Abstract
This study investigated the extraction and quantification of specific phenolic compounds, including chlorogenic acid and several pyranocoumarin derivatives, from the leaves and roots of Peucedanum japonicum. Using high-performance liquid chromatography, this study aimed to optimize extraction methodologies with different solvents to maximize the yield of bioactive compounds. The extraction process involved meticulous steps, including reflux extraction and solvent evaporation, and the total phenolic content was assessed using a spectrophotometric assay. The results demonstrated that ethanol and methanol were effective in extracting chlorogenic acid, yielding a total phenolic content of up to 47.71 mg/g tannic acid equivalent in MeOH extracts from roots. Conversely, acetone was superior for pyranocoumarin extraction, achieving a total coumarin content exceeding 100 mg/g in root extracts. Notably, pyranocoumarins were found to be more concentrated in the roots compared to leaves, supporting the hypothesis that roots are a reservoir for these bioactive compounds. This study emphasized the critical roles of solvent selections in profiling bioactive compounds from P. japonicum and provided valuable insights for future research into its pharmacological potential. The findings may serve as a foundation for further pharmacological studies, enhancing the understanding of the medicinal properties of P. japonicum in the context of traditional East Asian medicine.
1. Introduction
Secondary metabolites, also known as natural products, are compounds produced by organisms such as plants and bacteria that are not primarily involved in their survival and growth [1,2]. Instead, these compounds play crucial roles in the defense mechanisms of organisms, helping them combat pathogens, herbivory, and environmental stressors such as excessive sunlight, drought, and poor soil conditions [3,4]. These bioactive compounds have garnered significant attention in pharmacology and biotechnology due to their diverse biological activities [5]. Many secondary metabolites have been utilized in drug discovery, either through direct extraction from natural sources or synthetic replication in laboratories [6]. Notable examples include antibiotics, antifungal agents, anticancer drugs, and anti-inflammatory compounds derived from plants and microorganisms.
Among the vast array of secondary metabolites, phenolic compounds constitute one of the most ubiquitously distributed groups in nature [7]. These compounds are characterized by the presence of at least one phenol functional group and encompass a wide range of chemical subclasses, including lignans, flavonoids, stilbenes, phenolic acids, and coumarins [8,9]. Phenolic compounds are well documented for their antioxidant properties, which are crucial in plant defense and human health by preventing oxidative damage associated with chronic diseases [10,11].
Coumarins have been the subject of extensive research since their first isolation from tonka beans (Dipteryx odorata) in the early 19th century [12]. These compounds, derived from the phenylpropanoid biosynthetic pathway, are widely distributed in the plant kingdom, particularly in families such as Apiaceae, Rutaceae, Asteraceae, Fabaceae, Oleaceae, Moraceae, and Thymelaeaceae [13,14]. Coumarins exhibit a broad range of biological activities, including anticancer, antimicrobial, anticoagulant, anti-inflammatory, and immunomodulatory effects [15,16,17]. Structurally, they are classified into various subgroups, including simple coumarins, bicoumarins, phenyl coumarins, furanocoumarins, dihydrofuranocoumarins, and pyranocoumarins, which can exist in linear or angular forms [18]. Pyranocoumarins, a rare subclass, are distinguished by the presence of a fused pyran ring in their structure [19]. These specialized metabolites are predominantly found in plants belonging to the Rutaceae and Apiaceae families [20]. Given their unique chemical structures, pyranocoumarins have attracted considerable interest in medicinal chemistry and pharmacology due to their reported antimicrobial, cytotoxic, and anti-inflammatory activities [21]. However, despite their therapeutic potential, their phytochemical compositions and biological functions remain relatively underexplored.
One plant known to contain pyranocoumarins is Peucedanum japonicum, commonly known as coastal hog fennel [22]. This species, a member of the Apiaceae family, is widely cultivated in East Asia and the Philippines, where it has been traditionally used to alleviate symptoms of respiratory ailments such as coughs and colds [23]. In addition to its traditional uses, modern pharmacological studies have demonstrated that P. japonicum, along with other species of this genus, exhibits notable antioxidant, antibacterial, and anticancer properties [24,25]. Despite these promising findings, the specific distribution of pyranocoumarins within different plant organs, such as roots and leaves, has not been comprehensively investigated. Furthermore, the effects of different extraction solvents on the yields and compositions of these bioactive compounds remain largely unknown.
Research on natural products has been pivotal in identifying bioactive compounds with potential therapeutic applications [26]. The methodologies used in phytochemical studies, including solvent extraction and chromatographic analysis, play crucial roles in determining the effectiveness of target compound isolation [27]. The choice of solvent significantly influences the yield and composition of the bioactive compounds extracted from plant samples [28]. Polar solvents such as ethanol (EtOH) and methanol (MeOH) are known to extract a broad range of phytochemicals. Therefore, selecting an appropriate solvent system is essential for maximizing the recovery of biologically active compounds from medicinal plants.
In this study, the leaves and roots of P. japonicum were extracted using three solvents: EtOH, MeOH, and acetone (Ace). The objective was to evaluate the differences in phenolic content across these extracts using total polyphenol (TPC) and total coumarin content (TCC) assays as initial tests. More importantly, this study aimed to develop a high-performance liquid chromatography (HPLC) method for the quantification and distribution of pyranocoumarins in these extracts. Furthermore, by understanding the solvent-dependent extraction efficiency and phytochemical composition of P. japonicum, this study aimed to provide valuable insights into the optimal extraction conditions for harnessing its bioactive potential.
2. Materials and Methods
2.1. Plant Materials
The leaves and roots of P. japonicum Thunb. were cultivated and harvested on 22 March 2021, from a farmhouse (36.401466° N, 126.630675° E) in Boryeong, Republic of Korea. The species was identified by Dr. J. H. Kim at the Department of Herbal Crop Research, the National Institute of Horticultural and Herbal Science, the Rural Development Association, Eumseong, Republic of Korea (Figure 1). Voucher specimens (leaves: PJLB210322_1; roots: PJLB210322_2) were deposited in the herbarium of the Department of Herbal Crop Research at the National Institute of Horticultural and Herbal Science, Eumseong, Republic of Korea.

Figure 1.
Cultivation site of P. japonicum in Boryeong.
2.2. Instrumentation
For phytochemical extraction, an MTops EAM-MS heating mantle, obtained from Misung Scientific Equipment Co., Ltd. (Yangju, Republic of Korea), was used. A digital rotary Eyela evaporator was purchased from Sunil Eyela Ltd. (Seongnam, Republic of Korea). HPLC analysis was performed using a Waters Alliance e2695 separation module (Milford, MA, USA), equipped with a quaternary pump, an autosampler, and a Waters 2998 photodiode array (PDA) detector (Milford, MA, USA). Data acquisition and processing were carried out using Empower 3.8.0 Chromatography Data Software (Milford, MA, USA). A Primesep 200 column (4.6 × 250 mm, 5 μm) was obtained from SIELC Technologies (Wheeling, IL, USA). Sonication was conducted using a POWERSONIC 410 ultrasonic bath (Hwashin Instrument Co. Ltd., Seoul, Republic of Korea). Sample weights were measured using an Ohaus Pioneer analytical balance, purchased from Merck KGaA (Darmstadt, Germany). Centrifugation was performed using an N-Biotek MICRO-CENVAC centrifuge (Clayton, VIC, Australia).
2.3. Chemicals and Reagents
The extraction solvents, 95% EtOH, 99.5% MeOH, and Ace, were obtained from Samchun Chemicals (Pyeongtaek, Republic of Korea). HPLC-grade water, acetonitrile (ACN), and MeOH were supplied by Honeywell (Burdick and Jackson, Muskegon, MI, USA). Formic acid was purchased from J. T. Baker (Phillipsburg, PA, USA). The reference compounds, including chlorogenic acid (1), were provided and confirmed by the Natural Product Institute of Science and Technology, Anseong, Republic of Korea, whereas the pyranocoumarins isosamidin (2), 3′-acetoxy-4′-(2-methylbutyroyl)khellactone (3), 3′,4′-disencioylkhellactone (4), 3′-sencioyl-4′-isovalerylkhellactone (5), and 3′-isovaleryl-4′-(2-methylbutyroyl)khellactone (6) were isolated from P. japonicum and identified by one of the authors, Dr. Kim, from their previous study [29] (Figure 2).
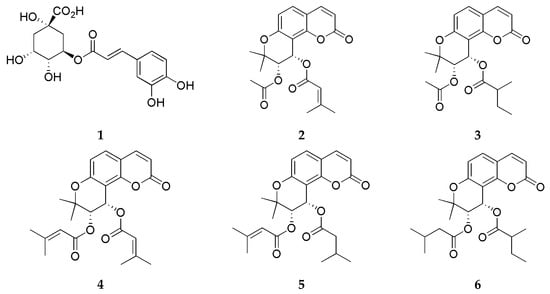
Figure 2.
Chemical structures of chlorogenic acid (1), isosamidin (2), 3′-acetoxy-4′-(2-methylbutyroyl)khellactone (3), 3′,4′-disencioylkhellactone (4), 3′-sencioyl-4′-isovalerylkhellactone (5), and 3′-isovaleryl-4′-(2-methylbutyroyl)khellactone (6).
2.4. Sample Extraction
For crude extraction, 10 g of each sample was carefully weighed and subjected to reflux extraction three times using 95% EtOH, 99.5% MeOH, and Ace in a heating mantle for 5 h. After each extraction, the extracts were filtered and concentrated using a rotary evaporator at 60 °C until all solvents had evaporated. The extraction yield was calculated after complete solvent evaporation using the following formula:
%yield = [(weight of vial with extract − weight of empty vial)/(dry sample weight)] × 100
2.5. Total Phenolic Content Assay
The TPC of P. japonicum extracts was measured using the procedure outlined in a previous study [30]. In a 96-well plate, 60 μL of each extract was added, followed by 40 μL of Folin–Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA) and 100 μL of 7.5% sodium carbonate (Na2CO3) solution. The plate was briefly shaken using a MicroMixer MX4 microplate reader (FINEPCR, Korea Science Co., Ltd., Gunpo, Republic of Korea) and incubated for 30 min at room temperature in the dark to allow the reaction to proceed. After incubation, the absorbance was recorded at 760 nm using an Epoch microplate reader (BioTek, Winooski, VT, USA). TPC, expressed as the mg tannic acid equivalent (TAE)/g of extract, was calculated based on a standard curve constructed using various concentrations of tannic acid.
2.6. Total Coumarin Content Assay
The TCC in the P. japonicum extracts was measured according to previously described methods, with modifications [31]. Briefly, a 2 μL aliquot of the extract was dispensed into a 96-well plate, followed by 8 μL of deionized water and 2 μL of 5% (w/v) lead acetate solution. The mixture was shaken, after which 28 μL of distilled water and 160 μL of 0.1 M hydrochloric acid were added to each well. The plate was briefly vortexed using a MicroMixer MX4 microplate reader (FINEPCR, Korea Science Co., Ltd., Gunpo, Republic of Korea) and incubated for 30 min at room temperature in the dark. The absorbance of the samples was measured at 320 nm using an Epoch microplate reader (BioTek, Winooski, VT, USA). TCC was determined using a standard curve derived from different concentrations of coumarin and expressed as the mg coumarin equivalent (CE)/g of extract.
2.7. HPLC Conditions
Each standard compound (1 mg) was dissolved in 1 mL of MeOH to obtain a stock solution with a concentration of 1 mg/mL. All test extracts were precisely weighed and mixed with the same solvent to achieve a concentration of 30 mg/mL. All standards and test extracts were sonicated for 15 min and subsequently filtered using a 0.45 µm polyvinylidene fluoride filter. The mobile phase of the gradient elution system consisted of 0.1% formic acid in water (A) and ACN (B), with the following gradient program: 90% A from 0 to 10 min, 0% A at 25 min until 30 min, and 10% B from 31 to 40 min. The sample injection volume was 10 µL, with a flow rate of 1.0 mL/min. Detection was performed at a wavelength of 320 nm. The limits of detection (LOD) and quantification (LOQ) were used to validate the HPLC method.
2.8. Calibration Curves
The quantitative results of the compounds were determined using calibration curves. The calibration functions of the standard compounds were calculated based on peak areas (Y), concentrations (X, μg/mL), and mean values ± standard deviation (SD) (n = 3).
2.9. Statistical Analysis
The results were expressed as mean ± SD, and all analyses were conducted in triplicate. The data were normalized using a one-way analysis of variance followed by Tukey’s post hoc test. All statistical analyses were performed using GraphPad Prism 8.0.2 software (GraphPad Software, Boston, MA, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Extraction Yield
The sample names, solvents used for extraction, plant parts utilized, and extraction yields are summarized in Table 1. After three reflux extraction runs, MeOH-based phytochemical extraction produced the highest yield, followed by EtOH and Ace. The extraction yield from the roots was higher when using MeOH and EtOH, whereas Ace resulted in a higher yield from the leaves. These findings reflect differences in extraction efficiency among the solvents in terms of total material obtained.

Table 1.
Overview of sample information for P. japonicum extracts from Boryeong, Korea.
3.2. TPC of the Different Extracts
The TPC of the P. japonicum extracts was evaluated using the Folin–Ciocalteu method. The results revealed that the leaf ethanolic extract (BEL) exhibited the highest TPC, measuring 42.3 mg TAE/g of extract (Figure 3a). This was followed by BML (27.2 mg TAE/g of extract), BER (13.3 mg TAE/g of extract), BAL (13.0 mg TAE/g of extract), BMR (10.91 mg TAE/g of extract), and BAR (6.54 mg TAE/g of extract). No clear trends were observed, except that the EtOH and MeOH extracts exhibited higher TPC values than the Ace extracts.
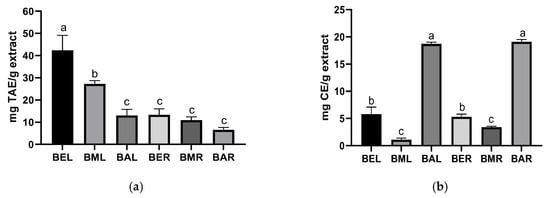
Figure 3.
TPC (a) and TCC (b) of P. japonicum extracts obtained using EtOH, MeOH, and Ace. Data are presented as mean ± SD. Different lowercase letters indicate significant differences (p < 0.05).
3.3. The TCC of the Different Extracts
Similarly, the TCC of the P. japonicum extracts was evaluated. The results demonstrated that the Ace extracts contained (p < 0.05) higher coumarin content than their EtOH and MeOH counterparts (Figure 3b). Specifically, BAR exhibited the highest TCC among the extracts, with a value of 19.1 mg CE/g of extract. This was followed by BAL (18.7 mg CE/g of extract), BEL (5.8 mg CE/g of extract), BER (5.3 mg CE/g of extract), BMR (3.4 mg CE/g of extract), and BML (1.1 mg CE/g of extract).
3.4. Content of Compounds 1–6 by HPLC Quantitative Analysis
The present study analyzed the contents of compounds 1–6 in the leaves and roots of P. japonicum extracted using EtOH, MeOH, and Ace (Figure 4). The standard compounds exhibited good separation and retention times under the applied chromatographic conditions (Table 2). The LOD and LOQ of each compound were also determined to validate the chromatographic separation.
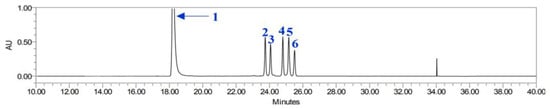
Figure 4.
HPLC chromatogram of chlorogenic acid (1), isosamidin (2), 3′-acetoxy-4′-(2-methylbutyroyl)khellactone (3), 3′,4′-disencioylkhellactone (4), 3′-sencioyl-4′-isovalerylkhellactone (5), and 3′-isovaleryl-4′-(2-methylbutyroyl)khellactone (6).

Table 2.
Calibration data for compounds 1–6.
The quantitative analysis of the different P. japonicum extracts revealed a significant (p < 0.05) difference in the distribution of these compounds between the leaves and roots of the plant (Table 3). A general observation was that, in terms of total content, both Ace extracts (BAL and BAR) were the only ones that exceeded 100 mg/g, whereas the other extracts contained less than 50 mg/g.

Table 3.
Content of compounds 1–6 in the P. japonicum extracts.
Chlorogenic acid (1) was predominantly found in the leaves, regardless of the extraction solvent used (Figure 5). EtOH and MeOH were more effective at extracting chlorogenic acid (1), with BEL and BML having the highest concentrations (28.76 and 24.29 mg/g, respectively). In contrast, their root counterparts, BER and BMR, contained only suboptimal levels (0.90 and 0.60 mg/g, respectively).
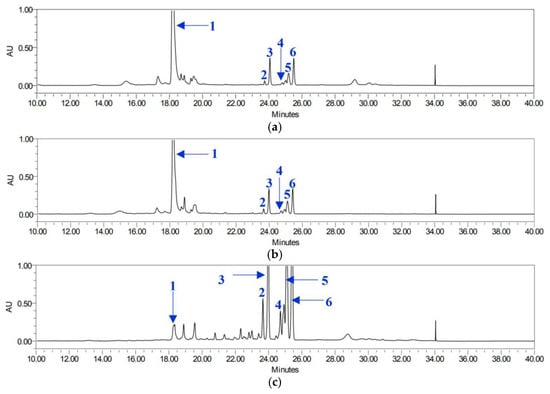
Figure 5.
HPLC chromatograms of P. japonicum leaves: BEL (a), BML (b), and BAL (c). Chlorogenic acid (1), isosamidin (2), 3′-acetoxy-4′-(2-methylbutyroyl)khellactone (3), 3′,4′-disencioylkhellactone (4), 3′-sencioyl-4′-isovalerylkhellactone (5), and 3′-isovaleryl-4′-(2-methylbutyroyl)khellactone (6).
The Ace extracts, BAL and BAR, exhibited relatively higher concentrations of chlorogenic acid (1) at 3.64 and 2.96 mg/g, respectively. Interestingly, compounds 2 and 3 in BEL and BML showed opposite trends, with EtOH being more effective at extracting compound 2, whereas MeOH was more efficient at extracting compound 3. The remaining compounds displayed similar trends for BEL and BML.
For BAL, only compounds 2 and 4 were detected at concentrations below 5 mg/g, whereas the concentrations of the remaining compounds exceeded 25 mg/g. In the BAR extract, all compounds were detected at higher concentrations than in the other extracts, except for compound 6, which was slightly lower than that in BAL. Overall, pyranocoumarins (compounds 2–6) were more abundant in the roots, regardless of the solvent used (Figure 6).
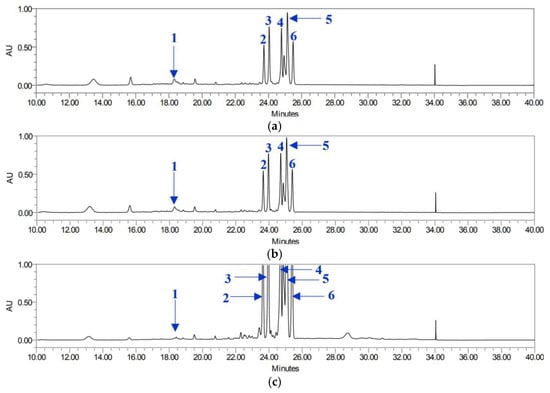
Figure 6.
HPLC chromatograms of P. japonicum roots: BER (a), BMR (b), and BAR (c). Chlorogenic acid (1), isosamidin (2), 3′-acetoxy-4′-(2-methylbutyroyl)khellactone (3), 3′,4′-disencioylkhellactone (4), 3′-sencioyl-4′-isovalerylkhellactone (5), and 3′-isovaleryl-4′-(2-methylbutyroyl)khellactone (6).
4. Discussion
In this study, the extraction yield, TPC, TCC, and quantitative HPLC results for P. japonicum were compared based on the solvents used (EtOH, MeOH, and Ace). The results demonstrate that the polarity of EtOH and MeOH influences the amount of crude extract obtained from plants [32]. Both EtOH and MeOH, as polar solvents, are particularly effective for extracting a broad range of compounds, especially polar and semi-polar compounds [33]. These solvents, due to their ability to dissolve various phenolic compounds such as flavonoids, lignans, and other polyphenolic substances, yield a complex mixture of bioactive compounds [34]. This aligns with previous findings highlighting the versatility of EtOH and MeOH in extracting compounds from plant material.
However, a clear distinction was observed in the extraction of pyranocoumarins, where Ace emerged as the superior solvent. This is not surprising, given that Ace is a non-polar solvent that can better dissolve non-polar compounds like pyranocoumarins, which have been identified in previous studies [35,36]. The ability of Ace to selectively extract these compounds suggests that it might be more advantageous when targeting specific bioactive compounds such as pyranocoumarins, which are known for their pharmacological properties.
The TPC results confirmed the presence of phenolic compounds in the extracts, indicating their contribution to the medicinal potential of P. japonicum. The high TPC observed in the EtOH and MeOH extracts may be attributed to the co-extraction of a wide array of phenolic compounds [37]. This broad-spectrum extraction method is advantageous for identifying multiple bioactive compounds in a single extraction process. In contrast, the TCC results confirmed the presence of coumarins, particularly in the root extracts. The higher pyranocoumarin content in the roots aligns with previous studies stating that coumarins tend to be more concentrated in the roots because plants utilize them for their allelopathic properties [38]. Allelopathy, the process by which plants release chemicals into their environment to inhibit the growth of competing plants, is an essential ecological strategy for ensuring access to limited resources such as water and nutrients [39]. This mechanism likely explains the accumulation of pyranocoumarins in the roots, where they may serve as chemical deterrents against herbivores and competing vegetation.
Interestingly, the extraction yield of the Ace extract was the lowest among all the solvents tested. This may initially seem counterintuitive given the specific targeting of pyranocoumarins in these extracts. However, the low yield of Ace may indicate its selectivity in isolating the target compounds. This finding aligns with the principle that nonpolar solvents such as Ace are less efficient at extracting a broad range of compounds compared to more polar solvents [40]. However, the advantage of using Ace lies in the purity and concentration of target compounds, such as pyranocoumarins. Although the overall crude extract yield was low, the high concentration of pyranocoumarins in the Ace extracts made this solvent particularly useful for isolating these compounds in a focused manner without interference from unnecessary plant constituents. This selective extraction approach is beneficial for studies aimed at isolating specific bioactive compounds for further analysis and pharmacological evaluation [41]. Additionally, this targeted extraction could reduce the need for additional purification steps, which are time-consuming and costly.
The high TPC observed in the EtOH and MeOH extracts could be attributed to the co-extraction of a broad range of phenolic compounds [42]. These compounds, including flavonoids, phenolic acids, lignans, and tannins, contribute to the overall antioxidant and antimicrobial properties of the extracts. However, the TCC assay, which specifically quantifies coumarins, revealed lower levels of these compounds in the EtOH and MeOH extracts compared to the Ace extract. This highlights the fact that different assays provide more nuanced insights into the specific bioactive compounds present in extracts [43]. The TPC assay measures the total amount of phenolic compounds, including those that do not belong to the coumarin category, which explains the higher TPC values observed in the EtOH and MeOH extracts. This distinction underscores the importance of selecting an appropriate analytical method to assess the presence of particular classes of compounds in plant extracts [44].
The standard compound chlorogenic acid (1) was predominantly found in the leaves rather than the roots. This compound belongs to the hydroxycinnamic acid group and is widely distributed in plants [45]. This is consistent with previous studies wherein chlorogenic acid and its derivatives are more prominent in the leaves of P. japonicum compared to its roots [46]. Chlorogenic acid (1) primarily serves as a natural antioxidant, protecting plants against oxidative stress induced by environmental factors such as sunlight, pathogens, and pollution [47]. Additionally, its bitter taste helps plants evade herbivory. The remaining compounds—isosamidin (2), 3′-acetoxy-4′-(2-methylbutyroyl)khellactone (3), 3′,4′-disencioylkhellactone (4), 3′-sencioyl-4′-isovalerylkhellactone (5), and 3′-isovaleryl-4′-(2-methylbutyroyl)khellactone (6)—are pyranocoumarins [20]. Unlike chlorogenic acid and other coumarins, the functions of these compounds in plants have not yet been fully characterized.
The choice of extraction solvent is crucial for isolating specific bioactive compounds, as it influences both the yield and composition of the extracted compounds. In this study, Ace was more effective at isolating pyranocoumarins, whereas EtOH and MeOH were better suited for chlorogenic acid (1) extraction. This difference can be attributed to the varying polarities of these solvents, which determine the solubility of compounds with different chemical characteristics. These findings emphasize the need to optimize extraction techniques to maximize the yield of desired bioactive compounds. By adjusting the solvent system, the selective extraction of target compounds can be enhanced, which is crucial for pharmacological testing and therapeutic applications. This optimization can lead to more efficient and cost-effective extraction methods, ensuring that the bioactive compounds of interest are obtained with high purity for subsequent use in drug development or other applications.
This study provides a detailed comparative analysis of the efficiency of different solvents in extracting pyranocoumarins from the different plant tissues, a notably under-explored area of research. To the best of the authors’ knowledge, this is the first study to quantify these specific pyranocoumarins in this plant species. The applications of this study go beyond the pharmacological importance of this plant. It further advances the understanding of pyranocoumarins, a relatively understudied group of phytochemicals. By highlighting the distinct extraction efficiency of different solvents, particularly the superior performance of acetone for isolating pyranocoumarins, this research addresses a critical gap in the phytochemical analysis of these compounds.
5. Conclusions
These results underscore the importance of selecting an appropriate solvent to optimize the extraction of bioactive compounds from P. japonicum. While EtOH and MeOH were effective for extracting chlorogenic acid, Ace was more suitable for obtaining pyranocoumarins. The insights gained from this study will be valuable for refining extraction methods in future research investigating the pharmacological potential of P. japonicum. The results suggest promising avenues for further pharmacological evaluation. P. japonicum should be explored as a potential candidate for inclusion in herbal formulations targeting respiratory ailments. The traditional use of this plant for cough relief reinforces its relevance in modern herbal medicine and highlights the need for clinical research to substantiate its efficacy and safety.
Author Contributions
HPLC/PDA analysis, N.P.U.; TPC and TCC analysis, S.Y.L.; resources and experimental design, J.H.K. and Y.H.Y.; supervision, writing—review, and editing, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Natural Product Institute of Science and Technology, Anseong, the Republic of Korea.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We express our gratitude to the National Institute of Horticultural and Herbal Science (Eumseong, Republic of Korea) for generously providing the samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Z.; Chen, K.; Rose, P.; Zhu, Y.Z. Natural products in drug discovery and development: Synthesis and medicinal perspective of leonurine. Front. Chem. 2022, 10, 1036329. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Baranwal, J.; Pati, S.; Barse, B.; Khan, R.H.; Kumar, A. Application of plant products in the synthesis and functionalisation of biopolymers. Int. J. Biol. Macromol. 2023, 237, 124174. [Google Scholar] [CrossRef] [PubMed]
- Asiminicesei, D.-M.; Fertu, D.I.; Gavrilescu, M. Impact of heavy metal pollution in the environment on the metabolic profile of medicinal plants and their therapeutic potential. Plants 2024, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, M.; Vassileva, V. Stress management in plants: Examining provisional and unique dose-dependent responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Chem. 2023, 14, 1132555. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Rispo, F.; De Negri Atanasio, G.; Demori, I.; Costa, G.; Marchese, E.; Perera-Del-Rosario, S.; Serrano-Candelas, E.; Palomino-Schätzlein, M.; Perata, E.; Robino, F.; et al. An extensive review on phenolic compounds and their potential estrogenic properties on skin physiology. Front. Cell Dev. Biol. 2024, 11, 1305835. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Chintha, P.; Sarkar, D.; Pecota, K.; Dogramaci, M.; Hatterman-Valenti, H.; Shetty, K. Phenolic bioactive-linked antioxidant, anti-hyperglycemic, and antihypertensive properties of sweet potato cultivars with different flesh color. Hortic. Environ. Biotechnol. 2023, 64, 877–893. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural coumarins: Exploring the pharmacological complexity and underlying molecular mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Saha, S. Coumarins: An important phytochemical with therapeutic potential. In Plant-Derived Bioactives; Springer: Singapore, 2020; pp. 205–222. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, G.; Shi, Q.; Zhu, H.; Zhou, N.; Kong, X.; Jiang, D.; Liu, C. Identification of a novel coumarins biosynthetic pathway in the endophytic fungus Fusarium oxysporum GU-7 with antioxidant activity. Appl. Environ. Microbiol. 2023, 89, e0160122. [Google Scholar] [CrossRef] [PubMed]
- Balewski, Ł.; Szulta, S.; Jalińska, A.; Kornicka, A. A mini-review: Recent advances in coumarin-metal complexes with biological properties. Front. Chem. 2021, 9, 781779. [Google Scholar] [CrossRef]
- Park, Y.J.; Choi, Y.B.; Oh, S.B.; Moon, J.; Truong, T.Q.; Huynh, P.K.; Kim, S.M. Development and application of a high-performance liquid chromatography diode-array detection (HPLC–DAD) method for the simultaneous quantification of phenolic compounds in the aerial part of Glehnia littoralis. Appl. Biol. Chem. 2024, 67, 34. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Do, T.H.; Nguyen, T.T.; Vu, T.D.; Tran, T.H.; Nguyen, H.T.; Doan, T.P.; Oh, W.K.; Park, J.H. Effects of coumarins from roots of Paramignya scandens (Griff.) Craib on LPS-induced IL-1β and IL-10 cytokine production in RAW 264.7 macrophages. Nat. Prod. Sci. 2024, 30, 30–38. [Google Scholar] [CrossRef]
- Zou, Y.; Teng, Y.; Li, J.; Yan, Y. Recent advances in the biosynthesis of coumarin and its derivatives. Green Chem. Eng. 2023, 5, 150–154. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. In Progress in the Chemistry of Organic Natural Products; Springer-Verlag: New York, NY, USA, 2017; pp. 241–304. [Google Scholar] [CrossRef]
- Khandy, M.T.; Grigorchuk, V.P.; Sofronova, A.K.; Gorpenchenko, T.Y. The different composition of coumarins and antibacterial activity of Phlojodicarpus sibiricus and Phlojodicarpus villosus root extracts. Plants 2024, 13, 601. [Google Scholar] [CrossRef]
- Min, S.J.; Lee, H.; Shin, M.-S.; Lee, J.W. Synthesis and biological properties of pyranocoumarin derivatives as potent anti-inflammatory agents. Int. J. Mol. Sci. 2023, 24, 10026. [Google Scholar] [CrossRef]
- Lee, C.-D.; Cho, H.; Shim, J.; Tran, G.H.; Lee, H.-D.; Ahn, K.H.; Yoo, E.; Chung, M.J.; Lee, S. Characteristics of phenolic compounds in Peucedanum japonicum according to various stem and seed colors. Molecules 2023, 28, 6266. [Google Scholar] [CrossRef]
- Joh, H.J.; Park, Y.S.; Kang, J.-S.; Kim, J.T.; Lado, J.P.; Han, S.I.; Chin, Y.-W.; Park, H.-S.; Park, J.Y.; Yang, T.-J. A recent large-scale intraspecific IR expansion and evolutionary dynamics of the plastome of Peucedanum japonicum. Sci. Rep. 2025, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.Q.; Huong, N.T.; Son, N. The medicinal plant Peucedanum japonicum Thunberg: A review of traditional use, phytochemistry, and pharmacology. Fitoterapia 2024, 179, 106270. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Yang, X. Qianhu (Peucedanum praeruptorum Dunn) improves exercise capacity in mice by regulating Nrf2/HO-1 oxidative stress signaling pathway. Appl. Biol. Chem. 2023, 66, 26. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, J.K.; Chanchal, D.K.; Shinde, M.G.; Kumar, S.; Jain, D.; Almarhoon, Z.M.; Alshahrani, A.M.; Calina, D.; Sharifi-Rad, J.; et al. Natural products as drug leads: Exploring their potential in drug discovery and development. Naunyn Schmiedebergs Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.D.; Cho, H.; Lee, C.D.; Tran, G.H.; Kim, H.; Moon, S.K.; Lee, S. Antioxidative phenolic compounds from the aerial parts of Cyperus exaltatus var. iwasakii and their HPLC analysis. Appl. Biol. Chem. 2023, 66, 61. [Google Scholar] [CrossRef]
- Yang, Z.; Yue, S.-J.; Gao, H.; Zhang, Q.; Xu, D.-Q.; Zhou, J.; Li, J.-J.; Tang, Y.-P. Natural deep eutectic solvent-ultrasound assisted extraction: A green approach for ellagic acid extraction from Geum japonicum. Front. Nutr. 2023, 9, 1079767. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.H.; Shin, J.Y.; Kang, E.S.; Cho, B.O. Anti-inflammatory effects of Peucedanum japonicum Thunberg leaves extract in Lipopolysaccharide-stimulated RAW264.7 cells. J. Ethnopharmacol. 2023, 309, 116362. [Google Scholar] [CrossRef]
- Tran, G.H.; Cho, H.; Lee, H.-D.; Lee, C.-D.; Shim, J.; Ahn, K.W.; Sung, J.S.; Yoo, E.; Lee, S. Analysis of the total polyphenol, flavonoid, and phenolic acid contents in three different leaf types of Lepidium sativum. Nat. Prod. Sci. 2023, 29, 235–241. [Google Scholar] [CrossRef]
- De Carvalho Osório, A.; Martins, J.L.S. Determinação de cumarina em extrato fluido e tintura de guaco por espectrofotometria derivada de primeira ordem. Braz. J. Pharm. Sci. 2004, 40, 481–486. [Google Scholar] [CrossRef]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis tinctoria. PeerJ 2019, 7, e7857. [Google Scholar] [CrossRef]
- Lee, J.-E.; Jayakody, J.; Kim, J.-I.; Jeong, J.-W.; Choi, K.-M.; Kim, T.-S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Shokoohinia, Y.; Hemmati, S. Isolation and identification of furanocoumarins and a phenylpropanoid from the acetone extract and identification of volatile constituents from the essential oil of Peucedanum pastinacifolium. Chem. Nat. Compd. 2012, 48, 668–671. [Google Scholar] [CrossRef]
- Khalil, N.; Bishr, M.; El-Degwy, M.; Abdelhady, M.; Amin, M.; Salama, O. Assessment of conventional solvent extraction vs. supercritical fluid extraction of Khella (Ammi visnaga L.) furanochromones and their cytotoxicity. Molecules 2021, 26, 1290. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, L.; Xu, Z.; Kong, Q.; Feng, S.; Chen, T.; Zhou, L.; Yang, H.; Xiao, Y.; Ding, C. Solvent effects on the phenolic compounds and antioxidant activity associated with Camellia polyodonta flower extracts. ACS Omega 2024, 9, 27192–27203. [Google Scholar] [CrossRef]
- Niro, E.; Marzaioli, R.; De Crescenzo, S.; D’Abrosca, B.; Castaldi, S.; Esposito, A.; Fiorentino, A.; Rutigliano, F.A. Effects of the allelochemical coumarin on plants and soil microbial community. Soil Biol. Biochem. 2015, 95, 30–39. [Google Scholar] [CrossRef]
- Shan, Z.; Zhou, S.; Shah, A.; Arafat, Y.; Rizvi, S.A.H.; Shao, H. Plant allelopathy in response to biotic and abiotic factors. Agronomy 2023, 13, 2358. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- Mokaizh, A.A.B.; Nour, A.H.; Ali, G.A.M.; Ukaegbu, C.I.; Hawege, E.F. Eco-friendly and efficient extraction of phenolic compounds from Commiphora gileadensis bark using microwave-assisted extraction. J. Ind. Eng. Chem. 2024, 142, 321–328. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; David, B.; Gafner, S.; Glasl, S. Botanical ingredient identification and quality assessment: Strengths and limitations of analytical techniques. Phytochem. Rev. 2019, 19, 1157–1177. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Uy, N.P.; Kim, J.T.; Lee, S.; Yang, T.J.; Lee, S. Comprehensive determination of the phenolic compound contents and antioxidant potentials of leaves and roots of Peucedanum japonicum harvested from different accessions and growth periods. ACS Omega 2024, 9, 41616–41628. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Sun, H.; Du, G.; Wang, X.; Lyu, D. Exogenous chlorogenic acid alleviates oxidative stress in apple leaves by enhancing antioxidant capacity. Sci. Hortic. 2020, 274, 109676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).