Effects of Soil–Sand Mixtures on Alchemilla mollis and Geranium psilostemon: A Multi-Criteria Performance Analysis Under Low-Altitude Conditions Using PROMETHEE
Abstract
1. Introduction
2. Material and Method
2.1. Plant Material
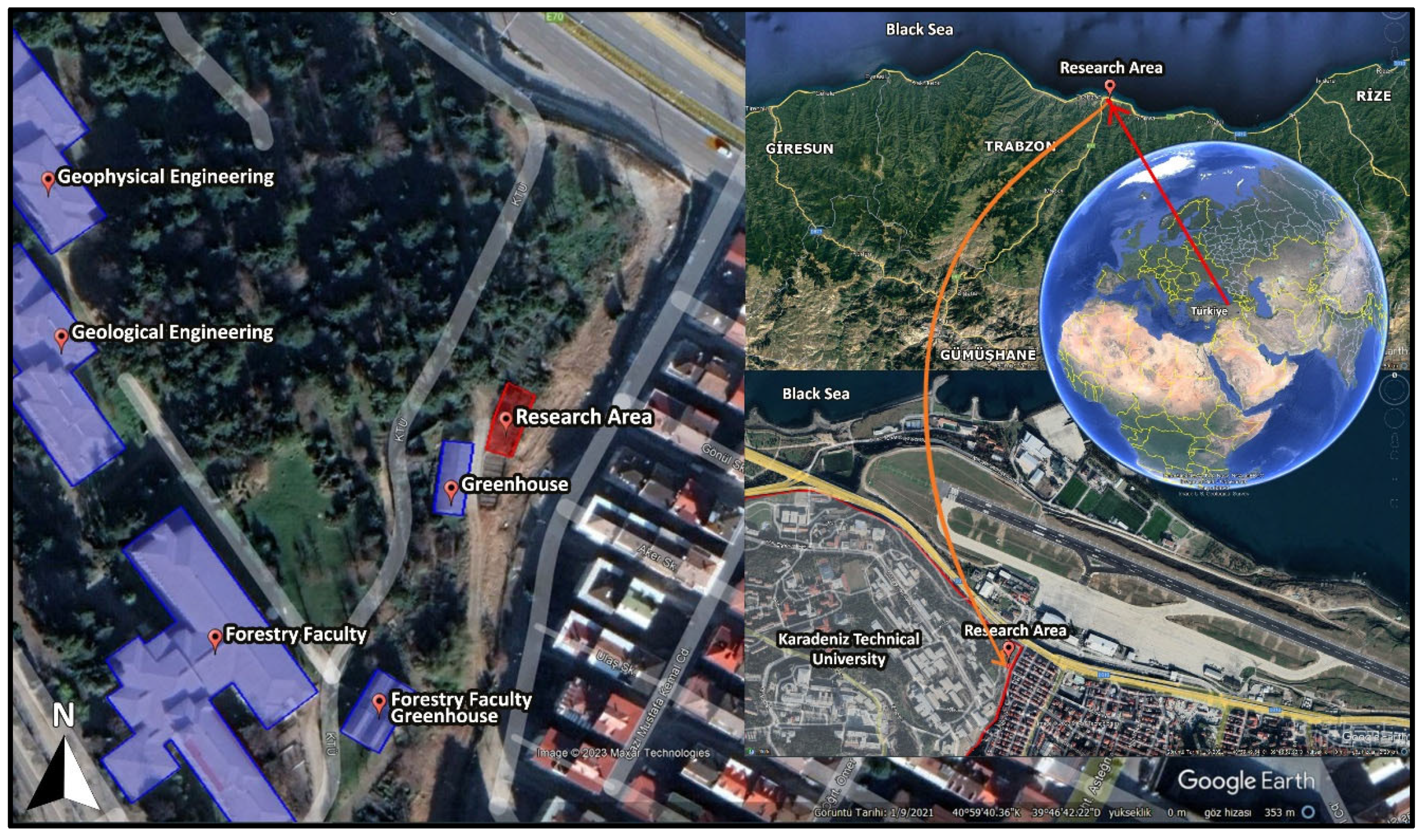
2.2. Excavation of Native Plants from the Field
2.3. Design of Growing Media and Soil Analysis
2.3.1. Preparation of Growing Media
- TA: 50% soil + 50% river sand;
- TB: 75% soil + 25% river sand;
- TC: 100% soil.
2.3.2. Soil Analysis and Experimental Setup
2.4. Measurement of Plant Growth Parameters
- First growing season (2022): May (Month 1), June (2), July (3), August (4), September (5), October (6), November (7), December (8);
- Dead (Dormant) season (2023): January (9), February (10);
- Second growing season (2023): March (11), April (12), May (13), June (14), July (15), August (16), September (17).
2.5. Statistical Analyses
2.6. Multi-Criteria Decision-Making (PROMETHEE) Analysis
2.7. Climatic Conditions of the Experimental Site
3. Result
3.1. Morphological Growth Performance and Soil Parameter Findings
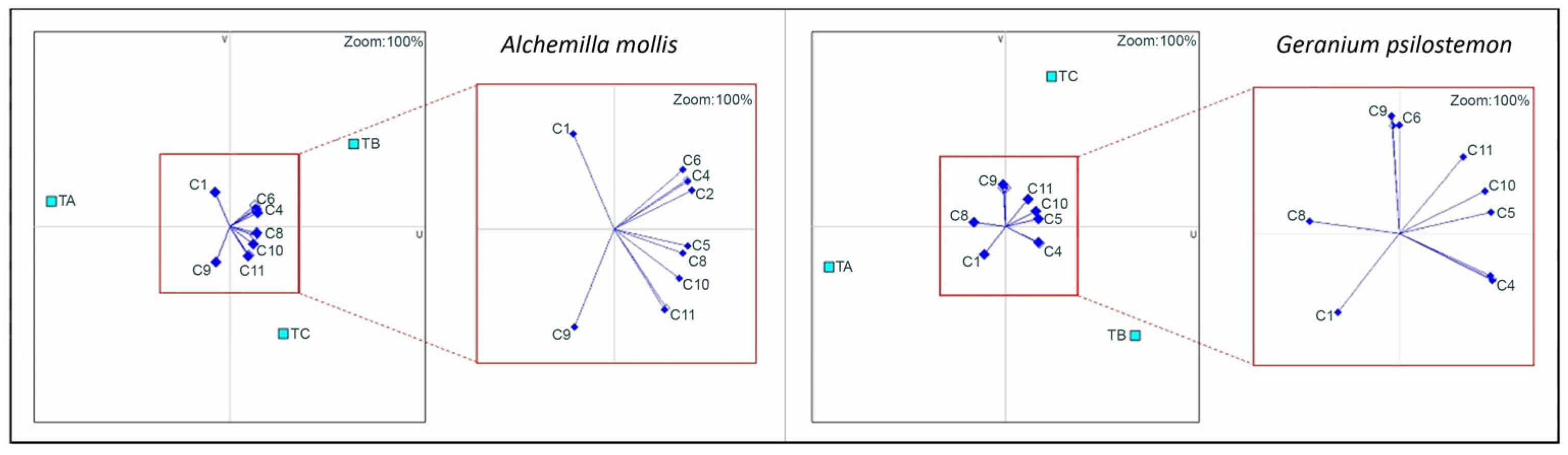
3.2. PROMETHEE Multi-Criteria Decision-Making (MCDM) Findings
- φ+ = 0.5273;
- φ− = 0.1005;
- φ (net flow) = 0.4268;
- Rank = 1.
- φ+ = 0.5511;
- φ− = 0.0963;
- φ (net flow) = 0.4548;
- Rank = 1.
- A. mollis: φ = 0.1805;
- G. psilostemon: φ = 0.0335.
4. Discussion
4.1. Effect of Growing Media on Morphological Development
4.2. Changes in Soil Parameters and Their Interaction with Plants
4.3. Effect of Growing Media According to PROMETHEE Analysis
4.4. The Role of Findings in Sustainable Landscape Design and Perennial Plant Selection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TA | 50% soil–50% sand mixture |
| TB | 75% soil–25% sand mixture |
| TC | 100% soil medium |
| MCDM | Multi-criteria decision-making |
| SPSS | Statistical Package for the Social Sciences |
| PROMETHEE | Preference Ranking Organization METHod for Enrichment Evaluation |
| EC | Electrical conductivity |
| mS/cm | MilliSiemens/centimeter |
| cm | Centimeter |
| μmol m−2 | Micromole per square meter |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| OM | Organic matter |
| OC | Organic carbon |
References
- Zhang, X.; Kuang, T.; Dong, W.; Qian, Z.; Zhang, H.; Landis, J.B.; Li, L.; Sun, Y.; Huang, J.; Deng, T.; et al. Genomic Convergence Underlying High-Altitude Adaptation in Alpine Plants. J. Integr. Plant Biol. 2023, 65, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Hao, R.; Fan, F.; Wang, Y.; Zhu, F. Adaptation of High-Altitude Plants to Plateau Abiotic Stresses: A Case Study of the Qinghai-Tibet Plateau. Int. J. Mol. Sci. 2025, 26, 2292. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, X.; Kong, X.; Galvan, J.V.; Li, X.; Yang, S.; Hu, X. Physiological, Biochemical and Proteomics Analysis Reveals the Adaptation Strategies of the Alpine Plant Potentilla saundersiana at Altitude Gradient of the Northwestern Tibetan Plateau. J. Proteom. 2015, 112, 63–82. [Google Scholar] [CrossRef]
- Gökçe, Z. Bitkilerin Kuraklık Stresine Tepkilerinde Bilinenler ve Yeni Yaklaşımlar. Turk. J. Agric. Food Sci. Technol. 2015, 3, 307. [Google Scholar] [CrossRef]
- Başaran, F.; Akçin, T. Sıcaklık Faktörünün Bitkiler Üzerindeki Etkileri ve Yüksek Sıcaklık Stresi. Bahçe 2022, 51, 139–147. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Cai, J.; Li, Y.; Zhou, Y.; Zhang, H.; Zheng, K. Altitudinal Effects on Soil Microbial Diversity and Composition in Moso Bamboo Forests of Wuyi Mountain. Plants 2024, 13, 2471. [Google Scholar] [CrossRef]
- Körner, C. Plant Adaptation to Cold Climates. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef]
- Billings, W.D.; Mooney, H.A. The Ecology of Arctic and Alpine Plants. Biol. Rev. 1968, 43, 481–529. [Google Scholar] [CrossRef]
- Wingler, A.; Juvany, M.; Cuthbert, C.; Munné-Bosch, S. Adaptation to Altitude Affects the Senescence Response to Chilling in the Perennial Plant Arabis alpina. J. Exp. Bot. 2015, 66, 355–367. [Google Scholar] [CrossRef]
- Peşkircioğlu, M.; Özaydın, K.; Özpınar, H.; Nadaroglu, Y.; Cankurtaran, G.; Ünal, S.; Şimşek, O. Bitkilerin Sıcağa ve Soğuğa Dayanıklılık Bölgelerinin Türkiye Ölçeğinde Coğrafi Bilgi Sistemleri ile Haritalanması. Tarla Bitk. Merk. Araştırma Enstitüsü Derg. 2016, 25, 11–25. [Google Scholar] [CrossRef][Green Version]
- Magaña Ugarte, R.; Escudero, A.; Gavilán, R.G. Metabolic and Physiological Responses of Mediterranean High-Mountain and Alpine Plants to Combined Abiotic Stresses. Physiol. Plant. 2019, 165, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fayetörbay, D.; Daşçı, M.; Çomaklı, B. Fosfor Çözücü Bakteri, Fosforlu Gübre ve Tavuk Gübresi Uygulamalarının Macar Fiğinde (Vicia pannonica Roth) Tohum Verimi ve Verim Unsurları Üzerine Etkileri. Tarım Bilim. Derg. 2014, 20, 345. [Google Scholar] [CrossRef]
- Esringü, A.; Sezen, İ. Türkiye Florasında Peyzaj Özelliği Gösteren Hiperakümülatör Bitkilerin Maden Alanlarının Onarımında Kullanımı. Türk. Doğa Fen Derg. 2021, 10, 327–334. [Google Scholar] [CrossRef]
- Öztürk, A.; Demirsoy, L. Investigating of the Effect of Different Shading Treatments on Growth in ‘Sweet Charlie’ Strawberry Variety with Quantitative Analyses. Anadolu J. Agric. Sci. 2014, 29, 87. [Google Scholar] [CrossRef]
- Devi, N.; Rani, K.; Kharb, P.; Kaushik, P. Bio-Fabrication of Euryale ferox (Makhana) Leaf Silver Nanoparticles and Their Antibacterial, Antioxidant and Cytotoxic Potential. Plants 2022, 11, 2766. [Google Scholar] [CrossRef]
- Trošt Sedej, T.; Turk, T. Alchemilla monticola Opiz. Functional Traits Respond to Diverse Alpine Environmental Conditions in Karavanke, Slovenia. Plants 2022, 11, 2527. [Google Scholar] [CrossRef]
- Deniz, B.; Şirin, U. Samson Dağı Doğal Bitki Örtüsünün Otsu Karakterdeki Bazı Örneklerinden Peyzaj Mimarlığı Uygulamalarında Yararlanma Olanaklarının İrdelenmesi. ADÜ Ziraat Fak. Derg. 2005, 2, 5–12. [Google Scholar]
- Simmons, M.T.; Venhaus, H.C.; Windhager, S. Exploiting the Attributes of Regional Ecosystems for Landscape Design: The Role of Ecological Restoration in Ecological Engineering. Ecol. Eng. 2007, 30, 201–205. [Google Scholar] [CrossRef]
- Labra, F.A.; Jaramillo, E. Biodiversity Dynamics in a Ramsar Wetland: Assessing How Climate and Hydrology Shape the Distribution of Dominant Native and Alien Macrophytes. Plants 2025, 14, 1116. [Google Scholar] [CrossRef]
- El-Hadidy, E.M.; Refat, O.G.; Halaby, M.S.; Elmetwaly, E.M.; Aya, A.A. Protective Effect on Lipids Profile of Lion’s Foot (Alchemilla vulgaris) Leaves against CCl4 Toxicity and Its Fortified to Guava and Mango Pulp. Int. J. Food Sci. Nutr. Diet. 2019, 8, 394–400. [Google Scholar] [CrossRef]
- İnci, Ş.; Eren, A.; Kirbağ, S. Determination of Antimicrobial and Antioxidant Activity of Alchemilla alpina L. Turk. J. Agric. Food Sci. Technol. 2021, 9, 2260–2264. [Google Scholar]
- Oğuztürk, T.; Acar, C. Perennial Garden Establishment, Karadeniz Technical University Herbaceous Perennial Garden Example. In Perennial Garden Establishment, Karadeniz Technical University Herbaceous Perennial Garden Example; Zenodo: Geneva, Switzerland, 2024. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B.; Celiński, R.; Bakalczuk, M.; Dos Santos Szewczyk, K. Phenolic Composition and Antioxidant Activity of Alchemilla Species. Plants 2022, 11, 2709. [Google Scholar] [CrossRef] [PubMed]
- Morales-Briones, D.F.; Gehrke, B.; Huang, C.H.; Liston, A.; Ma, H.; Marx, H.E.; Tank, D.C.; Yang, Y. Analysis of Paralogs in Target Enrichment Data Pinpoints Multiple Ancient Polyploidy Events in Alchemilla s.l. (Rosaceae). Syst. Biol. 2022, 71, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Ramírez, N.; Calzada, F.; Alquisiras-Burgos, I.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Ortiz-Plata, A.; Aguilera, P. Antioxidant Properties and Protective Effects of Some Species of the Annonaceae, Lamiaceae, and Geraniaceae Families Against Neuronal Damage Induced by Excitotoxicity and Cerebral Ischemia. Antioxidants 2020, 9, 253. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Zengin, G. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Balabanova, V.; Szakiel, A.; Zheleva-Dimitrova, D. Pelargonium graveolens: Towards In-Depth Metabolite Profiling, Antioxidant and Enzyme-Inhibitory Potential. Plants 2024, 13, 2612. [Google Scholar] [CrossRef]
- Oğuztürk, G.E.; Murat, C.; Yurtseven, M.; Oğuztürk, T. The Effects of AI-Supported Autonomous Irrigation Systems on Water Efficiency and Plant Quality: A Case Study of Geranium psilostemon Ledeb. Plants 2025, 14, 770. [Google Scholar] [CrossRef]
- Caf, A. Bingöl İli Doğal Vejetasyonundaki Bitkiler ile Kurakçıl Bahçe Oluşturulması Üzerine Bir Araştırma. Ph.D. Thesis, Atatürk Üniversitesi, Fen Bilimleri Enstitüsü, Peyzaj Mimarlığı Anabilim Dalı, Erzurum, Turkey, 2019. [Google Scholar]
- Ünver, H. Bitki Genetik Kaynaklarının Bahçe Bitkileri Açısından Değerlendirilmesi. Düzce Üniv. Süs Tıbbi Bitk. Bot. Bahçesi Derg. 2023, 2, 57–61. [Google Scholar]
- Oğuztürk, T. Evaluation of Natural and Exotic Perennial Plants in Plant Design. Ph.D. Thesis, Karadeniz Technical University, Trabzon, Turkey, 2023. [Google Scholar]
- Karadavut, U.; Sözen, Ö.; Yağmur, M. Estimation of Root Growth of Chickpea Plants Grown at Different Sowing Times with the Weibull Model. Turk. J. Agric. Nat. Sci. 2019, 6, 893–903. [Google Scholar]
- Ekberli, İ.; Dengiz, O. Bazı Inceptisol ve Entisol Alt Grup Topraklarının Fizikokimyasal Özellikleriyle Isısal Yayınım Katsayısı Arasındaki Regresyon İlişkilerin Belirlenmesi. Toprak Su Derg. 2016, 5, 1–10. [Google Scholar]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Kazemi, F.; Mohorko, R. Review on the Roles and Effects of Growing Media on Plant Performance in Green Roofs in World Climates. Urban For. Urban Green. 2017, 23, 13–26. [Google Scholar] [CrossRef]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Smith, A.; Jones, B. The Impact of Soil and Clay Mixtures on Plant Growth. J. Agric. Sci. 2010, 55, 123–131. [Google Scholar]
- Jørgensen, L.; Dresbøll, D.B.; Thorup-Kristensen, K. Root Growth of Perennials in Vertical Growing Media for Use in Green Walls. Sci. Hortic. 2014, 166, 31–41. [Google Scholar] [CrossRef]
- Schafer, G.; Lerner, B.L. Physical and Chemical Characteristics and Analysis of Plant Substrate. Ornam. Hortic. 2022, 28, 181–192. [Google Scholar] [CrossRef]
- Anjum, F.; Yaseen, M.; Rasul, E.; Wahid, A.; Anjum, S. Water Stress in Barley (Hordeum vulgare L.). II. Effect on Chemical Composition and Chlorophyll Contents. Pak. J. Agric. Sci. 2003, 40, 45–49. [Google Scholar]
- Taffouo, V.D.; Nouck, A.E.; Nyemene, K.P.; Tonfack, B.; Meguekam, T.L.; Youmbi, E. Effects of Salt Stress on Plant Growth, Nutrient Partitioning, Chlorophyll Content, Leaf Relative Water Content, Accumulation of Osmolytes and Antioxidant Compounds in Pepper (Capsicum annuum L.) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 481–490. [Google Scholar]
- Khayatnezhad, M.; Gholamin, R. The Effect of Drought Stress on Leaf Chlorophyll Content and Stress Resistance in Maize Cultivars (Zea mays). Afr. J. Microbiol. Res. 2012, 6, 2844–2848. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Shi, X.; Han, Y.; Liu, Y.; Jin, S. Effect of Simulated Organic–Inorganic N Deposition on Leaf Stoichiometry, Chlorophyll Content, and Chlorophyll Fluorescence in Torreya grandis. Horticulturae 2023, 9, 1042. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kara, M.; Eren, T. Evaluation of Drones That Can Be Assigned in Damage Assessment Tasks by the Fuzzy Decision-Making Methods. J. Polytech. 2024, 27, 2029–2041. [Google Scholar] [CrossRef]
- Minaz, M.; Er, A.; Ak, K.; Kurtoğlu, İ.Z.; Kayış, Ş. Determining the Appropriate Concentration of an Anesthetic Mixture in Three Different Fish Species with the PROMETHEE Decision Model. Front. Vet. Sci. 2024, 11, 1492769. [Google Scholar] [CrossRef] [PubMed]
- Arikan, A.; Sanlidag, T.; Sayan, M.; Uzun, B.; Uzun Ozsahin, D. Fuzzy-Based PROMETHEE Method for Performance Ranking of SARS-CoV-2 IgM Antibody Tests. Diagnostics 2022, 12, 2830. [Google Scholar] [CrossRef]
- Taherdoost, H.; Madanchian, M. Using PROMETHEE Method for Multi-Criteria Decision Making: Applications and Procedures. Iris J. Econ. Bus. Manag. 2023, 1, 1–9. [Google Scholar] [CrossRef]
- Gökalp, F. Comparing the Financial Performance of Banks in Turkey by Using PROMETHEE Method. Ege Strat. Araştırmalar Derg. 2015, 6, 63–82. [Google Scholar] [CrossRef][Green Version]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis, Part 3—Chemical Methods; Sparks, D.L., Ed.; ASA and SSSA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis, Part 2—Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; U.S. Government Printing Office: Washington, DC, USA, 1954.
- Ay, A.; Kızılkaya, R. Ordu ve Giresun İllerindeki Fındık Bahçelerinin Toprak Özellikleri ile Biyolojik Özellikleri Arasındaki İlişkiler. Toprak Bilim. Bitki Besleme Derg. 2021, 9, 71–78. [Google Scholar] [CrossRef]
- Passioura, J.B. Soil Structure and Plant Growth. Soil Res. 1991, 29, 717–728. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Guo, P.; Ji, G.; Zhang, X.; Qi, Q.; Xu, X.; Zhang, X.; Li, W.; Han, Z.; et al. Whole-Canopy Photosynthetic Characterization of Apple Tree and the Effects Induced by Grafting on Rootstocks with Different Vigor. Horticulturae 2022, 8, 816. [Google Scholar] [CrossRef]
- MacDonald, J.D.; Costello, L.R.; Lichter, J.M.; Quickert, D. Fill Soil Effects on Soil Aeration and Tree Growth. Arboric. Urban For. 2004, 30, 19–27. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Arisoy, R.Z. Doğrudan Ekim Sistemi.; T.C. Tarım ve Orman Bakanlığı, Tarımsal Araştırmalar ve Politikalar Genel Müdürlüğü, Bahri Dağdaş Uluslararası Tarımsal Araştırma Enstitüsü: Konya, Turkey, 2023. [Google Scholar]
- Zucco, M.A.; Walters, S.A.; Chong, S.K.; Klubek, B.P.; Masabni, J.G. Effect of Soil Type and Vermicompost Applications on Tomato Growth. Int. J. Recycl. Org. Waste Agric. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Nagase, A.; Dunnett, N. The Relationship between Percentage of Organic Matter in Substrate and Plant Growth in Extensive Green Roofs. Landsc. Urban Plan. 2011, 103, 230–236. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct Effects of Soil Organic Matter on Productivity Mirror Those Observed with Organic Amendments. Plant Soil 2018, 423, 363–373. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Barrow, N.J.; Hartemink, A.E. The Effects of pH on Nutrient Availability Depend on Both Soils and Plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Fageria, N.K.; Zimmermann, F.J.P. Influence of pH on Growth and Nutrient Uptake by Crop Species in an Oxisol. Commun. Soil Sci. Plant Anal. 1998, 29, 2675–2682. [Google Scholar] [CrossRef]
- Temel, S.; Keskin, B. Rüzgâr Erozyon Sahasında Gelişme Gösteren Dikenli Hölmez Otu (Noaea mucronata)’nun Toprağın Bazı Kimyasal Özelliklerine Etkisi. Atatürk Üniv. Ziraat Fak. Derg. 2019, 50, 167–173. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, S.R.; Singh, G. Soil Carbon, Microbial Activity and Nitrogen Availability in Agroforestry Systems on Moderately Alkaline Soils in Northern India. Appl. Soil Ecol. 2000, 15, 283–294. [Google Scholar] [CrossRef]
- Dalmonech, D.; Lagomarsino, A.; Moscatelli, M.C.; Chiti, T.; Valentini, R. Microbial Performance under Increasing Nitrogen Availability in a Mediterranean Forest Soil. Soil Biol. Biochem. 2010, 42, 1596–1606. [Google Scholar] [CrossRef]
- Montaño, N.M.; García-Oliva, F.; Jaramillo, V.J. Dissolved Organic Carbon Affects Soil Microbial Activity and Nitrogen Dynamics in a Mexican Tropical Deciduous Forest. Plant Soil 2007, 295, 265–277. [Google Scholar] [CrossRef]
- Yusran, F.H. Soil Organic Matter Decomposition: Effects of Organic Matter Addition on Phosphorus Dynamics in Lateritic Soils. Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2005. [Google Scholar]
- Hawkins, J.M.B.; Vermeiren, C.; Blackwell, M.S.A.; Darch, T.; Granger, S.J.; Dunham, S.J.; Hernandez-Allica, J.; Smolders, E.; McGrath, S. The Effect of Soil Organic Matter on Long-Term Availability of Phosphorus in Soil: Evaluation in a Biological P Mining Experiment. Geoderma 2022, 423, 115965. [Google Scholar] [CrossRef]
- Salas, A.M.; Elliott, E.T.; Westfall, D.G.; Cole, C.V.; Six, J. The Role of Particulate Organic Matter in Phosphorus Cycling. Soil Sci. Soc. Am. J. 2003, 67, 181–189. [Google Scholar] [CrossRef]
- Fisher, P.R.; Dickson, R.W.; Mohammad-Pour, G.S.; Huang, J. Effect of Solution Electrical Conductivity (EC) and Pre-Plant Nutrient Form on the pH of a Peat-Perlite Substrate. In Proceedings of the International Symposium on Growing Media and Soilless Cultivation 1034, Leiden, The Netherlands, 17–21 June 2013; pp. 249–254. [Google Scholar]
- Albano, J.P.; Altland, J.; Merhaut, D.J.; Wilson, S.B.; Wilson, P.C. Irrigation Water Acidification to Neutralize Alkalinity for Nursery Crop Production: Substrate pH, Electrical Conductivity, Nutrient Concentrations, and Plant Nutrition and Growth. HortScience 2017, 52, 1401–1405. [Google Scholar] [CrossRef]
- Angers, D.A.; Caron, J. Plant-Induced Changes in Soil Structure: Processes and Feedbacks. Biogeochemistry 1998, 42, 55–72. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S. Soil Structure and Plant Growth. In Soil Physical Environment and Plant Growth; Springer: Cham, Switzerland, 2023; pp. 125–154. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and Soil Structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Bengough, A.G. Root Growth and Function in Relation to Soil Structure, Composition, and Strength. In Root Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 151–171. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G.; Leavitt, B.; Ciganda, V.; Burba, G.G.; Suyker, A.E. Relationship between Gross Primary Production and Chlorophyll Content in Crops: Implications for the Synoptic Monitoring of Vegetation Productivity. J. Geophys. Res. Atmos. 2006, 111, D08S11. [Google Scholar] [CrossRef]
- Houborg, R.; Cescatti, A.; Migliavacca, M.; Kustas, W.P. Satellite Retrievals of Leaf Chlorophyll and Photosynthetic Capacity for Improved Modeling of GPP. Agric. For. Meteorol. 2013, 177, 10–23. [Google Scholar] [CrossRef]
- Uslu, A.; Shakouri, N. Kentsel Peyzajda Yeşil Altyapı ve Biyolojik Çeşitliliği Destekleyecek Olanaklar. Türk. Bilimsel Derlemeler Derg. 2013, 1, 46–50. [Google Scholar]
- Tırnakçı, A.; Aklıbaşında, M. Doğal Bitki Türlerinin Kentsel Alanlardaki Bitkisel Tasarımlarda Kullanımı. Artvin Çoruh Üniv. Orman Fak. Derg. 2023, 24, 167–177. [Google Scholar] [CrossRef]
- Yüksek, T.; Yüksek, F. Effects of Altitude, Aspect, and Soil Depth on Carbon Stocks and Properties of Soils in a Tea Plantation in the Humid Black Sea Region. Land Degrad. Dev. 2021, 32, 4267–4276. [Google Scholar] [CrossRef]
- Koester, H. Native Plants and Urban Sustainability. Nativ. Plants J. 2008, 9, 323–333. [Google Scholar] [CrossRef]
- Pham, M.A.; Scott, S.B.; Fyie, L.R.; Gardiner, M.M. Sustainable Landscaping Programs in the United States and Their Potential to Encourage Conservation and Support Ecosystem Services. Urban Ecosyst. 2022, 25, 1481–1490. [Google Scholar] [CrossRef]
- Smetana, S.M.; Crittenden, J.C. Sustainable Plants in Urban Parks: A Life Cycle Analysis of Traditional and Alternative Lawns in Georgia, USA. Landsc. Urban Plan. 2014, 122, 140–151. [Google Scholar] [CrossRef]
- Oz, B.; Ilhan, M.; Ozbilgin, S.; Akkol, E.; Acikara, O.; Saltan, G.; Keleş, H.; Suntar, I. Effects of Alchemilla mollis and Alchemilla persica on the Wound Healing Process. Bangladesh J. Pharmacol. 2016, 11, 717–724. [Google Scholar]
- Şöhretoğlu, D.; Sabuncuoğrlu, S.A.; Sakar, M.K.; Özgüneş, H.; Sterner, O. Antioxidant Effects of Secondary Metabolites from Geranium psilostemon. Nat. Prod. Commun. 2010, 5, 885–888. [Google Scholar]
- Castro, A.J.; Verburg, P.H.; Martín-López, B.; Garcia-Llorente, M.; Cabello, J.; Vaughn, C.C.; López, E. Ecosystem Service Trade-Offs from Supply to Social Demand: A Landscape-Scale Spatial Analysis. Landsc. Urban Plan. 2014, 132, 102–110. [Google Scholar] [CrossRef]
- Minaz, M. A new herbal anesthetic agent for common carp (Cyprinus carpio) sedation and anesthesia: Nutmeg (Myristica fragrans) essential oil. Front. Vet. Sci. 2024, 11, 1477357. [Google Scholar] [CrossRef]


| Perspective | Criteria Number | Evaluation Criteria | Weight Value | Preference Function |
|---|---|---|---|---|
| Soil | C1 | pH | 0.10 | Linear |
| C2 | Nitrogen (%) | 0.10 | V-shape | |
| C3 | Organic matter (%) | 0.09 | V-shape | |
| C4 | Organic carbon (%) | 0.09 | V-shape | |
| C5 | Phosphate (mg/L) | 0.09 | Linear | |
| C6 | Electrical conductivity (mS/cm) | 0.09 | V-shape | |
| Plant | C7 | Length (cm) | 0.10 | Linear |
| C8 | Diameter (cm) | 0.10 | Linear | |
| C9 | Chlorophyll (µmol/m−2) | 0.09 | Linear | |
| C10 | Number of leaves | 0.08 | Linear | |
| Economic | C11 | Cost (USD) | 0.07 | Linear |
| Climate Variable | Season | Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Annual Avg./Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monthly avg. max temperature (°C) | 1 | 24.0 | 19.2 | 18.4 | 15.2 | 11.0 | 12.8 | 8.9 | 18.0 | 18.5 | 24.2 | 26.3 | 29.4 | 18.8 |
| 2 | 25.9 | 20.7 | 18.6 | 15.4 | 13.3 | 11.1 | 14.2 | 17.4 | 18.3 | 24.3 | 28.1 | 29.5 | 19.7 | |
| Monthly avg. min temperature (°C) | 1 | 17.7 | 13.1 | 11.1 | 8.4 | 5.0 | 6.7 | 3.9 | 9.9 | 12.4 | 19.0 | 20.5 | 23.9 | 12.6 |
| 2 | 18.9 | 15.0 | 12.3 | 9.7 | 6.6 | 4.9 | 8.3 | 10.5 | 12.9 | 18.8 | 20.4 | 23.1 | 13.5 | |
| Monthly avg. temperature (°C) | 1 | 20.3 | 15.9 | 14.3 | 11.3 | 7.7 | 9.6 | 6.2 | 13.5 | 15.2 | 21.3 | 23.2 | 26.4 | 15.4 |
| 2 | 22.4 | 17.5 | 15.2 | 12.2 | 9.6 | 7.7 | 10.7 | 13.5 | 15.3 | 21.4 | 24.1 | 26.4 | 16.3 | |
| Monthly total precipitation (mm = kg÷m2) | 1 | 130.0 | 80.6 | 50.4 | 107.6 | 117.2 | 24.4 | 107.4 | 47.0 | 62.8 | 75.8 | 26.8 | 35.6 | 865.6 |
| 2 | 96.8 | 57.8 | 34.4 | 73.6 | 34.8 | 115.4 | 63.8 | 128.0 | 69.6 | 69.8 | 56.2 | 13.0 | 813.2 | |
| Monthly avg. relative humidity (%) | 1 | 74.2 | 75.4 | 69.0 | 57.4 | 62.8 | 64.7 | 72.0 | 65.9 | 73.1 | 79.3 | 68.9 | 76.4 | 69.9 |
| 2 | 65.9 | 73.8 | 65.6 | 66.3 | 58.9 | 60.8 | 75.3 | 71.2 | 79.6 | 79.3 | 65.5 | 70.0 | 69.3 |
| Species | Parameters | Source of Variance | Sum of Squares | Mean Square | F | η2 | p |
|---|---|---|---|---|---|---|---|
| Alchemilla mollis | Length | Group | 79.91 | 39.95 | 0.84 | 0.013 | >0.05 |
| Time | 9725.77 | 972.58 | 20.38 | 0.607 | <0.01 | ||
| Group × Time | 2184.63 | 109.23 | 2.29 | 0.258 | <0.01 | ||
| Error | 6298.00 | 47.71 | |||||
| Diameter | Group | 41.68 | 20.84 | 0.25 | 0.004 | >0.05 | |
| Time | 12,859.50 | 1285.85 | 15.80 | 0.545 | <0.01 | ||
| Group × Time | 1949.78 | 97.49 | 1.19 | 0.154 | >0.05 | ||
| Error | 10,743.20 | 81.39 | |||||
| Chlorophyll | Group | 3257.01 | 1628.51 | 1.37 | 0.020 | >0.05 | |
| Time | 94,100.94 | 9410.09 | 7.90 | 0.375 | <0.01 | ||
| Group × Time | 72,891.17 | 3644.56 | 3.06 | 0.317 | <0.01 | ||
| Error | 157,125.70 | 1190.35 | |||||
| Number of leaf | Group | 900.16 | 450.08 | 2.39 | 0.035 | >0.05 | |
| Time | 21,076.30 | 2107.63 | 11.19 | 0.459 | <0.01 | ||
| Group × Time | 1635.04 | 81.75 | 0.434 | 0.062 | >0.05 | ||
| Error | 24,843.20 | 188.21 | |||||
| Geranium psilostemon | Length | Group | 782.59 | 391.29 | 1.60 | 0.024 | >0.05 |
| Time | 30,817.39 | 2801.58 | 11.44 | 0.496 | <0.01 | ||
| Group × Time | 5734.18 | 318.56 | 1.30 | 0.155 | >0.05 | ||
| Error | 31,336.00 | 244.81 | |||||
| Diameter | Group | 1684.48 | 842.24 | 6.57 | 0.093 | <0.01 | |
| Time | 28,480.22 | 2589.11 | 20.20 | 0.634 | <0.01 | ||
| Group × Time | 2267.79 | 125.98 | 0.98 | 0.121 | >0.05 | ||
| Error | 16,406.40 | 128.17 | |||||
| Chlorophyll | Group | 183,885.91 | 91,942.96 | 126.40 | 0.664 | <0.01 | |
| Time | 283,954.24 | 25,814.02 | 35.49 | 0.753 | <0.01 | ||
| Group × Time | 139,091.51 | 7727.31 | 10.62 | 0.599 | <0.01 | ||
| Error | 93,105.99 | 727.39 | |||||
| Number of leaf | Group | 158.48 | 79.24 | 0.49 | 0.008 | >0.05 | |
| Time | 14,721.95 | 1338.36 | 8.38 | 0.419 | <0.01 | ||
| Group × Time | 3851.51 | 213.97 | 1.34 | 0.159 | >0.05 | ||
| Error | 20,444.40 | 159.72 |
| Alchemilla mollis | ||||
| TA | TB | TC | ||
| pH | Initial | 7.00 ± 0.70 a | 6.59 ± 0.30 ab | 5.87 ± 0.12 b |
| Final | 6.79 ± 0.24 a | 6.53 ± 0.08 ab | 6.11 ± 0.19 b | |
| N% | Initial | 0.05 ± 0.01 | 0.04 ± 0.02 | 0.05 ± 0.02 |
| Final | 0.03 ± 0.03 | 0.09 ± 0.07 | 0.06 ± 0.03 | |
| OM% | Initial | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.90 ± 0.20 |
| Final | 0.60 ± 0.70 | 1.83 ± 1.53 | 1.13 ± 0.50 | |
| OC% | Initial | 0.58 ± 0.12 | 0.50 ± 0.17 | 0.59 ± 0.21 |
| Final | 0.36 ± 0.39 | 1.05 ± 0.86 | 0.65 ± 0.33 | |
| P | Initial | 9.00 ± 7.00 | 37.0 ± 32.9 | 20.7 ± 20.1 |
| Final | 22.36 ± 18.2 | 76.19 ± 61.3 | 67.41 ± 47.8 | |
| EC | Initial | 0.06 ± 0.02 | 0.12 ± 0.09 | 0.08 ± 0.04 |
| Final | 0.06 ± 0.02 | 0.05 ± 0.03 | 0.06 ± 0.04 | |
| Geranium psilostemon | ||||
| TA | TB | TC | ||
| pH | Initial | 7.07 ± 0.61 a | 6.66 ± 0.40 ab | 5.84 ± 0.15 b |
| Final | 6.80 ± 0.25 a | 6.50 ± 0.06 ab | 6.18 ± 0.27 b | |
| N% | Initial | 0.04 ± 0.02 | 0.05 ± 0.02 | 0.04 ± 0.03 |
| Final | 0.06 ± 0.04 | 0.11 ± 0.05 | 0.08 ± 0.02 | |
| OM% | Initial | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.73 ± 0.46 |
| Final | 1.10 ± 0.89 | 2.23 ± 0.86 | 1.53 ± 0.38 | |
| OC% | Initial | 0.50 ± 0.24 | 0.54 ± 0.18 | 0.53 ± 0.31 |
| Final | 0.65 ± 0.51 | 1.27 ± 0.49 | 0.89 ± 0.21 | |
| P | Initial | 9.33 ± 6.66 | 36.67 ± 33.3 | 22.33 ± 18.1 |
| Final | 23.31 ± 17.4 | 75.42 ± 62.5 | 67.87 ± 47.1 | |
| EC | Initial | 0.08 ± 0.06 | 0.06 ± 0.05 | 0.02 ± 0.01 |
| Final | 0.07 ± 0.03 | 0.05 ± 0.02 | 0.11 ± 0.08 | |
| Groups | φ+ (i) | φ− (i) | φ (i) | Ranks | |
|---|---|---|---|---|---|
| Alchemilla mollis | TC | 0.5273 | 0.1005 | 0.4268 | 1 |
| TB | 0.3383 | 0.1579 | 0.1805 | 2 | |
| TA | 0.0452 | 0.0452 | 0.0452 | 3 | |
| Gerranium psilestemon | TC | 0.5511 | 0.0963 | 0.4548 | 1 |
| TB | 0.2677 | 0.2343 | 0.0335 | 2 | |
| TA | 0.0910 | 0.5792 | −0.4882 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oğuztürk, T.; Acar, C. Effects of Soil–Sand Mixtures on Alchemilla mollis and Geranium psilostemon: A Multi-Criteria Performance Analysis Under Low-Altitude Conditions Using PROMETHEE. Horticulturae 2025, 11, 653. https://doi.org/10.3390/horticulturae11060653
Oğuztürk T, Acar C. Effects of Soil–Sand Mixtures on Alchemilla mollis and Geranium psilostemon: A Multi-Criteria Performance Analysis Under Low-Altitude Conditions Using PROMETHEE. Horticulturae. 2025; 11(6):653. https://doi.org/10.3390/horticulturae11060653
Chicago/Turabian StyleOğuztürk, Türker, and Cengiz Acar. 2025. "Effects of Soil–Sand Mixtures on Alchemilla mollis and Geranium psilostemon: A Multi-Criteria Performance Analysis Under Low-Altitude Conditions Using PROMETHEE" Horticulturae 11, no. 6: 653. https://doi.org/10.3390/horticulturae11060653
APA StyleOğuztürk, T., & Acar, C. (2025). Effects of Soil–Sand Mixtures on Alchemilla mollis and Geranium psilostemon: A Multi-Criteria Performance Analysis Under Low-Altitude Conditions Using PROMETHEE. Horticulturae, 11(6), 653. https://doi.org/10.3390/horticulturae11060653








