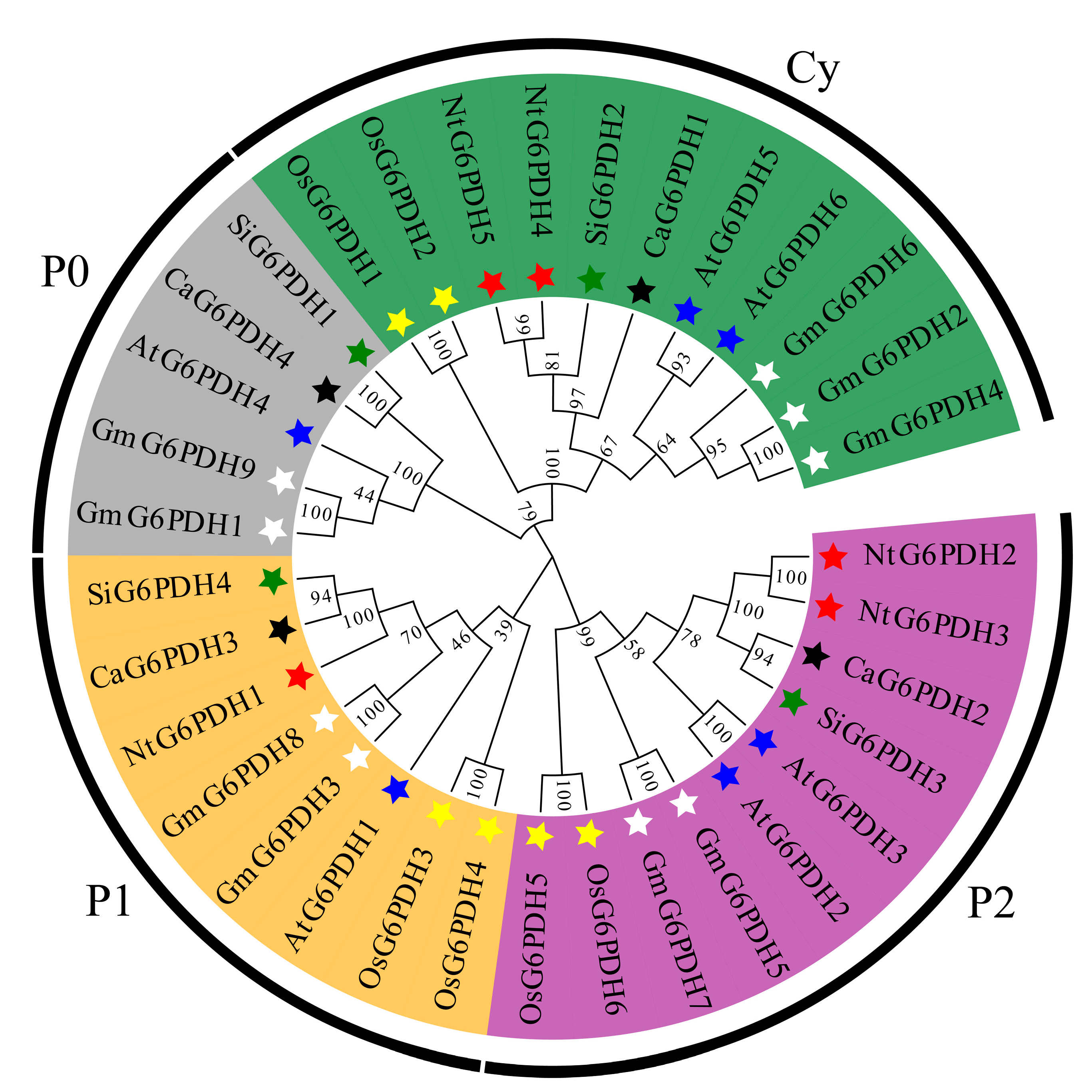
3.3. Phylogenetic and Collinearity Analysis
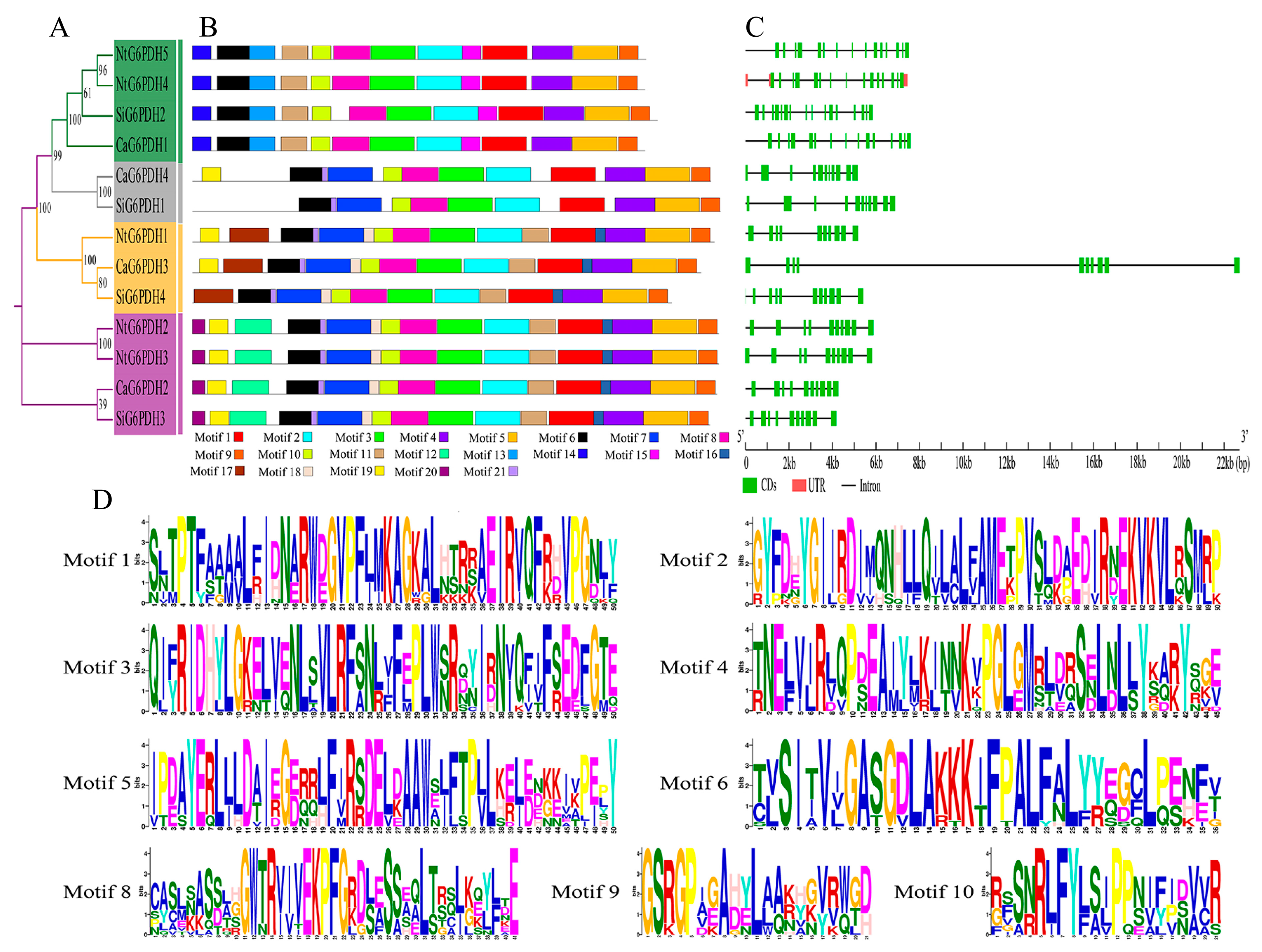
3.4. Motifs and Gene Structure Analysis of G6PDH in Solanaceae
3.5. Cis-Acting Element Analysis of CaG6PDH in Pepper
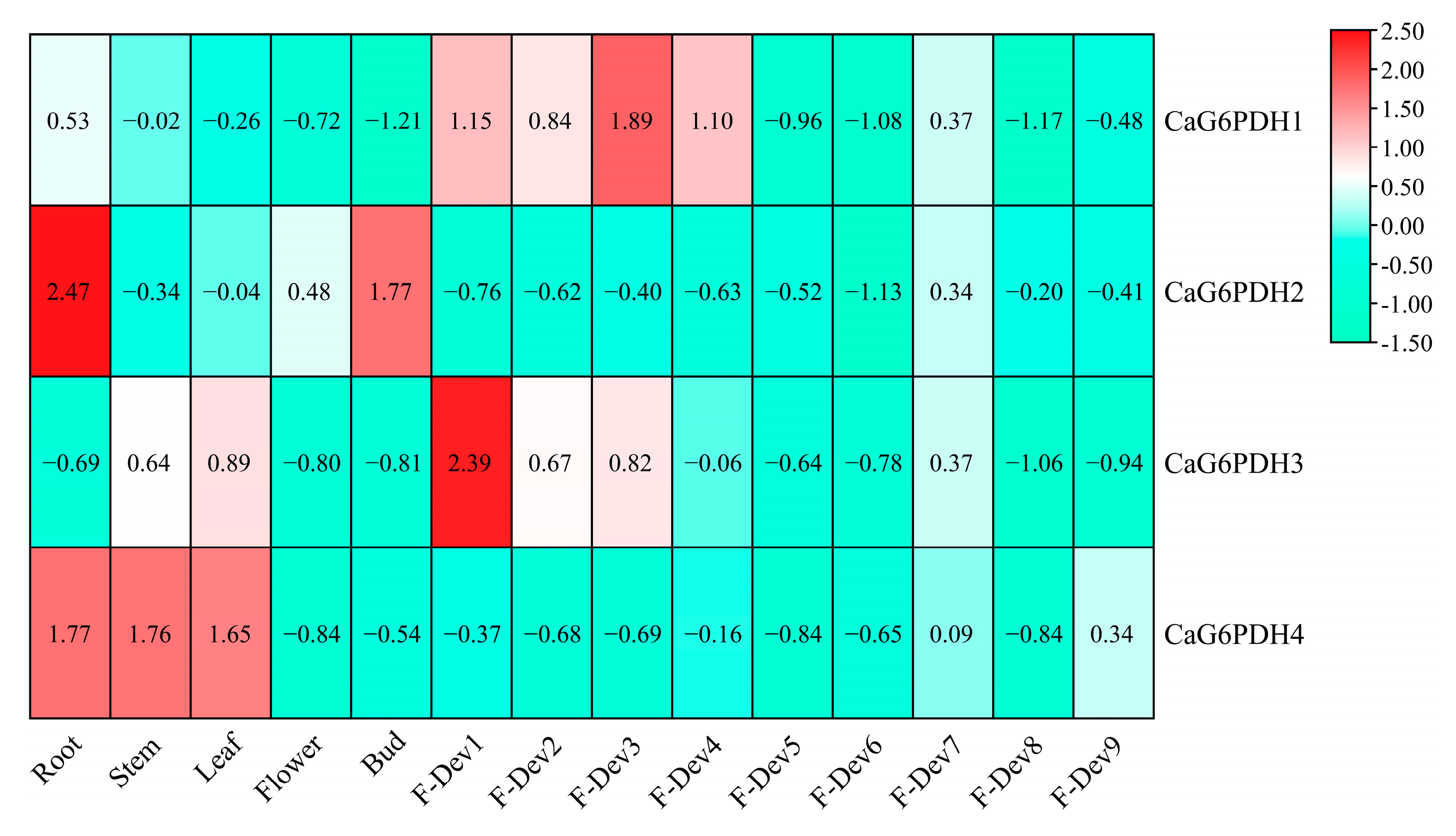
3.6. Expression Profile of CaG6PDHs in Different Tissues
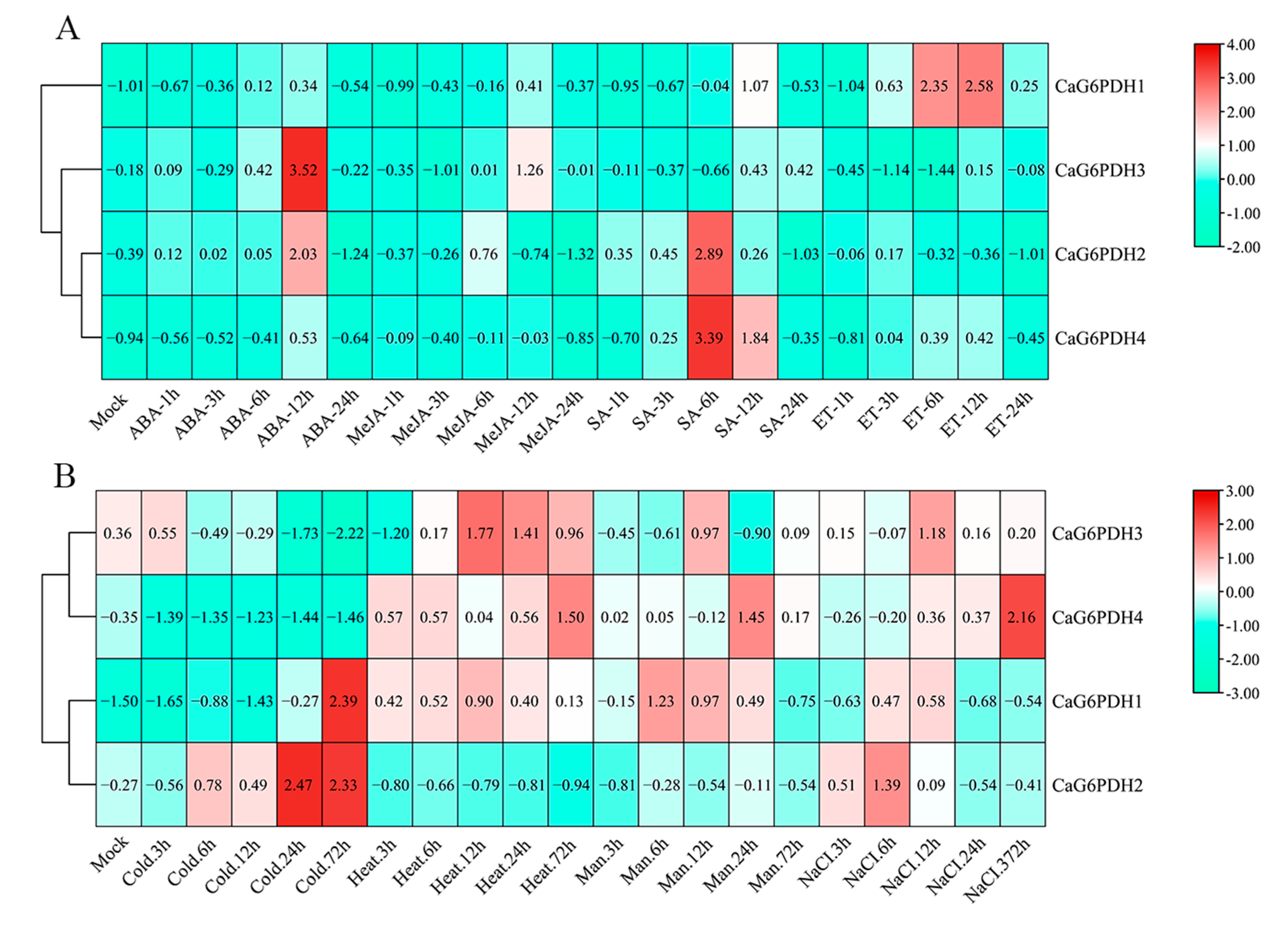
3.7. Expression Profiles of CaG6PDHs Under Different Phytohormone and Stresses Treatments
3.8. Analysis of CaG6PDH2 Subcellular Localization
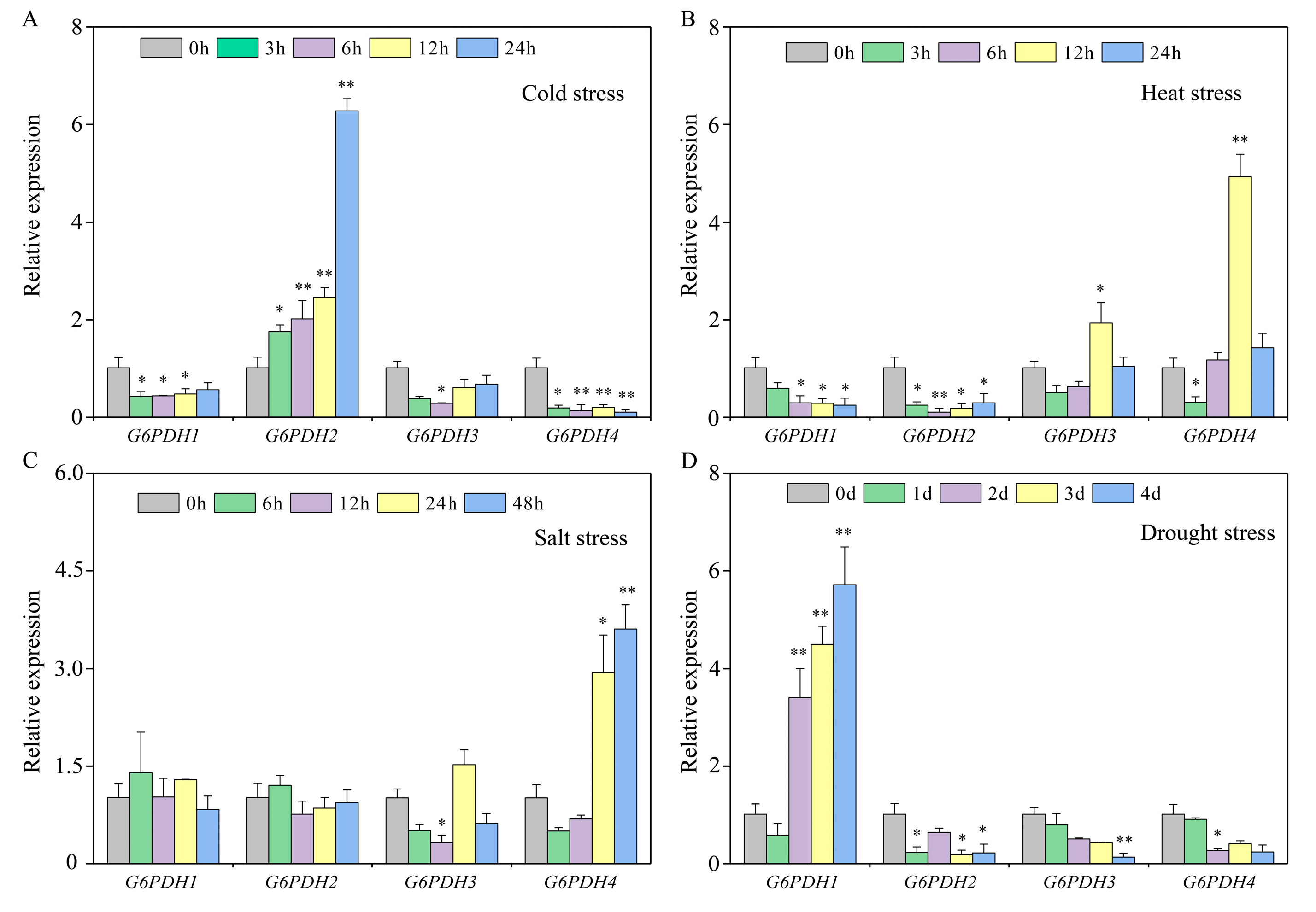
3.9. Identification of Tolerance of Pepper to Cold Stress Treatment
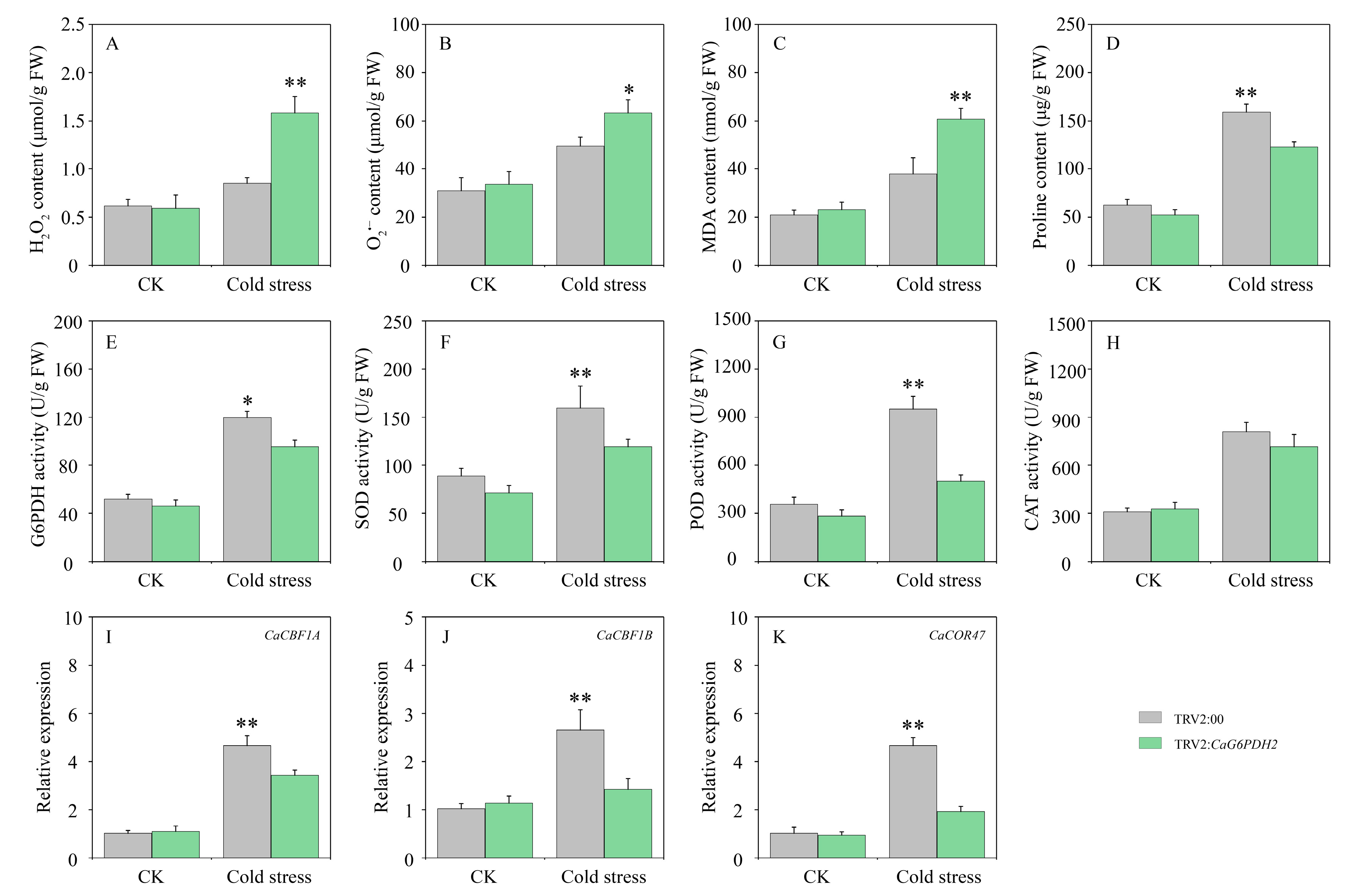
3.10. Determination of Physiological and Biochemical Indicators of Pepper Under Cold Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krüger, A.; Grüning, N.M.; Wamelink, M.M.; Kerick, M.; Kirpy, A.; Parkhomchuk, D.; Bluemlein, K.; Schweiger, M.R.; Soldatov, A.; Lehrach, H. The pentose phosphate pathway is a metabolic redox sensor and regulates transcription during the antioxidant response. Antioxid. Redox Signal. 2011, 15, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [PubMed]
- Wood, T. Physiological functions of the pentose phosphate pathway. Cell Biochem. Funct. 1986, 4, 241–247. [Google Scholar] [CrossRef]
- von Schaewen, A.; Langenkamper, G.; Graeve, K.; Wenderoth, I.; Scheibe, R. Molecular characterization of the plastidic glucose-6-phosphate-dehydrogenase from potato in comparison to its cytosolic counterpart. Plant Physiol. 1995, 109, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J.; von Schaewen, A. The oxidative pentose phosphate pathway: Structure and organisation. Curr. Opin. Plant Biol. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Chang, N.; Nan, W.; Wang, S.; Ruan, M.; Bi, Y. Cytosolic glucose-6-phosphate dehydrogenase is involved in seed germination and root growth under salinity in Arabidopsis. Front. Plant Sci. 2019, 10, 182. [Google Scholar] [CrossRef]
- Wakao, S.; Andre, C.; Benning, C. Functional analyses of cytosolic glucose-6-phosphate dehydrogenases and their contribution to seed oil accumulation in Arabidopsis. Plant Physiol. 2008, 146, 277–288. [Google Scholar] [CrossRef]
- Wakao, S.; Benning, C. Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 2005, 41, 243–256. [Google Scholar] [CrossRef]
- Siddappaji, M.H.; Scholes, D.R.; Bohn, M.; Paige, K.N. Overcompensation in response to herbivory in Arabidopsis thaliana: The role of glucose-6-phosphate dehydrogenase and the oxidative pentose-phosphate pathway. Genetics 2013, 195, 589–598. [Google Scholar] [CrossRef]
- Gong, H.; Chen, G.; Li, F.; Wang, X.; Hu, Y.; Bi, Y. Involvement of G6PDH in heat stress tolerance in the calli from Przewalskia tangutica and Nicotiana tabacum. Biol. Plant. 2012, 56, 422–430. [Google Scholar] [CrossRef]
- Lei, D.Y.; Lin, Y.X.; Luo, M.W.; Zhao, B.; Tang, H.L.; Zhou, X.; Yao, W.T.; Zhang, Y.T.; Wang, Y.; Li, M.Y.; et al. Genome-wide investigation of G6PDH gene in strawberry: Evolution and expression analysis during development and stress. Int. J. Mol. Sci. 2022, 23, 4728. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.J.; Wang, X.M.; He, L.; Wang, S.W.; Li, J.J.; Jin, J.; Bi, Y.R. Identification, characterization, and stress responsiveness of glucose-6-phosphate dehydrogenase genes in highland barley. Plants 2020, 9, 1800. [Google Scholar] [CrossRef] [PubMed]
- Long, X.Y.; He, B.; Fang, Y.J.; Tang, C.R. Identification and Characterization of the Glucose-6-Phosphate dehydrogenase gene family in the para rubber tree, Hevea brasiliensis. Front. Plant Sci. 2016, 7, 215. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.W.; Sun, L.L.; Ruan, M.J.; Li, S.F.; He, R.; Zhang, W.Y.; Liang, C.F.; Wang, X.M.; Bi, Y.R. Involvement of G6PD5 in ABA response during seed germination and root growth in Arabidopsis. BMC Plant Biol. 2019, 19, 44. [Google Scholar] [CrossRef]
- Kong, W.F.; Chen, J.Y.; Hou, Z.X.; Wen, P.F.; Zhan, J.C.; Pan, Q.H.; Huang, W.D. Activity and subcellular localization of glucose-6-phosphate dehydrogenase in peach fruits. J. Plant Physiol. 2007, 164, 934–944. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Taboada, J.; Palma, J.M.; Corpas, F.J. In Silico RNAseq and Biochemical Analyses of Glucose-6-Phosphate Dehydrogenase (G6PDH) from Sweet Pepper Fruits: Involvement of Nitric Oxide (NO) in Ripening and Modulation. Plants 2023, 12, 3408. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.L.; Ge, Y.H.; Li, C.Y.; Han, X.; Qin, S.C.; Chen, Y.R.; Tang, Q.; Li, J.R. G6PDH regulated NADPH production and reactive oxygen species metabolism to enhance disease resistance against blue mold in apple fruit by acibenzolar-S-methyl. Postharvest Biol. Technol. 2019, 148, 228–235. [Google Scholar] [CrossRef]
- Wei, X.B.; Huang, X.L.; Yang, W.L.; Wang, X.R.; Guan, T.; Kang, Z.S.; Liu, J. A chloroplast-localized Glucose-6-Phosphate dehydrogenase positively regulates stripe rust resistance in wheat. Int. J. Mol. Sci. 2022, 24, 459. [Google Scholar] [CrossRef]
- Hu, Y.F.; You, J.; Li, J.S.; Wang, C.L. Loss of cytosolic glucose-6-phosphate dehydrogenase increases the susceptibility of Arabidopsis thaliana to root-knot nematode infection. Ann. Bot. 2019, 123, 37–46. [Google Scholar] [CrossRef]
- Nemoto, Y.; Sasakuma, T. Specific expression of glucose-6-phosphate dehydrogenase (G6PDH) gene by salt stress in wheat (Triticum aestivum L.). Plant Sci. 2000, 158, 53–60. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Wang, X.M.; Bi, Y.R. Glucose-6-phosphate dehydrogenase acts as a regulator of cell redox balance in rice suspension cells under salt stress. Plant Growth Regul. 2013, 69, 139–148. [Google Scholar] [CrossRef]
- Wang, H.H.; Hou, J.J.; Li, Y.; Zhang, Y.Y.; Huang, J.J.; Liang, W.H. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 2017, 416, 39–52. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, S.; Guo, H.; Zhang, Z.; Chen, X. Functional analysis of PsG6PDH, a cytosolic glucose-6-phosphate dehydrogenase gene from Populus suaveolens, and its contribution to cold tolerance improvement in tobacco plants. Biotechnol. Lett. 2013, 35, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Luo, M.W.; Cheng, L.J.; Lin, Y.X.; Chen, Q.; Sun, B.; Gu, X.J.; Wang, Y.; Li, M.Y.; Luo, Y.; et al. Identification of the cytosolic Glucose-6-Phosphate dehydrogenase gene from strawberry involved in cold stress response. Int. J. Mol. Sci. 2020, 21, 7322. [Google Scholar] [CrossRef]
- Li, X.; Cai, Q.; Yu, T.; Li, S.J.; Li, S.N.; Li, Y.L.; Sun, Y.; Ren, H.L.; Zhang, J.J.; Zhao, Y.; et al. ZmG6PDH1 in glucose-6-phosphate dehydrogenase family enhances cold stress tolerance in maize. Front Plant Sci. 2023, 14, 1116237. [Google Scholar] [CrossRef]
- Zou, Z.Y.; Zou, X.X. Geographical and ecological differences in pepper cultivation and consumption in China. Front. Nutr. 2021, 8, 718517. [Google Scholar] [CrossRef]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins-Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.P.; Guo, A.Y.; Zhang, H.; Luo, J.C.; Gao, C. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative Toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, M.; Yeom, S.; Kim, Y.M.; Lee, J.M.; Lee, H.A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Qin, C.; Yu, C.S.; Shen, Y.O.; Fang, X.D.; Chen, L.; Min, J.M.; Cheng, J.W.; Zhao, S.C.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild pepper provides insights into capsicum domesti-cation and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef]
- Lee, J.; Nam, J.Y.; Jang, H.; Kim, N.; Kim, Y.M.; Kang, W.H.; Yeom, S.I. Comprehensive transcriptome resource for response to phytohormone-induced signaling in Capsicum annuum L. BMC Res. Notes 2020, 13, 440. [Google Scholar] [CrossRef]
- Kang, W.H.; Sim, Y.M.; Koo, N.; Nam, J.Y.; Lee, J.; Kim, N.; Jang, H.; Kim, Y.M.; Yeom, S.I. Transcriptome profiling of abiotic responses to heat, cold, salt, and osmotic stress of Capsicum annuum L. Sci. Data 2020, 7, 17. [Google Scholar] [CrossRef]
- Yin, Y.X.; Wang, S.B.; Xiao, H.J.; Zhang, H.X.; Zhang, Z.; Jing, H.; Zhang, Y.L.; Chen, R.G.; Gong, Z.H. Overexpression of the CaTIP1-1 pepper gene in tobacco enhances resistance to osmotic stresses. Int. J. Mol. Sci. 2014, 15, 20101–20116. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fernandez-Pozo, N.; Rosli, H.G.; Martin, G.B.; Mueller, L.A. The SGN VIGS Tool: User-friendly software to design virus-induced gene silencing (VIGS) constructs for functional genomics. Mol. Plant 2014, 8, 486–488. [Google Scholar] [CrossRef]
- Velásquez, A.; Chakravarthy, S.; Martin, G. Virus-induced gene silencing (VIGS) in Nicotiana benthamiana and tomato. J. Vis. Exp. 2009, 28, e1292. [Google Scholar] [CrossRef]
- Zhang, J.W.; Chen, Y.; He, J.; Wang, D.; Jiang, Y.; Chen, X.J.; Yang, Q.; Li, H.X. Genome-wide identification of LACS family genes and functional characterization of CaLACS6/9 in response to cold stress in pepper (Capsicum annuum L.). Agronomy 2025, 15, 970. [Google Scholar] [CrossRef]
- Landi, S.; Nurcato, B.; Lillo, A.D.; Lentini, M.; Grillo, S.; Esposito, S. Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato (Solanum lycopersicum) plants to short and long-term drought. Plant Physiol. Biochem. 2016, 105, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Han, R.Z.; Ji, F.; Yu, Y.Y.; Wang, R.Y.; Hai, Z.X.; Liang, W.H.; Wang, H.H. Glucose-6-phosphate dehydrogenase and abscisic acid mediate programmed cell death induced by aluminum toxicity in soybean root tips. J. Hazard. Mater. 2022, 425, 127964. [Google Scholar] [CrossRef]
- Jin, J.; Li, K.K.; Qin, J.; Yan, L.L.; Wang, S.W.; Zhang, G.H.; Wang, X.M.; Bi, Y.R. The response mechanism to salt stress in Arabidopsis transgenic lines over-expressing of GmG6PD. Plant Physiol. Biochem. 2021, 162, 74–85. [Google Scholar] [CrossRef]
- Ito, T.M.; Trevizan, C.B.; Santos, T.B.D.; Souza, S.G.H.D. Genome-wide identification and characterization of the Dof transcription factor gene family in Phaseolus vulgaris L. Am. J. Plant Sci. 2017, 8, 3233–3257. [Google Scholar] [CrossRef]
- Consortium, T.G. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217, 109–119. [Google Scholar] [CrossRef]
- Islam, T.; Manna, M.; Kaul, T.; Pandey, S.; Reddy, C.S.; Reddy, M. Genome-wide dissection of Arabidopsis and rice for the identification and expression analysis of glutathione peroxidases reveals their stress-specific and overlapping response patterns. Plant Mol. Biol. Rep. 2015, 33, 1413–1427. [Google Scholar] [CrossRef]
- Esposito, S. Nitrogen assimilation, abiotic stress and Glucose 6-Phosphate dehydrogenase: The full circle of reductants. Plants 2016, 5, 24. [Google Scholar] [CrossRef]
- Dimitrios, T. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Baxter, A.; Suzuki, N.; Mittler, R. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, K.K.; Bao, Y.Z.; Zhang, D.; Meng, J.; Wang, D.J.; Wang, X.N.; Cang, J. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase genes of winter wheat enhance the cold tolerance of transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 161, 86–97. [Google Scholar] [CrossRef] [PubMed]
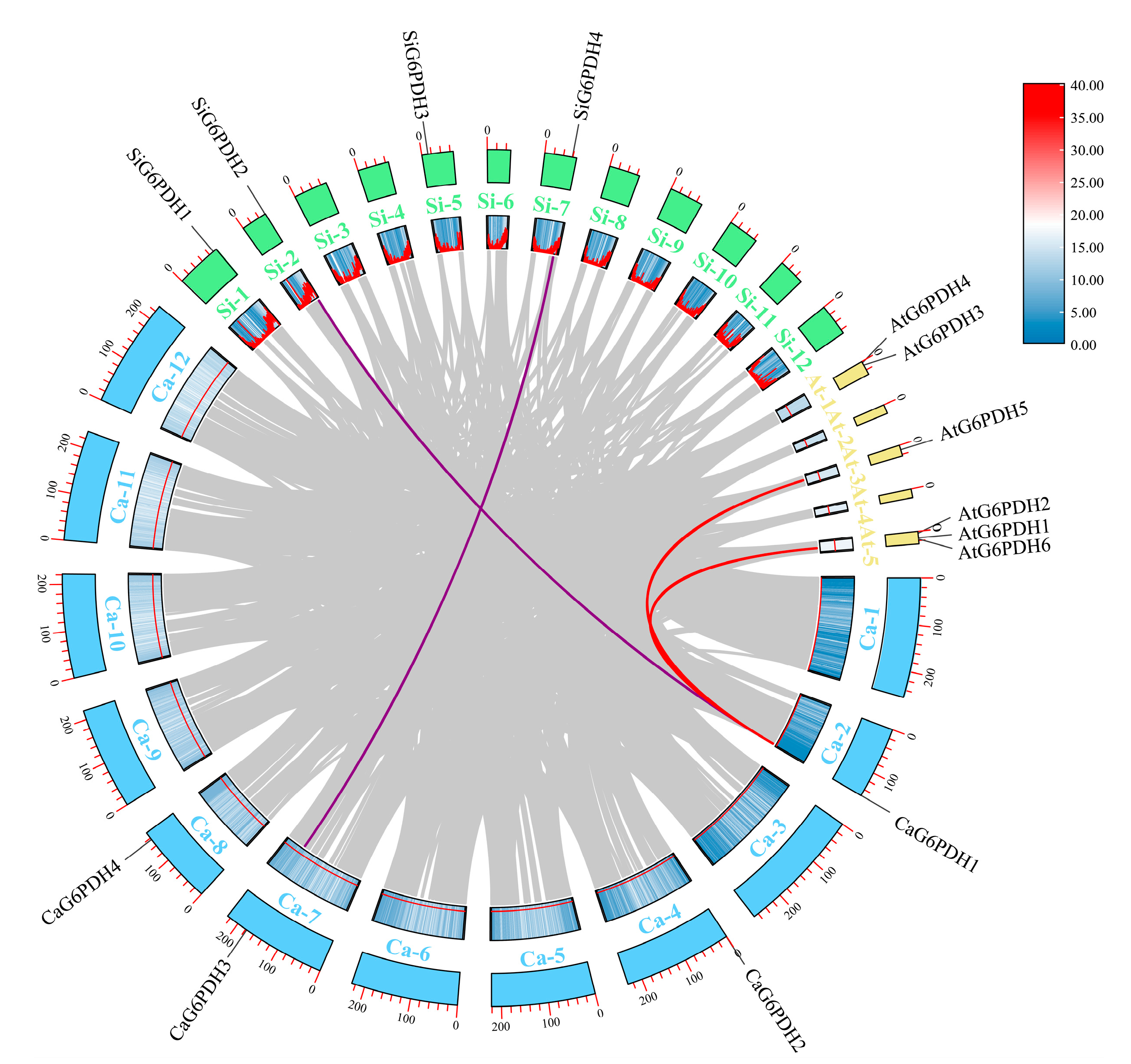
| Species | Homologous Gene Pairs | Ka | Ks | Ka/Ks | Divergent Time (Mya) |
|---|---|---|---|---|---|
| Pepper/Arabidopsis | CaG6PDH1/AtG6PDH5 | 0.115 | 1.82 | 0.063 | 116.15 |
| CaG6PDH1/AtG6PDH6 | 0.095 | 2.22 | 0.043 | 141.35 | |
| Pepper/Tomato | CaG6PDH1/SiG6PDH2 | 0.022 | 0.22 | 0.10 | 13.75 |
| CaG6PDH3/SiG6PDH4 | 0.018 | 0.16 | 0.11 | 10.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, J.; Tan, Z.; Jiang, Y.; Chen, X.; Yang, Q.; Li, H. Identification of Glucose-6-Phosphate Dehydrogenase Family Members Associated with Cold Stress in Pepper (Capsicum annuum L.). Horticulturae 2025, 11, 719. https://doi.org/10.3390/horticulturae11070719
Zhang J, Fan J, Tan Z, Jiang Y, Chen X, Yang Q, Li H. Identification of Glucose-6-Phosphate Dehydrogenase Family Members Associated with Cold Stress in Pepper (Capsicum annuum L.). Horticulturae. 2025; 11(7):719. https://doi.org/10.3390/horticulturae11070719
Chicago/Turabian StyleZhang, Jianwei, Jianxin Fan, Zhiying Tan, Yao Jiang, Xianjun Chen, Qin Yang, and Huanxiu Li. 2025. "Identification of Glucose-6-Phosphate Dehydrogenase Family Members Associated with Cold Stress in Pepper (Capsicum annuum L.)" Horticulturae 11, no. 7: 719. https://doi.org/10.3390/horticulturae11070719
APA StyleZhang, J., Fan, J., Tan, Z., Jiang, Y., Chen, X., Yang, Q., & Li, H. (2025). Identification of Glucose-6-Phosphate Dehydrogenase Family Members Associated with Cold Stress in Pepper (Capsicum annuum L.). Horticulturae, 11(7), 719. https://doi.org/10.3390/horticulturae11070719






