Identification and Morphophysiological Characterization of Oryzalin-Induced Polyploids and Variants in Lysimachia xiangxiensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Preparation
2.2. Oryzalin Treatments and Experimental Design
2.3. Plant Establishment and Visual Screening
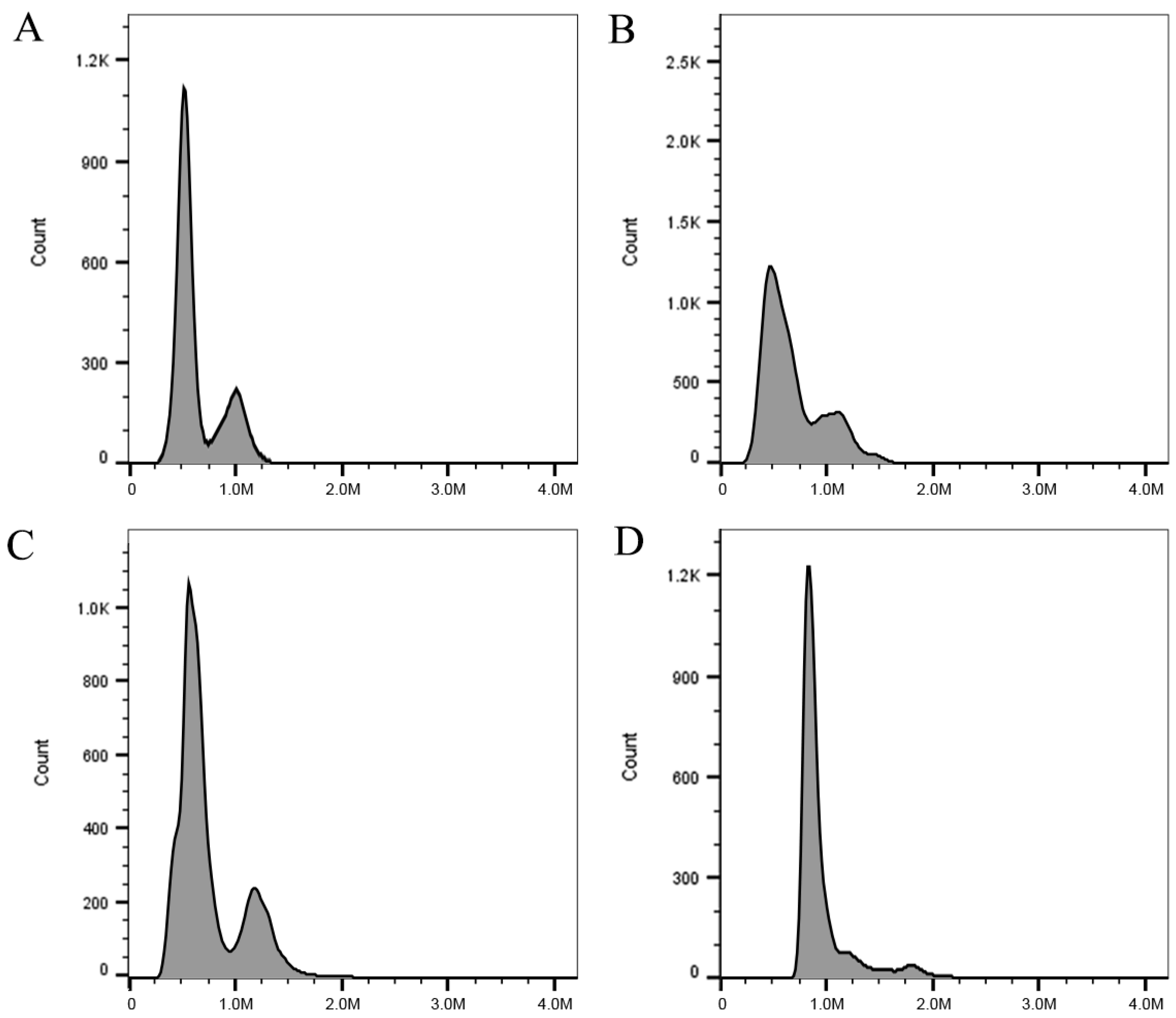
2.4. Flow Cytometry Analysis
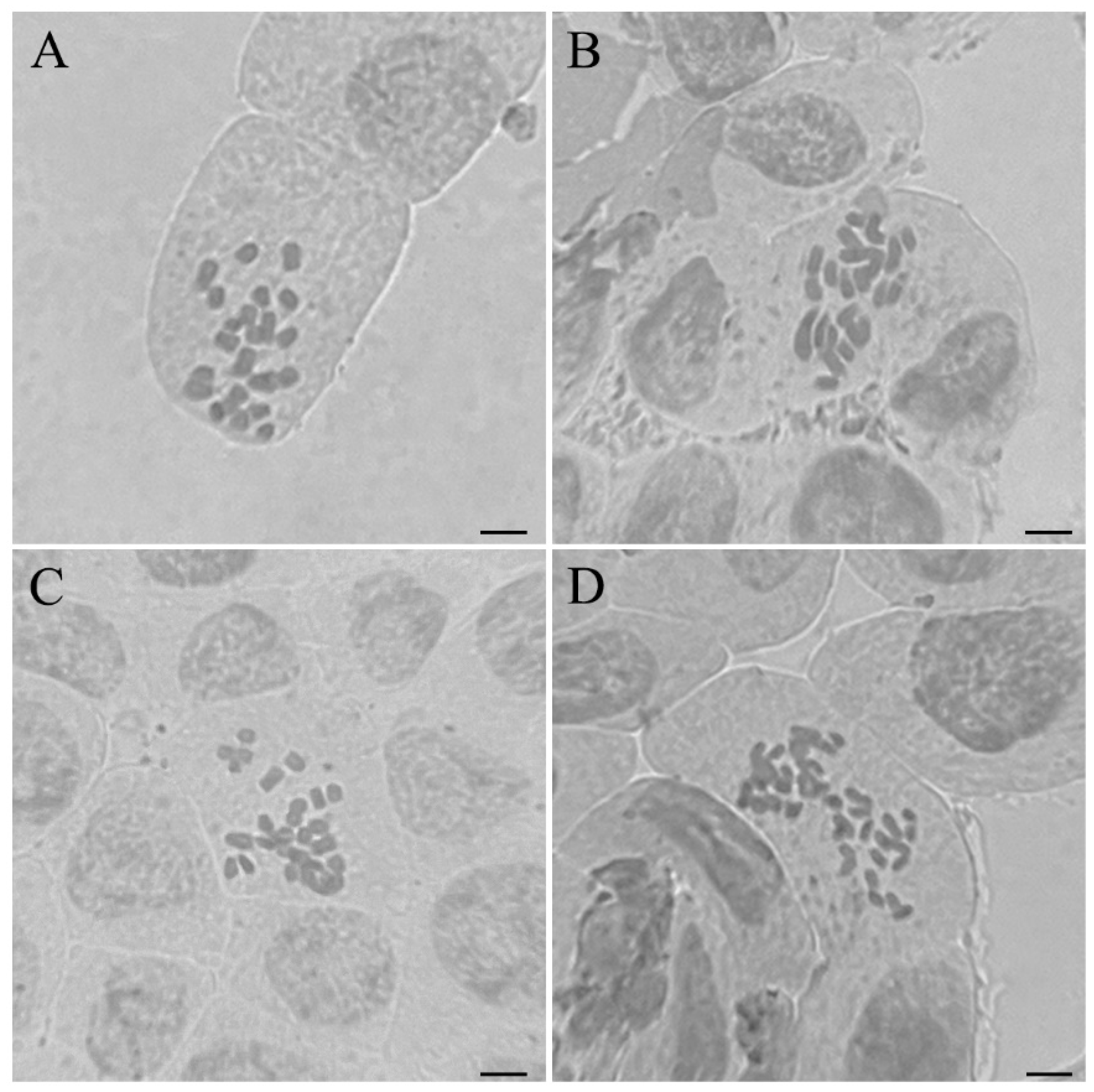
2.5. Chromosome Counting
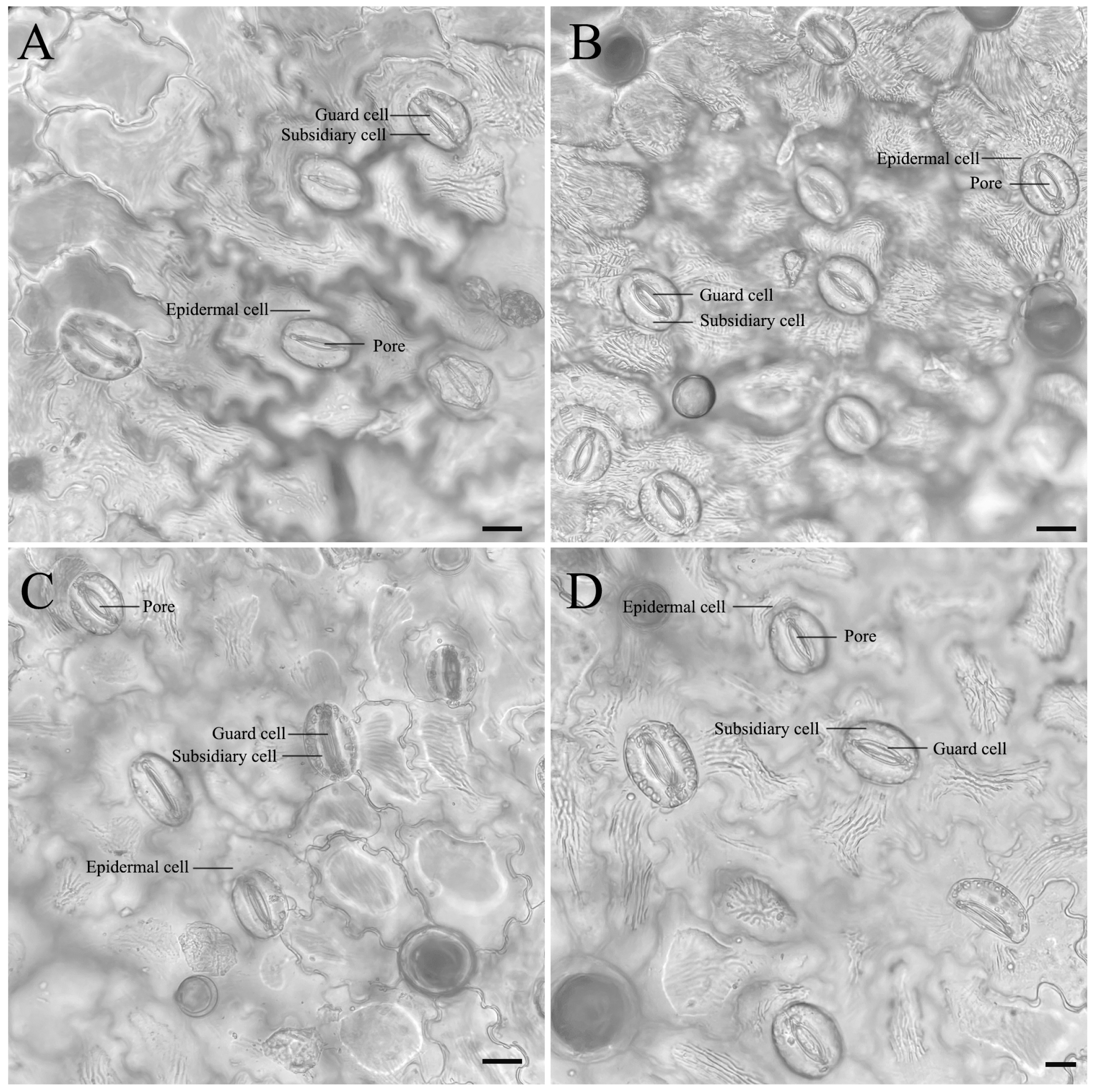
2.6. Stomatal Analysis
2.7. Morphological Characterization Observations
2.8. Physiological Characteristics Analysis
2.9. Gas Exchange Measurements
2.10. Statistical Analysis
3. Results
3.1. Plant Establishment and Identification of Three Variant Types
3.2. Effects of Oryzalin on the Survival and Polyploid Induction Rates of Regenerated Plants
3.3. Changes in Ploidy Level and Chromosome Numbers
3.4. Differences in Morphological and Stomatal Characteristics
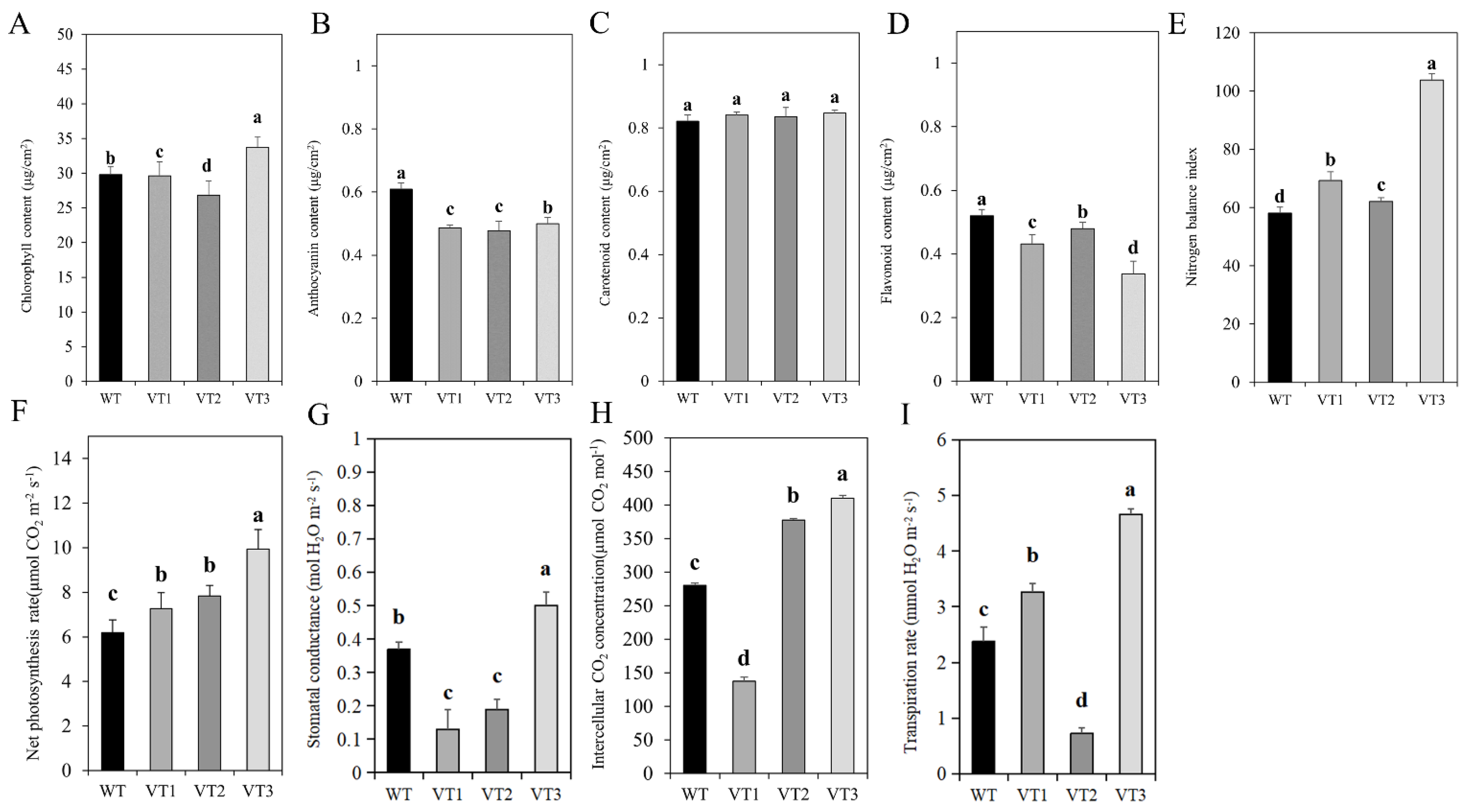
3.5. Analysis of Changes in Physiological Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Mou, C.; Wu, Y.; Xiang, L.; Xiang, X.-M.; Zhang, D.-G. Lysimachia xiangxiensis (Primulaceae), a New Species from Limestone Area in Hunan Province, Central China. PhytoKeys 2020, 140, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.-C.; Anderberg, A.-L.; Schönenberger, J.; Anderberg, A.A. Comparative Seed Morphology and Character Evolution in the Genus lysimachia (Myrsinaceae) and Related Taxa. Plant Syst. Evol. 2008, 271, 177–197. [Google Scholar] [CrossRef]
- Hu, X. Physiological Responses of Lysimachia alfredii to Cadmium Stress. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2023. [Google Scholar]
- Zheng, W. Studies on Germplasm Characteristics and Enhancement of Four Lysimachia Landscape Species. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2015. [Google Scholar]
- Eng, W.-H.; Ho, W.-S. Polyploidization Using Colchicine in Horticultural Plants: A Review. Sci. Hortic. 2019, 246, 604–617. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Ureshino, K.; Miyajima, I.; Ozaki, Y.; Okubo, H. Induction of Tetraploids in Ornamental Alocasia through Colchicine and Oryzalin Treatments. Plant Cell Tiss Organ Cult. 2003, 72, 19–25. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, P.; Zeng, F.; Geng, X.; Zhou, J.; Sun, J. Induction and Identification of Polyploids in Four Rhododendron Species. Plant Cell Tiss Organ Cult. 2024, 158, 11. [Google Scholar] [CrossRef]
- Winkelmann, T.; Braun, P.; Dhooghe, E.; Huylenbroeck, J. van Advances in Conventional Breeding Techniques for Ornamentals. In Achieving Sustainable Cultivation of Ornamental Plants; Burleigh Dodds Science Publishing: Sawston, UK, 2020; ISBN 978-1-003-04776-6. [Google Scholar]
- Bharati, R.; Gupta, A.; Novy, P.; Severová, L.; Šrédl, K.; Žiarovská, J.; Fernández-Cusimamani, E. Synthetic Polyploid Induction Influences Morphological, Physiological, and Photosynthetic Characteristics in Melissa officinalis L. Front. Plant Sci. 2023, 14, 1332428. [Google Scholar] [CrossRef]
- Fakhrzad, F.; Jowkar, A.; Shekafandeh, A.; Kermani, M.J.; Moghadam, A. Tetraploidy Induction Enhances Morphological, Physiological and Biochemical Characteristics of Wallflower (Erysimum cheiri (L.) Crantz). Sci. Hortic. 2023, 308, 111596. [Google Scholar] [CrossRef]
- Allum, J.F.; Bringloe, D.H.; Roberts, A.V. Chromosome Doubling in a Rosa Rugosa Thunb. Hybrid by Exposure of in Vitro Nodes to Oryzalin: The Effects of Node Length, Oryzalin Concentration and Exposure Time. Plant Cell Rep. 2007, 26, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, L.; Li, J.; Lu, M.; An, H. Polyploid Induction and Identification of Rosa roxburghii f. eseiosa. Plants 2023, 12, 2194. [Google Scholar] [CrossRef]
- Contreras, R.N.; Ranney, T.G.; Tallury, S.P. Reproductive Behavior of Diploid and Allotetraploid rhododendron L. ‘Fragrant Affinity’. HortScience 2007, 42, 31–34. [Google Scholar] [CrossRef]
- Zhang, Y.-S.; Chen, J.-J.; Cao, Y.-M.; Duan, J.-X.; Cai, X.-D. Induction of Tetraploids in ‘Red Flash’ Caladium Using Colchicine and Oryzalin: Morphological, Cytological, Photosynthetic and Chilling Tolerance Analysis. Sci. Hortic. 2020, 272, 109524. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhang, Y.-S.; Duan, J.-X.; Cao, Y.-M.; Cai, X.-D. Morphological, Cytological, and Pigment Analysis of Leaf Color Variants Regenerated from Long-Term Subcultured Caladium Callus. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 60–71. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Pei, H.; Zhang, J.; Zhang, J.; Luo, J. Variations in Colchicine-Induced Autotetraploid Plants of Lilium davidii var. unicolor. Plant Cell Tiss Organ Cult. 2020, 141, 479–488. [Google Scholar] [CrossRef]
- Wang, L.-J.; Cao, Q.-Z.; Zhang, X.-Q.; Jia, G.-X. Effects of Polyploidization on Photosynthetic Characteristics in Three Lilium Species. Sci. Hortic. 2021, 284, 110098. [Google Scholar] [CrossRef]
- Miri, S.M. Artificial Polyploidy in the Improvement of Horticultural Crops. J. Plant Physiol. Breed. 2020, 10, 1–28. [Google Scholar] [CrossRef]
- Kermani, M.J.; Sarasan, V.; Roberts, A.V.; Yokoya, K.; Wentworth, J.; Sieber, V.K. Oryzalin-Induced Chromosome Doubling in Rosa and Its Effect on Plant Morphology and Pollen Viability. Theor. Appl. Genet. 2003, 107, 1195–1200. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Sowik, I.; Machlańska, A.; Kruczyńska, D.; Dyki, B. In Vitro Tetraploid Induction of Malus × Domestica Borkh. Using Leaf or Shoot Explants. Sci. Hortic. 2017, 226, 379–388. [Google Scholar] [CrossRef]
- Kirchner, S.; Pianowski, Z. Photopharmacology of Antimitotic Agents. Int. J. Mol. Sci. 2022, 23, 5657. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, X.-D.; Dai, H.; Chen, L.-Q. Direct Regeneration of Plants Derived from in Vitro Cultured Shoot Tips and Leaves of Three Lysimachia Species. Sci. Hortic. 2009, 122, 138–141. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of Nuclear DNA Content in Plants Using Flow Cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Xu, S.; Deng, Z. Induction, Regeneration and Characterization of Tetraploids and Variants in ‘Tapestry’ Caladium. Plant Cell Tissue Organ Cult. 2015, 120, 689–700. [Google Scholar] [CrossRef]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic Chromosome Doubling of Plant Tissues in Vitro. Plant Cell Tiss Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Chauvin, J.E.; Souchet, C.; Dantec, J.P.; Ellissèche, D. Chromosome Doubling of 2x Solanum Species by Oryzalin: Method Development and Comparison with Spontaneous Chromosome Doubling in Vitro. Plant Cell Tissue Organ Cult. 2003, 73, 65–73. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Z.; Du, K.; Kang, X. Oryzalin-Induced Chromosome Doubling in Triploid Populus and Its Effect on Plant Morphology and Anatomy. Plant Cell Tiss Organ Cult. 2019, 138, 571–581. [Google Scholar] [CrossRef]
- Fauzan, Y.S.A.; Supriyanto; Mulyono, J.; Tajuddin, T. The Effectiveness of Colchicine and Oryzalin on Polyploidy Induction in Teak (Tectona grandis Linn. f.) in vitro. Indones. J. For. Res. 2024, 11, 1–15. [Google Scholar] [CrossRef]
- Shmeit, Y.H. Autopolyploidy Effect on Morphological Variation and Essential Oil Content in Thymus vulgaris L. Sci. Hortic. 2020, 263, 109095. [Google Scholar] [CrossRef]
- Bharati, R.; Fernández-Cusimamani, E.; Gupta, A.; Novy, P.; Moses, O.; Severová, L.; Svoboda, R.; Šrédl, K. Oryzalin Induces Polyploids with Superior Morphology and Increased Levels of Essential Oil Production in Mentha spicata L. Ind. Crops Prod. 2023, 198, 116683. [Google Scholar] [CrossRef]
- Denaeghel, H.E.R.; Van Laere, K.; Leus, L.; Lootens, P.; Van Huylenbroeck, J.; Van Labeke, M.-C. The Variable Effect of Polyploidization on the Phenotype in Escallonia. Front. Plant Sci. 2018, 9, 354. [Google Scholar] [CrossRef]
- Shariat, A.; Sefidkon, F. Tetraploid Induction in Savory (Satureja khuzistanica): Cytological, Morphological, Phytochemical and Physiological Changes. Plant Cell Tiss Organ Cult. 2021, 146, 137–148. [Google Scholar] [CrossRef]
- Roux, N.; Toloza, A.; Radecki, Z.; Zapata-Arias, F.J.; Dolezel, J. Rapid Detection of Aneuploidy in Musa Using Flow Cytometry. Plant Cell Rep. 2003, 21, 483–490. [Google Scholar] [CrossRef]
- Sanaei-Hoveida, Z.; Mortazavian, S.M.M.; Norouzi, M.; Sadat-Noori, S.A. Exploring the Potential of Polyploidization as a Breeding Tool for Medicinal Plants: A Case Study on Cumin (Cuminum cyminum L.). Plant Cell Tiss Organ Cult. 2023, 156, 23. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, Z.; Chen, X.; Cao, J.; Zhang, W.; Sun, R.; Teixeira Da Silva, J.A.; Yu, X. Induction of 2n Pollen by Colchicine during Microsporogenesis to Produce Polyploids in Herbaceous Peony (Paeonia lactiflora Pall.). Sci. Hortic. 2022, 304, 111264. [Google Scholar] [CrossRef]
- Liu, K.; Hong, X.; Zhou, S.-B.; Cheng, Y.-S.; Tang, C.-F.; Xu, H.-J. A New Species of Lysimachia (Myrsinaceae) from Dabieshan Mountain, China. Plant Syst. Evol. 2014, 300, 1615–1620. [Google Scholar] [CrossRef]
- Gerard, A.; Naciri, Y.; Peignon, J.-M.; Ledu, C.; Phelipot, P. Optimization of Triploid Induction by the Use of 6-DMAP for the Oyster Crassostrea Gigas (Thunberg). Aquac. Res. 1994, 25, 709–719. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Banadka, A.; Dubey, S.; Al-Khayri, J.M.; Nagella, P. Advances in Triploid Plant Production: Techniques, Benefits, and Applications. Plant Cell Tiss Organ Cult. 2025, 160, 70. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Yang, W.; Li, X.; Hou, L.; Li, M.; Pang, X.; Li, Y. Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus Jujuba Mill.) ‘Maya’ and Its Characteristics. Forests 2021, 12, 112. [Google Scholar] [CrossRef]
- Hong, L.; Dumond, M.; Zhu, M.; Tsugawa, S.; Li, C.-B.; Boudaoud, A.; Hamant, O.; Roeder, A.H.K. Heterogeneity and Robustness in Plant Morphogenesis: From Cells to Organs. Annu. Rev. Plant Biol. 2018, 69, 469–495. [Google Scholar] [CrossRef]
- Zhang, F.; Rathod, B.; Jones, J.B.; Wang, Q.; Bernhard, E.; Godyn, J.; Studzinski, G.P. Increased Stringency of the 1,25-dihydroxyvitamin D3-induced G1 to S Phase Block in Polyploid HL60 Cells. J. Cell. Physiol. 1996, 168, 18–25. [Google Scholar] [CrossRef]
- Trojak-Goluch, A.; Kawka-Lipińska, M.; Wielgusz, K.; Praczyk, M. Polyploidy in Industrial Crops: Applications and Perspectives in Plant Breeding. Agronomy 2021, 11, 2574. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, X.; Gao, X.; Wang, L.; Jia, G. Effects of Ploidy Level on the Cellular, Photochemical and Photosynthetic Characteristics in Lilium FO Hybrids. Plant Physiol. Biochem. 2018, 133, 50–56. [Google Scholar] [CrossRef]
- Rahmawati, K.; Yunianta, Y.; Risjani, Y. Polyploid Induction Enhance Pigment Production in Nannochoropsis Oculata: A Study Using a Mutagenesis Agent [Preprint]. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of Polyploidy on Plant Tolerance to Abiotic and Biotic Stresses. Front. Plant Sci. 2022, 13, 869423. [Google Scholar] [CrossRef]
- Fasano, C.; Diretto, G.; Aversano, R.; D’Agostino, N.; Di Matteo, A.; Frusciante, L.; Giuliano, G.; Carputo, D. Transcriptome and Metabolome of Synthetic Solanum Autotetraploids Reveal Key Genomic Stress Events Following Polyploidization. New Phytol. 2016, 210, 1382–1394. [Google Scholar] [CrossRef]
- Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Muñoz, R.; Khojasteh, A.; Palazon, J. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules 2021, 11, 899. [Google Scholar] [CrossRef]
- Baker, R.L.; Brock, G.L.; Newsome, E.L.; Zhao, M. Polyploidy and the Evolution of Phenotypic Integration: Network Analysis Reveals Relationships among Anatomy, Morphology, and Physiology. Appl. Plant Sci. 2024, 12, e11605. [Google Scholar] [CrossRef]
- Yao, P.-Q.; Chen, J.-H.; Ma, P.-F.; Xie, L.-H.; Cheng, S.-P. Stomata Variation in the Process of Polyploidization in Chinese Chive (Allium tuberosum). BMC Plant Biol. 2023, 23, 595. [Google Scholar] [CrossRef]
- Dunn, J.; Hunt, L.; Afsharinafar, M.; Meselmani, M.A.; Mitchell, A.; Howells, R.; Wallington, E.; Fleming, A.J.; Gray, J.E. Reduced Stomatal Density in Bread Wheat Leads to Increased Water-Use Efficiency. J. Exp. Bot. 2019, 70, 4737–4748. [Google Scholar] [CrossRef]
- Lourkisti, R.; Oustric, J.; Quilichini, Y.; Froelicher, Y.; Herbette, S.; Morillon, R.; Berti, L.; Santini, J. Improved Response of Triploid Citrus Varieties to Water Deficit Is Related to Anatomical and Cytological Properties. Plant Physiol. Biochem. 2021, 162, 762–775. [Google Scholar] [CrossRef]
- Misiukevičius, E.; Mažeikienė, I.; Stanys, V. Ploidy’s Role in Daylily Plant Resilience to Drought Stress Challenges. Biology 2024, 13, 289. [Google Scholar] [CrossRef]
- Khazaei, H.; Monneveux, P.; Hongbo, S.; Mohammady, S. Variation for Stomatal Characteristics and Water Use Efficiency among Diploid, Tetraploid and Hexaploid Iranian Wheat Landraces. Genet. Resour. Crop Evol. 2010, 57, 307–314. [Google Scholar] [CrossRef]

| Indicator | Plant Survival Rate | Total Mutation-Induced Plant Rate | Polyploid Plant Induction Rate |
|---|---|---|---|
| Error distribution | Binomial | Binomial | Binomial |
| Link function | Complementary Log-log | Complementary Log-log | Logit |
| Deviance | 18.552 | 7.835 | 0 |
| Residual df (df_res) 1 | 20 | 13 | 13 |
| Deviance/df-res | 0.928 | 0.603 | 0 |
| Pearson χ2 | 15.707 | 5.992 | 0 |
| Pearson χ2/df | 0.785 | 0.461 | 0 |
| Likelihood Ratio χ2 | 61.995 | 18.196 | 16.875 |
| Model df (df_model) 2 | 9 | 9 | 9 |
| p-value | <0.001 | 0.033 | 0.051 |
| Model Fit | Good | Good | Apparently good |
| Overall significance | Significant | Significant | Borderline significant |
| Source | Plant Survival Rate | Total Mutation-Induced Plant Rate | Polyploid Plant Induction Rate |
|---|---|---|---|
| χ2 (df), p-value | χ2 (df), p-value | χ2 (df), p-value | |
| Treatment Concentration | 108,207.811 (3), <0.001 | 0.000 (3), 1.000 | 0.000 (3), 1.000 |
| Treatment Duration | 2.756 (2), 0.252 | 0.003 (2), 0.998 | 0.000 (2), 1.000 |
| Treatment Conc. * Duration | 4.376 (4), 0.358 | 0.008 (3), 1.000 | 0.000 (2), 1.000 |
| Treatment Conc. (%) | Treatment Duration (d) | Plant Survival Rate (%) Estimated Marginal Mean (±SE) | Total Mutation-Induced Plant Rate (%) Estimated Marginal Mean (±SE) | Polyploid Plant Induction Rate (%) Estimated Marginal Mean (±SE) | Total Number of Plants Treated | Number of Survival Plants | Number of Mutation-Induced Plants |
|---|---|---|---|---|---|---|---|
| 0 (CK) | 6 | 100.00 ± 0.00 | - | - | 18 | 18 | - |
| Conc. mean | 100.00 ± 0.00 A | - | - | - | - | - | |
| 0.001 | 2 | 27.78 ± 9.80 | 1.92 ± 22.93 | 0.00 ± 0.00 | 18 | 5 | 1 |
| 4 | 11.11 ± 7.40 | 100.00 ± 0.00 | 100.00 ± 0.00 | 18 | 2 | 2 | |
| 6 | 38.89 ± 11.50 | 28.57 ± 17.08 | 0.00 ± 0.00 | 18 | 7 | 2 | |
| Conc. mean | 25.00 ± 6.00 B | 76.00 ± 15.80 | 0.00 ± 0.00 | ||||
| 0.002 | 2 | 16.67 ± 8.80 | 33.33 ± 27.22 | 0.00 ± 0.00 | 18 | 3 | 1 |
| 4 | 27.78 ± 10.60 | 20.00 ± 17.90 | 0.00 ± 0.00 | 18 | 5 | 1 | |
| 6 | 33.33 ± 11.10 | 33.33 ± 19.20 | 0.00 ± 0.00 | 18 | 6 | 2 | |
| Conc. mean | 26.00 ± 6.00 B | 28.00 ± 12.60 | 0.00 ± 0.00 | ||||
| 0.003 | 2 | 5.56 ± 5.40 | 0.00 ± 0.00 | 0.00 ± 0.00 | 18 | 1 | 0 |
| 4 | 16.67 ± 8.80 | 0.00 ± 0.00 | 0.00 ± 0.00 | 18 | 3 | 0 | |
| 6 | 11.11 ± 7.40 | 0.00 ± 0.00 | 0.00 ± 0.00 | 18 | 2 | 0 | |
| Conc. mean | 11.00 ± 4.30 C | 0.00 ± 0.00 | 0.00 ± 0.00 | - | - | - |
| Plant Types | Estimated Ploidy Level | Chromosome Number (2n) | Change in Chromosome Number |
|---|---|---|---|
| WT | 2.05 ± 0.08 c | 24 | 2x |
| VT1 | 1.76 ± 0.03 d | 22 | 2x − 2 |
| VT2 | 2.40 ± 0.17 b | 26 | 2x + 2 |
| VT3 | 3.33 ± 0.05 a | 36 | 3x |
| Phenotypic Characteristics | Wild Type (2n = 2x) | Diploid Aneuploid (VT1, 2n = 2x − 2) | Diploid Aneuploid (VT2, 2n = 2x + 2) | Polyploid (VT3, 2n = 3x) |
|---|---|---|---|---|
| Leaf edge | Slightly wavy, not curled | Slightly wavy, slightly curled | Slightly wavy, not curled | Slightly wavy, not curled |
| Stolon characteristics | Light-green | Light-green, thinner veins | Light-green, thinner veins | Light-green, thicker veins |
| Leaf color | Green | Dark-green | Light-green | Muted-green with red-purple patches |
| Spot color | Dark-purple | Dark-purple | Dark-purple | Red-purple |
| Spot shape | Irregular, scattered | Irregular, more prominent | Irregular, less defined | Irregular, varied patches |
| Stolon length (cm) | 5.65 ± 0.26 bc | 6.33 ± 0.6 ab | 4.90 ± 1.08 c | 7.33 ± 1.30 a |
| Leaf length (cm) | 3.26 ± 0.35 b | 3.13 ± 0.50 b | 2.38 ± 0.23 c | 4.98 ± 0.22 a |
| Leaf width (cm) | 1.64 ± 0.32 b | 1.60 ± 0.08 b | 1.45 ± 0.23 b | 2.75 ± 0.17 a |
| Leaf length/width ratio | 2.04 ± 0.35 a | 1.95 ± 0.22 a | 1.68 ± 0.26 a | 1.81 ± 0.12 a |
| Leaf thickness (cm) | 0.04 ± 0.03 ab | 0.05 ± 0.02 ab | 0.03 ± 0.01 b | 0.06 ± 0.02 a |
| Stolon diameter (cm) | 0.16 ± 0.03 b | 0.17 ± 0.01 ab | 0.15 ± 0.03 b | 0.20 ± 0.01 a |
| Stomatal number | 9.39 ± 0.2 b | 14.52 ± 0.32 a | 8.22 ± 0.24 c | 6.12 ± 0.09 d |
| Stomatal guard cell length (μm) | 76.18 ± 0.03 b | 60.08 ± 0.2 d | 75.86 ± 0.1 c | 82.43 ± 0.35 a |
| Stomatal guard cell width (μm) | 55.49 ± 0.41 b | 54.81 ± 0.2 c | 53.1 ± 0.3 d | 67.46 ± 0.1 a |
| Stomatal density (no./mm2) | 41.1 ± 0.02 b | 63.93 ± 0.3 a | 36.53 ± 0.24 c | 27.4 ± 0.31 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, G.; Shen, R.; Li, Q.; Shen, X. Identification and Morphophysiological Characterization of Oryzalin-Induced Polyploids and Variants in Lysimachia xiangxiensis. Horticulturae 2025, 11, 654. https://doi.org/10.3390/horticulturae11060654
Zhang Y, Chen G, Shen R, Li Q, Shen X. Identification and Morphophysiological Characterization of Oryzalin-Induced Polyploids and Variants in Lysimachia xiangxiensis. Horticulturae. 2025; 11(6):654. https://doi.org/10.3390/horticulturae11060654
Chicago/Turabian StyleZhang, Yuanshan, Guanqun Chen, Ruixue Shen, Qiujing Li, and Xiaohui Shen. 2025. "Identification and Morphophysiological Characterization of Oryzalin-Induced Polyploids and Variants in Lysimachia xiangxiensis" Horticulturae 11, no. 6: 654. https://doi.org/10.3390/horticulturae11060654
APA StyleZhang, Y., Chen, G., Shen, R., Li, Q., & Shen, X. (2025). Identification and Morphophysiological Characterization of Oryzalin-Induced Polyploids and Variants in Lysimachia xiangxiensis. Horticulturae, 11(6), 654. https://doi.org/10.3390/horticulturae11060654






