Effect of Ozonated Avocado and High-Oleic Palm Oils on “Bolo Verde” Variety Squash
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Ozonation Process
2.3. Peroxide Value (PV) Determination
2.4. Formulation of Ozonated Oil Concentrations
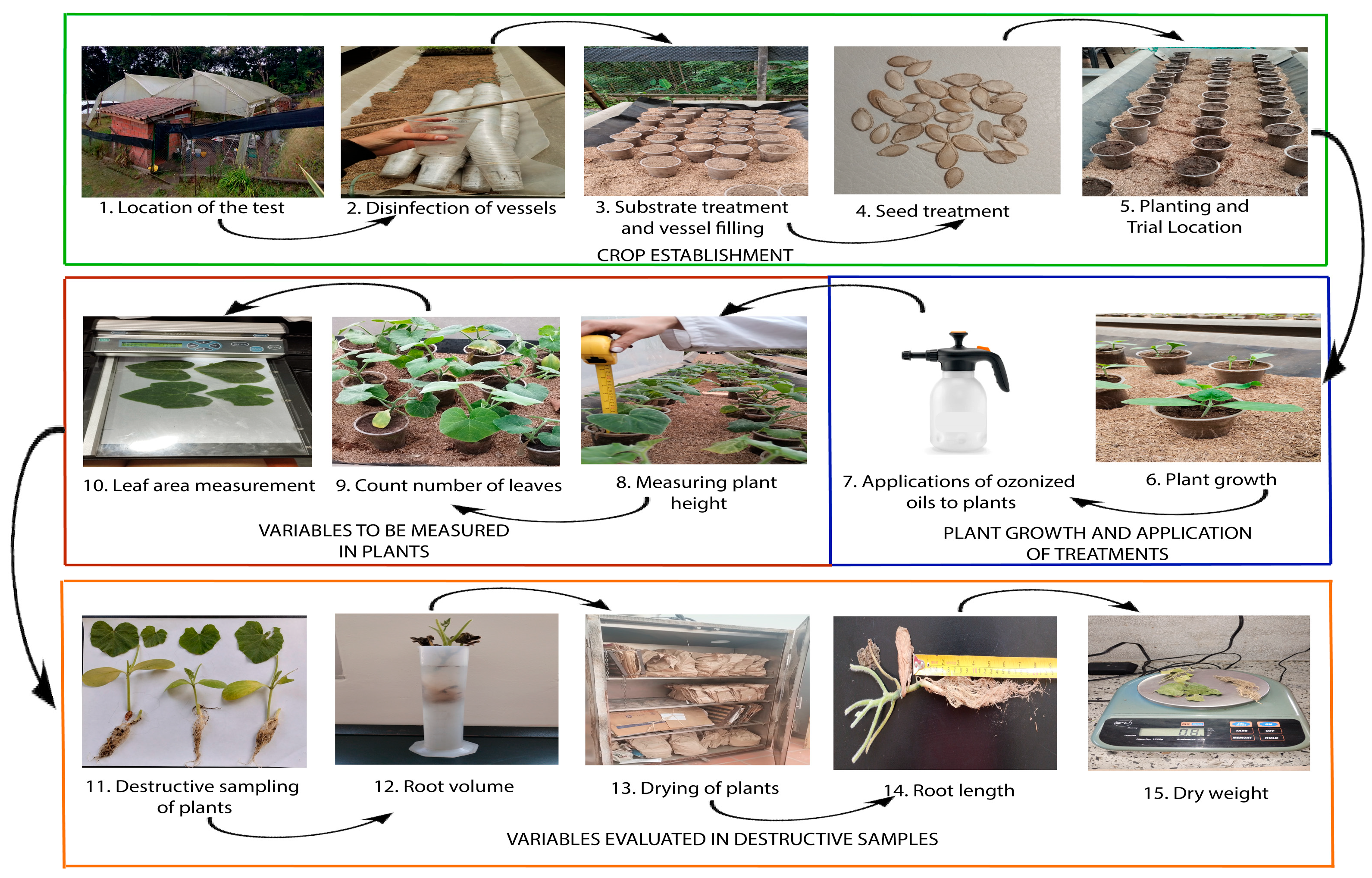
2.5. Experimental Design and Establishment
2.6. Crop Management
2.7. Evaluated Variables
2.8. Lipid Profile Using HS–SPME with GC–MS
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RGR | Relative Growth Rate |
| CGR | Crop Growth Rate |
| NAR | Net Assimilation Rate |
| LAI | Leaf Area Index |
References
- Men, X.; Choi, S.I.; Han, X.; Han, X.; Kwon, H.Y.; Jang, G.W.; Choi, Y.E.; Park, S.; Lee, O. Physicochemical, nutritional and functional properties of Cucurbita moschata. Food Sci. Biotechnol. 2021, 30, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.; Paz, E.; Echeverria, S.P.; Ruiz, D.C.; Mosquera, S.A. Efecto del sistema de producción en la maduración fisiológica de Cucurbita moschata var. bolo verde. Biotecnol. Sect. Agropecu. Agroind. 2016, 14, 29–37. [Google Scholar] [CrossRef][Green Version]
- Díaz, M.F.; Cumsille-Escándar, S.; Hidalgo, A.; Rodríguez-Díaz, M. Los aceites ozonizados en la agricultura ecológica: Uso y perspectiva de los aceites eozonizados en la ecoagricultura. Cuad. Médico Soc. 2024, 64, 83–86. [Google Scholar] [CrossRef]
- Sadowska, J.; Johansson, B.; Johannessen, E.; Friman, R.; Broniarz-Press, L.; Rosenholm, J. Characterization of ozonated vegetable oils by spectroscopic and chromatographic methods. Chem. Phys. Lipids 2008, 151, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.F.; Gavín, J.A.; Gómez, M.; Curtielles, V.; Hernández, F. Study of Ozonated Sunflower Oil Using 1H NMR and Microbiological Analysis. Ozone Sci. Eng. 2006, 28, 59–63. [Google Scholar] [CrossRef]
- Fedepalma. Available online: https://fedepalma.org/international/ (accessed on 29 May 2025).
- Torres, M.C.; Trochez, J. El mercado del aguacate en Colombia. Apunt. Cenes 2023, 42, 273–292. [Google Scholar] [CrossRef]
- Díaz, M.F.; Martínez, G.; Meneau, R.I.; Alaiz, M.; Garcés, R. Estudio analítico de especies oxigenadas en el aceite de teobroma ozonizado. Química Nova 2008, 31, 610–613. [Google Scholar] [CrossRef]
- Ledea-Lozano, O.E.; Fernández-García, L.A.; Gil-Ibarra, D.; Bootello, M.Á.; Garcés, R.; Martínez-Force, E.; Salas, J.J. Characterization of different ozonized sunflower oils II. Triacylglycerol condensation and physical properties. Grasas Aceites 2019, 70, e330. [Google Scholar] [CrossRef]
- Díaz, M.F.; Ledea, O.E.; Gómez, M.; Garcés, R.; Alaiz, M.S.; Martínez, E. Estudio comparativo de la ozonización de aceites de girasol modificados genéticamente y sin modificar. Química Nova 2009, 32, 2467–2472. [Google Scholar] [CrossRef]
- Yánez, J.J.; Valle Naranjo, G.D.; Veloz Naranjo, W.O.; Tamayo, J.D.; Leiva-Mora, M. Efecto de diferentes combinaciones de Agrozoil con Delphinium spp. sobre el porcentaje de prendimiento e índice de producción. Rev. Científica Arbitr. Multidiscip. Pentaciencias 2023, 5, 70–76. [Google Scholar]
- Vijay, S.; Sudhakar, D.V.; Sharma, R.R.; Preethi, P.; Pandiselvam, R. Role of Ozone in Post-Harvest Disinfection and Processing of Horticultural Crops: A Review. Ozone Sci. Eng. 2021, 44, 127–146. [Google Scholar] [CrossRef]
- Skalska, K.; Ledakowicz, S.; Perkowski, J.; Sencio, B. Germicidal Properties of Ozonated Sunflower Oil. Ozone Sci. Eng. 2009, 31, 232–237. [Google Scholar] [CrossRef]
- Martínez- Sánchez, G.; Re, L.; Perez-Davison, G.; Horwat, R. Las aplicaciones médicas de los aceites ozonizados, actualización. Rev. Española Ozonoterapia 2012, 2, 121–139. [Google Scholar]
- Lee, J.H.; Goto, E. Ozone control as a novel method to improve health-promoting bioactive compounds in red leaf lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1045239. [Google Scholar] [CrossRef] [PubMed]
- Anisha, G.S.; Reeta, R.S.; Patel, A.K. Chapter 30: Green solutions: Ozone applications for sustainable food industry practices. In Advances and Technology Development in Greenhouse Gases: Emission, Capture and Conversion; Reza, M., Amin, M., Makarem, M.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 337–355. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone application in different industries: A review of recent developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef]
- Singh, G.; Arya, S.K.; Bibra, M.; Bhalla, A.; Aggarwal, A.; Singh, J. Antimicrobial potential of ozone for the storage of grains: Special focus on inhibition of bacterial contamination. Arch. Phytopathol. Plant Prot. 2022, 55, 1625–1637. [Google Scholar] [CrossRef]
- Voltolini, L.C.; Bastos, R.G.; Souza, C.F. A simple system for ozone application in domestic sewage for agricultural reuse. Rev. Ambiente Água 2022, 17, e2861. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Etman, A.A.; Yassin, A.M.; Elsayed, S.; Scholz, M.; Yaseen, Z.M. Mitigation of Chilling Stress by Ozone Pretreatment and Acclimation of Sweet Pepper Grown under Unheated Greenhouse Conditions. Horticulturae 2022, 8, 1189. [Google Scholar] [CrossRef]
- Pastacaldi, C.; Gaudioso, D.; Beltrami, C.; Gunnella, B.; Tegli, S. On the Effectiveness of Ozone Treatments: A Silver Bullet for Plant Health? Agronomy 2025, 15, 567. [Google Scholar] [CrossRef]
- Moreno, M.J.; Pineda, J.; Colinas, M.T.; Sahagun, J. El oxígeno en la zona radical y su efecto en las plantas. Rev. Mex. Cienc. Agrícolas 2020, 11, 931–943. [Google Scholar] [CrossRef]
- NTC 236; Grasas y Aceites Vegetales y Animales. Determinación del Índice de Peróxido. Segunda Actualización. ICONTEC–Norma Técnica Colombiana, 2011. Available online: https://es.scribd.com/document/487681916/NTC-236-PEROXIDOS-EN-ACEITES (accessed on 29 May 2025).
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO-International Standards Organization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:3960:ed-5:v1:en (accessed on 29 May 2025).
- Silva-Marrufo, O.; Ortega-Ramirez, A.T.; Alaniz-Villanueva, O.G.; Herrera-Gamboa, J.; Marin-Tinoco, R.I.; Hurtado-Salazar, A.; Garcia-Andrade, M. Cobalt oxide nanoparticles and their effect on melon (Cucumis melo L.) yield and quality. Not. Sci. Biol. 2024, 16, 12175. [Google Scholar] [CrossRef]
- Bucio, C.M.; Díaz Serrano, F.R.; Martínez Jaime, O.A.; Torres Morales, J.J. Efecto del ozono sobre la población microbiana del suelo y el crecimiento de plantas de fresa. Terra Latinoam. 2016, 34, 229–237. [Google Scholar]
- Mixquititla, G.; Villegas Torres, O.G.; Andrade Rodríguez, M.; Sotelo Nava, H.; Cardoso Taketa, A.T. Crecimiento, rendimiento y calidad de fresa por efecto del régimen nutrimental. Rev. Mex. Cienc. Agrícolas 2020, 11, 1337–1348. [Google Scholar] [CrossRef]
- Lopes, E.C.S.; Dos Santos, M.S.; Allaman, I.B.; Dalmolin, Â.C.; Sousa-Santos, C.; Freitas, A.; Netto, D.L.; Schramm, M. Growth and biomass-allocation responses of arabica coffee young plants subjected to the interactive effects of root deformation and light availability. Acta Physiol. Plant. 2025, 47, 4. [Google Scholar] [CrossRef]
- Quintero, M.; Alvarez, E.; Ceballos, N.; Duno, D.; Taborda, G. Volatilomic profile of the tree tomato (Solanum betaceum Cav.) pulp during ripening and senescence using HS–SPME with GC–MS. LWT 2023, 186, 115213. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef]
- Batista, D.; Murillo, B.; Nieto, A.; Alcaráz, L.; Troyo, E.; Hernández, L.; Ojeda, C.M.; Mazón, J.M.; Agüero, Y.M. Bioestimulante derivado de caña de azúcar mitiga los efectos del estrés por NaCl en Ocimum basilicum L. Ecosistemas Recur. Agropecu. 2019, 6, 297–306. [Google Scholar] [CrossRef]
- Tamaoki, M. The role of phytohormone signaling in ozone-induced cell death in plants. Plant Signal. Behav. 2008, 3, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Heath, R. Modification of the biochemical pathways of plants induced by ozone: What are the varied routes to change? Environ. Pollut. 2008, 155, 453–463. [Google Scholar] [CrossRef]
- Heath, R.L. Alterations of the biochemical pathways of plants by the air pollutant ozone: Which are the true gauges of injury? Sci. World J. 2007, 7, 110–118. [Google Scholar] [CrossRef][Green Version]
- Scimone, G.; Carucci, M.G.; Risoli, S.; Pisuttu, C.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Petersen, M. Ozone Treatment as an Approach to Induce Specialized Compounds in Melissa officinalis Plants. Plants 2024, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Valverde, J.C.; Dagoberto, A. Evaluación del índice de área foliar con método indirecto y directo en distintas condiciones ambientales en plantaciones dendroenergéticas de Eucalyptus tereticornis Sm. Madera Bosques 2020, 26, 1–12. [Google Scholar] [CrossRef]
- da Silva, D.F.P.; de Souza, P.H.M.; Cruz, S.C.S.; Gomes, F.R.; Ragagnin, A.L.S.L. Leaf area estimation of Anacardium humile. Rev. Bras. Frutic. 2020, 42, e-628. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N. Chapter 4. Effect of elevated O3 on plants growth, active constituents, and production. In Plants and Their Interaction to Environmental Pollution; Husen, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–97. [Google Scholar] [CrossRef]
- Duque, L.; Poelman, E.H.; Steffan-Dewenter, I. Effects of ozone stress on flowering phenology, plant-pollinator interactions and plant reproductive success. Environ. Pollut. 2021, 272, 115953. [Google Scholar] [CrossRef]
- Leisner, C.P.; Ainsworth, E.A. Quantifying the effects of ozone on plant reproductive growth and development. Glob. Change Biol. 2012, 18, 606–616. [Google Scholar] [CrossRef]
- Montes, C.M.; Demler, H.J.; Li, S.; Martin, D.G.; Ainsworth, E.A. Approaches to investigate crop responses to ozone pollution: From O3-FACE to satellite-enabled modeling. Plant J. Cell Mol. Biol. 2022, 109, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Singh, A.A.; Agrawal, S.B.; Agrawal, M. Tropospheric O3: A Cause of Concern for Terrestrial Plants. In Plant Responses to Air Pollution; Kulshrestha, U., Saxena, P., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Daripa, A.; Bhatia, A.; Ojha, S.; Tomer, R.; Chattaraj, S.; Singh, K.P.; Singh, S.D. Chemical and natural plant extract in ameliorating negative impact of tropospheric ozone on wheat crop: A case study in a part of semiarid north west India. Aerosol AirQual. Res. 2016, 16, 1742–1756. [Google Scholar] [CrossRef]
- Wattanapenpaiboon, N.; Wahlqvist, M.L. Phytonutrient deficiency: The place of palm fruit. Asia Pac. J. Clin. Nutr. 2003, 12, 363–368. [Google Scholar]
- Iha, O.K.; Alves, F.C.S.C.; Suarez, P.A.Z.; de Oliveira, M.B.F.; Meneghetti, S.M.P.; Santos, B.P.T.; Soletti, J.I. Physicochemical properties of Syagrus coronata and Acrocomia aculeata oils for biofuel production. Ind. Crops Prod. 2014, 62, 318–322. [Google Scholar] [CrossRef]
| Concentration meqO2 kg−1 | |||||
|---|---|---|---|---|---|
| Treatment | Oil Type | Application Type | Application 1 (V3 Stage) | Application 2 (V5 Stage) | |
| 1 | Avocado | Foliar | Low | 50 | 50 |
| 2 | Drench | ||||
| 3 | Foliar | Medium | 100 | 100 | |
| 4 | Drench | ||||
| 5 | Foliar | High | 200 | 200 | |
| 6 | Drench | ||||
| 7 | High-oleic Palm | Foliar | Low | 5 | 5 |
| 8 | Drench | ||||
| 9 | Foliar | Medium | 10 | 10 | |
| 10 | Drench | ||||
| 11 | Foliar | High | 20 | 20 | |
| 12 | Drench | ||||
| 13 | Commercial Oxyzhen | Foliar | Commercial dose 5 cm3 L−1 | Commercial dose 5 cm3 L−1 | |
| 14 | Drench | ||||
| 15 | Control (water) | --- | --- | --- | |
| Ozonated Oil Concentration | Application | Height (cm) | Number of Leaves | Root Length (cm) | Root Volume (cm3) | Total Dry Weight (g) | |
|---|---|---|---|---|---|---|---|
| Avocado | Low | (1) Foliar | 11.81 bc | 4.66 bcde | 36.45 abc | 3.20 bcd | 2.22 bcd |
| (2) Drench | 11.76 bcd | 4.53 bcedf | 41.50 abc | 3.00 cd | 1.92 cdef | ||
| Average | (3) Foliar | 10.83 bcde | 4.50 cdef | 36.80 abc | 2.30 d | 2.05 cdef | |
| (4) Drench | 10.73 cde | 4.56 bcdef | 35.55 bc | 3.30 bcd | 2.05 cdef | ||
| High | (5) Foliar | 7.81 gh | 2.84 h | 34.15 bc | 2.40 d | 2.14 cde | |
| (6) Drench | 14.64 a | 5.19 a | 44.55 ab | 4.20 ab | 2.60 ab | ||
| Palm | Low | (7) Foliar | 11.05 bcde | 4.16 efg | 36.75 abc | 2.90 cd | 2.29 bc |
| (8) Drench | 12.68 b | 5.00 abc | 35.15 bc | 4.20 ab | 2.80 a | ||
| Average | (9) Foliar | 7.69 h | 3.78 g | 37.47 abc | 3.20 bcd | 2.27 bc | |
| (10) Drench | 8.79 fgh | 4.66 bcde | 31.75 c | 2.40 d | 1.73 ef | ||
| High | (11) Foliar | 9.21 efgh | 4.06 fg | 37.43 abc | 3.10 bcd | 2.28 bc | |
| (12) Drench | 9.86 def | 4.34 def | 33.35 c | 3.60 bc | 1.94 cdef | ||
| Commercial | (13) Foliar | 9.68 efg | 4.50 cdef | 46.84 a | 3.40 bcd | 1.84 def | |
| (14) Drench | 11.10 bcde | 5.03 ab | 30.92 c | 5.10 a | 2.21 bcd | ||
| Control | 9.94 cdef | 4.78 abcd | 35.84 bc | 3.90 bc | 1.69 f | ||
| Ozonated Oil Concentration | Application | Crop Growth Rate (g cm2 day−1) | Relative Growth Rate (g g day−1) | Net Assimilation Rate (g cm2 day−1) | Leaf Area Index | |
|---|---|---|---|---|---|---|
| Avocado | Low | (1) Foliar | 0.000566802 bcd | 0.0405122 bc | 2.62203 cd | 4989.03 fg |
| (2) Drench | 0.000480046 cdef | 0.0376485 cd | 2.72693 cd | 6509.65 bcde | ||
| Average | (3) Foliar | 0.00051764 cdef | 0.0390575 cd | 2.92534 bcd | 6496.09 bcde | |
| (4) Drench | 0.00051764 cdef | 0.0391068 cd | 2.94884 bcd | 6573.79 bcde | ||
| High | (5) Foliar | 0.000543667 cde | 0.040213 c | 2.35326 cd | 4645.19 g | |
| (6) Drench | 0.000676692 ab | 0.0441953 ab | 4.29069 a | 7957.99 a | ||
| Palm | Low | (7) Foliar | 0.000587045 bc | 0.0412687 bc | 3.07454 bc | 6048.57 cdef |
| (8) Drench | 0.000734529 a | 0.0454216 a | 4.36514 a | 7208.40 ab | ||
| Average | (9) Foliar | 0.000581261 bc | 0.0411 bc | 3.18034 bc | 6268.99 bcde | |
| (10) Drench | 0.000425101 ef | 0.0358173 d | 2.10982 cd | 5536.66 efg | ||
| High | (11) Foliar | 0.000584153 bc | 0.0412926 bc | 2.96081 bcd | 5794.78 def | |
| (12) Drench | 0.00048583 cdef | 0.0383872 cd | 2.86018 bcd | 7104.83 abc | ||
| Commercial | (13) Foliar | 0.000456911 def | 0.0374252 cd | 2.32656 cd | 5814.03 def | |
| (14) Drench | 0.00056391 bcd | 0.0407485 bc | 3.69219 ab | 8075.03 a | ||
| Control | 0.000413534 f | 0.0357569 d | 2.35711 cd | 6780.24 bcd | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aura, R.; Santiago, A.; Alejandro, H.-S.; Nelson, C.-A. Effect of Ozonated Avocado and High-Oleic Palm Oils on “Bolo Verde” Variety Squash. Horticulturae 2025, 11, 676. https://doi.org/10.3390/horticulturae11060676
Aura R, Santiago A, Alejandro H-S, Nelson C-A. Effect of Ozonated Avocado and High-Oleic Palm Oils on “Bolo Verde” Variety Squash. Horticulturae. 2025; 11(6):676. https://doi.org/10.3390/horticulturae11060676
Chicago/Turabian StyleAura, Ramírez, Amariles Santiago, Hurtado-Salazar Alejandro, and Ceballos-Aguirre Nelson. 2025. "Effect of Ozonated Avocado and High-Oleic Palm Oils on “Bolo Verde” Variety Squash" Horticulturae 11, no. 6: 676. https://doi.org/10.3390/horticulturae11060676
APA StyleAura, R., Santiago, A., Alejandro, H.-S., & Nelson, C.-A. (2025). Effect of Ozonated Avocado and High-Oleic Palm Oils on “Bolo Verde” Variety Squash. Horticulturae, 11(6), 676. https://doi.org/10.3390/horticulturae11060676






