Comparative Effects of Nitrogen Fertigation and Granular Fertilizer Application on Pepper Yield and Soil GHGs Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Materials and Experimental Design
2.2. Biometrical Determinations
2.3. Automated Chamber Greenhouse Gases Fluxes Measurements
2.4. Data Managing and Filtering
2.5. Statistical Analysis
3. Results
3.1. CO2 and N2O Daily Fluxes and Environmental Drivers over the Season
3.2. CO2 and N2O Fluxes Diel Trends by Fertilization Period
3.3. Growing Season Cumulative Fluxes and Yield-Scaled Global Warming Potential
3.4. Hot Moments Contribution Importance Across the Season
4. Discussion
4.1. Fertilization Effects on Yield and GHG Emission
4.2. GHGs Emission Temporal Variability Driven by Hot Moments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilizers in agriculture. Sci. Rep. 2022, 12, 19777. [Google Scholar] [CrossRef]
- Alasinrin, S.Y.; Salako, F.K.; Busari, M.A.; Sainju, U.M.; Badmus, B.S.; Isimikalu, T.O. Greenhouse gas emissions in response to tillage, nitrogen fertilization, and manure application in the tropics. Soil Tillage Res. 2025, 245, 106296. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergov-Ernmental Panel on Climate Change; Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; 151p, ISBN 978-92-9169-143-2. [Google Scholar]
- Kim, D.G.; Hernandez-Ramirez, G.; Donna, G. Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 53–65. [Google Scholar] [CrossRef]
- Lin, S.; Hernandez-Ramirez, G. Nitrous oxide emissions from manured soils as a function of various nitrification inhibitor rates and soil moisture contents. Sci. Total Environ. 2020, 738, 139669. [Google Scholar] [CrossRef]
- Manco, A.; Giaccone, M.; Zenone, T.; Onofri, A.; Tei, F.; Farneselli, M.; Gabbrielli, M.; Allegrezza, M.; Perego, A.; Magliulo, V.; et al. An Overview of N2O Emissions from Cropping Systems and Current Strategies to Improve Nitrogen Use Efficiency. Horticulturae 2024, 10, 754. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; Van Groenigen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Kennedy, T.L.; Suddick, E.C.; Six, J. Reduced nitrous oxide emissions and increased yields in California tomato cropping systems under drip irrigation and fertigation. Agric. Ecosyst. Environ. 2013, 170, 16–27. [Google Scholar] [CrossRef]
- David, C.; Lemke, R.; Helgason, W.; Farrell, R.E. Current inventory approach overestimates the effect of irrigated crop management on soil-derived greenhouse gas emissions in the semi-arid Canadian prairies. Agric. Water Manag. 2018, 208, 19–32. [Google Scholar] [CrossRef]
- Smith, D.R.; Hernandez-Ramirez, G.; Shalamar, S.D.; Bucholtz, D.L.; Stott, D.E. Nitrogen fertilizer and tillage management impacts on non-CO2 greenhouse emissions in corn/soybean and biomass cropping systems. Soil Sci. Soc. Am. J. 2011, 75, 1070–1082. [Google Scholar] [CrossRef]
- Maharjan, B.; Venterea, R.T.; Rosen, C. Fertilizer and irrigation management effects on nitrous oxide emissions and nitrate leaching. Agron. J. 2014, 106, 703–714. [Google Scholar] [CrossRef]
- Lin, S.; Hernandez Ramirez, G.; Kryzanowski, L.; Wallace, T.; Grant, R.; Degenhardt, R.; Berger, N.; Sprout, C.; Lohstraeter, G.; Powers, L.A. Timing of manure injection and nitrification inhibitors impacts on nitrous oxide emissions and nitrogen transformations in a barley crop. Soil Sci. Soc. Am. J. 2017, 81, 1595–1605. [Google Scholar] [CrossRef]
- Hanson, B.R.; Šimůnek, J.; Hopmans, J.W. Evaluation of urea–ammonium–nitrate fertigation with drip irrigation using numerical modeling. Agric. Water Manag. 2006, 86, 102–113. [Google Scholar] [CrossRef]
- Machiwal, D.; Jha, M.K.; Mal, B.C. Modelling infiltration and quantifying spatial soil variability in a wasteland of Kharagpur, India. Biosyst. Eng. 2006, 95, 569–582. [Google Scholar] [CrossRef]
- Karandish, F.; Šimůnek, J. Two-dimensional modeling of nitrogen and water dynamics for various N-managed water-saving irrigation strategies using HYDRUS. Agric. Water Manag. 2017, 193, 174–190. [Google Scholar] [CrossRef]
- Alaoui, A.; Lipiec, J.; Gerke, H.H. A review of the changes in the soil pore system due to soil deformation: A hydrodynamic perspective. Soil Tillage Res. 2011, 115–116, 1–15. [Google Scholar] [CrossRef]
- Liu, K.; Huang, G.H.; Xu, X.; Xiong, Y.Y.; Huang, Q.Z.; Šimůnek, J. A coupled model for simulating water flow and solute transport in furrow irrigation. Agric. Water Manag. 2019, 213, 792–802. [Google Scholar] [CrossRef]
- Wen, T.D.; Chen, X.S.; Luo, Y.W.; Shao, L.T.; Niu, G. Three-dimensional pore structure characteristics of granite residual soil and their relationship with hydraulic properties under different particle gradation by X-ray computed tomography. J. Hydrol. 2023, 618, 129230. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, G.; Brouder, S.M.; Smith, D.R.; Van Scoyoc, G.E.; Michalski, G. Nitrous oxide production in an eastern corn belt soil: Sources and redox range. Soil Sci. Soc. Am. J. 2009, 73, 1182–1191. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef]
- Li, H.R.; Mei, X.R.; Wang, J.D.; Huang, F.; Hao, W.P.; Li, B.G. Drip fertigation significantly increased crop yield, water productivity and nitrogen use efficiency with respect to traditional irrigation and fertilization practices: A meta-analysis in China. Agric. Water Manag. 2021, 244, 106534. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, Y.; Mu, Y.; Zhou, Y.; Zhang, C.; Liu, J. The effect of drip irrigation and drip fertigation on N2O and NO emissions, water saving and grain yields in a maize field in the North China Plain. Sci. Total Environ. 2017, 575, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Liang, Y.P.; Zhang, Q.; Jha, S.K.; Gao, Y.; Shen, X.J.; Sun, J.S.; Duan, A.W. Mitigated CH4 and N2O emissions and improved irrigation water use efficiency in winter wheat field with surface drip irrigation in the North China Plain. Agric. Water Manag. 2016, 163, 403–407. [Google Scholar] [CrossRef]
- Fentabil, M.M.; Nichol, C.F.; Jones, M.D.; Neilsen, G.H.; Neilsen, D.; Hannam, K.D. Effect of drip irrigation frequency, nitrogen rate and mulching on nitrous oxide emissions in a semi-arid climate: An assessment across two years in an apple orchard. Agric. Ecosyst. Environ. 2016, 235, 242–252. [Google Scholar] [CrossRef]
- Marino, S.; Aria, M.; Basso, B.; Leone, A.P.; Alvino, A. Use of soil and vegetation spectroradiometry to investigate crop water use efficiency of a drip irrigated tomato. Eur. J. Agron. 2014, 59, 67–77. [Google Scholar] [CrossRef]
- Zhang, H.M.; Xiong, Y.W.; Huang, G.H.; Xu, X.; Huang, Q.Z. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Lamm, F.R.; Schlegel, A.J.; Clark, G.A. Development of a best management practice for nitrogen fertigation of corn using SDI. Appl. Eng. Agric. 2004, 20, 211–220. [Google Scholar] [CrossRef]
- Abbasi, F.; Shooshtari, M.; Feyen, J. Evaluation of Various Surface Irrigation Numerical Simulation Models. J. Irrig. Drain. Eng. 2003, 129, 208–213. [Google Scholar] [CrossRef]
- Brunetti, G.; Šimůnek, J.; Bautista, E. A hybrid finite volume-finite element model for the numerical analysis of furrow irrigation and fertigation. Comput. Electron. Agric. 2018, 150, 312–327. [Google Scholar] [CrossRef]
- Zhu, Y.; Houping, Z.; Li, R.; Wendong, Z.; Kang, Y. Nitrogen fertigation affects crop yield, nitrogen loss and gaseous emissions: A meta-analysis. Nutr. Cycl. Agroecosyst. 2023, 127, 359–373. [Google Scholar] [CrossRef]
- Yao, Z.S.; Yan, G.X.; Wang, R.; Zheng, X.H.; Liu, C.Y.; Butterbach-Bahl, K. Drip irrigation or reduced N-fertilizer rate can mitigate the high annual N2O + NO fluxes from Chinese intensive greenhouse vegetable systems. Atmos. Environ. 2019, 212, 183–193. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, G.; Xie, H.; Chang, N.; Zhang, J.; Zhang, J.; Li, G.; Li, H. Balancing high yields and low N2O emissions from greenhouse vegetable fields with large water and fertilizer input: A case study of multiple-year irrigation and nitrogen fertilizer regimes. Plant Soil 2023, 483, 131–152. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Alsina, M.M.; Fanton-Borges, A.C.; Smart, D.R. Spatiotemporal variation of event related N2O and CH4 emissions during fertigation in a California almond orchard. Ecosphere 2013, 4, 1. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Alva, A. High water regime can reduce ammonia volatilization from soils under potato production. Comm. Soil Sci. Plant Anal. 2007, 38, 1203–1220. [Google Scholar] [CrossRef]
- Gagnon, B.; Ziadi, N.; Rochette, P.; Chantigny, M.H.; Angers, D.A. Fertilizer Source Influenced Nitrous Oxide Emissions from a Clay Soil under Corn. Soil Sci. Soc. Am. J. 2011, 75, 595–604. [Google Scholar] [CrossRef]
- Inselsbacher, E.; Wanek, W.; Ripka, K.; Hackl, E.; Sessitsch, A.; Strauss, J.; Zechmeister-Boltenstern, S. Greenhouse gas fluxes respond to different N fertilizer types due to altered plant-soil-microbe interactions. Plant Soil 2011, 343, 17–35. [Google Scholar] [CrossRef]
- Wolff, M.W.; Hopmans, J.W.; Stockert, C.M.; Sanden, M.B.B.L.; Smart, D.R. Effects of drip fertigation frequency and N-source on soil N2O production in almonds. Agric. Ecosyst. Environ. 2017, 238, 67–77. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Hamad, A.A.A.; Wei, Q.; Xu, J.; Hamoud, Y.A.; He, M.; Shaghaleh, H.; Wei’, Q.; Li, X.; Qi, Z. Managing Fertigation Frequency and Level to Mitigate N2O and CO2 Emissions and NH3 Volatilization from Subsurface Drip-Fertigated Field in a Greenhouse. Agronomy 2022, 12, 1414. [Google Scholar] [CrossRef]
- Grace, P.R.; van der Weerden, T.J.; Rowlings, D.W.; Scheer, C.; Brunk, C.; Kiese, R.; Skiba, U.M. Global Research Alliance N2O chamber methodology guidelines: Considerations for automated flux measurement. J. Environ. Qual. 2020, 49, 1126–1140. [Google Scholar] [CrossRef]
- McClain, M.E.; Boyer, E.W.; Dent, C.L.; Gergel, S.E.; Grimm, N.B.; Groffman, P.M.; Hart, S.C.; Harvey, J.W.; Johnston, C.A.; Mayorga, E.; et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 2003, 6, 301–312. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Blaszczak, J.R.; Ficken, C.D.; Fork, M.L.; Kaiser, K.E.; Seybold, E.C. Control points in ecosystems: Moving beyond the hot spot hot moment concept. Ecosystems 2017, 20, 665–682. [Google Scholar] [CrossRef]
- Sihi, D.; Davidson, E.A.; Savage, K.E.; Liang, D. Simultaneous numerical representation of soil microsite production and consumption of carbon dioxide, methane, and nitrous oxide using probability distribution functions. Glob. Change Biol. 2020, 26, 200–218. [Google Scholar] [CrossRef]
- Molodovskaya, M.; Singurindy, O.; Richards, B.K.; Warland, J.; Johnson, M.S.; Steenhuis, T.S. Temporal variability of nitrous oxide from fertilized croplands: Hot moment analysis. Soil Sci. Soc. Am. J. 2012, 76, 1728. [Google Scholar] [CrossRef]
- Savage, K.; Phillips, R.; Davidson, E. High temporal frequency measurements of greenhouse gas emissions from soils. Biogeosciences 2014, 11, 2709–2720. [Google Scholar] [CrossRef]
- Krichels, A.H.; Yang, W.H. Dynamic controls on field-scale soil nitrous oxide hot spots and hot moments across a microtopographic gradient. J. Geophys. Res. Biogeosci. 2019, 124, 3618–3634. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 1996, 60, 609–640. [Google Scholar] [CrossRef] [PubMed]
- McNicol, G.; Silver, W.L. Separate effects of flooding and anaerobiosis on soil greenhouse gas emissions and redox sensitive biogeochemistry. J. Geophys. Res. Biogeosci. 2014, 119, 557–566. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Congreves, K.A.; Abalos, D. Globally important nitrous oxide emissions from croplands induced by freeze–thaw cycles. Nat. Geosci. 2017, 10, 279–283. [Google Scholar] [CrossRef]
- Hall, S.J.; Tenesaca, C.G.; Lawrence, N.C.; Green, D.I.S.; Helmers, M.J.; Crumpton, W.G.; Heaton, E.A.; VanLoocke, A. Poorly drained depressions can be hotspots of nutrient leaching from agricultural soils. J. Environ. Qual. 2023, 52, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Silver, W.L. Hot moments drive extreme nitrous oxide and methane emissions from agricultural peatlands. Glob. Change Biol. 2021, 27, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Szutu, D.J.; Verfaillie, J.G.; Baldocchi, D.D.; Silver, W.L. Carbon-sink potential of continuous alfalfa agriculture lowered by short-term nitrous oxide emission events. Nat. Commun. 2023, 14, 1926. [Google Scholar] [CrossRef]
- Lintott, P.R.; Mathews, F. Basic mathematical errors may make ecological assessments unreliable. Biodivers. Conserv. 2018, 27, 265–267. [Google Scholar] [CrossRef]
- Macharia, J.M.; Pelster, D.E.; Ngetich, F.K.; Shisanya, C.A.; Mucheru-Muna, M.; Mugendi, D.N. Soil greenhouse gas fluxes from maize production under different soil fertility management practices in East Africa. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005427. [Google Scholar] [CrossRef]
- Wiggins, B.C. Detecting and dealing with outliers in univariate and multivariate contexts. In In Proceedings of the Annual Meeting of the Mid-South Educational Research Association, Bowling Green, KY, USA, 15–17 November 2000. [Google Scholar]
- Benhadi-Marín, J. A conceptual framework to deal with outliers in ecology. Biodivers. Conserv. 2018, 27, 3295–3300. [Google Scholar] [CrossRef]
- Holst, J.; Liu, C.; Yao, Z.; Brüggemann, N.; Zheng, X.; Giese, M.; Butterbach-Bahl, K. Fluxes of nitrous oxide, methane and carbon dioxide during freezing–thawing cycles in an Inner Mongolian steppe. Plant Soil 2008, 308, 105–117. [Google Scholar] [CrossRef]
- Barton, L.; Wolf, B.; Rowlings, D.; Scheer, C.; Kiese, R.; Grace, P.; Stefanova, K.; Butterbach-Bahl, K. Sampling frequency affects estimates of annual nitrous oxide fluxes. Sci. Rep. 2015, 5, 15912–15919. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Silver, W.L. Hot spots and hot moments of greenhouse gas emissions in agricultural peatlands. Biogeochemistry 2024, 167, 461–477. [Google Scholar] [CrossRef]
- de Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Padilla, F.M. Assessing Performance of Vegetation Indices to Estimate Nitrogen Nutrition Index in Pepper. Remote Sens. 2020, 12, 763. [Google Scholar] [CrossRef]
- Palma, J.; Terán Calle, F.; Contreras-Ruiz, A.; Ruiz, M.; Corpas, F. Antioxidant Profile of Pepper (Capsicum annuum L.) Fruits Containing Diverse Levels of Capsaicinoids. Antioxidants 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Diodato, N.; Bellocchi, G. Long-term winter temperatures in central Mediterranean: Forecast skill of an ensemble statistical model. Theor. Appl. Climatol. 2014, 116, 131–146. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef]
- Seo, Y.; Kim, G.; Park, K.; Kim, k.; Jung, Y. Nitrous Oxide Emissions from Red Pepper, Chinese Cabbage, and Potato Fields in Gangwon-do, Korea. Korean J. Soil Sci. Fertil. 2013, 46, 463–468. [Google Scholar] [CrossRef]
- Regione Campania. Disciplinare di Produzione Integrata: Peperone. 2024. Available online: https://agricoltura.regione.campania.it/disciplinari/2024/peperone.pdf (accessed on 11 June 2025).
- Caccavello, G.; Giaccone, M.; Scognamiglio, P.; Mataffo, A.; Teobaldelli, M.; Basile, B. Vegetative, Yield, and Berry Quality Response of Aglianico to Shoot Trimming Applied at Three Stages of Berry Ripening. Am. J. Enol. Vitic. 2019, 70, 351–359. [Google Scholar] [CrossRef]
- Roman-Perez, C.C.; Hernandez-Ramirez, G. Sources and priming of N2O production across a range of moisture contents in a soil with high organic matter. J. Environ. Qual. 2021, 50, 94–109. [Google Scholar] [CrossRef]
- Eosense Website—Application Article. Available online: https://eosense.com/application-article-using-the-eosmx-recirculating-multiplexer-with-manual-chambers/ (accessed on 1 March 2025).
- Macharia, J.M.; Ngetich, F.K.; Shisanya, C.A. Parameterization, calibration and validation of the DNDC model for carbon dioxide, nitrous oxide and maize crop performance estimation in East Africa. Heliyon 2020, 7, e06977. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Anthropogenic and Natural Radiative Forcing. In Climate Change 2013–The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 659–740. [Google Scholar]
- Silber, A.; Bruner, M.; Kenig, E.; Reshef, G.; Zohar, H.; Posalski, I.; Yehezkel, H.; Shmuel, D.; Cohen, S.; Dinar, M.; et al. High fertigation frequency and phosphorus level: Effects on summer-grown bell pepper growth and blossom-end rot incidence. Plant Soil 2005, 270, 135–146. [Google Scholar] [CrossRef]
- Sourav, S.K.; Subbarayappa, C.T.; Hanumanthappa, D.C.; Mudalagiriyappa; Vazhacharickal, P.J.; Mock, A.; Ingold, M.; Buerkert, A. Soil respiration under different N fertilization and irrigation regimes in Bengaluru, S-India. Nutr. Cycl. Agroecosyst. 2023, 127, 333–345. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Andrew Black, T.; Griffis, T.J.; Barr, A.G.; Jassal, R.S.; Nesic, Z. Interpreting the dependence of soil respiration on soil temperature and water content in a boreal aspen stand. Agric. For. Meteorol. 2006, 140, 220–235. [Google Scholar] [CrossRef]
- Themistokleous, G.; Philippou, K.; Ioannides, I.M.; Omirou, M. A high-frequency greenhouse-gas flux analysis tool: Insights from automated non-steady-state transparent soil chambers. Eur. J. Soil Sci. 2024, 75, e13560. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.; Liu, Y.; Wang, D.; Chen, S.; Qian, W.; He, M.; Chen, P.; Zhou, X.; Qi, Z. Nitrogen losses from soil as affected by water and fertilizer management under drip irrigation: Development, hotspots and future perspectives. Agric. Water Manag. 2024, 296, 108791. [Google Scholar] [CrossRef]
- Feng, Z.-J.; Weibo, N.; Ma, Y.-P.; Li, Y.; Ma, X.-Y.; Zhu, H.-Y. Effects of urea solution concentration on soil hydraulic properties and water infiltration capacity. Sci. Total Environ. 2023, 898, 165471. [Google Scholar] [CrossRef] [PubMed]
- Todman, L.; Chhang, A.; Riordan, H.; Brooks, D.; Butler, A.; Templeton, M. Soil Osmotic Potential and Its Effect on Vapor Flow from a Pervaporative Irrigation Membrane. J. Environ. Eng. 2018, 144, 04018048. [Google Scholar] [CrossRef]
- Sher, Y.; Baker, N.R.; Herman, D.; Fossum, C.; Hale, L.; Zhang, X.; Nuccio, E.; Saha, M.; Zhou, J.; Pett-Ridge, J.; et al. Microbial extracellular polysaccharide production and aggregate stability controlled by switchgrass (Panicum virgatum) root biomass and soil water potential. Soil Biol. Biochem. 2020, 143, 107742. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Z.; Zhuang, D. Urea fertilization decreases soil bacterial diversity but increases microbial biomass, respiration and N-cycling potential in a semiarid grassland. Biol. Fertil. Soils 2019, 55, 303–315. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qu, C.; Huang, Q.; Cai, P. Microbial extracellular polymeric substances (EPS) in soil: From interfacial behaviour to ecological multifunctionality. Geo-Bio Interfaces 2024, 1, e4. [Google Scholar] [CrossRef]
- Ribeiro, P.L.; Pitann, B.; Banedjschafie, S.; Mühling, K.H. Effectiveness of three nitrification inhibitors on mitigating trace gas emissions from different soil textures under surface and subsurface drip irrigation. J. Environ. Manag. 2024, 359, 120969. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Zhang, H.; Wang, M.; Xu, J.; Yu, L.; Cai, H. Deficit irrigation interacting with biochar mitigates N2O emissions from farmland in a wheat–maize rotation system. Agric. Water Manag. 2024, 297, 108843. [Google Scholar] [CrossRef]


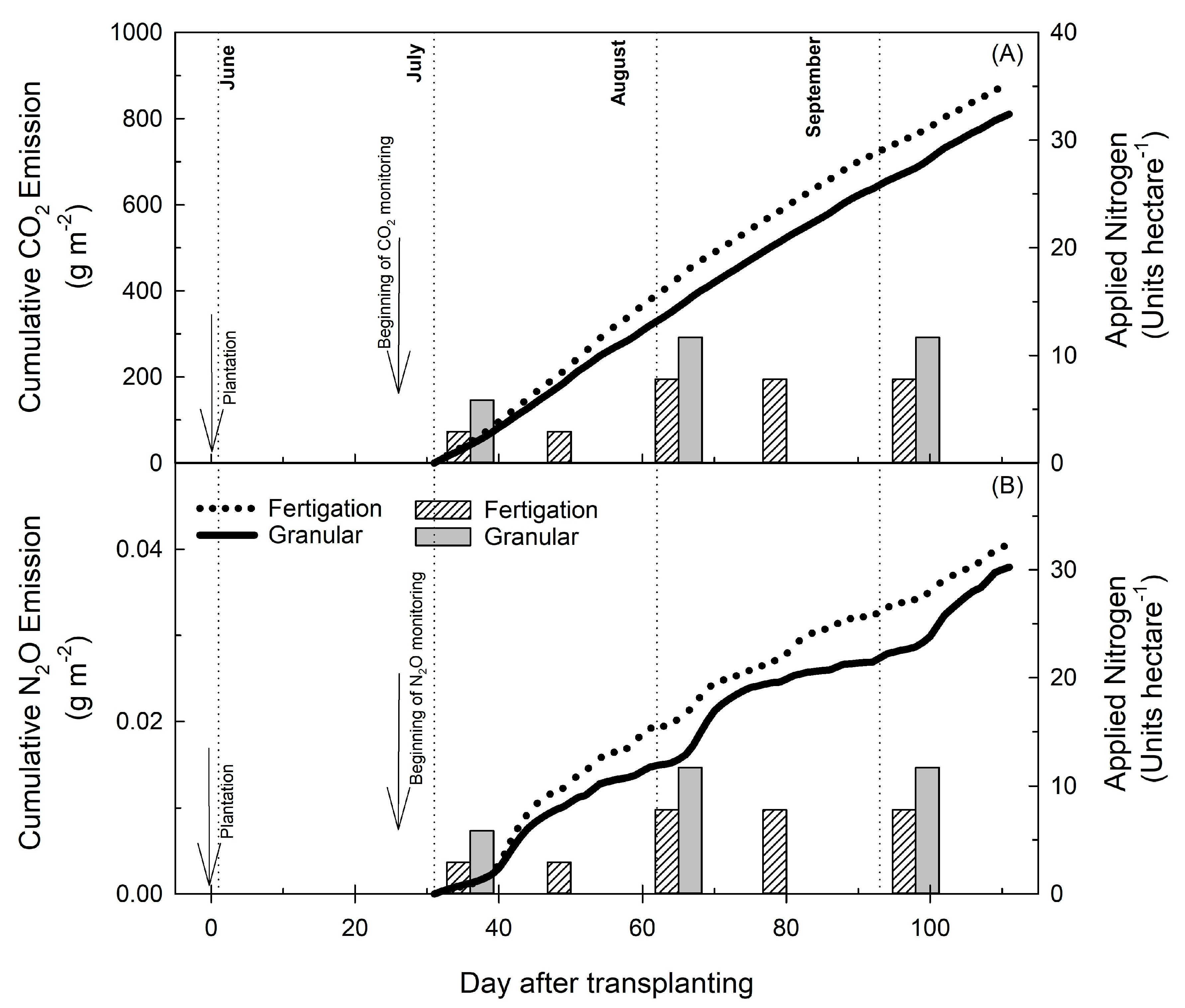
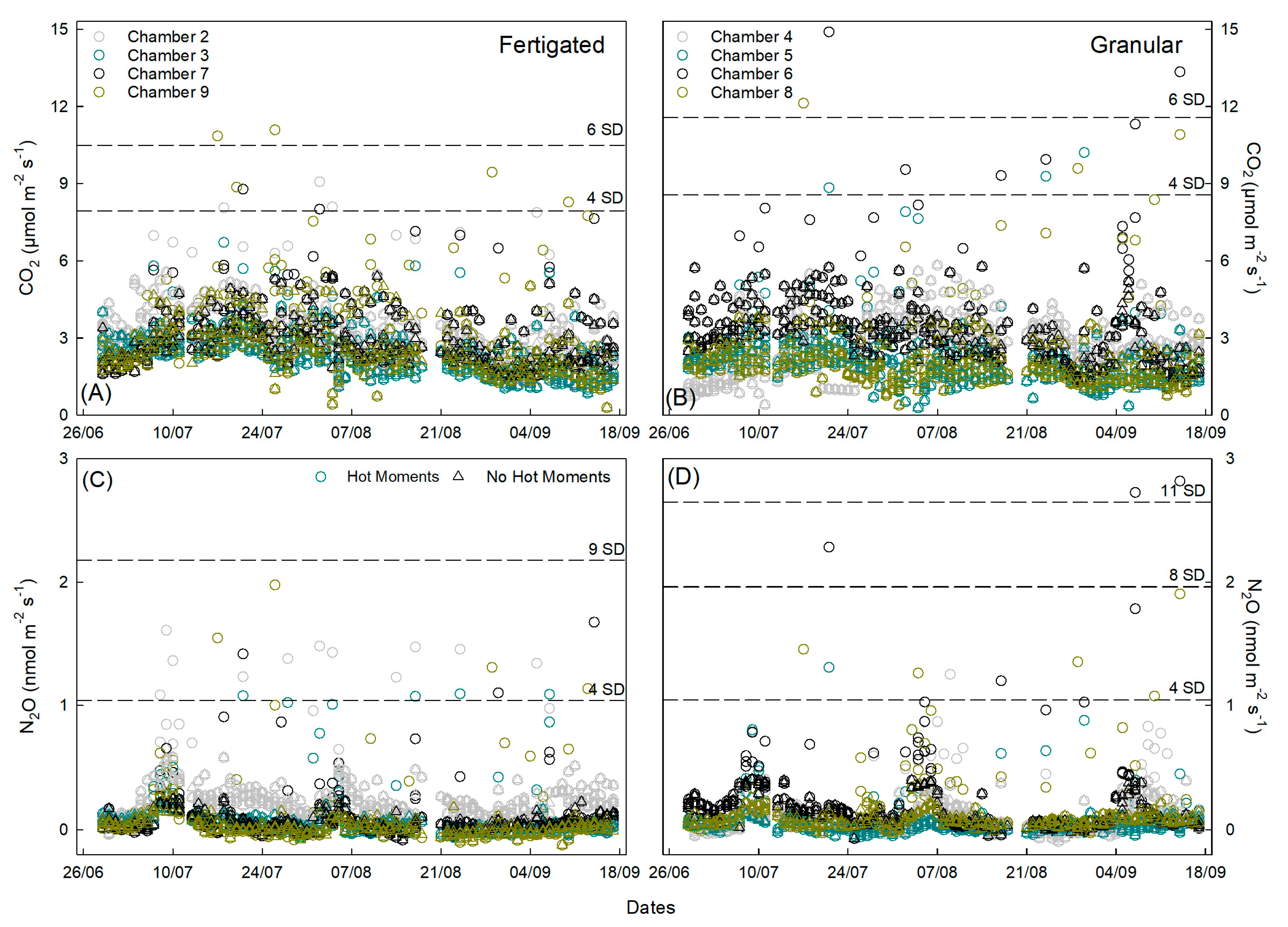

| Treatment | CO2 Flux (µmol m−2 s−1) | CO2 Cumulative Emission (g m−2) | Yield-Scaled CO2 (kg CO2-eq ha−1) | Flux (n) | Hot Moment Frequency (%) | Hot Moment % of Flux |
|---|---|---|---|---|---|---|
| G | 2.65 ± 0.55 * | 810.53 ± 187.4 ns | 0.72 ± 0.006 ns | 1212 | 0.99% | 4.0% |
| F | 2.88 ± 0.67 * | 880.92 ± 113.2 ns | 0.80 ± 0.003 ns | 1212 | 0.82% | 2.6% |
| Treatment | N2O Flux (nmol m−2 s−1) | N2O Cumulative Emission (g m−2) | Yield-Scaled N2O (kg CO2-eq ha−1) | Flux (n) | Hot Moment Frequency (%) | Hot Moment % of Flux |
| G | 0.12 ± 0.10 ns | 0.038 ± 0.02 ns | 0.010 ± 0.001 ns | 1212 | 0.99% | 13.3% |
| F | 0.13 ± 0.10 ns | 0.041 ± 0.03 ns | 0.011 ± 0.002 ns | 1212 | 1.81% | 18.8% |
| G | F | |||||
|---|---|---|---|---|---|---|
| GHG | R2 | p | Equation | R2 | p | Equation |
| CO2 | 0.0272 | 0.004 | 1.924 + (0.0128WFPS) | 0.0639 | <0.00 | 1.166 + (0.0642Ts) |
| N2O | 0.0453 | <0.001 | 0.134 − (0.0100Ts) + (0.00182WFPS) | 0.158 | <0.001 | −0.255 + (0.00511WFPS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manco, A.; Giaccone, M.; Vitale, L.; Maglione, G.; Riccardi, M.; Matteo, B.D.; Esposito, A.; Magliulo, V.; Tedeschi, A. Comparative Effects of Nitrogen Fertigation and Granular Fertilizer Application on Pepper Yield and Soil GHGs Emissions. Horticulturae 2025, 11, 708. https://doi.org/10.3390/horticulturae11060708
Manco A, Giaccone M, Vitale L, Maglione G, Riccardi M, Matteo BD, Esposito A, Magliulo V, Tedeschi A. Comparative Effects of Nitrogen Fertigation and Granular Fertilizer Application on Pepper Yield and Soil GHGs Emissions. Horticulturae. 2025; 11(6):708. https://doi.org/10.3390/horticulturae11060708
Chicago/Turabian StyleManco, Antonio, Matteo Giaccone, Luca Vitale, Giuseppe Maglione, Maria Riccardi, Bruno Di Matteo, Andrea Esposito, Vincenzo Magliulo, and Anna Tedeschi. 2025. "Comparative Effects of Nitrogen Fertigation and Granular Fertilizer Application on Pepper Yield and Soil GHGs Emissions" Horticulturae 11, no. 6: 708. https://doi.org/10.3390/horticulturae11060708
APA StyleManco, A., Giaccone, M., Vitale, L., Maglione, G., Riccardi, M., Matteo, B. D., Esposito, A., Magliulo, V., & Tedeschi, A. (2025). Comparative Effects of Nitrogen Fertigation and Granular Fertilizer Application on Pepper Yield and Soil GHGs Emissions. Horticulturae, 11(6), 708. https://doi.org/10.3390/horticulturae11060708









