Abstract
This study reports seven new records of Curcuma L. (Zingiberaceae) for the flora of Laos: Curcuma comosa Roxb., C. pedicellata (Chaveer. & Mokkamul) Škorničk., C. phrayawan Boonma & Saensouk, C. pierreana Gagnep., C. rangjued Saensouk & Boonma, C. sabhasrii Saensouk, Maknoi, Wongnak & Rakarcha, and C. wanenlueanga Saensouk, Thomudtha & Boonma. Field surveys were conducted across various habitats in Laos, and species identification was confirmed through morphological comparisons with type specimens and protologues. An identification key for Curcuma species in Laos is provided to facilitate future taxonomic studies. Additionally, the lectotypification of C. comosa Roxb. is designated to ensure nomenclatural stability and clarify its taxonomic placement. The discovery of these species expands the known distribution of Curcuma in Indochina and highlights the importance of continued floristic exploration in understudied regions. These findings emphasize the need for biodiversity conservation, particularly in the nnatural habitats where these species occur. Furthermore, some of the newly recorded species exhibit attractive floral characteristics, making them valuable for sustainable horticultural applications, especially in ornamental plant cultivation. This study underscores the significance of taxonomic research in documenting regional flora, supporting conservation efforts, and promoting the sustainable utilization of plant diversity.
Keywords:
Curcuma; diversity; Ecomata; Hitcheniopsis; Laos; lectotypification; taxonomy; turmeric; Zingibereae; Zingiberoideae 1. Introduction
The genus Curcuma L. (Zingiberaceae) is an ecologically and economically significant group of flowering plants distributed across tropical and subtropical Asia [1]. Many species within this genus are valued for their ornamental appeal, medicinal applications, and commercial cultivation [2,3,4,5,6,7,8]. Despite its broad distribution, the diversity of Curcuma in some regions remains underexplored. In Laos, only 13 native species have been recorded [1]. Additional introduced species, such as Curcuma aurantiaca Zijp., C. longa L., C. mangga Valeton & Zijp, C. zanthorrhiza Roxb., and C. zedoaria (Christm.) Roscoe, have been reported for use and cultivation. These are commonly found in urban areas and markets but not in the wild [5]. This suggests that the diversity of Curcuma in Laos is still not fully understood, especially in terms of wild species, with many potentially remaining undocumented. Furthermore, the number of species recorded in Laos is significantly lower than that in neighboring countries such as Thailand (92 species [1]) and Vietnam (36 species [1]), underscoring the need for more comprehensive botanical surveys to uncover additional taxa and expand the knowledge of the genus in Indochina.
Previous studies on Curcuma in the region have primarily focused on Thailand and Vietnam, where extensive field explorations have led to the discovery of new species and additional distribution records [9,10,11,12,13,14,15,16,17,18,19,20,21]. In contrast, the diversity of Curcuma in Laos has received limited attention, with only a few reports documenting species occurrences. For instance, C. alismatifolia Gagnep. and C. singularis Gagnep. are widely distributed across Indochina [22,23], while others, such as C. corniculata Škorničk. and C. flammea Škorničk., have been documented exclusively in Laos [24]. These patterns indicate that Laos may harbor more undocumented species, highlighting the need for continued taxonomic exploration.
During recent field investigations in various parts of Laos, seven additional Curcuma species were identified and recorded, increasing the known diversity of the genus in the country. Many Curcuma species possess medicinal properties and are cultivated for commercial purposes [5,6], making them valuable for sustainable management and conservation.
The aim of this study is to document the morphological characteristics of the newly recorded Curcuma species in Laos. In addition, this research provides an updated identification key for native species, presents new distributional data, and offers ethnobotanical notes on their economic uses, medicinal applications, and horticultural potential. These findings support the sustainable utilization and conservation of Curcuma as part of Laos’s rich biological resources.
2. Materials and Methods
2.1. Plant Materials and Study Area
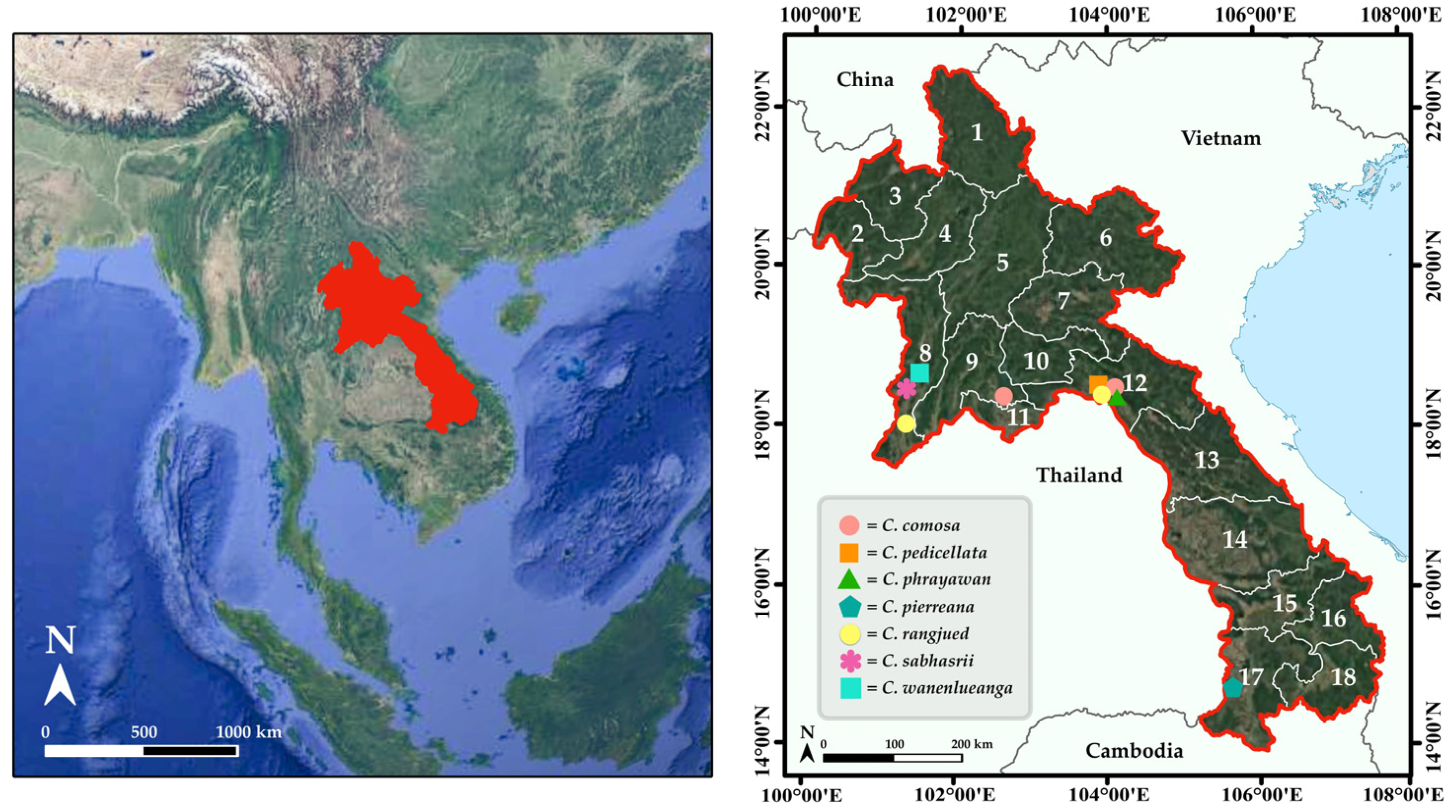
Whole-plant specimens were gathered from their natural habitats across several provinces in Laos (Figure 1), in January 2024 to January 2025. Ecological and phenological observations were specific to plants growing in Laos.
Figure 1.
Map of Laos and administrative divisions of Laos: 1 = Phôngsali, 2 = Bokèo, 3 = Louangnamtha, 4 = Oudômxay, 5 = Louangphrabang, 6 = Houaphan, 7 = Xiangkhoang, 8 = Xaignabouli, 9 = Vientiane (Province), 10 = Xaisomboun, 11 = Vientiane Prefecture, 12 = Bolikhamxai, 13 = Khammouan, 14 = Savannakhét, 15 = Salavan, 16 = Xékong, 17 = Champasak, and 18 = Attapeu.
2.2. Procedures
To minimize the impact on natural populations in Laos, morphological assessments were conducted in their natural habitats. Measurements were recorded using 20 living plants of each species with a Vernier caliper (Series 536–Moving Jaw Type, Mitutoyo Corporation, Kawasaki, Japan), while finer details were examined with a stereomicroscope (Stemi 2000-C, ZEISS, Oberkochen, Germany). Herbarium specimens and spirit-preserved collections (in 70% alcohol) were stored at the Faculty of Forestry (FOF), National University of Laos, and the Vascular Plant Herbarium, Mahasarakham University (VMSU).
Species identification was carried out by the authors through a detailed examination of the specimens’ morphological features, comparing these with previously published descriptions of Zingiberaceae species, especially with respect to their occurrence in Laos and neighboring countries, as well as other parts of Asia, referencing studies [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Additional information was gathered from the Zingiberaceae Resource Center (ZRC) and the Global Biodiversity Information Facility (GBIF). Furthermore, we consulted digital images and data from several herbarium collections, including those at the Aarhus University Herbarium (AAU), the Department of Agriculture (BK), the Natural History Museum (BM), the Department of National Parks, Wildlife and Plant Conservation (BKF), the Botanical Survey of India (CAL), the University of Calicut (CALI), Chiang Mai University (CMU), Chiang Mai University’s Department of Biology (CMUB), the Royal Botanic Garden Edinburgh (E), the National University of Laos (FOF), the South China Botanical Garden (IBSC), the Royal Botanic Gardens (K), Khon Kaen University (KKU), the Naturalis Biodiversity Center (L), the Makino Botanical Garden (MBK), the Tamil Nadu Agricultural University Campus at Coimbatore (MH), Muséum National d’Histoire Naturelle (P), Prince of Songkla University (PSU), the Queen Sirikit Botanic Garden, Botanical Garden Organization (QBG), the Forest Research Institute (RAF), Singapore Botanic Gardens, National Parks Board (SING), the National Museum of Nature and Science (TNS), Universiti Kebangsaan Malaysia (UKMB), and the Smithsonian Institution (US), along with additional herbarium records from Vietnam and China. This thorough approach allowed for accurate and well-supported comparisons.
Dissection images of each species were not captured during fieldwork due to the primary focus on confirming the occurrence of species in the study area. Taxonomic identifications were supported by referenced voucher specimens deposited in herbaria and additional examined materials.
The lectotypification was carried out based on the relevant literature, the examination of digitized specimens and illustrations housed at the Central National Herbarium Botanical Survey of India (CAL) and the Royal Botanic Gardens, Kew (K), and in accordance with Articles 9.3, 9.11, and 9.12 of the International Code of Nomenclature for algae, fungi, and plants [25].
Graphic Design and Illustrations: All graphics designed in this study were created using the Pixelmator Pro Program (Version 3.6.15 (Archipelago), 2025, Pixelmator Team, Vilnius, Lithuania) on a MacBook Pro (13-inch, M1, 2020, Apple Inc., Cupertino, CA, USA) by Thawatphong Boonma.
3. Results
This study documented seven new records of Curcuma L. in Laos: Curcuma comosa Roxb. [26,27], C. pedicellata (Chaveer. & Mokkamul) Škorničk. [28,29], C. phrayawan Boonma & Saensouk [30], C. pierreana Gagnep. [31], C. rangjued Saensouk & Boonma [32], C. sabhasrii Saensouk, Maknoi, Wongnak & Rakarcha [13], and C. wanenlueanga Saensouk, Thomudtha & Boonma [33] (Figure 2). An identification key for the Curcuma species native to Laos is provided (Table 1). Additionally, the lectotypification of C. comosa Roxb. is designated.

Figure 2.
Photographs of Curcuma spp. in Lao PDR. (a) Curcuma comosa Roxb. from Vientiane; (b) C. pedicellata (Chaveer. & Mokkamul) Škorničk. from Bolikhamxai; (c) C. phrayawan Boonma & Saensouk from Bolikhamxai; (d) C. pierreana Gagnep. from Champasak; (e) C. rangjued Saensouk & Boonma from Xaignabouli; (f) C. sabhasrii Saensouk, Maknoi, Wongnak & Rakarcha from Xaignabouli; and (g) C. wanenlueanga Saensouk, Thomudtha & Boonma from Xaignabouli. Photograph (b) is by Surapon Saensouk; photographs (a,c–g) are by Thawatphong Boonma.

Table 1.
Identification key for the Curcuma species native to Laos.
3.1. Taxonomic Treatments
3.1.1. Curcuma comosa Roxb. [26]
Type: Curcuma comosa Roxb., Icones Roxburghianae Ineditae No. 1925 (lectotype, K, designated here).
- Specimens from Laos examined: Lao PDR, Vientiane, P. Saensouk, S. Saensouk, T. Boonma, A. Sengthong, and K. Phengmala 2401 (FOF!); Lao PDR, Vientiane, P. Saensouk, S. Saensouk, T. Boonma, A. Sengthong, and K. Phengmala 2402 (VMSU!); Lao PDR, Bolikhamxai Province, Pakkading District, Phengmala KC017 (FOF!, VMSU!).
Curcuma comosa was originally described by Roxburgh based on material collected from Pegu, Burma, which was sent by F. Carey to the Calcutta Botanic Garden in 1809, where it later flowered [26]. No original specimens of Curcuma comosa Roxb. have been located. Considering the unpublished drawings as part of the original material on which the name was based, we designated a lectotype for Curcuma comosa Roxb. using Roxburgh’s illustration. Duplicate sets exist in Calcutta (CAL) and Kew (K); however, Leong-Škorničková et al. [27] referred only to the illustration of Curcuma comosa in K as original material and did not designate a lectotype. Here, we select the illustration of Curcuma comosa in K as the lectotype, as it is better preserved and more completely colored than the one in CAL. Hay [34] treated the duplicate drawings collectively as the “type” (sic), but since both elements were included equally without distinguishing one as the lectotype, this did not constitute effective typification.
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid to globose, measuring 8–10 × 6–9 cm, internally pale brown to white, and rarely branched. The rhizome branches are very short. The leafy shoot reaches 0.7–1.6 m in height. The leaf sheaths are 25–40 cm long and green; the ligule is indistinct; the petiole is either sessile or up to 5 cm long; the lamina measures 18–65 × 10–24 cm, green with red patches on either side of the midrib when young and plain green in mature plants. It is glabrous on both surfaces, with a cuneate base and an acute apex.
The inflorescence is lateral, with a peduncle 5–12 cm long and glabrous. The thyrse is 14–22 cm long. The fertile bracts are ovate with an obtuse to round apex, white with pink apices, and sparsely puberulent to glabrous above. The cincinni contain 6–8 flowers at the basal bracts. The coma bracts have an obtuse to round apex and are pink. The bracteoles are broadly obovate with an acute to retuse apex, measuring 1–1.8 × 1.5 cm, semitranslucent white with a pink tinge at the apex, and glabrous.
The flowers are in a closed form; the calyx is 8–9 mm long, with a tridentate apex and a unilateral incision 3–4 mm long. It is semitranslucent white and glabrous. The floral tube is narrowly cylindrical at the base and funnel-shaped in the distal 1/3 of its length, measuring 2.5–3 cm long. Externally, it is cream-white and glabrous, while internally, it is cream-white with yellow patches in the uppermost part ventrally. It is glabrous but has a ring of hairs around the constriction area. The dorsal corolla lobe is ovate to triangular, prominently concave, hooded with a mucronate apex, and measures 1.3–1.5 × 1–1.1 cm. It is white with a pale pink tinge at the apex, glabrous with a few hairs at the mucro. The lateral corolla lobes are triangular with a blunt apex, shallowly concave, and measure 1.2–1.4 × 0.9–1 cm. They are white, sometimes with a slight pale pink tinge at the apex, and glabrous. The lateral staminodes are irregularly elliptic to obovate with an obtuse apex folded inwards at the center, measuring 1–1.1 × 0.6–0.8 cm. They are pale yellow at the base, darker towards the apex, and have glandular hairs above. The labellum is obovate and trilobed, measuring approximately 1.5 × 1.5 cm, with the midlobe bilobed and an incision of approximately 3 mm. The side lobes fold upwards and are pale yellow with a bright yellow median band. It is glabrous but has glandular hairs along the sides of the median band.
The stamen is single, 7–8 mm long, with a filament measuring 3–4 mm long and 3 mm wide at the base, tapering to about 1.5 mm at the point of attachment. It is pale yellow and glabrous. The anther is approximately 6 × 3 mm with pale yellow connective tissue and glabrous. The anther crest is negligible, being merely a thickened ridge. The spurs are narrowly conical, approximately 2.5 mm long, pale yellow, and glabrous. The thecae are 3–4 mm long and dehisce throughout their length, with cream-white pollen. The epigynous glands are cylindrical with an acute apex, approximately 5 mm long, pale yellow at the base and darker distally. The stigma is irregularly capitate with two blunt dorsal bulges and a lateral ostiole, ciliate. The ovary is barrel-shaped, approximately 4 × 3 mm, cream-white to white, glabrous at the base, and pubescent distally. The fruit was not seen.
- Vernacular name in Laos: Wan Chak Mot Luk or Kajiaow Khao.
- Ecology: Deciduous forest.
- Phenology: Flowering in late March to June.
- Distribution: Native to Assam, Laos, Myanmar, and Thailand.
- Utilization: The rhizome is used in traditional medicine to treat uterine symptoms [5,6].
3.1.2. Curcuma phrayawan Boonma & Saensouk [30]
Type: Thailand. Nakhon Nayok Province, Ban Na District, 1 September 2019, Boonma 5 (holotype: KKU!) [30].
- Specimens from Laos examined: Lao PDR, Bolikhamxai Province, P. Saensouk, S. Saensouk, K. Chanthavongsa, A. Sengthong, K. Phengmala, and T. Boonma 2403 (FOF!, VMSU!).
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid, measuring 6–9 × 4–6 cm, internally deep yellow, with branches, fragrant. The rhizome branches are yellowish with darker yellow core, 2–2.5 cm in diameter. The leafy shoot reaches 1.5–2 m in height, with 7–9 leaves. The leaf sheaths are alternate, 45–50 cm long and brownish-red, minutely pubescent; the ligule is bilobed triangular, 2.5–4 × 2–3 mm, translucent white, apex acute, pubescent; the petiole is 20–35 cm long, red, glabrous; the lamina is oblanceolate, measures 60–80 × 12–18 cm, upper green with red along the midrib almost reaching the apex, lower pale green with red along the midrib almost reaching the apex. It is glabrous on both surfaces, with an attenuate base and caudate apex.
The inflorescence is terminal, with a reddish peduncle 40–50 cm long, minutely pubescent, with or without sterile bract. The thyrse is 15–25 cm long. The fertile bracts are broadly elliptic with rounded apex, pale green with pale pink in the middle and at the distal part, and minutely pubescent on both surfaces. The cincinni contain 3–5 flowers. The coma bracts have an obtuse apex and are white with pink at the distal part. The bracteoles are obovate with an obtuse apex (slightly hooded), measuring 2.1–2.5 × 1.5–1.8 cm, pale yellow, and pubescent.
The flowers are in a closed form; the calyx is 12–13 mm long, translucent white, pubescent. The floral tube is narrowly cylindrical at the base and funnel-shaped in the distal 1/3 of its length. Externally, it is pale yellow and glabrous but has a ring of hairs around the constriction area. The dorsal corolla lobe is ovate–triangular, hooded with a mucronate apex, and measures 1.4–1.5 × 1.2–1.3 cm. It is white to light pale yellow with a pale pink tinge at the apex, pubescent. The lateral corolla lobes are ovate–triangular with an obtuse apex, shallowly concave, and measure 1.4–1.5 × 1.2–1.3 cm. They are white to light pale yellow with a pale pink tinge at the apex and glabrous. The lateral staminodes are obovate with a rounded apex, pale yellow, measuring 1–1.1 × 0.7–0.8 cm. The labellum is obovate with emarginate apex, measuring approximately 1.6–1.7 × 1.7–1.8 cm, with the midlobe bilobed and an incision of approximately 2 mm. The side lobes fold upwards and are pale yellow with a yellow median band.
The stamen is single, 4.5–5 mm long, glabrous, with a filament measuring 2.5–3 mm long and 4–5 mm wide at the base, tapering to about 2 mm at the point of attachment. It is pale yellow and glabrous. The anther is approximately 2.8–3 × 2.5 mm with pale yellow connective tissue and glabrous. The anther crest is present but not prominent. The spurs are narrowly conical, pointing downward, approximately 3.5 mm long, pale yellow, and glabrous, and the tips of the spurs point out in the opposite direction. The thecae are 3–4 mm long and dehisce throughout their length, with cream-white pollen. The epigynous glands are cylindrical, approximately 4 mm long, pale yellow. The stigma is white. The ovary is subglobose, approximately 3 × 3 mm, pale yellow, pubescent. The fruit was not seen.
- Vernacular name in Laos: Phaya Wan.
- Ecology: Deciduous to dry evergreen forest.
- Phenology: Flowering in rainy season between late June and September.
- Distribution: Native to Laos and Thailand.
- Utilization: The rhizome is used in traditional medicine, and whole plants are cultivated as auspicious ornamental plants, with the belief that they protect growers and their homes from misfortune.
3.1.3. Curcuma pedicellata (Chaveer. & Mokkamul) Škorničk. [28]
Basionym: Stahlianthus pedicellatus Chaveer. & Mokkamul [29].
Type: Thailand. Nakhon Phanom Province, Pla Pak District, Wang Krabao Village, P. Mokkamul and A. Chaveerach 315 (holotype: BKF!; isotype: BK!) [28].
- Specimens from Laos examined: Lao PDR, Bolikhamxai Province, P. Saensouk, S. Saensouk, K. Chanthavongsa, A. Sengthong, K. Phengmala, and T. Boonma 2404 (FOF!, VMSU!).
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid, short, measuring 1.5–2.8 × 1–2 cm, without branches. The leafy shoot reaches 20–35 cm in height, with 2–3 leaves. Sheaths are alternate, 4–9 cm long and red, greenish-red, or green with red stripes, glabrous; the ligule is bilobed, very short c. 2 mm long, apex rounded to obtuse, ciliate; the petiole is 4–7 cm long, green, reddish-green, or red, glabrous; the lamina is linear to narrowly obovate–oblong, or narrowly oblanceolate, measures 10–19 × 1–1.7 cm, upper green with red along the midrib almost reaching the apex, glabrous, lower green, purplish-green, or purplish, pubescent, with a narrowly cuneate base and acute apex.
The inflorescence is lateral, direct from the rhizome, erect, with a peduncle 3–6 cm long. The bracts are campanulate with obtuse to acute apex, reflexed, green with dense stripes from the base to apex, or red, and glabrous. The cincinni contain 10–30 flowers. The bracteoles are linear with an obtuse apex, membranous, measuring 0.8–1.2 × 0.1–0.15 cm, glabrous.
The flowers are white, in an open form, staminodes free from dorsal corolla lobe; the calyx is 7–9 × c. 3 mm, translucent white, apex trilobed, each lobe of apex rounded, glabrous. The floral tube is narrowly cylindrical, white, glabrous. The dorsal corolla lobe is ovate–oblong, hooded with a mucronate apex, and measures 1–1.2 × 0.4–0.5 cm, white and glabrous. The lateral corolla lobes are ovate–oblong with rounded apex and measure 1–1.2 × 0.4–0.5 cm, white and glabrous. The lateral staminodes are obovate with a rounded apex, white with yellow at the base, inner side hairy, measuring 1.2–1.3 × 0.5 cm. The labellum is spatulate with emarginate apex or shortly bilobed, measuring approximately 1.7–2.2 × 1.2–1.6 cm, lobe apex rounded and crenate, white with central part yellow and with a red blotch at the base, pubescent.
The stamen is single, with a short filament measuring c. 2 mm, white and glabrous. The anther is approximately 2–3 × 1–2 mm, white and glabrous. The anther crest is ovate, 3–4 × 2–3 mm, apex obtuse to rounded, white, glabrous. The spurs are absent. The thecae are 3–4 mm long and dehisce throughout their length, with cream-white pollen. The epigynous glands are absent. The stigma is white and glabrous. The ovary is ellipsoid, approximately 3 × 2 mm, white, glabrous. The fruits are trilocular, ellipsoid, 7–9 × 4–5 mm, white and glabrous. Seeds are ovoid–oblong, 3 × 1 mm, brownish.
- Vernacular name in Laos: Waan Dak Dae.
- Ecology: Deciduous forest.
- Phenology: Flowering between late March and April.
- Distribution: Native to Laos and Thailand.
- Utilization: The rhizome is used as a carminative.
3.1.4. Curcuma pierreana Gagnep. [31]
Syntypes: Pierre s.n., Cochinchine (syntype P!); Vietnam, Hue, August 1904, Cadière s.n. (syntype P!) [31].
- Specimens from Laos examined: Lao PDR, Champasak Province, P. Saensouk, S. Saensouk, A. Sengthong, K. Phengmala, and T. Boonma 2405 (FOF!, VMSU!).
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid to globose, measuring 1–2 × 1–2 cm, internally pale yellow, with numerous branches, horizontal, fragrant. The rhizome branches are pale yellow, 0.5–1.5 cm in diameter. The leafy shoot reaches 20–46 cm in height, with 2–5 leaves. Leaf sheaths are alternate, 8–22 cm long, green or green with reddish tinge, puberulent; the ligule is bilobed, c. 2 mm, translucent greenish, apex obtuse, pubescent; the petiole is 1–14 cm long, green, puberulent; the lamina is ovate–lanceolate, measures 15–35 × 5–8 cm, upper green or green with narrow red patch along the midrib, lower pale green. It is glabrous on both surfaces, or abaxial surface is puberulent to densely puberulent, with an obtuse to attenuate base and acuminate apex.
The inflorescence is terminal, with a peduncle 2.5–6 cm long, puberulent, with or without sterile bract. The thyrse is 3–6 cm long. The fertile bracts are broadly ovate with acute to obtuse apex, pale tawny-pink, pale green, or cream, puberulent. The cincinni contain 3–5 flowers. The coma bract is absent. The bracteoles are narrowly ovate or absent.
The flowers are exerted, longer than the bracts; the calyx is 15–16 mm long, semitranslucent white, pubescent. The floral tube is narrowly cylindrical at the base and funnel-shaped in the distal 1/2 of its length. Externally, it is white to cream, sparsely puberulent, internally white, glabrous at the base, and has a ring of hairs around the constriction area. The dorsal corolla lobe is ovate, hooded with a mucronate apex, and measures 1.5–1.6 × 1.2–1.3 cm. It is white to cream, glabrous. The lateral corolla lobes are ovate–triangular with an obtuse apex, shallowly concave, and measure 1.4–1.5 × 1.1–1.2 cm. They are white to cream and glabrous. The lateral staminodes are broadly ovate to broadly elliptic, somewhat oblique with obtuse to rounded apex, white to cream with purplish-red apex, measuring 1.5–1.7 × 1.2–1.4 cm, with glandular hair above. The labellum is broadly obovate with emarginate apex, measuring approximately 1.3–1.6 × 1.2–1.5 cm, white to cream with bright yellow median band, sometimes with purplish-red patch at the apex, with glandular hair above, senser along edges of median band.
The stamen is single, 9–11 mm long, with a filament measuring 2–3 mm long and 6–7 mm wide at the base, tapering to about 2 mm at the point of attachment. It is white to cream with glandular hairs. The anther is approximately 7–8 mm long, with white to cream connective tissue and with glandular hairs. Anther crest with rounded, slightly reflexed apex, 1–1.5 × 1.5–2 mm, white to pale yellow. The spurs are filamentous, c. 1 mm long, incurved, white. The thecae are dehisced throughout their length, with cream-white pollen. The epigynous glands are cylindrical with blunt apex, approximately 5 mm long, pale yellow. The stigma is white. The ovary is globose to subglobose, approximately 3 × 2–3 mm, pale yellow, pubescent. The fruit was not seen.
- Vernacular name in Laos: Kajiaow Dok Daeng.
- Ecology: Deciduous forest.
- Phenology: Flowering in rainy season between late June and September.
- Distribution: Native to Cambodia, Laos, Thailand, and Vietnam [1,35].
- Utilization: Young inflorescences are eaten fresh or blanched and dipped in chili paste.
3.1.5. Curcuma rangjued Saensouk & Boonma [32]
Type: Mae Hong Son Province, Mae Sariang District, 4 August 2017, Boonma 9 (holotype: KKU!) [32].
- Specimens from Laos examined: Lao PDR, Xaignabouli Province, P. Saensouk, S. Saensouk, A. Sengthong, K. Phengmala, and T. Boonma 2406 (FOF!, VMSU!). Lao PDR, Bolikhamxai Province, P. Saensouk, S. Saensouk, A. Sengthong, K. Phengmala, and T. Boonma 2409 (FOF!, VMSU!).
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid, measuring 4–8 × 3–5 cm, internally pale yellowish-white to white with yellowish core, with 2–4 branches on opposite sides, non-aromatic. The rhizome branches pale yellowish-white to white, 1–2.3 cm in diameter. The leafy shoot reaches 60–120 cm in height, with 5–7 leaves. The leaf sheaths are alternate, 10–40 cm long and green, glabrous; the ligule is thin and short, 1–2 mm long, translucent white; the petiole is 12–25 cm long, green, glabrous; the lamina is narrowly elliptic to oblanceolate, measures 20–60 × 10–17 cm, green, lower pale green, midrib green; the leaves are sparsely hairy along the leaf veins on adaxial side while glabrous on abaxial side, with an attenuate base, and acuminate with slightly cordate apex.
The inflorescence is terminal, with a pale green peduncle 25–35 cm long, very short fine hairs, with or without sterile bract. The thyrse is 15–20 cm long. The fertile bracts are ovate with obtuse apex, very pale green with very pale pink tinge at the distal part, and finely hairy on both surfaces. The cincinni contain 3–5 flowers. The coma bracts are narrowly obovate; lower bracts have an obtuse apex and are white with pale pink tinge at distal part, upper bracts slightly mucronate apex, white with pinkish-purple at distal part. The bracteoles are obovate with an obtuse apex and hooded, measuring 1–3 cm long, very pale yellow, and sparsely hairy (hairier at distal part).
The flowers are in a closed form; the calyx is 16–18 mm long, semitranslucent pale yellowish-white, sparsely hairy. The floral tube is narrowly cylindrical at the base and funnel-shaped in the distal 1/3 of its length. Externally, it is pale yellow and glabrous but has a ring of hairs around the constriction area. The dorsal corolla lobe is ovate–triangular, hooded with a mucronate apex, and corolla lobe measures 1.8–2.1 × 1.7–2 cm. It is white to light pale yellow, sparsely hairy at distal part. The lateral corolla lobes are ovate–triangular with an obtuse apex, shallowly concave, and measure 1.3–1.6 × 1–1.4 cm. They are white to light pale yellow. The lateral staminodes are asymmetrical obovate with an obtuse to rounded apex, pale yellow, measuring 1.7–2.1 × 0.9–1.1 cm. The labellum is obovate with emarginate apex, labellum measuring approximately 2.2–2.5 × 1.5–1.8 cm, with an incision of 1.5–2.5 mm long. The side lobes fold upwards and are pale yellow with a yellow median band.
The stamen is single, with a filament measuring 4–4.2 mm long. The anther is approximately 5.5–6 mm long, with pale yellow connective tissue. The anther crest is yellow with an obtuse apex. The spurs are narrowly conical, pointing downward, approximately 3.5–4 mm long, pale yellow. The thecae are 4–4.5 mm long and dehisce throughout their length, with cream-white pollen. The epigynous glands are cylindrical, approximately 5–6 mm long, pale yellow. The stigma is very pale yellowish-white. The ovary is subglobose, approximately 2.5–3 × 2.5–3 mm, pale yellow, pubescent. The fruit was not seen.
- Vernacular name in Laos: Wan-Jued.
- Ecology: Deciduous to dry evergreen forest.
- Phenology: Flowering in rainy season between late June and October.
- Distribution: Native to Laos and Thailand.
- Utilization: Rhizome used to treat poisoning, as an anti-inflammatory, and in detoxification [5,6,32].
3.1.6. Curcuma sabhasrii Saensouk, Maknoi, Wongnak & Rakarcha [13]
Type: Phitsanulok Province, Chat Trakan District, 15 May 2021, P. Phaosrichai & M. Wongnak 2641 (holotype: QBG!; isotypes: BKF!, KKU!) [13].
- Specimens from Laos examined: Lao PDR, Xaignabouli Province, P. Saensouk, S. Saensouk, A. Sengthong, K. Phengmala, and T. Boonma 2407 (FOF!, VMSU!).
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid to elliptic, measuring 2–3.5 × 1.5–2.5 cm, internally pale yellow to creamy white, with creeping branches, fragrant. The rhizome branches are white internally with fibers (the fibers can be seen when they break apart from each other), 0.8–1.5 cm in diameter. The leafy shoot is 30–50 cm in height, with 2–5 leaves. The leaf sheaths are alternate, green, reddish at the base or red; the ligule is bilobed, 2–3 mm long, translucent white, apex obtuse; the petiole is 9–12 cm long, green, pubescent; the lamina is narrowly elliptic, measures 25–30 × 8–12 cm, upper green with green midrib, lower pale green, adaxial surface glabrous, abaxial surface pubescent, with an oblique base and apiculate apex.
The inflorescence is terminal, with a green peduncle 4–6 cm long. The thyrse is 4–7 cm long. The fertile bracts are lanceolate with acute apex, red or green with reddish-brown distal part, and pubescent on both surfaces. The cincinni contain 3–5 flowers. The coma bract is absent. The bracteoles are narrowly lanceolate to linear or absent, measuring 1.5–8 × 1.5–2 mm, translucent white.
The flowers are in an open form; the calyx is 13–15 mm long, white to light pale pink, glabrous. The floral tube is narrowly cylindrical at the base and slightly funnel-shaped in the distal 1/3 of its length. Externally, it is white with purple tinge at the distal part and densely puberulent. The dorsal corolla lobe is narrowly ovate, hooded with a mucronate apex, and measures 1.8–2.2 × 0.6–0.8 cm. It is pale reddish-purple at the base with a gradually increasing reddish-purple tinge towards the distal part, glabrous. The lateral corolla lobes are narrowly ovate with an obtuse apex, shallowly concave, and measure 1.6–1.9 × 0.5–0.7 cm. They are pale reddish-purple at the base with a gradually increasing reddish-purple tinge towards the distal part and glabrous. The lateral staminodes are ovate to rhomboid to elliptic-rhomboid with an acute apex, ruby pink to pale pink with white in the middle, measuring 1.8–2.0 × 1.2–1.5 cm. The labellum is obovate with emarginate apex, measuring approximately 1.7–1.8 × 1.5 cm, with the midlobe bilobed and an incision of approximately 3.5–5 mm. The lobe is ruby pink with yellow median band in the middle and reddish-orange at the base.
The stamen is single, 12–14 mm long, with a flattened filament measuring 4–5 mm long and 4–5 mm wide at the base, tapering to about 3 mm at the point of attachment. It is white with reddish-purple mottling and densely puberulent. The anther is approximately 6–8 mm long, white, and densely puberulent. The anther crest is present, c. 2 mm long, white to pale reddish-purple, apex emarginate, densely puberulent. The spurs are narrowly conical, pointing downward, densely puberulent, approximately 3.2–4 mm long, white, and glabrous. The thecae are 7–8 mm long and dehisce throughout their length, with cream-white pollen. The epigynous glands are cylindrical, 4–5 mm long. The stigma is white. The ovary is subglobose, approximately 2.5–3 × 2.5–3 mm, whitish, pubescent. The fruit is subglobose, 8–12 mm, pubescent. Seeds pale brown with white laciniate arils, 1.5–2 mm in diameter and c. 5 mm long.
- Vernacular name in Laos: Kajiaow Tab Tim.
- Ecology: In deciduous forest and dry evergreen forest.
- Phenology: Flowering in May to September and fruiting in late June to October.
- Distribution: Native to Laos and Thailand.
- Utilization: Auspicious ornamental plants.
3.1.7. Curcuma wanenlueanga Saensouk, Thomudtha & Boonma [33]
Type: Mae Hong Son Province, Sob Moei District, 28 August 2017, Boonma 60 (holotype: KKU!; isotypes: BK!, BKF!, BK!) [33].
- Specimens from Laos examined: Lao PDR, Xaignabouli Province, P. Saensouk, S. Saensouk, A. Sengthong, K. Phengmala, and T. Boonma 2408 (FOF!, VMSU!).
Description of specimens collected in Laos: Perennial Herb with rhizome. The rhizome is ovoid, measuring 5–9 × 3–5 cm, internally yellow with darker core, with 3–4 branches on opposite sides, bitter tasting, slightly aromatic. The rhizome branches are yellow with sub-branching, 1–2 cm in diameter. The leafy shoot reaches 100–150 cm in height, with 7–10 leaves. The leaf sheaths are alternate, 10–60 cm long and green with reddish-brown tinge, pubescent; the ligule is up to 1 cm long, bilobed, green with a reddish tinge; the petiole is 15–35 cm long, canaliculate, green with a reddish tinge, sparsely pubescent; the lamina is narrowly lanceolate, measures 50–70 × 15–18 cm, green with a reddish-purple tinge along the midrib (the reddish-purple tinge may fade when the leaves grow old), lower pale green, with an attenuate base and acuminate apex.
The inflorescence is terminal, with a pale green peduncle 30–40 cm long, pubescent, with or without sterile bract. The thyrse is 8–10 cm long, 4–6 cm in diam. The fertile bracts are ovate with acute to slightly mucronate apex, and the upper-most bracts are slightly hooded, pubescent, pale green with a reddish tinge at the distal part, except for veins which are pale green. The cincinni contain 3–5 flowers. The coma bracts are narrowly obovate, hooded at the distal part, apex acute with slightly mucronate, pink at distal part and gradually fade to pale green at the base, pubescent. The bracteoles are narrowly obovate–oblong with a mucronate apex and hooded, measuring 2–2.4 cm long, pale greenish-white with pale pink at the distal part, and pubescent.
The flowers are in a closed form; the calyx is 13–15 mm long, pale yellowish-white, pubescent. The floral tube is narrowly cylindrical at the base and funnel-shaped in the distal 1/3 of its length. Externally, it is pale yellow with pink at the distal part. The dorsal corolla lobe is ovate, hooded with a mucronate apex, and corolla lobe measures 1.4–1.6 × 9–11 mm. It is pink and sparsely hairy at the distal part. The lateral corolla lobes are ovate with hooded apex, concave, and pink and measure 1.3–1.5 × 0.8–0.9 cm. The lateral staminodes are irregularly obovate with a rounded apex, yellow, measuring 1.3–1.4 × 0.7–0.8 cm. The labellum is obovate with emarginate apex, measuring approximately 1.5–1.7 × 1.5–1.6 cm, with an incision of about 5 mm long. The side lobes fold upwards and are yellow with a yellow median band.
The stamen is single, with a filament measuring about 4 mm long. The anther is approximately 8 mm long, with pale yellow connective tissue. The anther crest is not obvious. The spurs are narrowly conical, pointing downward, approximately 4 mm long, pale yellow. The thecae are about 4 mm long and dehisce throughout their length, with cream-white pollen. The epigynous glands are cylindrical, approximately 4–5 mm long, yellow. The stigma is exerted above the anther lobes. The ovary is subglobose, approximately 3 × 3 mm, pubescent. The fruit was not seen.
- Vernacular name in Laos: Wan En Lueang.
- Ecology: In mixed deciduous forest.
- Phenology: Flowering in July to October.
- Distribution: Native to Laos and Thailand.
- Utilization: Herbal medicine.
Notes: In cultivation, the thyrse is longer and larger, with more well-developed coma bracts (8–12), which are longer, larger, and more numerous than described in the protologue of this species (5–6 coma bracts).
4. Discussion
4.1. Species Diversity and Ecological Adaptation
This study significantly enhances our understanding of Curcuma species in Laos by documenting seven new species distributed across various ecosystems, ranging from deciduous to dry evergreen forests. The presence of Curcuma species in such diverse habitats reflects their remarkable ecological adaptability and resilience. Curcuma comosa was found in Vientiane Province and Bolikhamxai Province, C. pedicellata in Bolikhamxai Province, C. phrayawan in Bolikhamxai Province, C. pierreana in Champasak Province, C. rangjued in Xaignabouli Province and Bolikhamxai Province, C. sabhasrii in Xaignabouli Province, and C. wanenlueanga in Xaignabouli Province. Moreover, the variation in flowering phenology—occurring across different seasons—indicates that these species are ecologically flexible and well-adapted to a range of climatic conditions. These findings not only expand the current floristic knowledge of Laos but also underscore its role as a critical biodiversity hotspot in Southeast Asia. The identification of new species suggests that many more undocumented taxa may exist, highlighting the need for continued botanical surveys in underexplored areas.
4.2. Horticultural and Medicinal Potential
The newly recorded Curcuma species also exhibit promising horticultural and medicinal potential. Ornamental species such as Curcuma pierreana and C. sabhasrii are valued for their attractive floral structures and aesthetic appeal. These features make them suitable candidates for the ornamental plant market, where demand for novel and visually appealing species continues to grow [2,7,8,9,15,17,18,19,20,21].
Meanwhile, species like C. comosa, C. pedicellata, and C. phrayawan are traditionally used for medicinal purposes, including in the treatment of uterine disorders and as carminatives [3,4,5,6,7]. These uses not only underscore their ethnobotanical importance but also suggest potential for broader application in herbal medicine industries.
However, promoting sustainable cultivation is vital to prevent the overexploitation of wild populations. Propagation via rhizome division, combined with suitable growing conditions such as well-drained soil and adequate moisture, can help meet horticultural demand without degrading natural habitats.
4.3. Conservation Implications and Strategies for Curcuma Species in Laos
The discovery of seven new records of Curcuma species in Laos carries significant implications for biodiversity conservation. Many of these species are habitat specialists, confined to ecosystems such as deciduous and dry evergreen forests, which are increasingly under threat from deforestation, land conversion, and climate change. Species like Curcuma comosa and C. pedicellata are particularly vulnerable due to their narrow ecological specificity and limited distribution, increasing the risk of population decline in the face of environmental disturbances.
Based on field surveys and the restricted number of known populations, we propose that all seven Curcuma species be categorized as Near Threatened (NT) under the IUCN Red List Categories and Criteria [36]. Although their Extent of Occurrence (EOO) and Area of Occupancy (AOO) do not currently meet the thresholds for Vulnerable (VU) status, ongoing threats and the limited number of localities justify a precautionary approach. The continued monitoring of population trends and habitat conditions is essential, and reassessment may be needed if conditions worsen.
To address these risks, effective conservation must integrate in situ and ex situ strategies. In situ measures should focus on habitat preservation through legal protection, sustainable forest management, and ecological restoration, especially in ecologically sensitive zones. Ex situ methods—such as seed banking and cultivation in botanical gardens—can safeguard genetic diversity and serve as insurance against habitat loss. These conservation efforts should be incorporated into national biodiversity action plans and aligned with global frameworks like the IUCN Red List [36].
Community involvement is crucial to the success of these conservation initiatives. Education programs and the promotion of sustainable harvesting practices can empower local communities to act as stewards of their natural resources. Community-based conservation models, particularly those involving the sustainable use of Curcuma species, can reduce overharvesting pressures while enhancing conservation outcomes.
Furthermore, the integration of Curcuma species into local agricultural systems offers a promising strategy to balance biodiversity conservation with sustainable development. Community-driven cultivation projects and the establishment of local nurseries can provide alternative income sources while lessening collection pressure on wild populations. The cultivation of Curcuma for ornamental [2,7,8,9,15,17,18,19,20,21] and medicinal purposes [3,4,5,6,7] can support economic development while conserving biodiversity.
To ensure long-term sustainability, the following horticultural practices should be promoted:
- (1)
- Sustainable Propagation and Nursery Development: Establishing nurseries using vegetative propagation or seed collection helps conserve genetic diversity and reduce reliance on wild populations. This controlled approach supports healthy plant growth and habitat protection [37].
- (2)
- Agroforestry Systems: Integrating Curcuma into agroforestry systems mimicking natural ecosystems can restore and protect habitats, promote biodiversity, and support resilient farming practices. These systems benefit both agriculture and wildlife by fostering diverse ecological communities [19,38,39,40].
- (3)
- Soil and Water Conservation: Practices such as mulching, proper irrigation, and organic fertilization maintain soil fertility and water retention while minimizing environmental harm. These methods ensure sustainable Curcuma cultivation and reduce ecosystem degradation [41,42,43,44].
- (4)
- Education and Community Engagement: Training programs and outreach initiatives can equip farmers with knowledge of sustainable cultivation and conservation techniques. When communities recognize the long-term value of conservation, they become more invested in protecting both the species and their habitats [45,46,47,48,49].
- (5)
- Monitoring and Research: The ongoing assessment of wild and cultivated populations is necessary to evaluate conservation success and guide adaptive management. Research supports the continuous improvement in propagation and habitat protection strategies [50,51,52,53,54,55].
By combining conservation and development goals through sustainable cultivation, legal protection, and active community participation, Curcuma conservation in Laos can contribute to preserving biodiversity while supporting local livelihoods. These efforts promote a balanced relationship between cultural heritage, economic progress, and ecological sustainability [56,57,58,59,60,61,62,63].
4.4. Cultivation Tips for Curcuma Species
Cultivating Curcuma species successfully requires attention to their specific environmental and care needs. The following are some key cultivation tips to help ensure the healthy growth and optimal development of Curcuma species:
(1). Site Selection
Light: Curcuma species prefer partial shade, though they can tolerate some sunlight. In areas with hot, intense sun, it is best to plant them where they can receive filtered light or afternoon shade [62,63,64,65].
Temperature: These plants thrive in warm climates with temperatures of between 25 °C and 30 °C. They do best in tropical and subtropical environments but can be grown in temperate regions with suitable protection during colder months [66,67,68,69,70].
(2). Soil Requirements
Well-draining soil: Curcuma species prefer rich, loamy, and well-draining soil. Heavy or waterlogged soils should be avoided as they can lead to root rot [68,71,72,73,74].
Soil pH: A slightly acidic to neutral pH (5.5 to 7.0) is ideal for Curcuma species. You can amend the soil with organic matter or compost to improve its structure and nutrient content [73].
(3). Planting
Planting Depth: Curcuma species are grown from rhizomes (underground stems). Plant the rhizomes about 5–10 cm deep in the soil. Space them about 30 cm apart to allow for proper growth [75,76,77].
Planting Time: In tropical areas, Curcuma can be planted year-round. However, in cooler regions, it is best to plant these plants in the early spring or after the last frost to give them a full growing season [78,79,80].
(4). Watering
Consistent Moisture: Curcuma species require regular watering, especially during the growing season, but they should not sit in waterlogged soil. Keep the soil consistently moist but allow it to dry out slightly before watering [64,67,81,82].
Avoid Overwatering: While moisture is essential, overwatering can lead to root rot. Make sure the soil has good drainage and avoid water stagnation in the planting area [80,83].
(5). Fertilization
Organic Fertilizer: Curcuma species benefit from regular feeding during the growing season. Use organic fertilizers like compost or well-rotted manure to provide essential nutrients [84,85,86,87,88].
Slow-release Fertilizer: A slow-release balanced fertilizer can also be used, especially during the active growing phase. Avoid high-nitrogen fertilizers, as they can lead to excessive foliage growth at the expense of flowers and rhizomes [89,90].
(6). Mulching
Apply a layer of organic mulch around the base of the plants to retain moisture, regulate soil temperature, and suppress weeds. Mulch also improves the soil structure as its decomposes over time [91,92].
(7). Pest and Disease Control
Common Pests: Curcuma species are susceptible to pests such as aphids, caterpillars, and scale insects. Regularly check the plants for signs of infestation and treat them with insecticidal soap or neem oil when necessary. Curcuma is prone to fungal diseases like root rot, particularly in poorly draining soils. Ensure proper watering practices and avoid overwatering to prevent fungal infections. Use fungicides if necessary but always follow the manufacturer’s instructions [74].
(8). Propagation
Vegetative Propagation: The most common method of propagation for Curcuma species is by dividing rhizomes. Each rhizome should have at least one growing bud, which will develop into a new plant. This should be conducted in early spring to allow the plants to establish before the growing season begins [93,94].
Seed Propagation: Although less common, some Curcuma species can be propagated by seed. However, this method takes longer and requires more patience, as the seeds must be sown in well-drained seed trays and kept moist until germination [95].
(9). Harvesting
Harvesting Rhizomes: Curcuma species are typically harvested when the leaves begin to yellow and die back. This signals that the rhizomes have matured. Carefully dig up the rhizomes, taking care not to damage them, and allow them to dry out for a few days before storage. After harvesting, rhizomes can be stored in a cool, dry place until ready for use or replanting. If you plan to use them immediately, fresh rhizomes can be used in cooking, for medicinal applications, or for ornamental purposes [96,97,98].
(10). Climate Adaptation
Cold Protection: If you are growing Curcuma species in a region that experiences frost, it is crucial to protect the plants during the colder months. Consider growing them in containers that can be moved indoors or covered with frost cloth during the winter.
By following these cultivation tips, you can grow healthy Curcuma species, whether for ornamental, culinary, or medicinal purposes. Proper care and attention to their needs will ensure that they thrive and contribute to both the beauty and biodiversity of your garden or farm.
4.5. Limitations and Future Research
While this study contributes valuable insights into Curcuma diversity in Laos, certain limitations remain. Detailed ecological data, reproductive biology, population dynamics, and conservation assessments are still needed for each newly identified species. Future research should also evaluate the plants’ propagation methods, habitat specificity, and responses to environmental changes. Such studies will be essential for informing effective conservation and utilization strategies.
Further studies on the ecology and distribution of Curcuma species are necessary to better understand their specific habitat requirements and the environmental factors influencing their occurrence. The collection of additional field data, particularly on population size, phenology, and microhabitat conditions, will be essential for formulating effective conservation strategies and ensuring the long-term survival of these species in the wild.
In addition, current findings on the distribution patterns of Curcuma species in the study area contribute valuable insights into the broader biogeography of the genus. These data may help clarify historical dispersal routes, centers of endemism, and ecological adaptations that have shaped the present distribution of Curcuma across Southeast Asia. Understanding these patterns is fundamental not only for biodiversity conservation but also for unraveling the evolutionary origins of the genus.
Future research should also focus on the genetic diversity and phylogenetic relationships among Curcuma species. Such studies could provide critical information on speciation processes, evolutionary history, and the extent of gene flow among populations. Integrating ecological, biogeographical, and molecular approaches will enhance our understanding of the genus and support evidence-based conservation planning.
5. Conclusions
This study documents seven Curcuma species in Laos, contributing to baseline data on the region’s plant diversity, bringing the total to 20 recorded species. The findings enhance our understanding of Curcuma distribution in Indochina and underscore the need for continued floristic surveys in Laos, where botanical exploration remains limited. Although this study provides valuable taxonomic information, its scope is preliminary. Future research should focus on detailed ecological assessments, conservation status evaluations, and the potential applications of these species. In alignment with the study’s objectives, the results serve as a foundation for further taxonomic and ecological investigations in the region.
Author Contributions
Conceptualization, P.S., S.S. and T.B.; methodology, P.S., S.S., K.C., A.S., K.P., C.M., S.R. and T.B.; software, T.B.; validation, P.S., S.S. and T.B.; formal analysis, P.S., S.S. and T.B.; investigation, P.S., S.S., K.C., A.S., K.P., C.M., S.R. and T.B.; resources, P.S., S.S. and T.B.; data curation, P.S., S.S. and T.B.; writing—original draft preparation, T.B.; writing—review and editing, P.S., S.S., K.C., A.S., K.P., C.M., S.R. and T.B.; visualization, T.B.; supervision, P.S. and S.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Mahasarakham University.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely thank all who supported us during our field trip and manuscript preparation. Additionally, we acknowledge the invaluable support of all the curators of the visited herbarium collections.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plant of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: www.plantsoftheworldonline.org/ (accessed on 14 February 2025).
- Boonma, T.; Saensouk, S.; Saensouk, P. Diversity and traditional utilization of the Zingiberaceae plants in Nakhon Nayok Province, Central Thailand. Diversity 2023, 15, 904. [Google Scholar] [CrossRef]
- Chen, T.V.; Truong, M.N.; Quynh, T.T.T.; Nhi, N.T.T.; Linh, N.H.K. GC-MS analysis and cytotoxic activity of the n-hexane fraction from Curcuma sahuynhensis Škorničk. & N.S.Lý leaves collected in Vietnam. Plant Sci. Today 2024, 11, 308–315. [Google Scholar] [CrossRef]
- Chen, T.V.; Tu, V.L.; Nghia, N.T.; Truong, M.N.; Quynh, T.T.T.; Tham, V.M.; Hien, N.T.T. Volatile components of ethyl acetate extracts from the leaves and rhizomes of Curcuma sahuynhensis and their cytotoxic activity: In vitro and in silico studies. Egypt. J. Chem. 2024, 67, 253–267. [Google Scholar] [CrossRef]
- Phengmala, K.; Saensouk, S.; Saensouk, P.; Souladeth, P. Ethnobotany of Hmong ethnic groups in Bolikhamxay province, central Laos PDR. Not. Bot. Horti. Agrobo. 2023, 51, 13284. [Google Scholar] [CrossRef]
- Phengmala, K.; Saensouk, S.; Saensouk, P.; Souladeth, P. Ethnobotanical study of medicinal plants used by Lao ethnic group in Central Laos. Not. Bot. Horti. Agrobo. 2024, 52, 13633. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Biogeography, conservation status, and traditional uses of Zingiberaceae in Saraburi Province, Thailand, with Kaempferia chaveerachiae sp. nov. Horticulturae 2024, 10, 934. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Maknoi, C.; Boonma, T. Curcuma borealis sp. nov. and C. retrocalcaria sp. nov. (Zingiberaceae): Two novel taxa from Northern Thailand. Horticulturae 2024, 10, 787. [Google Scholar] [CrossRef]
- Saensouk, P.; Boonma, T.; Saensouk, S. Curcuma pulcherrima (Zingiberaceae), a new rare species of Curcuma subgen. Ecomata from eastern Thailand. Biodiversitas 2022, 23, 6635–6644. [Google Scholar] [CrossRef]
- Saensouk, P.; Boonma, T.; Saensouk, S. Curcuma nakhonphanomensis (Zingiberaceae), a new species from lower Mekong River basin, northeastern Thailand. Biodiversitas 2022, 23, 6040–6048. [Google Scholar] [CrossRef]
- Saensouk, S.; Boonma, T.; Saensouk, P. Curcuma achrae (Zingiberaceae), a new species from Central Thailand. Rheedea 2022, 32, 30–45. [Google Scholar]
- Saensouk, P.; Boonma, T.; Rakarcha, S.; Maknoi, C.; Wongnak, M.; Saensouk, S. Two new species of Curcuma subgenus Ecomata (Zingiberaceae: Zingibereae), from Central and Southwestern Thailand. Biodiversitas 2022, 23, 4578–4588. [Google Scholar] [CrossRef]
- Rakarcha, S.; Saensouk, S.; Maknoi, C.; Wongnak, M.; Thammarong, W.; Saensouk, P. Curcuma lampangensis and C. sabhasrii, two new species of the family Zingiberaceae from Northern Thailand. Biodiversitas 2022, 23, 4448–4459. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Le, T.A.; Hoang, Q.H.; Le, Q.T.; Nguyen, E. Two new taxa of Curcuma subgen. Ecomata (Zingiberaceae: Zingibereae), from coastal Central Vietnam. Biodiversitas 2022, 23, 2512–2519. [Google Scholar] [CrossRef]
- Saensouk, P.; Boonma, T.; Maknoi, C.; Saensouk, S. Curcuma ubonensis (Zingiberaceae), a new species of Curcuma subgen. Hitcheniopsis from Eastern Thailand. Not. Bot. Horti. Agrobo. 2023, 51, 13374. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, N.A.; Averyanov, L.; Nguyen, D.D.; Le, C.T. Curcuma tuanii (Zingiberaceae) a new species of subgenus Ecomata from Vietnam based on morphological and molecular evidence. Acta Bot. Bras. 2023, 37, e20230028. [Google Scholar] [CrossRef]
- Boonma, T. Curcuma suraponii sp. nov. (Zingiberaceae), a new species of Curcuma subgen. Curcuma from Thailand. Biodiversitas 2023, 24, 4885–4895. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Boonma, T.; Ragsasilp, A.; Maknoi, C.; Techa, C.; Srisuk, P.; Souladeth, P. Curcuma sumonii sp. nov., and C. wanchaii sp. nov. (Zingiberaceae), two new taxa of Curcuma subgen. Curcuma from Thailand. Sci. Rep. 2024, 14, 27541. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Maknoi, C.; Setyawan, A.D.; Boonma, T. A horticultural Gem Unveiled: Curcuma peninsularis sp. nov. (Zingiberaceae), a new species from Peninsular Thailand, previously misidentified as Curcuma aurantiaca Zijp. Horticulturae 2024, 10, 950. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S.; Maknoi, C.; Nguyen, D.D.; Boonma, T. Curcuma roseobracteata sp. nov. (Zingiberaceae), a new species from Thailand and distribution notes on the recently described Curcuma suraponii Boonma. Biodiversitas 2024, 25, 3368–3375. [Google Scholar] [CrossRef]
- Boonma, T.; Saensouk, S.; Saensouk, P. Kaempferia sipraiana (Zingiberaceae), a new species from Thailand and a new record of Kaempferia pseudoparviflora for Myanmar. Biodiversitas 2022, 23, 2203–2211. [Google Scholar] [CrossRef]
- Leong-Škorničková, J.; Newman, M. Gingers of Cambodia, Laos and Vietnam; Singapore Botanic Gardens, National Parks Board: Singapore, 2015; Volume 26. [Google Scholar]
- Binh, N.Q.; Tuan, H.A.; Dat, N.V.; Thanh, T.T.V.; Hanh, N.P.; Cuong, N.M.; Thanh, N.T. A new record species for Flora of Vietnam—Curcuma singularis Gagnep. (Zingiberaceae). VNU J. Sci. Nat. Sci. Technol. 2017, 33, 25–29. [Google Scholar]
- Leong-Škorničková, J.; Šída, O.; Bouamanivong, S.; Souvannakhoummane, K.; Phathavong, K. Three new ginger species (Zingiberaceae) from Laos. Blumea 2014, 59, 106–112. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar]
- Roxburgh, W. Descriptions of Several of the Monandrous Plants of India. Asiat. Res. 1810, 11, 318–362. [Google Scholar]
- Leong-Škorničková, J.; Šída, O.; Marhold, K. Back to types! Towards stability of names in Indian Curcuma L. (Zingiberaceae). Taxon 2010, 59, 269–282. [Google Scholar] [CrossRef]
- Leong-Škorničková, J.; Šída, O.; Záveská, E.; Marhold, K. History of infrageneric classification, typification of supraspecific names and outstanding transfers in Curcuma (Zingiberaceae). Taxon 2015, 64, 362–373. [Google Scholar] [CrossRef]
- Chaveerach, A.; Mokkamul, P.; Sudmoon, R.; Tanee, T.; Garcia, V.F. A new species of Stahlianthus (Zingiberaceae) from Northeastern Thailand. Taiwania 2007, 52, 315–319. [Google Scholar]
- Saensouk, S.; Boonma, T.; Saensouk, P. Six new species and a new record of Curcuma L. (Zingiberaceae) from Thailand. Biodiversitas 2021, 22, 1658–1685. [Google Scholar] [CrossRef]
- Gagnepain, F. Zingibéracées, Marantacées et Musacées Nouvelles de L’Herbier du Muséum. Bull. Mus. Natl. Hist. Nat. 1907, 54, 403–405. [Google Scholar] [CrossRef]
- Saensouk, S.; Boonma, T.; Saensouk, P. A new species and a new record of Curcuma subgen. Curcuma (Zingiberaceae) from Northern Thailand. Biodiversitas 2021, 22, 3617–3626. [Google Scholar] [CrossRef]
- Saensouk, S.; Boonma, T.; Thomudtha, A.; Thomudtha, P.; Saensouk, P. Short Communication: Curcuma wanenlueanga (Zingiberaceae), a new species of subgenus Curcuma from Thailand. Biodiversitas 2021, 22, 2988–2994. [Google Scholar] [CrossRef]
- Hay, A. The genus Alocasia (Araceae-Colocasieae) in West Malesia and Sulawesi. Gard. Bull. Sing. 1998, 50, 221–334. [Google Scholar]
- Nguyen, T.N.H.; Cao, N.G.; Le, B.S.; Vu, T.T.; Le, A.T. Botanical characteristics and ITS sequence of Curcuma pierreana Gagnep.—Zingiberaceae. Res. J. Biotech. 2025, 20, 100–110. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria Version 16. 2024. Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf (accessed on 28 February 2025).
- Brijesh, H.; Ajjappala, B. Micropropagation strategies in medicinally important turmeric (Curcuma sp.): Current research and future challenges. J. Appl. Biol. Biotechnol. 2023, 11, 1–8. [Google Scholar] [CrossRef]
- Wang Mei Hua, M.; Warren-Thomas, E.; Wanger, T.C. Rubber Agroforestry: Feasibility at Scale; Mighty Earth: Washington, DC, USA, 2021; p. 130. [Google Scholar]
- Kumar, P.; Dagar, J.C.; Gupta, S.R.; Sileshi, G.W. Achieving Biodiversity Conservation, Livelihood Security and Sustainable Development Goals Through Agroforestry in Coastal and Island Regions of India and Southeast Asia. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa; Dagar, J.C., Gupta, S.R., Sileshi, G.W., Eds.; Sustainability Sciences in Asia and Africa; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Josephrajkumar, A.; Mani, M.; Anes, K.M.; Mohan, C. Ecological Engineering in Pest Management in Horticultural and Agricultural Crops. In Trends in Horticultural Entomology; Mani, M., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Soliman, Y.M.; Soliman, W.S.; Abbas, A.M. Alley Cropping and Organic Compost: An Efficient and Sustainable Agro-Ecological Strategy for Improving Turmeric (Curcuma longa L.) Growth and Attributes. Agriculture 2023, 13, 149. [Google Scholar] [CrossRef]
- Udhaya Nandhini, D.; Janaki, P.; Venkatesan, S.; Senthilraja, K.; Somasundaram, E.; Meena, S. Assessing changes in soil quality indicators, turmeric (Curcuma longa L.) yield, and monetary returns under different years of organic nutrient management. Org. Agr. 2023, 13, 443–460. [Google Scholar] [CrossRef]
- Singh, P.; Nayak, S.L.; Sharkar, R.; Sarkar, A.; Dutta, S.; Paul, D. Application of modern techniques in cultivation of medicinal plants: A review. Plant Arch. 2025, 25, 1947–1962. [Google Scholar] [CrossRef]
- Islam, K.K.; Hossain, M.B.; Mostakim, G.M.; Ashraf, S.M.K.; Ripta, S.K. Moving to conservation agriculture: (1) evidence of rhizome crops performance in existing agroforestry practices of Madhupur Garh, Bangladesh. J. Agrofor. Environ. 2023, 16, 147–153. [Google Scholar] [CrossRef]
- Ikendi, S. Ecological conservation, biodiversity, and agricultural education as integrated approaches for envisioning the future of sustainable agriculture in North America. Int. J. Sustain. Dev. World Ecol. 2022, 30, 152–163. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, K.; Singh, S.; Kumar, S.; Rai, A.; Kumar, R.; Yadav, S.; Kumar, A. Sustainable horticulture practices: An environmental-friendly approach. Pharm. Innov. J. 2023, 12, 4777–4782. [Google Scholar]
- Srinivasu, P.; Parkavi, S.; Ragul, P.; Panotra, N.; Thrilekha, D.; Upadhyay, L.; Sai Meghana, B.; Sakhamo, K. A critical review on fostering community involvement in sustainable horticulture initiatives. J. Sci. Res. Rep. 2024, 30, 394–404. [Google Scholar] [CrossRef]
- Santos, M.; Moreira, H.; Cabral, J.A.; Gabriel, R.; Teixeira, A.; Bastos, R.; Aires, A. Contribution of Home Gardens to Sustainable Development: Perspectives from A Supported Opinion Essay. Int. J. Environ. Res. Public Health 2022, 19, 13715. [Google Scholar] [CrossRef] [PubMed]
- Kee, T.; Zhang, H. Digital Experiential Learning for Sustainable Horticulture and Landscape Management Education. Sustainability 2022, 14, 9116. [Google Scholar] [CrossRef]
- Moussy, C.; Burfield, I.J.; Stephenson, P.J.; Newton, A.F.E.; Butchart, S.H.M.; Sutherland, W.J.; Gregory, R.D.; McRae, L.; Bubb, P.; Roesler, I.; et al. A quantitative global review of species population monitoring. Conserv. Biol. 2022, 36, e13721. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, P.; Pons, V.; Letourneau, A.; Klesczewski, M.; Papuga, G.; Thompson, J.D. Combining population monitoring with habitat vulnerability to assess conservation status in populations of rare and endangered plants. J. Nat. Conserv. 2017, 37, 83–95. [Google Scholar] [CrossRef]
- Bubac, C.M.; Johnson, A.C.; Fox, J.A.; Cullingham, C.I. Conservation translocations and post-release monitoring: Identifying trends in failures, biases, and challenges from around the world. Biol. Conserv. 2019, 238, 108239. [Google Scholar] [CrossRef]
- Wrege, P.H.; Rowland, E.D.; Keen, S.; Shiu, Y. Acoustic monitoring for conservation in tropical forests: Examples from forest elephants. Methods Ecol. Evol. 2017, 8, 1292–1301. [Google Scholar] [CrossRef]
- Danovaro, R.; Fanelli, E.; Aguzzi, J.; Billett, D.; Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Gjerde, K.; Jamieson, A.J.; Kark, S.; et al. Ecological variables for developing a global deep-ocean monitoring and conservation strategy. Nat. Ecol. Evol. 2020, 4, 181–192. [Google Scholar] [CrossRef]
- Leroy, G.; Carroll, E.L.; Bruford, M.W.; DeWoody, J.A.; Strand, A.; Waits, L.; Wang, J. Next-generation metrics for monitoring genetic erosion within populations of conservation concern. Evol. Appl. 2018, 11, 1066–1083. [Google Scholar] [CrossRef]
- Corlett, R.T. Safeguarding our future by protecting biodiversity. Plant Divers. 2020, 42, 221–228. [Google Scholar] [CrossRef]
- Borelli, T.; Hunter, D.; Powell, B.; Ulian, T.; Mattana, E.; Termote, C.; Pawera, L.; Beltrame, D.; Penafiel, D.; Tan, A.; et al. Born to Eat Wild: An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition. Plants 2020, 9, 1299. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, J.-M.; Emery, M.R.; Donaldson, J.; Balachander, G.; Barron, E.S.; Chaudhary, R.P.; Danner, M.-C.; Gasalla, M.A.; Hallosserie, A.; Halmy, M.; et al. Status, challenges and pathways to the sustainable use of wild species. Global Environ. Change 2023, 81, 102692. [Google Scholar] [CrossRef]
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Hassan, F.; Zahoor, I.; Majeed, U.; Khanday, F.A. Biodiversity, Management and Sustainable Use of Medicinal and Aromatic Plant Resources. In Medicinal and Aromatic Plants; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar] [CrossRef]
- Sharangi, A.B.; Gowda, M.P.; Das, S. Responses of turmeric to light intensities and nutrients in a forest ecosystem: Retrospective insight. Trees For. People 2022, 7, 100208. [Google Scholar] [CrossRef]
- Harish, B.S.; Umesha, K.; Venugopalan, R.; Maruthi Prasad, B.N. Photo-selective nets influence physiology, growth, yield and quality of turmeric (Curcuma longa L.). Ind. Crops Prod. 2022, 186, 115202. [Google Scholar] [CrossRef]
- Vidanapathirana, N.P.; Subasinghe, S.; Sunil, K.; Ketipearachchi, K.G.; Siriwardana, A.J.M.C.M.; Bandusekara, B.S. Growth and yield performances of turmeric (Curcuma longa) grown in the dry zone of Sri Lanka as affected by planting space, growing media, and shade. J. Agro-Technol. Rural Sci. 2022, 1, 27–31. [Google Scholar] [CrossRef]
- Murwani, Z.A.; Artika, I.M.; Syaefudin; Nurcholis, W. Evaluation of growth, chlorophyll content, and photosynthesis rate of Curcuma xanthorrhiza with different shade levels. Curr. Appl. Sci. Technol. 2024, 24, e0256871. [Google Scholar] [CrossRef]
- Retana-Cordero, M.; Fisher, P.R.; Gómez, C. Modeling the Effect of Temperature on Ginger and Turmeric Rhizome Sprouting. Agronomy 2021, 11, 1931. [Google Scholar] [CrossRef]
- Chintakovid, N.; Tisarum, R.; Samphumphuang, T.; Sotesaritkul, T.; Cha-um, S. Evaluation of curcuminoids, physiological adaptation, and growth of Curcuma longa under water deficit and controlled temperature. Protoplasma 2022, 259, 301–315. [Google Scholar] [CrossRef]
- Dolase, P.; Chaudhari, V. Review on cultivation practices of Haridra (Curcuma longa Linn.). Int. J. Ayurveda Pharm. Res. 2024, 12, 56–61. [Google Scholar] [CrossRef]
- Bustami, K.; Saifrizal, M.; Maulana, M.; Chadafi, M.F.; Abdullah, A. Development of Curcuma caesia (Black Turmeric) cultivation as a leading local traditional medicine plant. J. Jurma: J. Program Mhs. Kreat. Univ. Ibn Khaldun Bogor 2022, 6, 442–450. [Google Scholar] [CrossRef]
- Shannon, D.A.; van Santen, E.; Zehtab Salmasi, S.; Murray, T.J.; Duong, L.T.; Greenfield, J.T.; Gonzales, T.; Foshee, W. Shade, establishment method, and varietal effects on rhizome yield and curcumin content in turmeric in Alabama. Agronomy 2019, 59, 2701–2710. [Google Scholar] [CrossRef]
- Boontiang, K.; Siritrakulsak, T.; Nontaswatsri, C. A strategic approach to achieve healthy plant growth and decontaminated rhizome of Curcuma alismatifolia Gagnep. cultivation in modified substrate on raised-bed planting. J. Applied Hort. 2024, 26, 202–205. [Google Scholar] [CrossRef]
- Ingudam, B.; Chongtham, S.K.; Basumatary, A.; Singh, A.H.; Das, A.; Choudhary, A.K.; Kamei, G.; Sinyorita, S.; Singh, L.K.; Devi, E.L.; et al. Changes in soil properties, productivity and profitability as influenced by the adoption of site-specific integrated crop management technology in turmeric (Curcuma longa L.) in Eastern Himalayan acidic Inceptisol. Ind. Crops Prod. 2022, 180, 114745. [Google Scholar] [CrossRef]
- Sontsa-Donhoung, A.M.; Hawaou, B.; Bahdjolbe, M.; Nekou, G.N.; Tadjouo, I.K.; Nwaga, D. Growing Curcuma longa for rhizome production on diverse arable soil types in Cameroon: Agronomic and microbial parameters. Agric. Sci. 2021, 12, 464–480. [Google Scholar] [CrossRef]
- Kumar, A. Diseases of Ginger and Turmeric. In Handbook of Spices in India: 75 Years of Research and Development; Ravindran, P.N., Sivaraman, K., Devasahayam, S., Babu, K.N., Eds.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Retana-Cordero, M.; Flores, S.J.; Fisher, P.R.; Freyre, R.; Gómez, C. Effect of Container Volume and Planting Density on Ginger and Turmeric Growth and Yield. HortTechnology 2022, 32, 425–434. [Google Scholar] [CrossRef]
- Divya, V.U.; Sindhu, P.V. Spacing and propagule size on yield and quality of Curcuma aromatica Salisb. J. Crop Weed 2023, 19, 266–270. [Google Scholar] [CrossRef]
- Kittur, B.H.; Sudhakara, K.; Mohan Kumar, B.; Kunhamu, T.K.; Sureshkumar, P. Bamboo-Based Agroforestry Systems in Kerala, India: Performance of Turmeric (Curcuma longa L.) in the Subcanopy of Differentially Spaced Seven-Year-Old Bamboo Stand. Agrofor. Syst. 2016, 90, 237–250. [Google Scholar] [CrossRef]
- Flores, S.; Retana-Cordero, M.; Fisher, P.R.; Freyre, R.; Gómez, C. Effect of Photoperiod, Propagative Material, and Production Period on Greenhouse-Grown Ginger and Turmeric Plants. HortScience 2021, 56, 1476–1485. [Google Scholar] [CrossRef]
- Anitha, M.; Hore, J.K. Production Technology of Some Major and Minor Spice Crops. In Indian Spices; Sharangi, A., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Nair, M.B.; Groot, M.J. Medicinal Plants for Home Herbal Gardens, Institutional Gardens, and Animal Health; Natural Livestock Farming India. 2021. Available online: https://edepot.wur.nl/561732 (accessed on 31 March 2025).
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of Water-Deficit Stress and Paclobutrazol on Growth, Relative Water Content, Electrolyte Leakage, Proline Content and Some Antioxidant Changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef]
- Chungloo, D.; Tisarum, R.; Pinruan, U.; Sotesaritkul, T.; Saimi, K.; Praseartkul, P.; Himanshu, S.K.; Datta, A.; Cha-Um, S. Alleviation of Water-Deficit Stress in Turmeric Plant (Curcuma longa L.) Using Phosphate Solubilizing Rhizo-Microbes Inoculation. 3 Biotech 2024, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, D. A Guide to Good Agricultural Practices for Commercial Production of Ginger Under Field Conditions in Jamaica; FAO: Kingston, UK, 2021. [Google Scholar] [CrossRef]
- Kadam, J.H.; Kamble, B.M. Effect of organic manures on growth, yield, and quality of turmeric (Curcuma longa L). J. Appl. Nat. Sci. 2020, 12, 91–97. [Google Scholar] [CrossRef]
- Ben, T.T.; Linh, L.N.T.; Giang, T.T.L.; Dao, V.Q.; Oanh, N.T.T.; Dang, L.V.; Thach, B.D. The effects of organic fertilizer and planting type on growth and yield of Curcuma aromatica. Indian J. Agric. Res. 2022, 56, 712–716. [Google Scholar] [CrossRef]
- Datta, S.; Jana, J.C.; Bhaisare, P.T.; Nimbalkar, K.H. Effect of organic source of nutrients and biofertilizers on growth, yield, and quality of turmeric (Curcuma longa L). J. Appl. Nat. Sci. 2017, 9, 1981–1986. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Tripathy, S.; Samal, H.B.; Mahalik, G. The impact of organic fertilizers on growth, yield, and rhizospheric bacterial diversity in black turmeric (Curcuma caesia). J. Angiotherapy 2024, 8, 1–12. [Google Scholar]
- Chandana, M.; Padma, M.; Prabhakar, B.N.; Joshi, V.; Mahender, B.; Gouthami, P.; Sathish, G. Studies on effect of organic manures and biofertilizers on growth, yield, and economics of turmeric (Curcuma longa L.) varieties. Pharma Innov. J. 2022, 11, 824–832. [Google Scholar]
- Bahar, F.A.; Lone, A.A.; Makhdoomi, M.I.; Dar, E.A.; Ahmad, M.; Akhone, M.M.; Hussain, N.; Mushtaq, T.; Bhat, F.N.; Aziz, M.A. Slow release nitrogen fertilizers—An ideal approach for reducing nitrogen losses and improving crop yields. Chem. Sci. Rev. Lett. 2019, 8, 159–172. [Google Scholar]
- Barłóg, P.; Grzebisz, W.; Łukowiak, R. Fertilizers and Fertilization Strategies Mitigating Soil Factors Constraining Efficiency of Nitrogen in Plant Production. Plants 2022, 11, 1855. [Google Scholar] [CrossRef]
- Prem, M.; Ranjan, P.; Seth, N.; Patle, G.T. Mulching techniques to conserve the soil water and advance the crop production—A review. Curr. World Environ. 2020, 15, 10–30. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Basit, A.; Mohamed, H.I.; Ali, I.; Ullah, S.; Kamel, E.A.R.; Shalaby, T.A.; Ramadan, K.M.A.; Alkhateeb, A.A.; Ghazzawy, H.S. Mulching as a Sustainable Water and Soil Saving Practice in Agriculture: A Review. Agronomy 2022, 12, 1881. [Google Scholar] [CrossRef]
- Haida, Z.; Sinniah, U.R.; Nakasha, J.J.; Hakiman, M. Shoot Induction, Multiplication, Rooting and Acclimatization of Black Turmeric (Curcuma caesia Roxb.): An Important and Endangered Curcuma Species. Horticulturae 2022, 8, 740. [Google Scholar] [CrossRef]
- Bandara, M.M.N.T.; Dahanayake, N.; Subasinghe, S.; Perera, P.C.D. A review on in vitro propagation of turmeric (Curcuma longa L.). J. Univ. Ruhuna 2021, 9, 39–46. [Google Scholar] [CrossRef]
- Babu, K.N.; Divakaran, M.; Pillai, G.S.; Sumathi, V.; Praveen, K.; Raj, R.P.; Akshita, H.J.; Ravindran, P.N.; Peter, K.V. Protocols for In Vitro Propagation, Conservation, Synthetic Seed Production, Microrhizome Production, and Molecular Profiling in Turmeric (Curcuma longa L.). In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, 2nd ed.; Jain, S., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1391, pp. 333–350. [Google Scholar] [CrossRef]
- Hailemichael, G.; Zakir, M. Pre- and post-harvest practices influencing yield and quality of turmeric (Curcuma longa L.) in Southwestern Ethiopia: A review. African J. Agric. Res. 2021, 17, 1096–1105. [Google Scholar] [CrossRef]
- Thakur, N.; Sharma, P.; Govender, P.P.; Shukla, S.K. Analysis and extraction of curcumin at mid and late phase harvested Curcuma longa samples collected from Western Himalayan regions. Chem. Afr. 2022, 5, 1733–1742. [Google Scholar] [CrossRef]
- Nair, K.P. Harvesting and Postharvest Management of Turmeric. In Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices; Springer: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).