Strawberry Fruit Deformity Detection and Symmetry Quantification Using Deep Learning and Geometric Feature Analysis
Abstract
1. Introduction
2. Materials and Methods
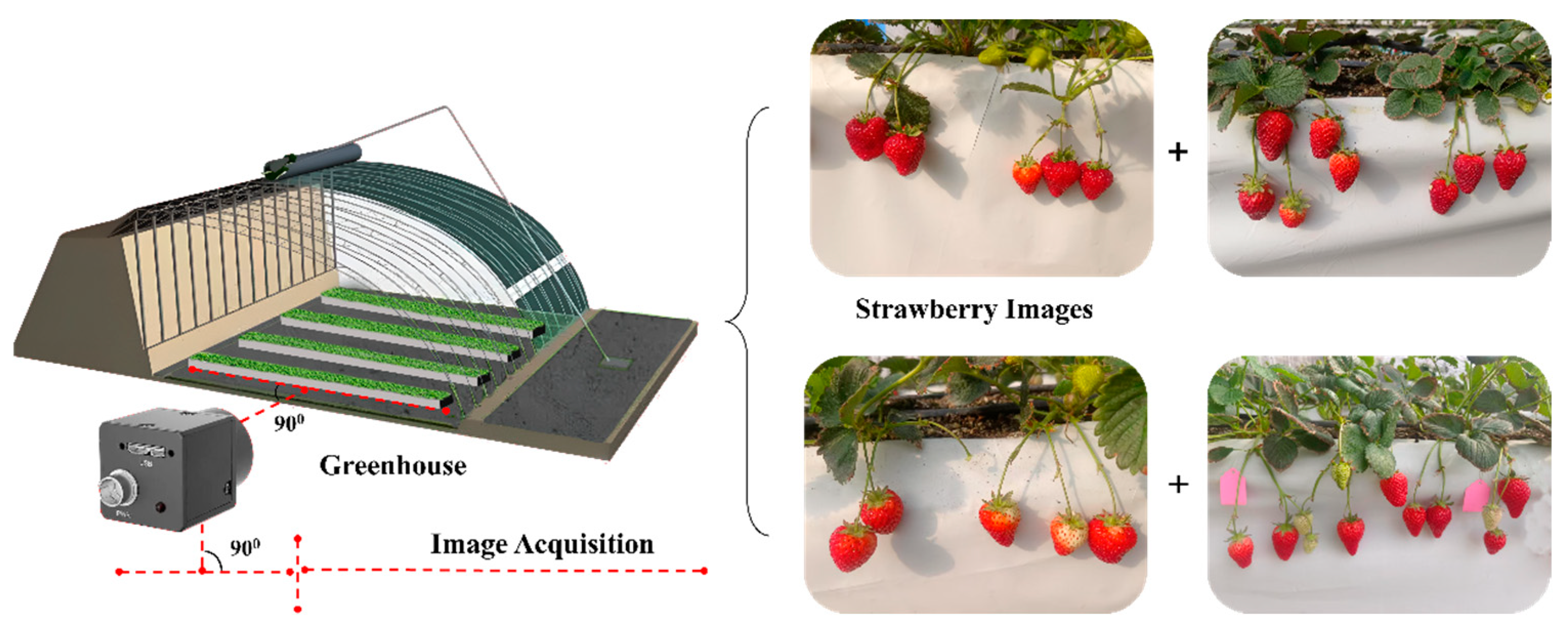
2.1. Image Acquisition and Data Processing
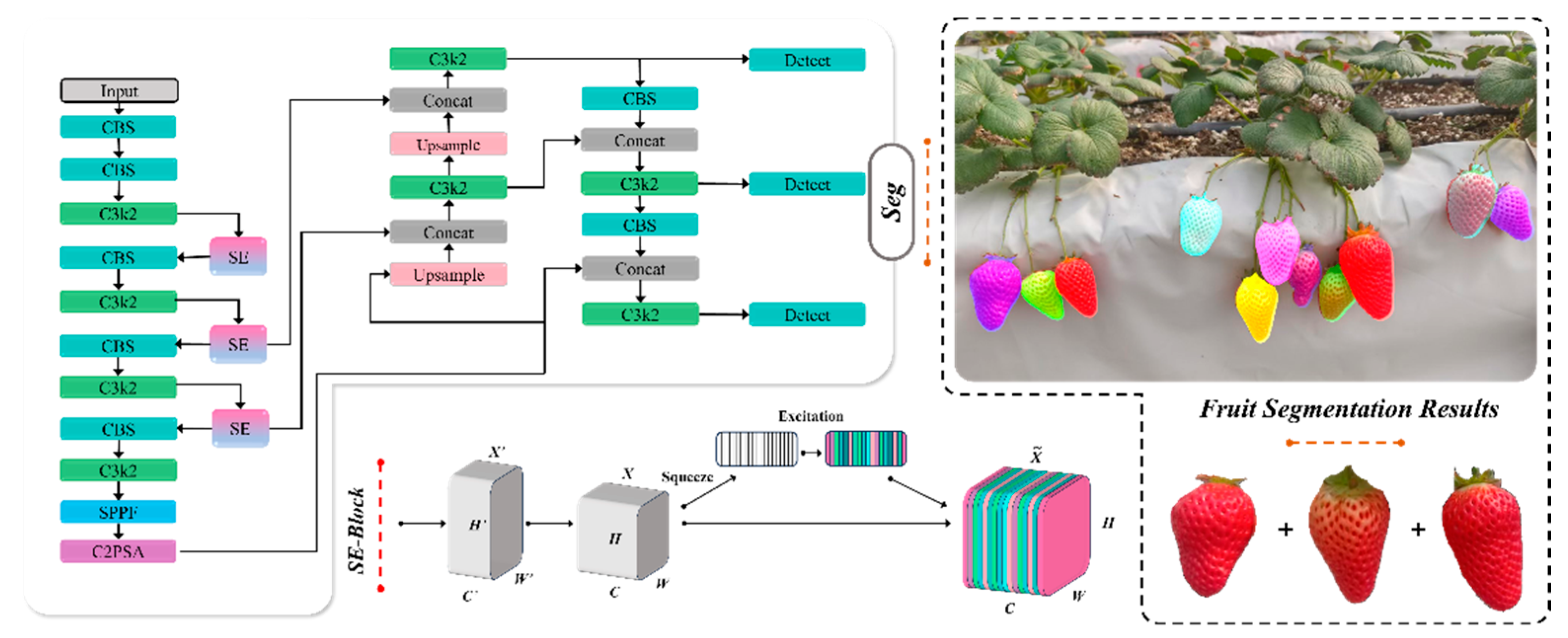
2.2. Strawberry Segmentation Model Based on Improved YOLOv11
2.2.1. Model Selection
2.2.2. Improved YOLOv11-Based Strawberry Segmentation Model
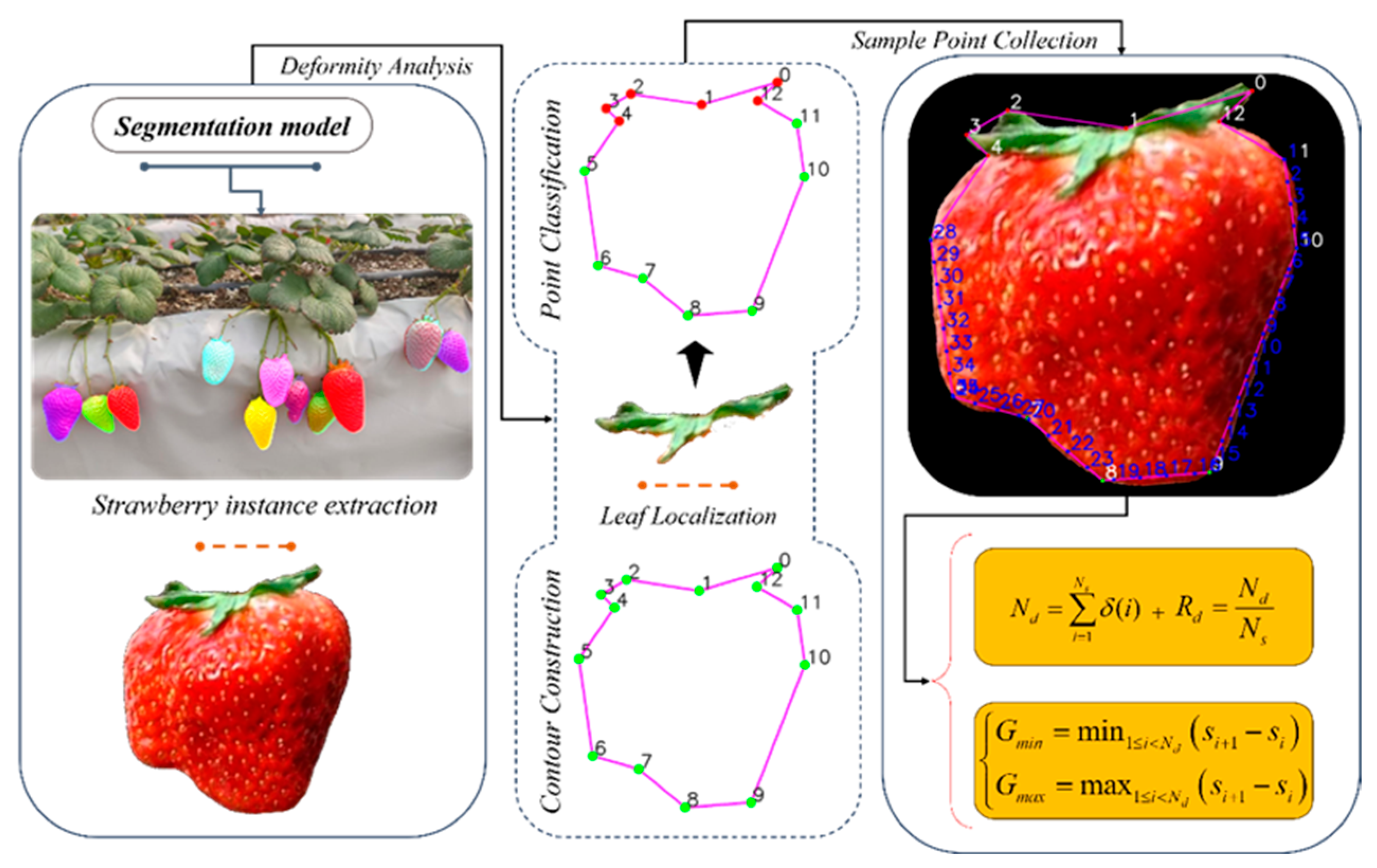
2.3. Strawberry Deformity Detection and Symmetry Quantification Analysis
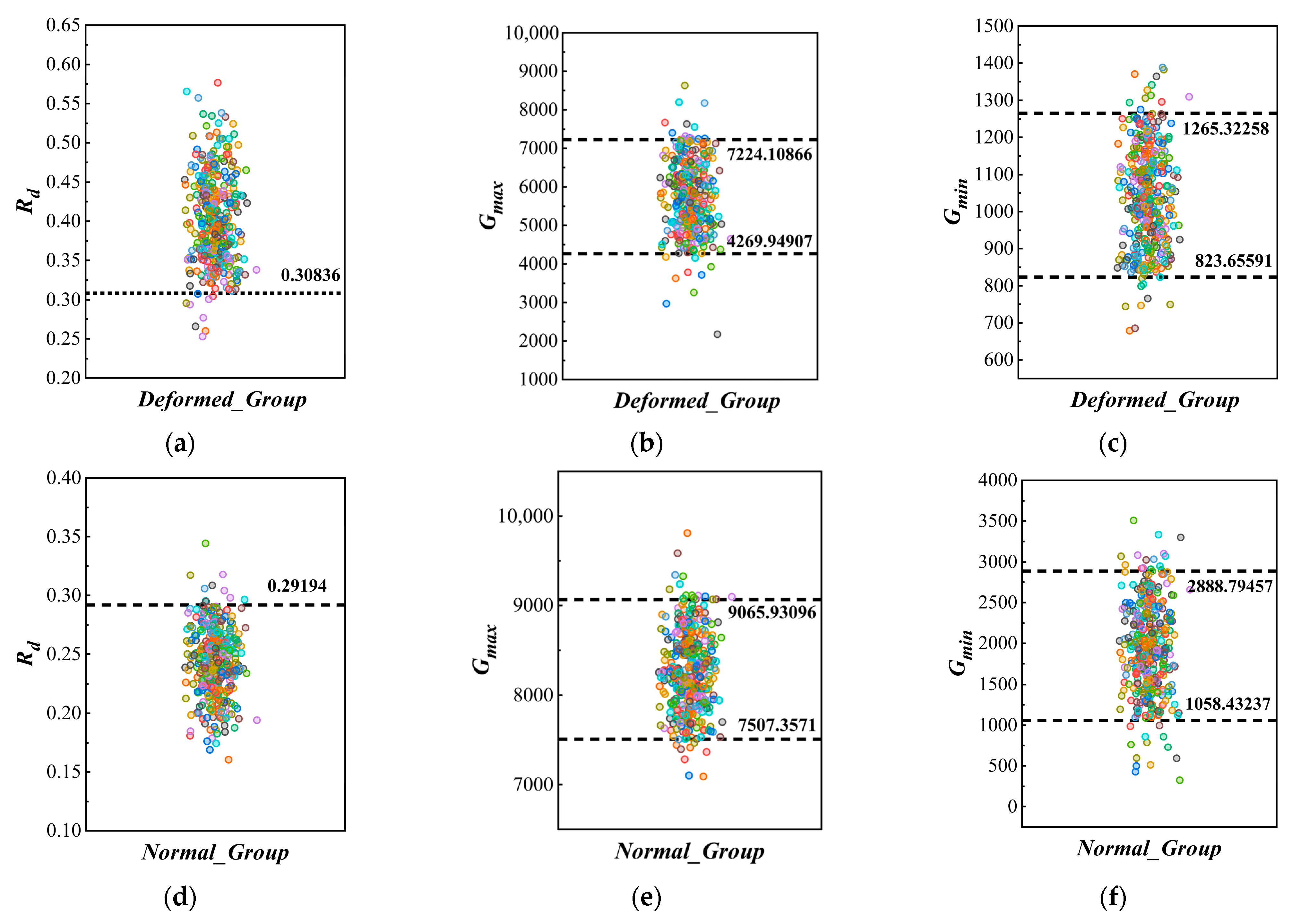
2.3.1. Classification and Quantitative Analysis of Deformed Strawberries
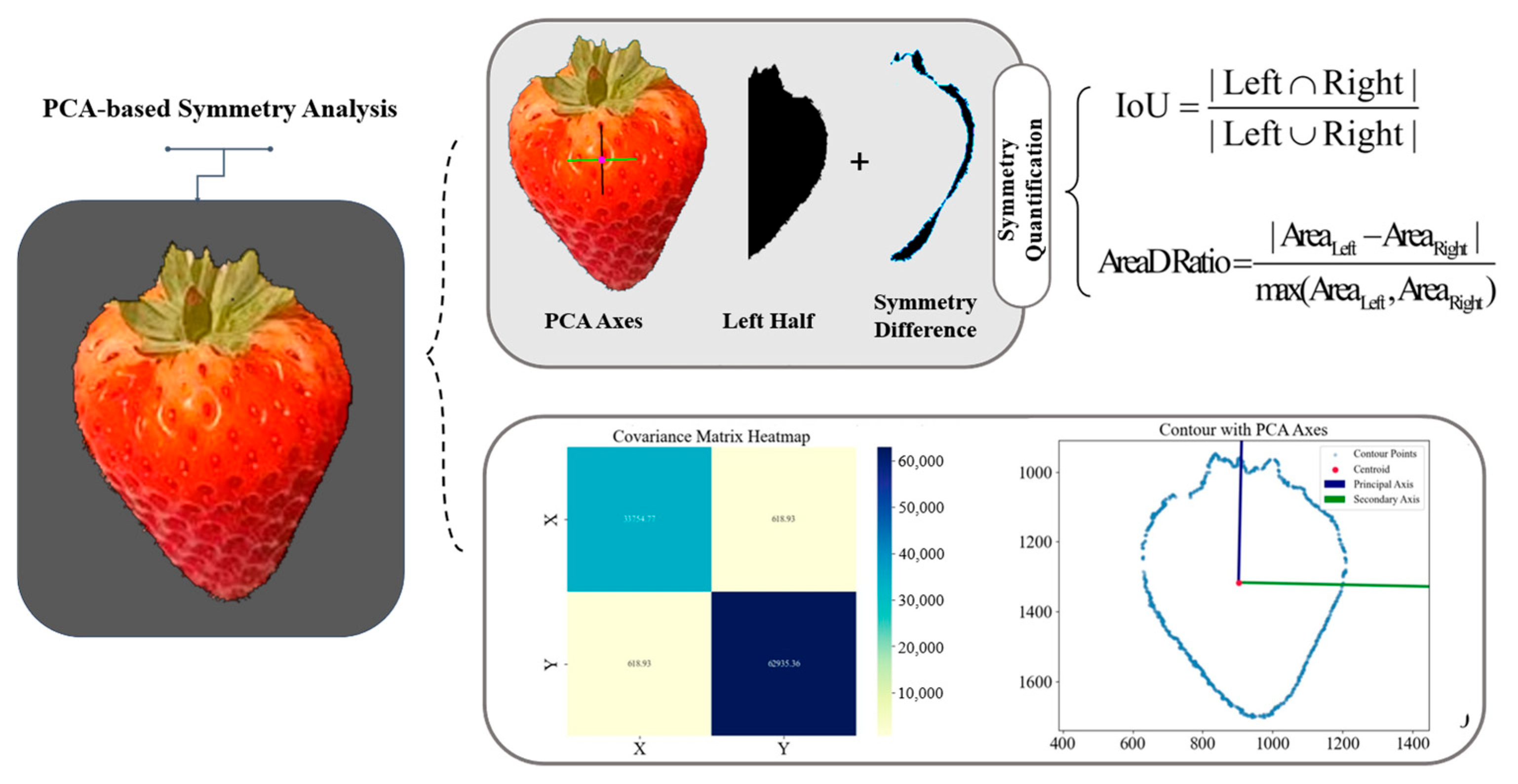
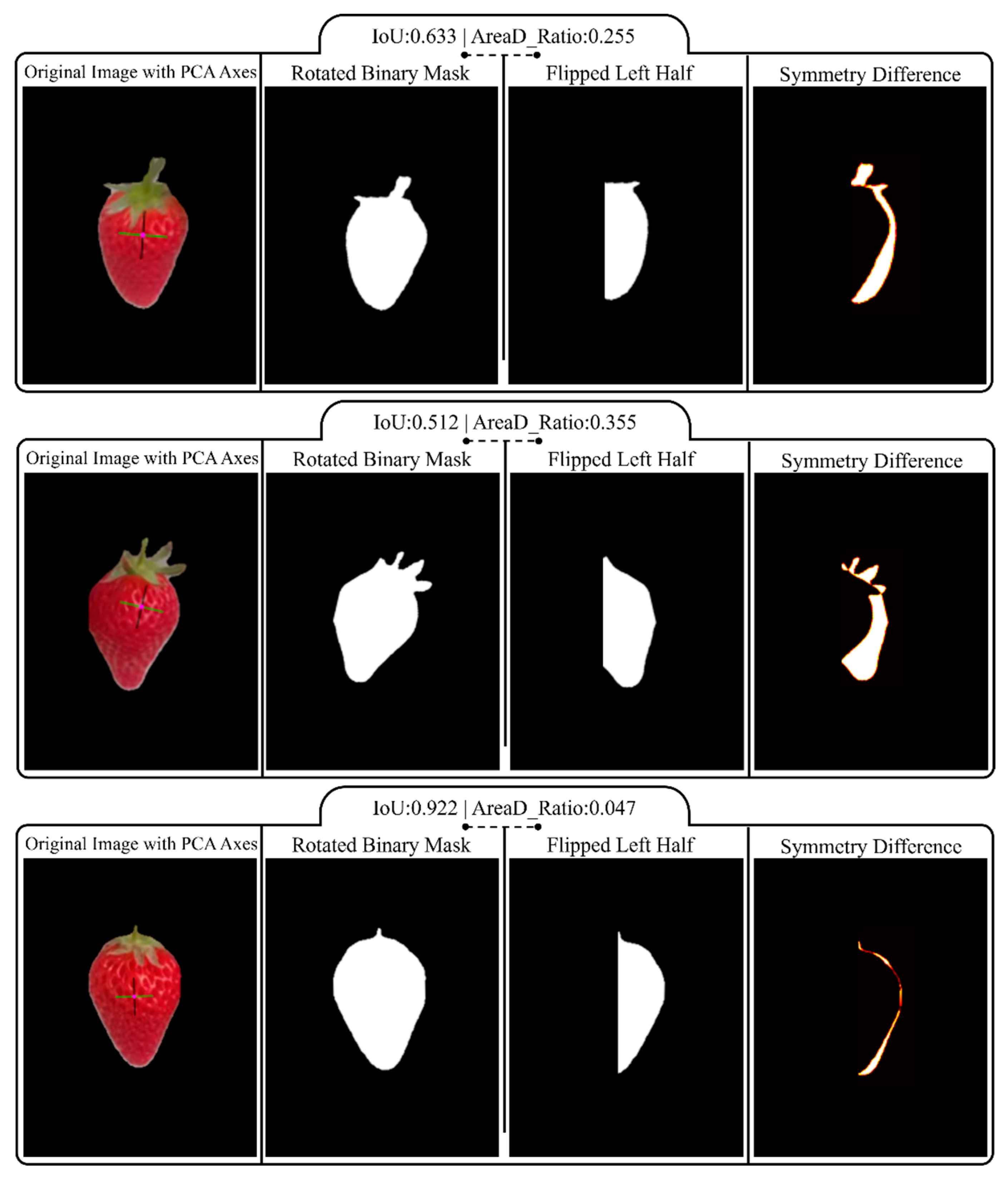
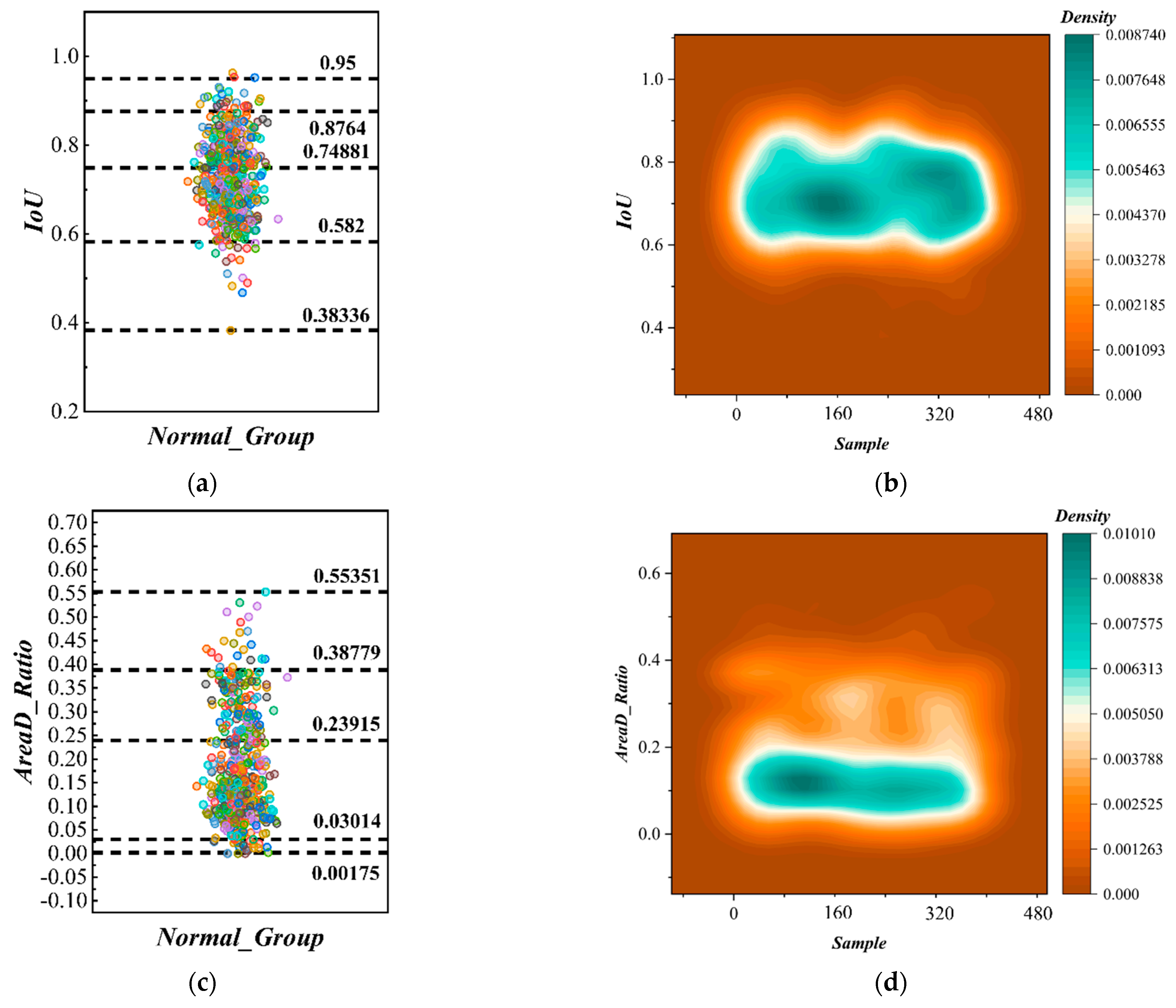
2.3.2. Symmetry Analysis of Normal Strawberries
3. Results and Discussion
3.1. Strawberry Segmentation Experiments and Result Analysis
3.2. Experimental Validation of Strawberry Deformity Detection and Symmetry Quantification
3.2.1. Deformity Classification and Quantification Experiments for Strawberries
3.2.2. Symmetry Quantification Experiments for Normal Strawberries
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, B.; Feng, M.; Chitrakar, B.; Cheng, J.; Wei, B.; Wang, B.; Ma, H. Multi-frequency power thermosonication treatments of clear strawberry juice: Impact on color, bioactive compounds, flavor volatiles, microbial and polyphenol oxidase inactivation. Innov. Food Sci. Emerg. Technol. 2023, 84, 103295. [Google Scholar] [CrossRef]
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. Technol. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Yuan, L.M.; Cai, J.R.; Sun, L.; Han, E.; Ernest, T. Nondestructive measurement of soluble solids content in apples by a portable fruit analyzer. Food Anal. Methods 2016, 9, 785–794. [Google Scholar] [CrossRef]
- Garcia-Palazon, A.; Suthanthangjai, W.; Kajda, P.; Zabetakis, I. The effects of high hydrostatic pressure on β-glucosidase, peroxidase and polyphenoloxidase in red raspberry (Rubus idaeus) and strawberry (Fragaria× ananassa). Food Chem. 2004, 88, 7–10. [Google Scholar] [CrossRef]
- Zheng, Z.; He, Y.; He, Y.; Zhan, J.; Shi, C.; Xu, Y.; Zhang, C. Micro-nano bubble water subsurface drip irrigation affects strawberry yield and quality by modulation of microbial communities. Agric. Water Manag. 2025, 307, 109228. [Google Scholar] [CrossRef]
- Sabbadini, S.; Capocasa, F.; Battino, M.; Mazzoni, L.; Mezzetti, B. Improved nutritional quality in fruit tree species through traditional and biotechnological approaches. Trends Food Sci. Technol. 2021, 117, 125–138. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jiyong, S.; Mahunu, G.K.; Zhai, X.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Feng, M.; Chitrakar, B.; Chen, J.; Islam, M.N.; Wei, B.; Wang, B.; Xu, B. Effect of multi-mode thermosonication on the microbial inhibition and quality retention of strawberry clear juice during storage at varied temperatures. Foods 2022, 11, 2593. [Google Scholar] [CrossRef]
- Barikloo, H.; Ahmadi, E. Shelf life extension of strawberry by temperatures conditioning, chitosan coating, modified atmosphere, and clay and silica nanocomposite packaging. Sci. Hortic. 2018, 240, 496–508. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef]
- Xu, B.; Chen, J.; Azam, S.R.; Feng, M.; Wei, B.; Yan, W.; Duan, X. Flat dual-frequency sweeping ultrasound enhances the inactivation of polyphenol oxidase in strawberry juice. J. Food Meas. Charact. 2022, 16, 762–771. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, J.; Li, Z.; Hu, D.; Sun, Y.; Jin, H.; Fang, H. Recent Advances in Light Penetration Depth for Postharvest Quality Evaluation of Fruits and Vegetables. Foods 2024, 13, 2688. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gong, M.; Li, J.; Ma, J. Strawberry Defect Identification Using Deep Learning Infrared–Visible Image Fusion. Agronomy 2023, 13, 2217. [Google Scholar] [CrossRef]
- Meng, L.; Audenaert, K.; Van Labeke, M.C.; Höfte, M. Detection of Botrytis cinerea on strawberry leaves upon mycelial infection through imaging technique. Sci. Hortic. 2024, 330, 113071. [Google Scholar] [CrossRef]
- Yang, S.; Wang, W.; Gao, S.; Deng, Z. Strawberry ripeness detection based on YOLOv8 algorithm fused with LW-Swin Transformer. Comput. Electron. Agric. 2023, 215, 108360. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Zhu, W.; Wei, X.; Sun, H. Maturity assessment of tomato fruit based on electrical impedance spectroscopy. Int. J. Agric. Biol. Eng. 2019, 12, 154–161. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Han, Q.; Zhang, Z.; Kong, D.; Zou, X. Strawberry Detection and Ripeness Classification Using YOLOv8+ Model and Image Processing Method. Agriculture 2024, 14, 751. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Zhang, Y.; Sun, C.; Jayan, H.; Majeed, U.; Zou, X. Dynamic nondestructive detection models of apple quality in critical harvest period based on near-infrared spectroscopy and intelligent algorithms. Foods 2024, 13, 1698. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, X.; Zhao, B.; Wang, H.; Ma, X. Strawsnake: A real-time strawberry instance segmentation network based on the contour learning approach. Electronics 2024, 13, 3103. [Google Scholar] [CrossRef]
- Liming, X.; Yanchao, Z. Automated strawberry grading system based on image processing. Comput. Electron. Agric. 2010, 71, S32–S39. [Google Scholar] [CrossRef]
- Jeong, H.; Moon, H.; Jeong, Y.; Kwon, H.; Kim, C.; Lee, Y.; Kim, S. Automated Technology for Strawberry Size Measurement and Weight Prediction Using AI. IEEE Access 2024, 12, 14157–14167. [Google Scholar] [CrossRef]
- Ariza, M.T.; Soria, C.; Medina, J.J.; Martínez-Ferri, E. Fruit misshapen in strawberry cultivars (Fragaria× ananassa) is related to achenes functionality. Ann. Appl. Biol. 2011, 158, 130–138. [Google Scholar] [CrossRef]
- Oo, L.M.; Aung, N.Z. A simple and efficient method for automatic strawberry shape and size estimation and classification. Biosyst. Eng. 2018, 170, 96–107. [Google Scholar] [CrossRef]
- Zhou, X.; Ampatzidis, Y.; Lee, W.S.; Zhou, C.; Agehara, S.; Schueller, J.K. Deep learning-based postharvest strawberry bruise detection under UV and incandescent light. Comput. Electron. Agric. 2022, 202, 107389. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Wu, S. Multiple disease detection method for greenhouse-cultivated strawberry based on multiscale feature fusion Faster R_CNN. Comput. Electron. Agric. 2022, 199, 107176. [Google Scholar] [CrossRef]
- Li, G.; Jiao, L.; Chen, P.; Liu, K.; Wang, R.; Dong, S.; Kang, C. Spatial convolutional self-attention-based transformer module for strawberry disease identification under complex background. Comput. Electron. Agric. 2023, 212, 108121. [Google Scholar] [CrossRef]
- Liu, J.; Guo, J.; Zhang, S. YOLOv11-HRS: An Improved Model for Strawberry Ripeness Detection. Agronomy 2025, 15, 1026. [Google Scholar] [CrossRef]
- Ye, R.; Shao, G.; Gao, Q.; Zhang, H.; Li, T. CR-YOLOv9: Improved YOLOv9 multi-stage strawberry fruit maturity detection application integrated with CRNET. Foods 2024, 13, 2571. [Google Scholar] [CrossRef]
- He, L.H.; Zhou, Y.Z.; Liu, L.; Cao, W.; Ma, J.H. Research on object detection and recognition in remote sensing images based on YOLOv11. Sci. Rep. 2025, 15, 14032. [Google Scholar] [CrossRef]
- He, L.H.; Zhou, Y.Z.; Liu, L.; Zhang, Y.Q.; Ma, J.H. Research on the directional bounding box algorithm of YOLO11 in tailings pond identification. Measurement 2025, 253, 117674. [Google Scholar] [CrossRef]




| Model | Precision/% | Recall/% | AP50/% | F1 |
|---|---|---|---|---|
| YOLOv9e-seg | 88.50 | 85.15 | 91.30 | 86.79 |
| YOLOv8x-seg | 88.81 | 83.62 | 90.20 | 86.31 |
| YOLOv10x-seg | 89.56 | 85.14 | 91.15 | 86.98 |
| YOLOv11-seg | 90.11 | 86.21 | 91.24 | 87.16 |
| Improved YOLOv11 | 91.11 | 87.46 | 92.90 | 88.45 |
| Groups | Nd | Rd | Gmin | Gmax |
|---|---|---|---|---|
| Deformed_Group1 | 13.4894 | 0.3966 | 1023.7706 | 5726.1149 |
| Deformed_Group2 | 13.5109 | 0.3932 | 1019.6861 | 5752.0336 |
| Deformed_Group3 | 13.9474 | 0.4058 | 1039.6707 | 5815.4124 |
| Deformed_Group4 | 13.7895 | 0.3973 | 1050.3308 | 5775.0839 |
| Normal_Group1 | 6.7292 | 0.2446 | 1913.9930 | 8293.9072 |
| Normal_Group2 | 6.8229 | 0.2479 | 1828.5643 | 8275.3413 |
| Normal_Group3 | 6.7396 | 0.2452 | 1905.2040 | 8274.5819 |
| Normal_Group4 | 6.8000 | 0.2490 | 1956.4432 | 8229.4651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wang, Y.; Yan, H.; Yin, Y.; Wu, C. Strawberry Fruit Deformity Detection and Symmetry Quantification Using Deep Learning and Geometric Feature Analysis. Horticulturae 2025, 11, 652. https://doi.org/10.3390/horticulturae11060652
Jiang L, Wang Y, Yan H, Yin Y, Wu C. Strawberry Fruit Deformity Detection and Symmetry Quantification Using Deep Learning and Geometric Feature Analysis. Horticulturae. 2025; 11(6):652. https://doi.org/10.3390/horticulturae11060652
Chicago/Turabian StyleJiang, Lili, Yunfei Wang, Haohao Yan, Yingzi Yin, and Chong Wu. 2025. "Strawberry Fruit Deformity Detection and Symmetry Quantification Using Deep Learning and Geometric Feature Analysis" Horticulturae 11, no. 6: 652. https://doi.org/10.3390/horticulturae11060652
APA StyleJiang, L., Wang, Y., Yan, H., Yin, Y., & Wu, C. (2025). Strawberry Fruit Deformity Detection and Symmetry Quantification Using Deep Learning and Geometric Feature Analysis. Horticulturae, 11(6), 652. https://doi.org/10.3390/horticulturae11060652






